The Eh-pH Diagram and Its Advances
Abstract
:1. Introduction
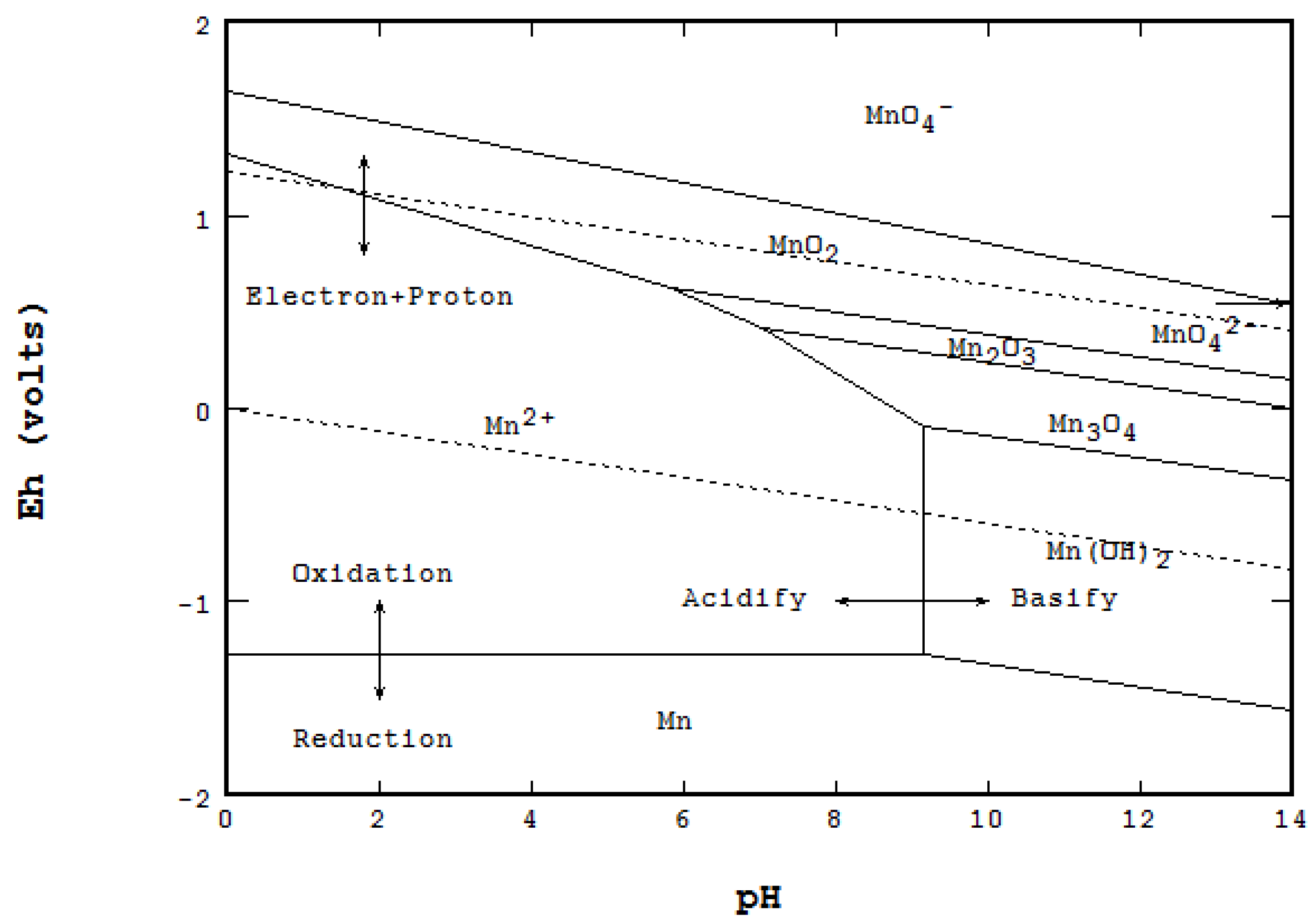
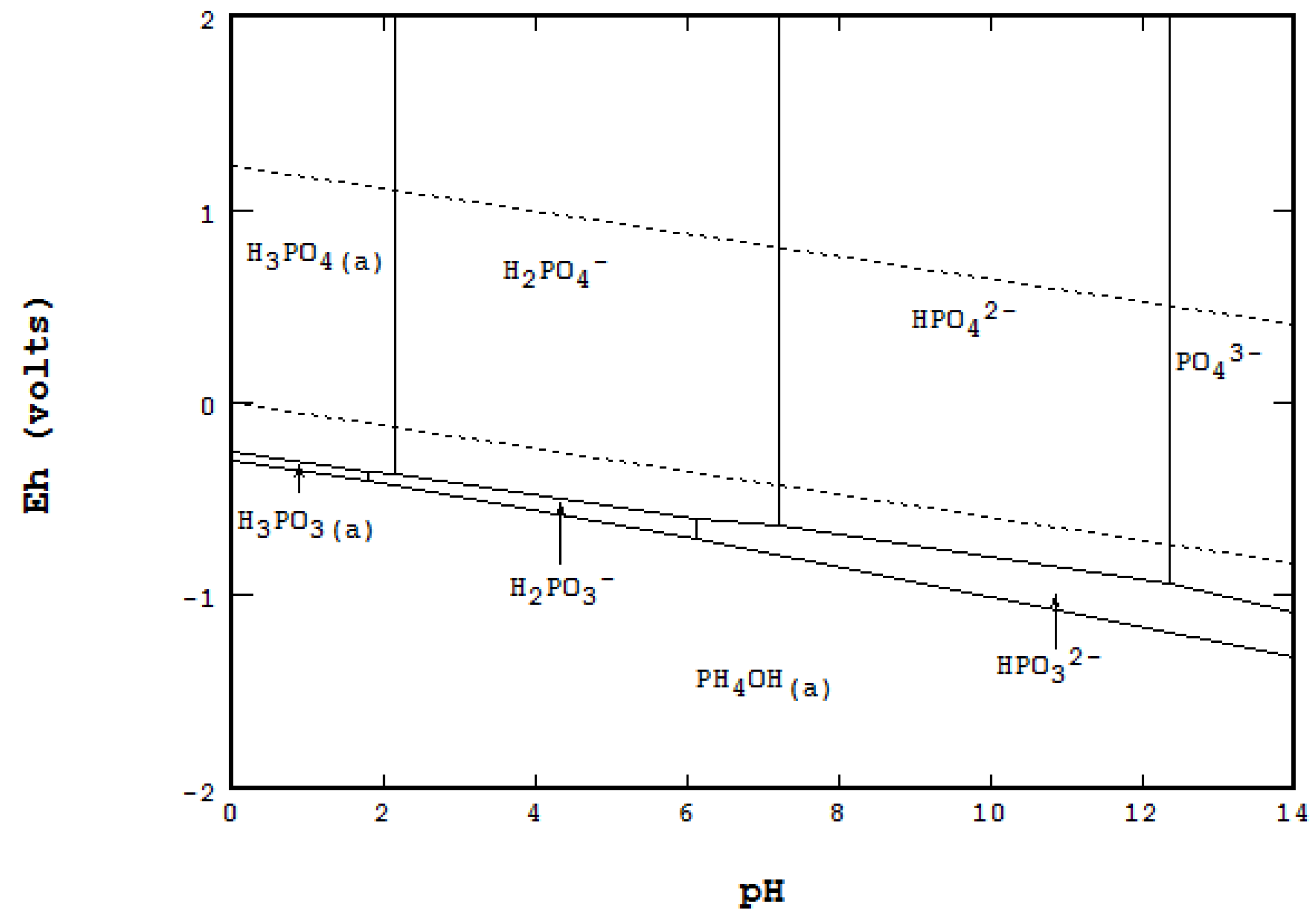
Scope of the Paper
- Examples of applications: geochemical formation, corrosion and passivation, leaching and metal recovery, water treatment precipitation and adsorption.
- Development of equilibrium line and mass balance point methods to handle ligand component(s): the theory, illustration and result comparison are presented; both methods satisfy the Gibbs phase rule derived for the Eh-pH diagram.
- Examples by merging two or more diagrams for better illustration of the overall reactions involved in a process.
- Demonstrations using a third party program to produce 3D diagrams with the addition of a third axis. The axis can represent the solubility of stable solids, ligand concentration or temperature. Two 3D wireframe volume plots of the Uranium-carbonate system based on a classic Garrels and Christ [2] work were used to verify the Eh-pH calculation and the presentation from ParaView.
- Comparison among existent computer programs listed from the literature that directly or indirectly construct an Eh-pH diagram.
- Effects from temperature, pressure, ionic strength and surface complexation for aqueous chemistry.
- The algorithm and flow sheet to construct the diagram used by the author: they are available and referenced elsewhere; no source codes of the programs are presented.
- Comparison or comments on third party 3D programs used by the author.
2. Crucial Developments of the Eh-pH Diagram
2.1. Development of the Equilibrium Line Method
2.2. Development of the Mass Balance Point Method
3. Applications for the Diagrams
3.1. Geochemical Formation
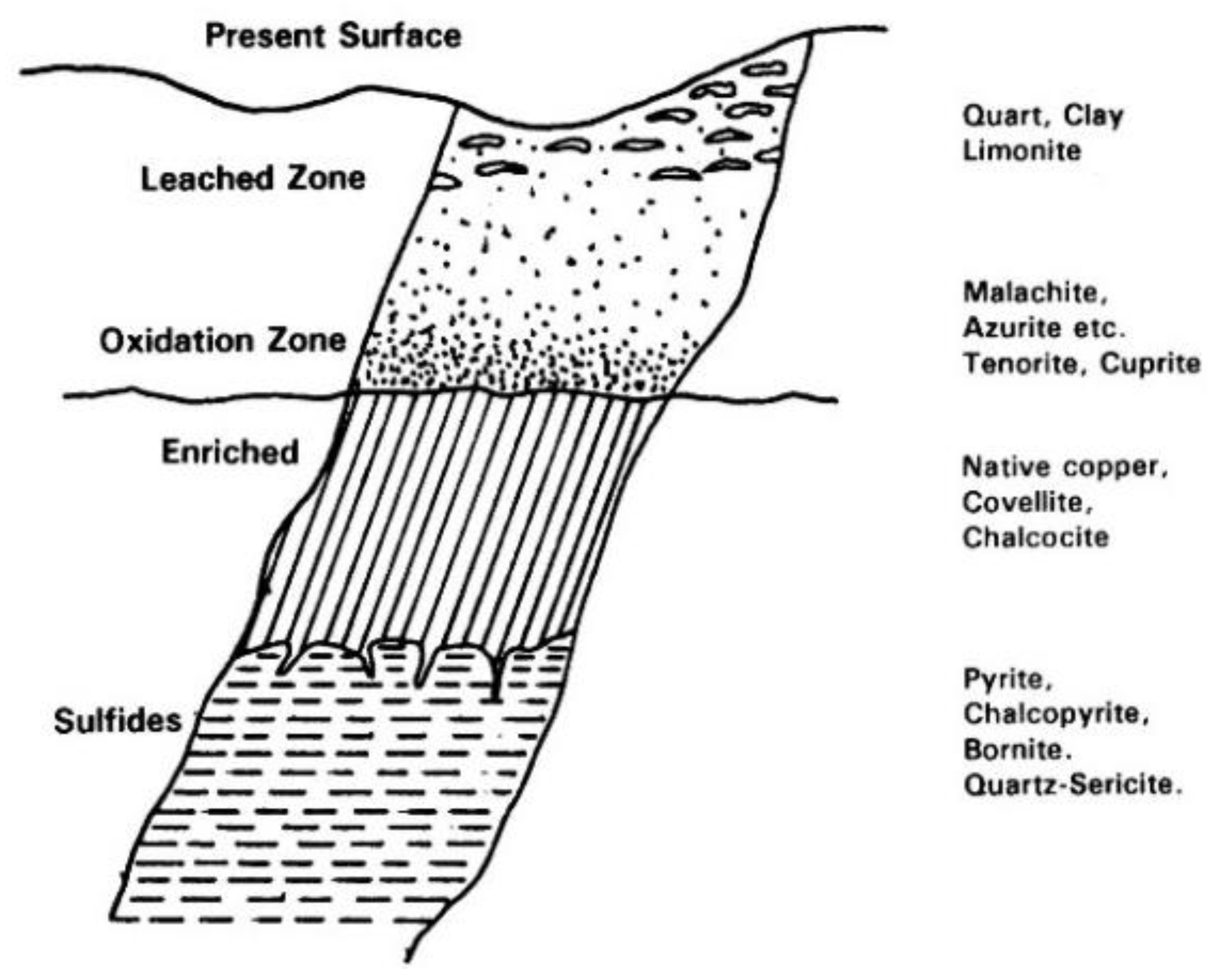
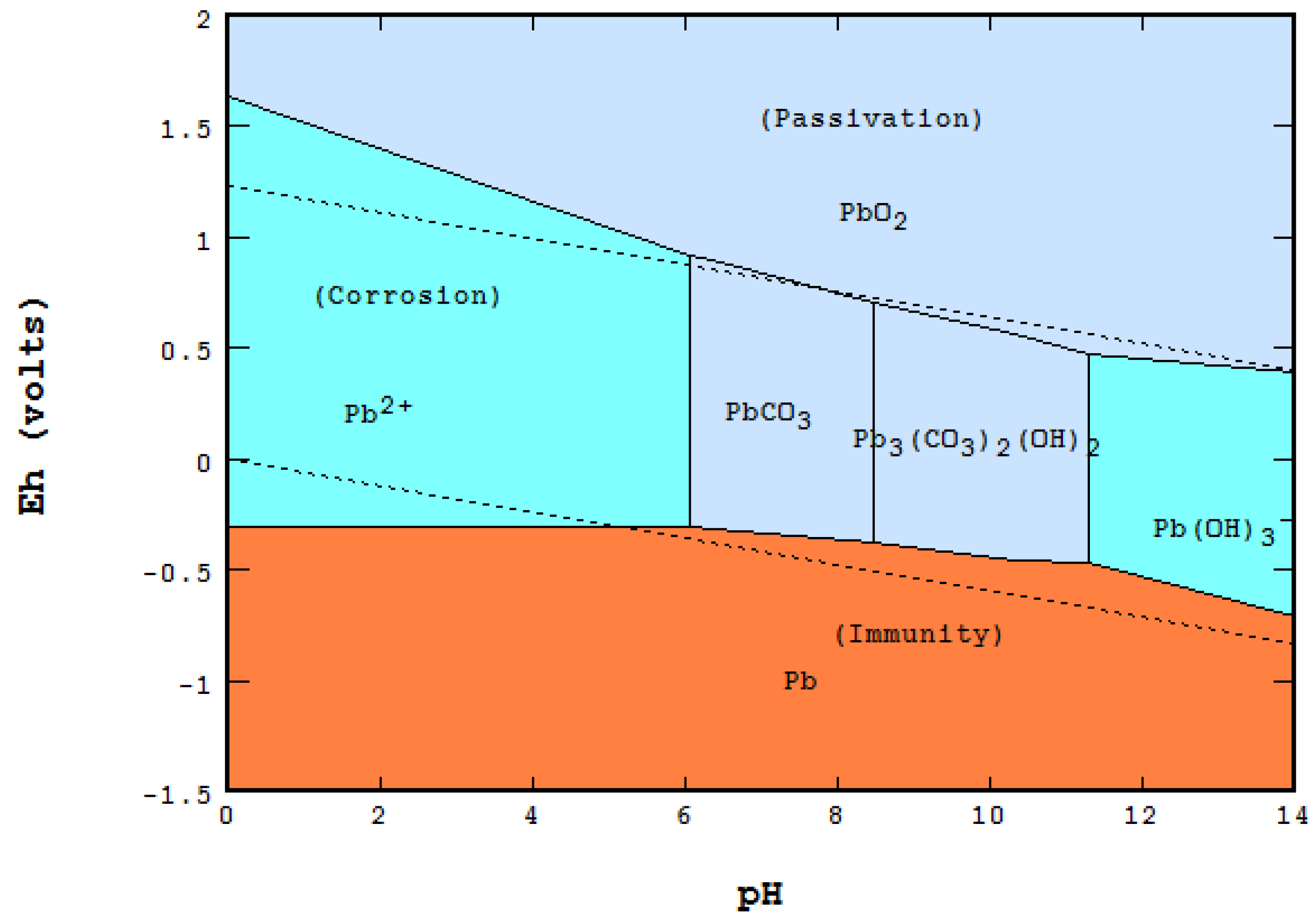
3.2. Corrosion and Passivation
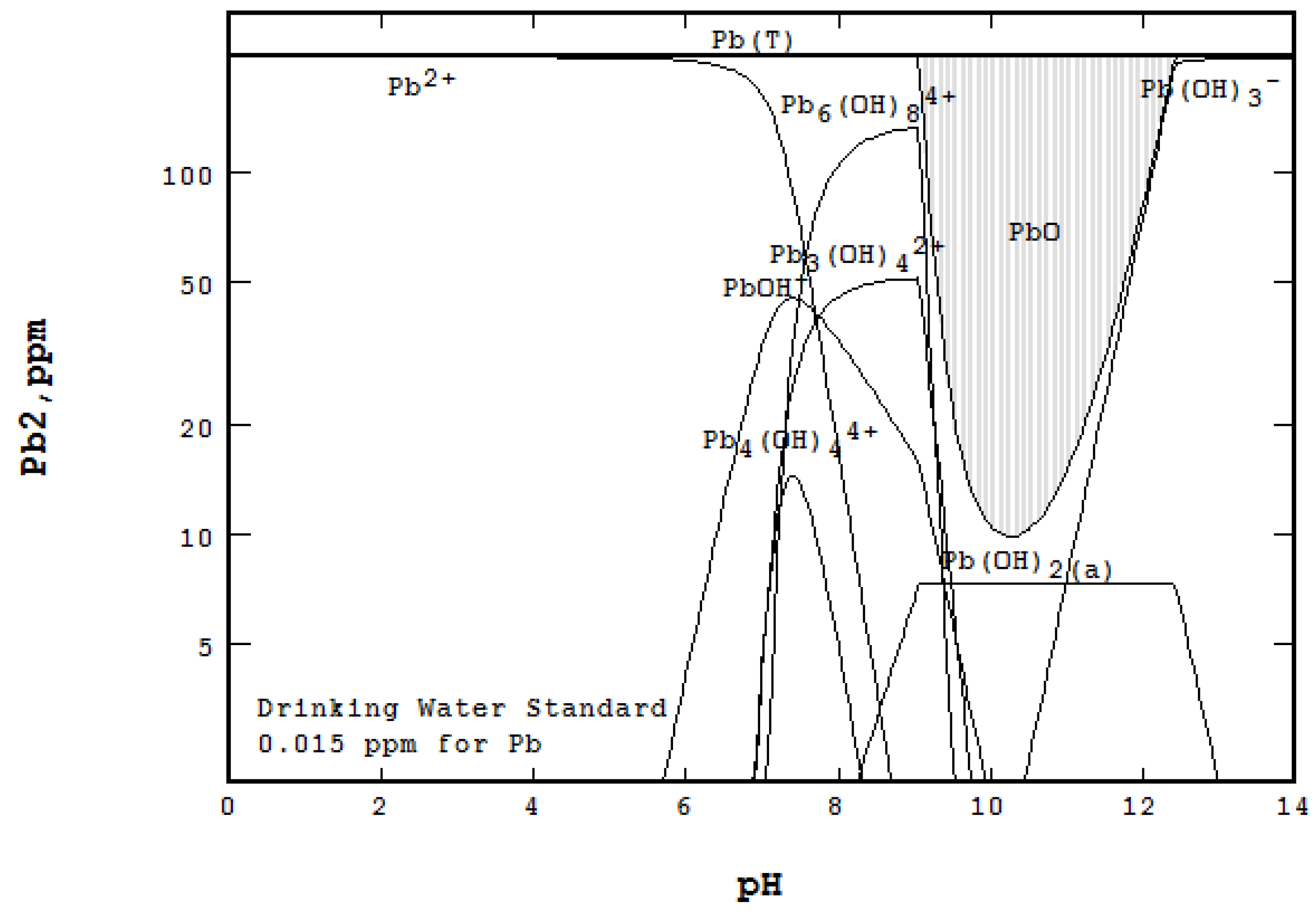
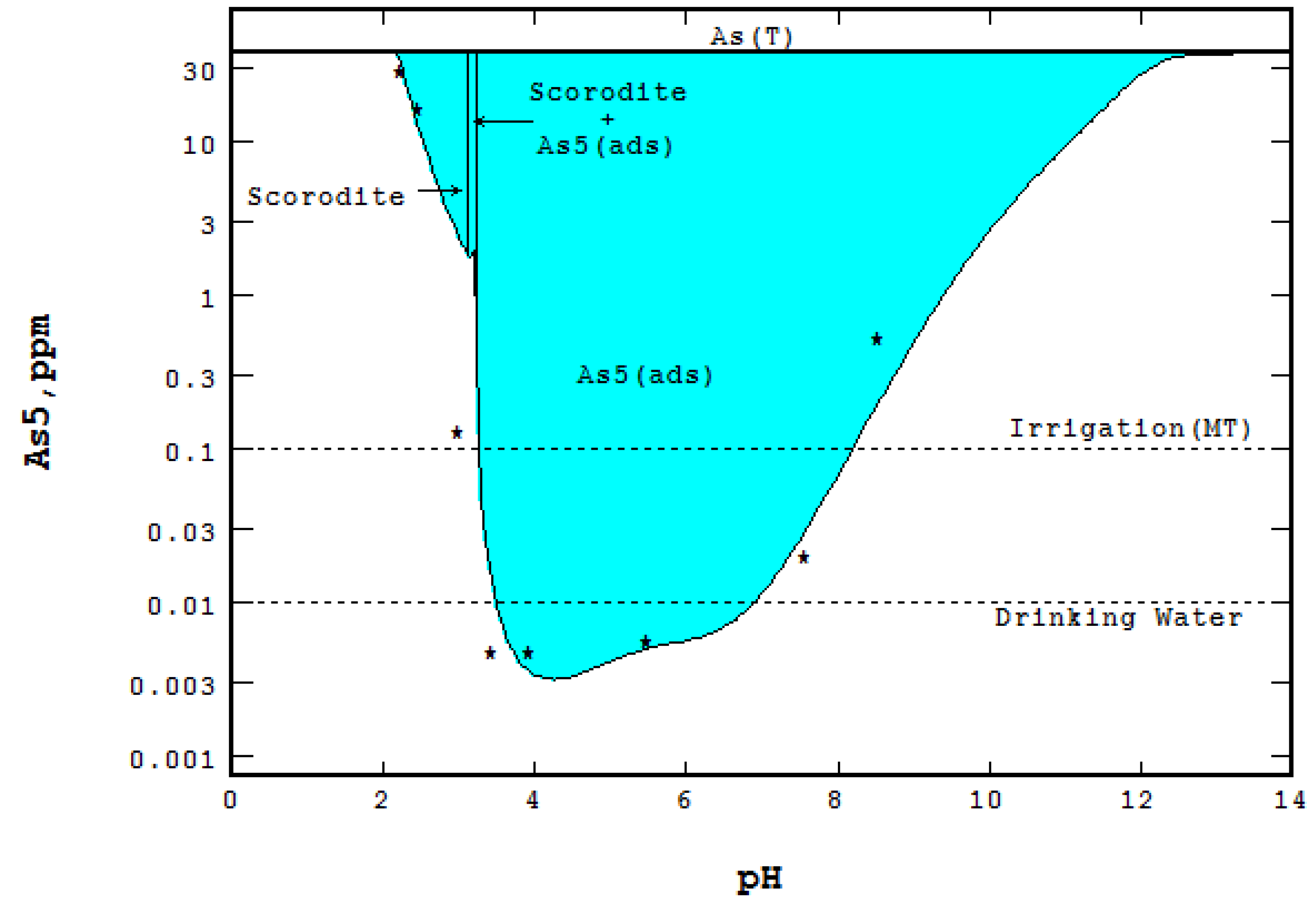
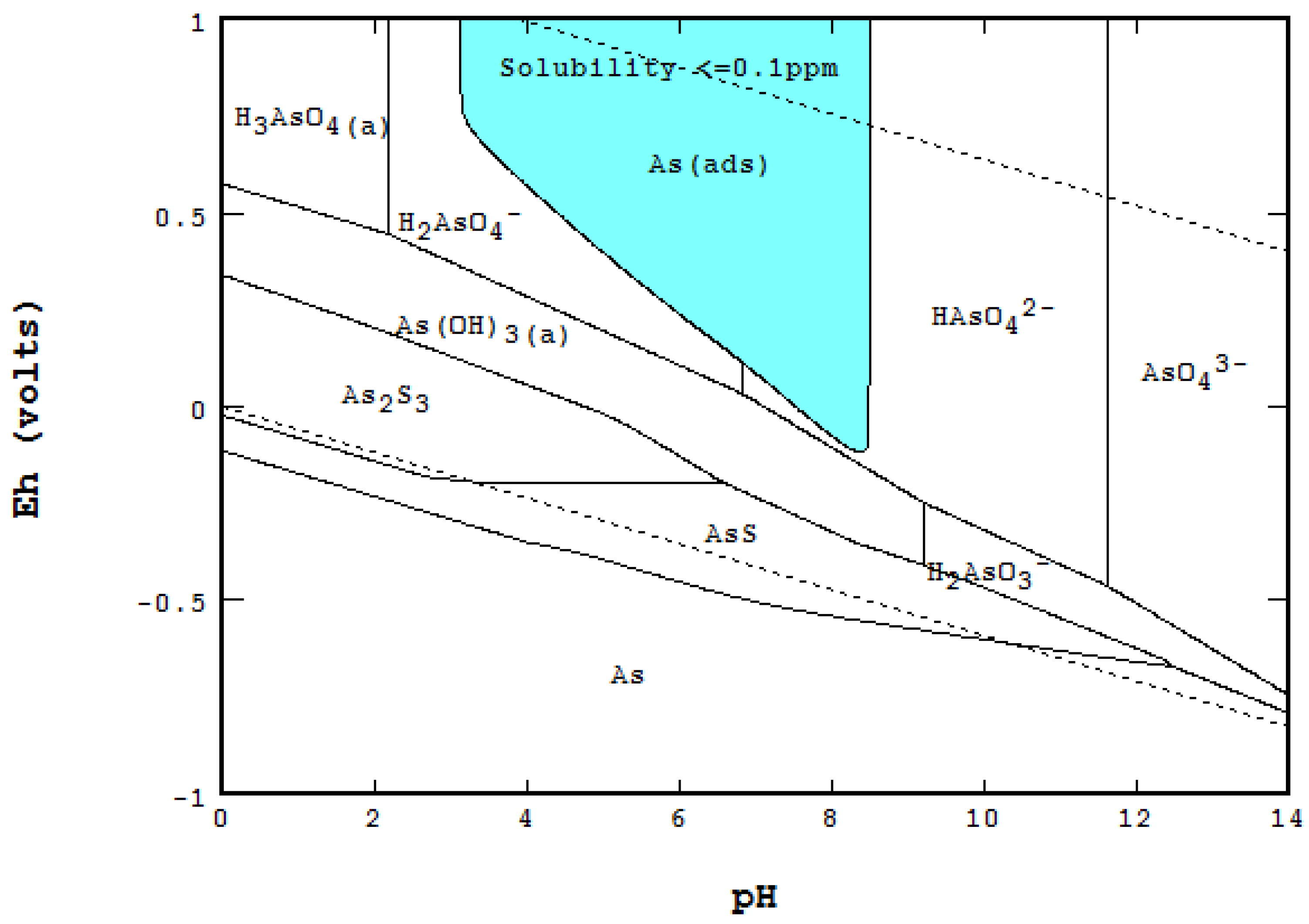
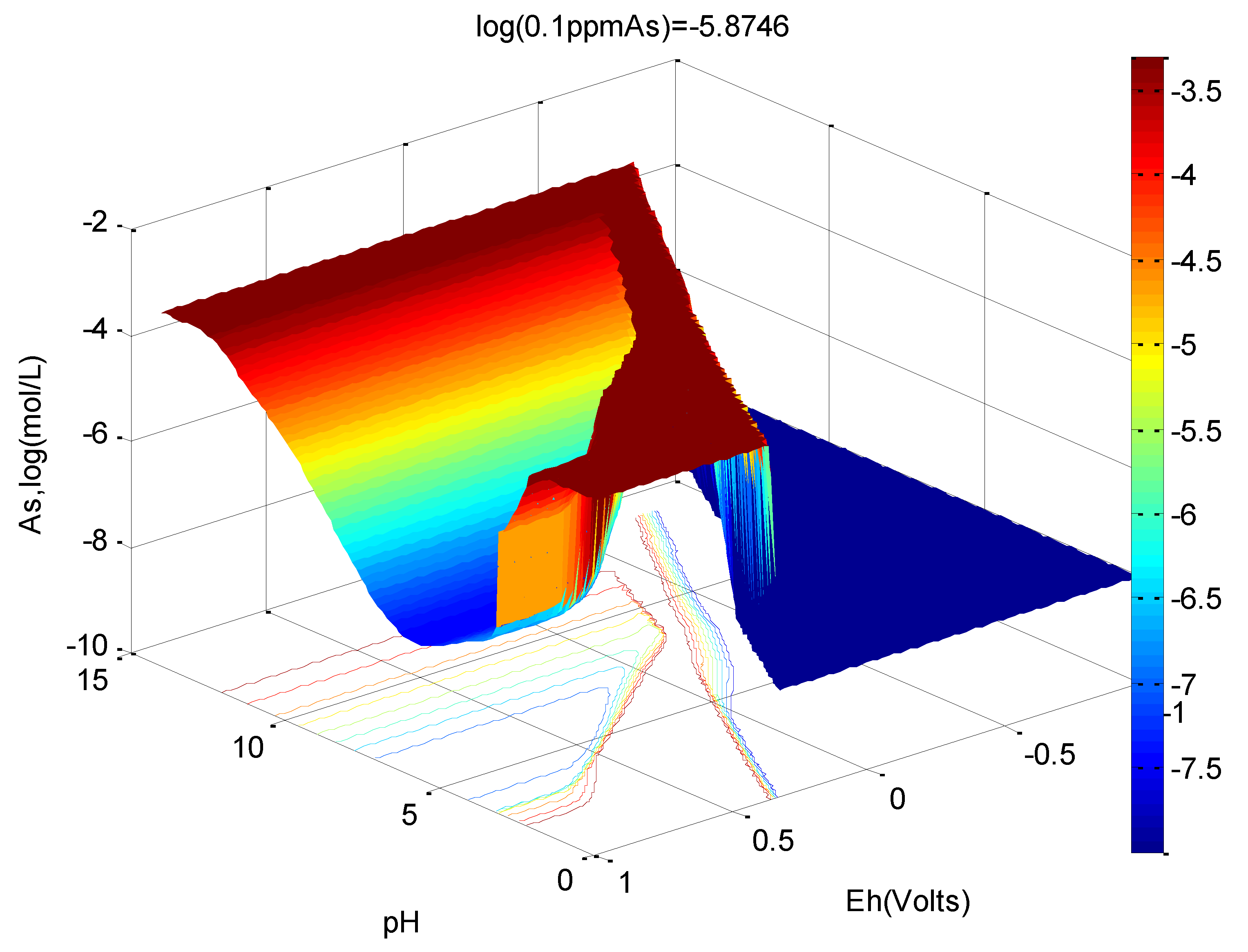
3.3. Water Treatment and Adsorption
- Type 2 site density for ferrihydrite was changed from 0.2 to 0.3 mole As/mole Fe due to co-precipitation,
- The logK1int for adsorbed species ≡FeH2AsO4 was changed from 29.31 to 31.67,
- The adsorbed species ≡FeAsO42− and its logK3int = 21.404 were added and
- Solid scorodite (FeAsO4:2H2O) and its ΔG025C = −297.5 kcal/mole were included with the LLnL dbase.
3.4. Hydrometallurgical Leaching and Metal Recovery
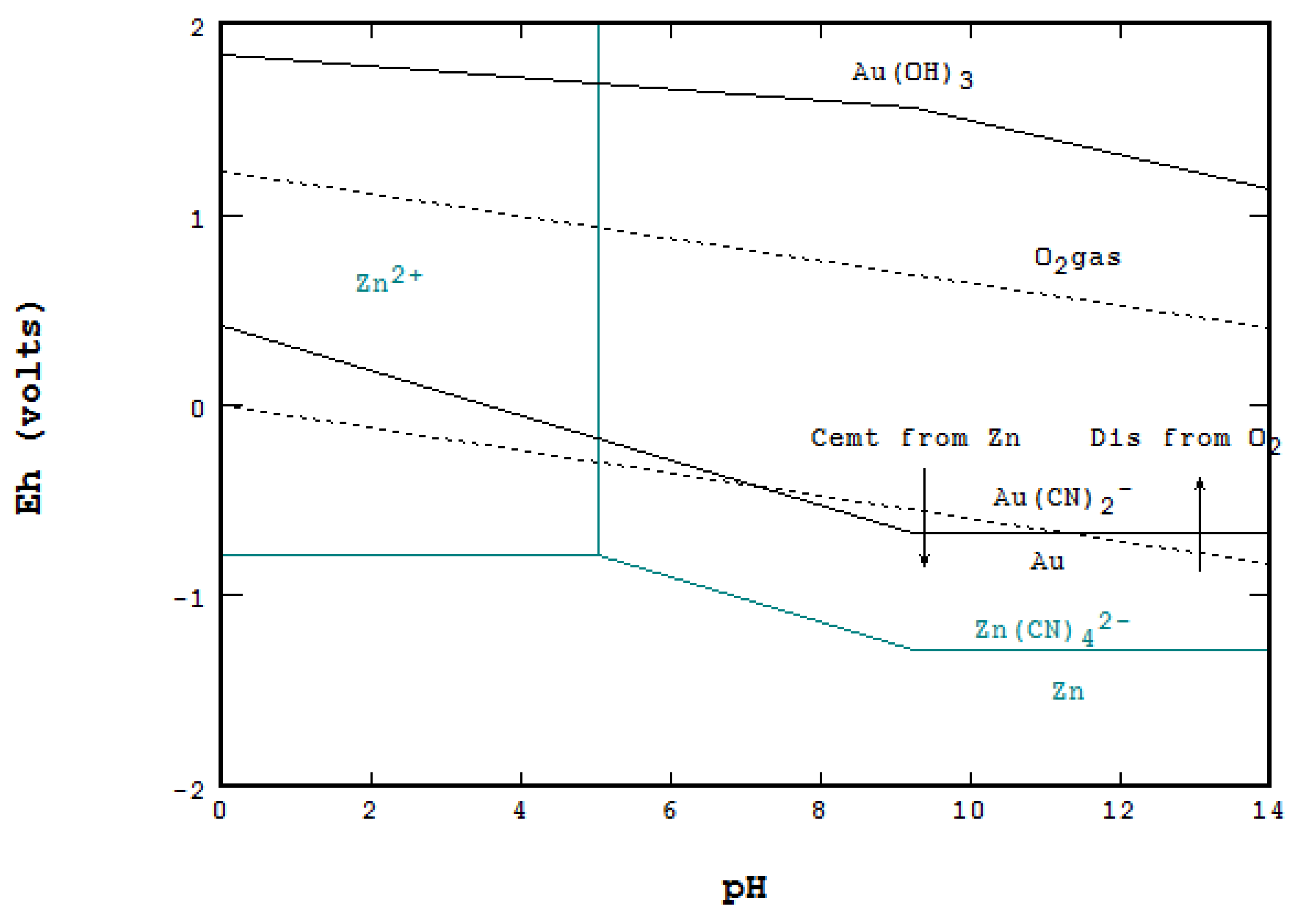
- Cyanidation of Au and cementation with Zn Metal,
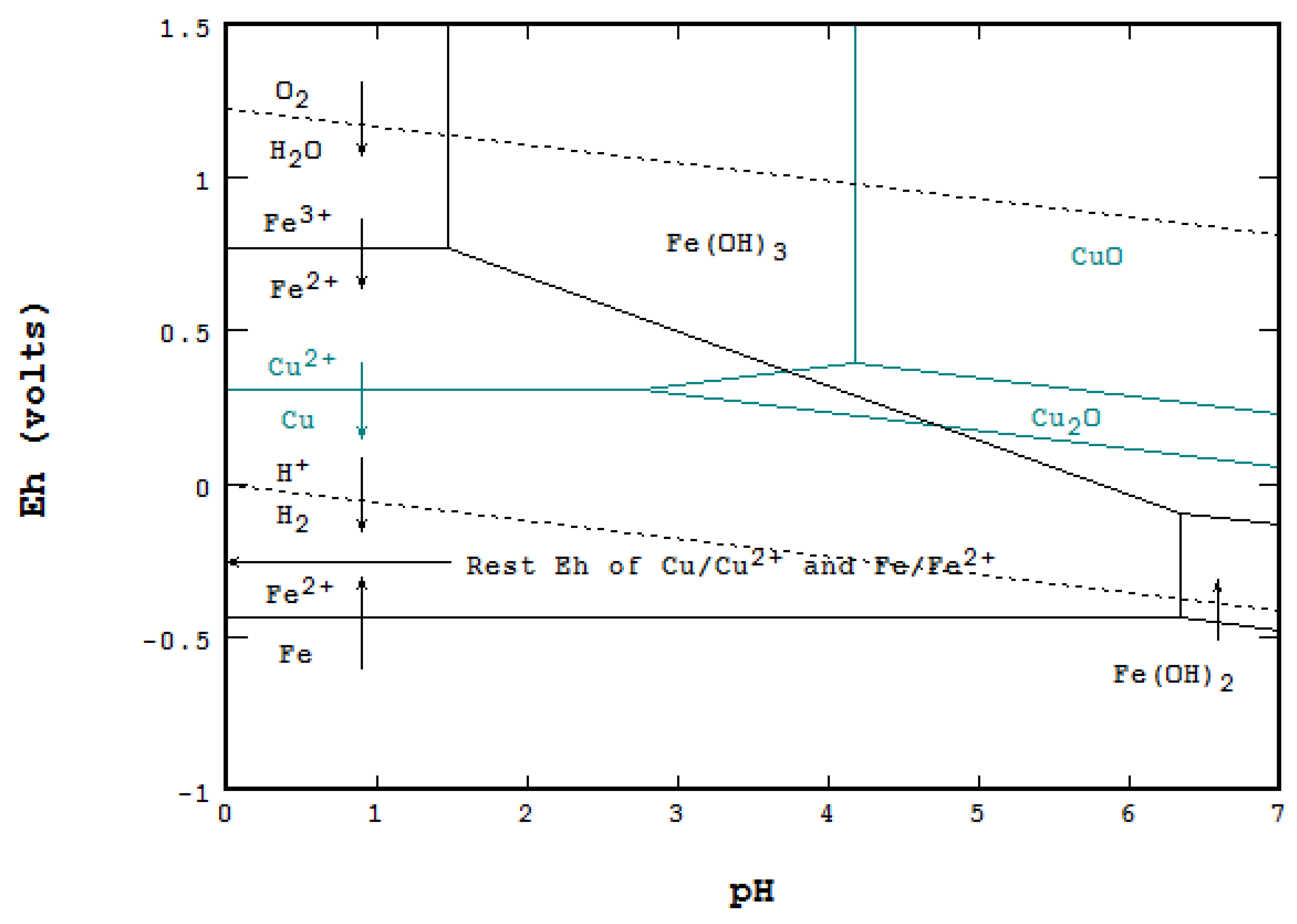
- Cementation of copper with elemental Fe, and
- Galvanic conversion of chalcopyrite with Cu metal with two construction methods for Eh-pH diagrams to handle ligand components.
4. Descriptions and Comparison between These Two Crucial Methods
4.1. Equilibrium Equations for Eh-pH Diagrams
4.2. Line Method Using Equilibrium Concentration [5]
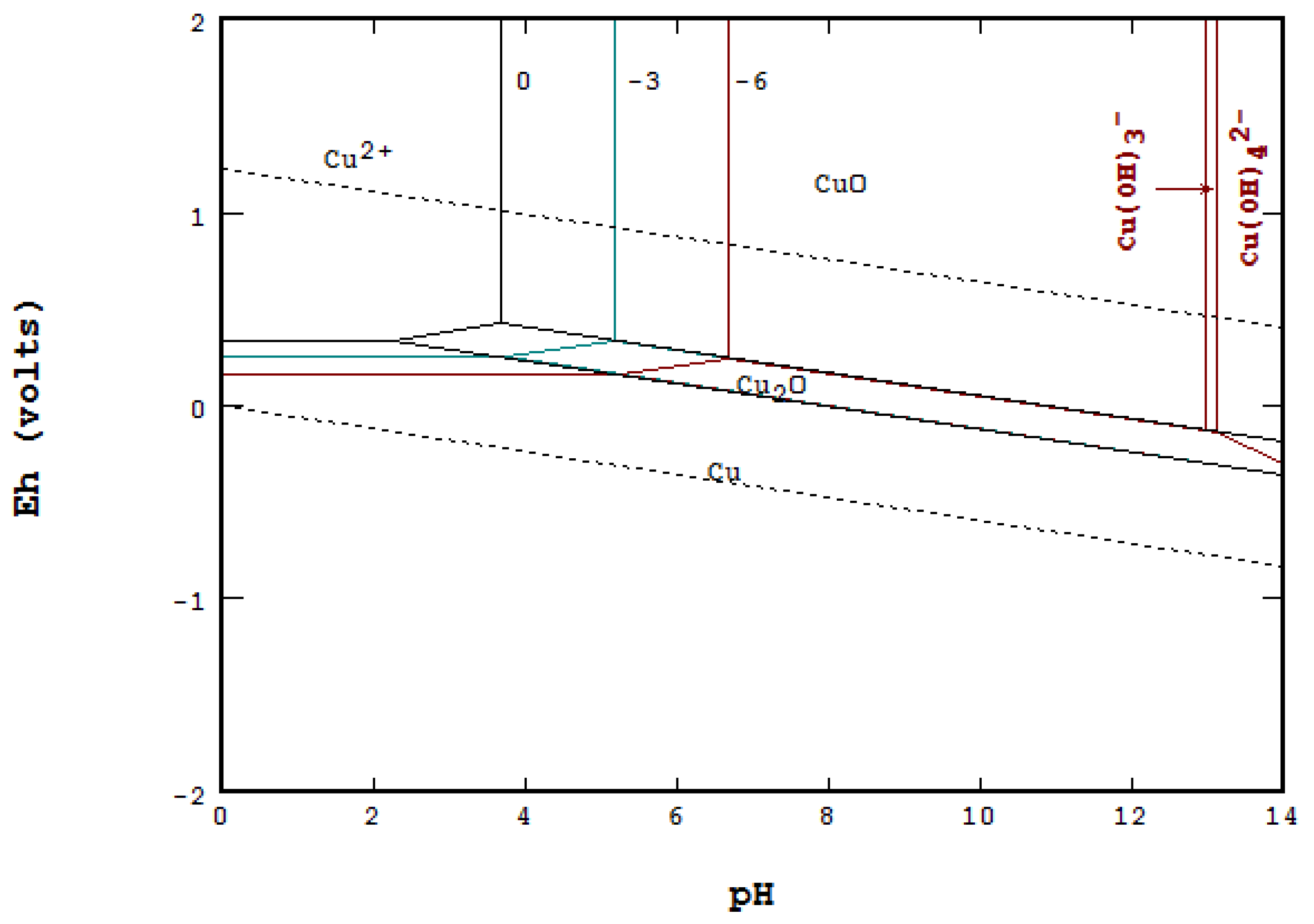
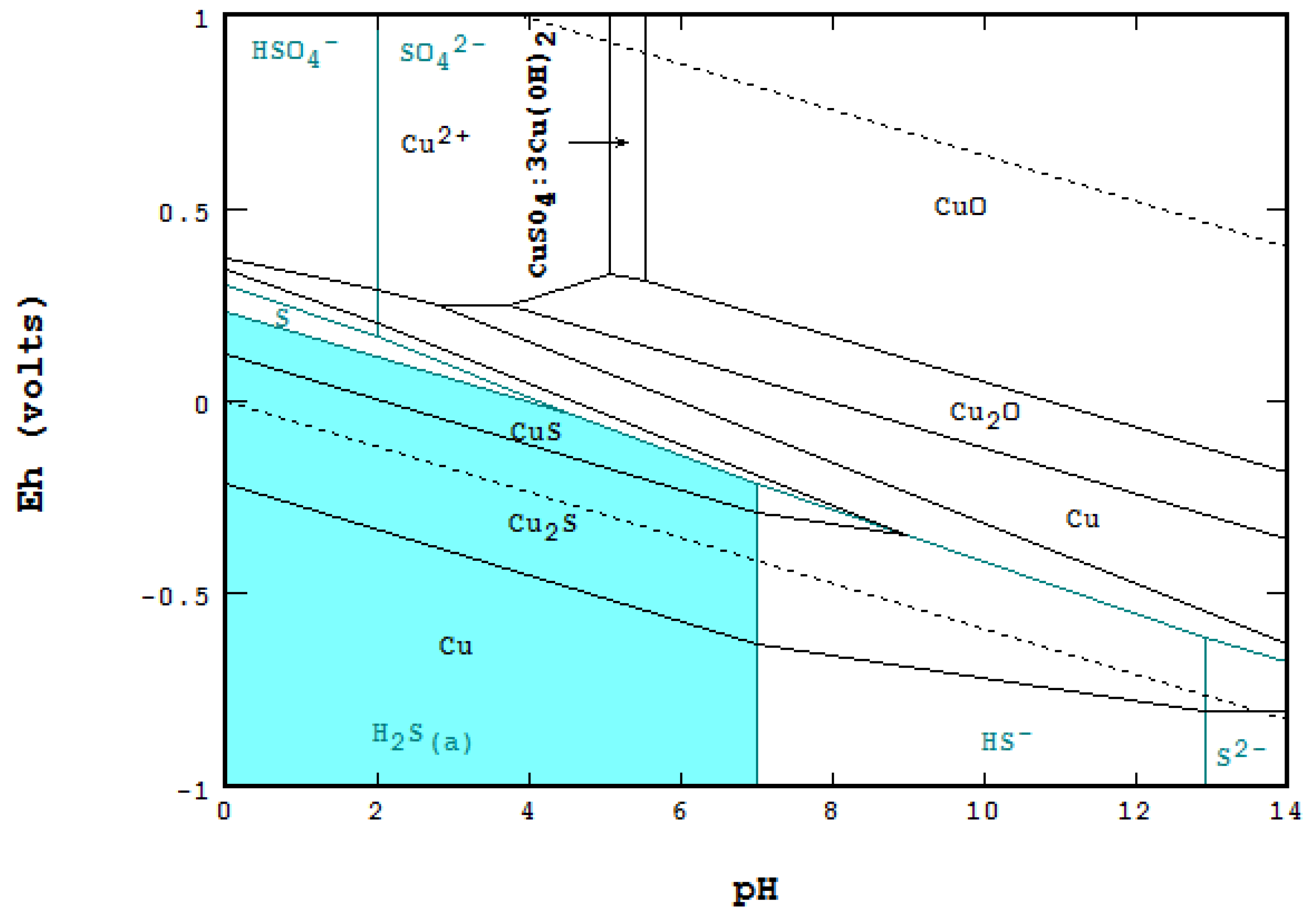
4.3. Point-by-Point Method Using Mass Balance [9,18]
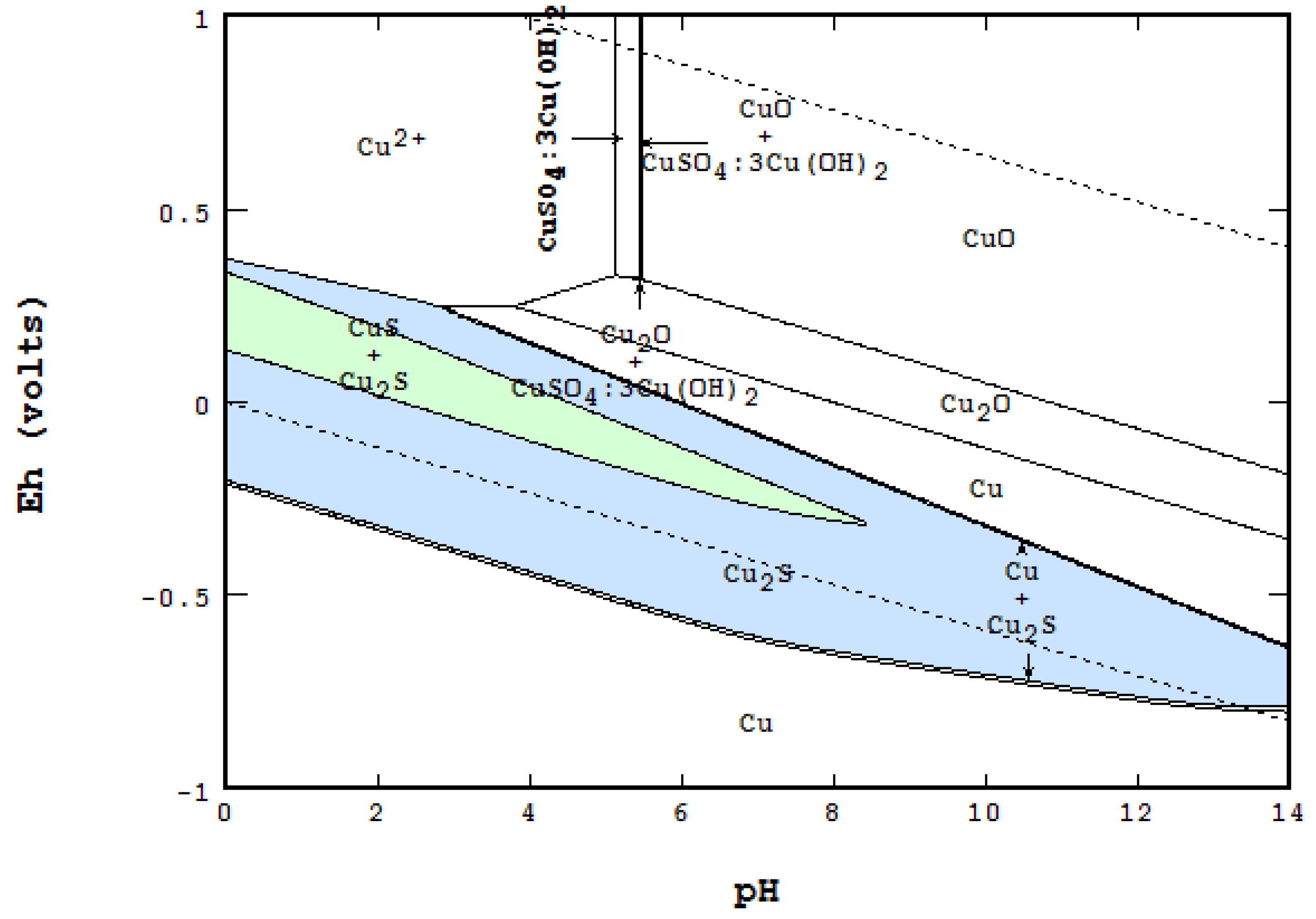
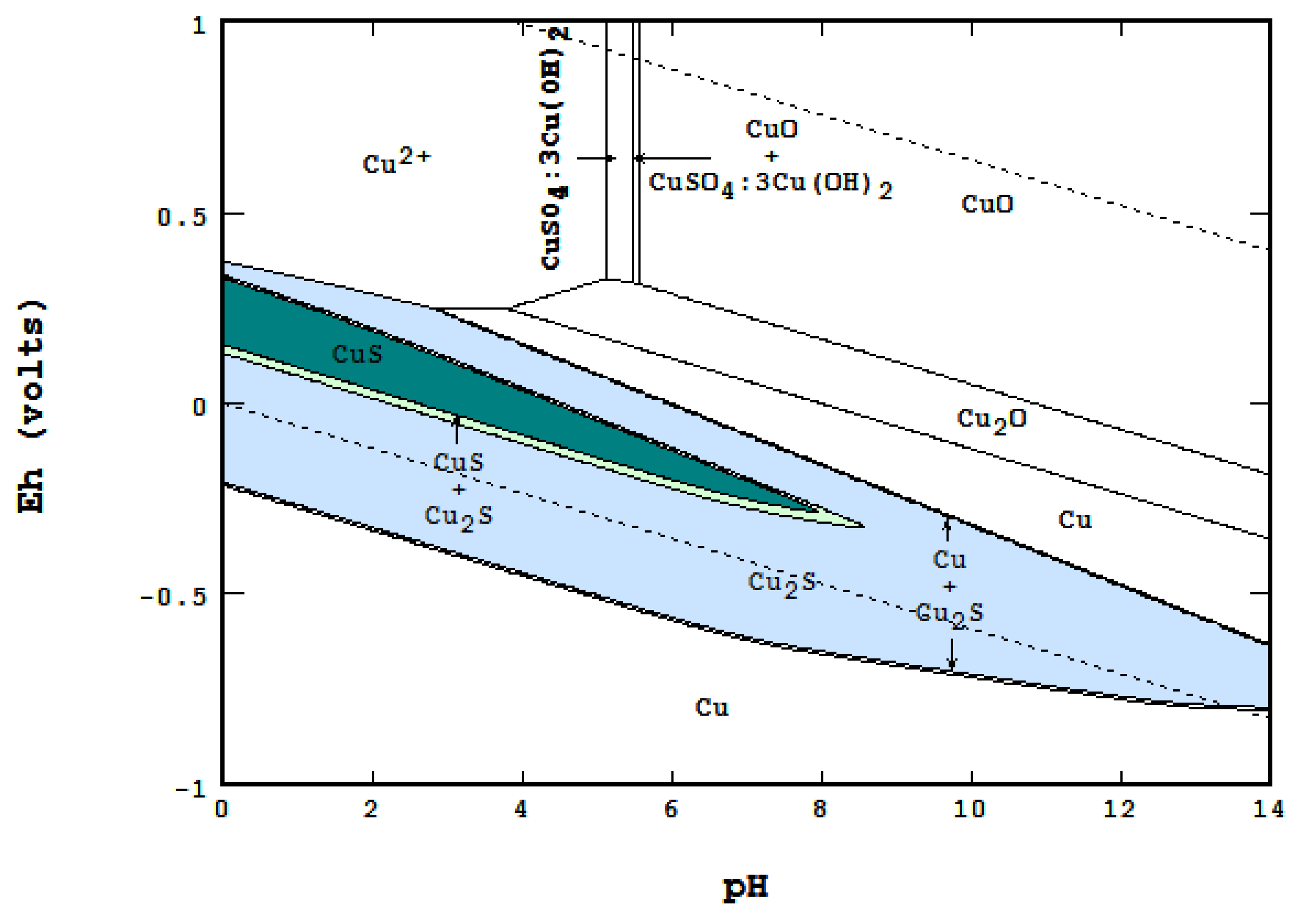
4.4. Differences and Comparison between the Methods
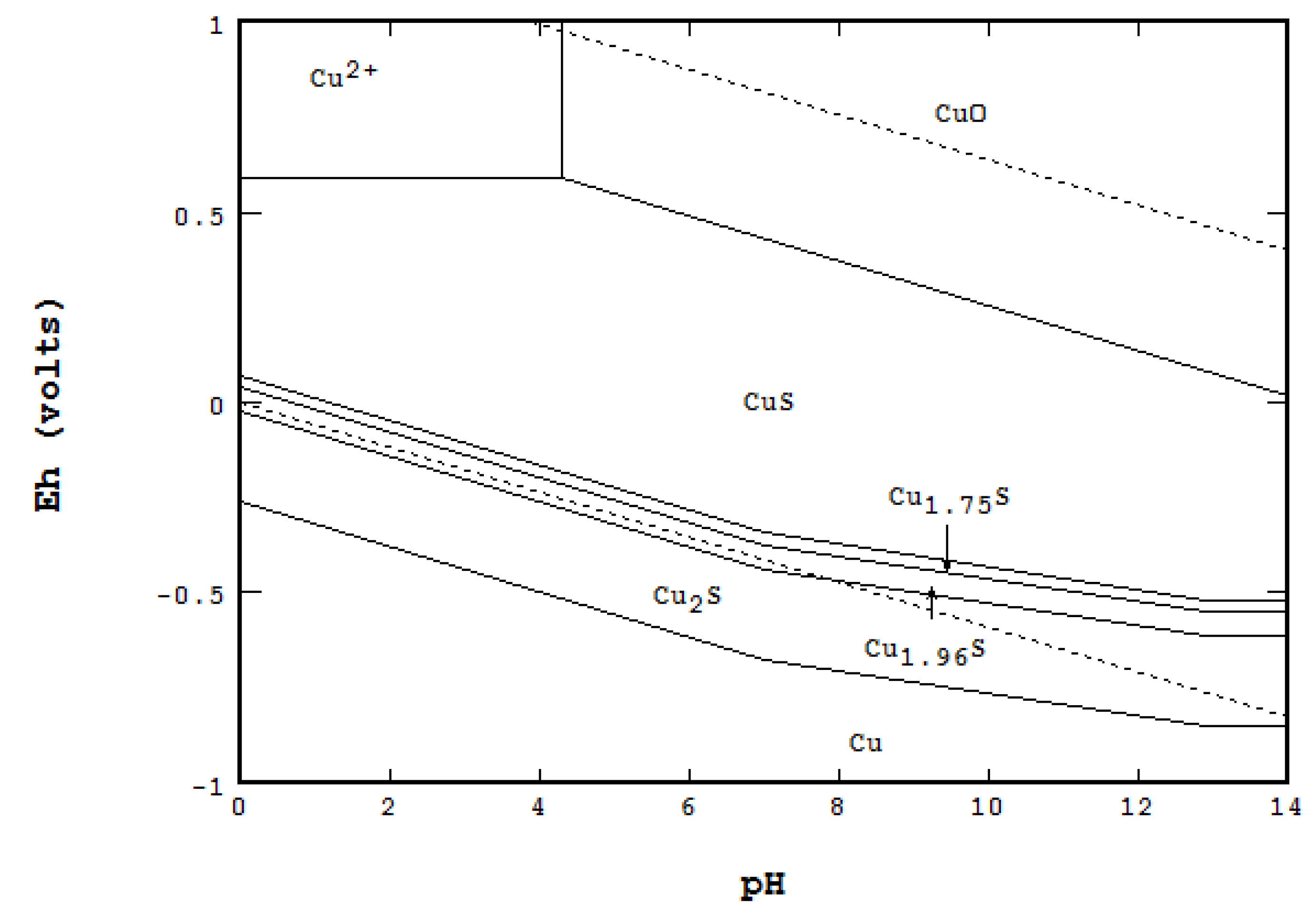
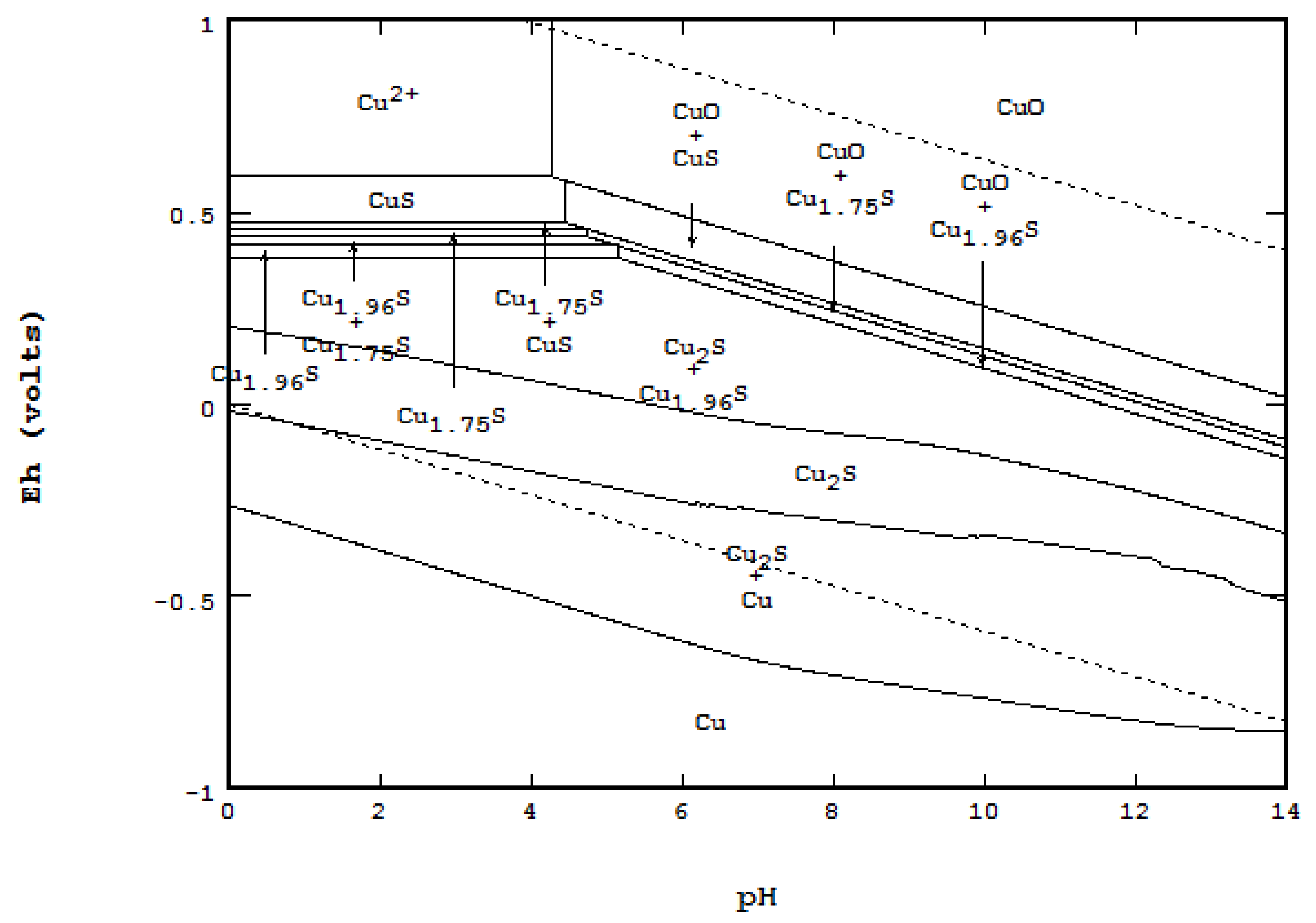
- When a system is required to specify total concentration, not equilibrium concentration nor activity.
- When the adsorption by solids, such as ferrihydrite, is considered (refer to Figure 8).
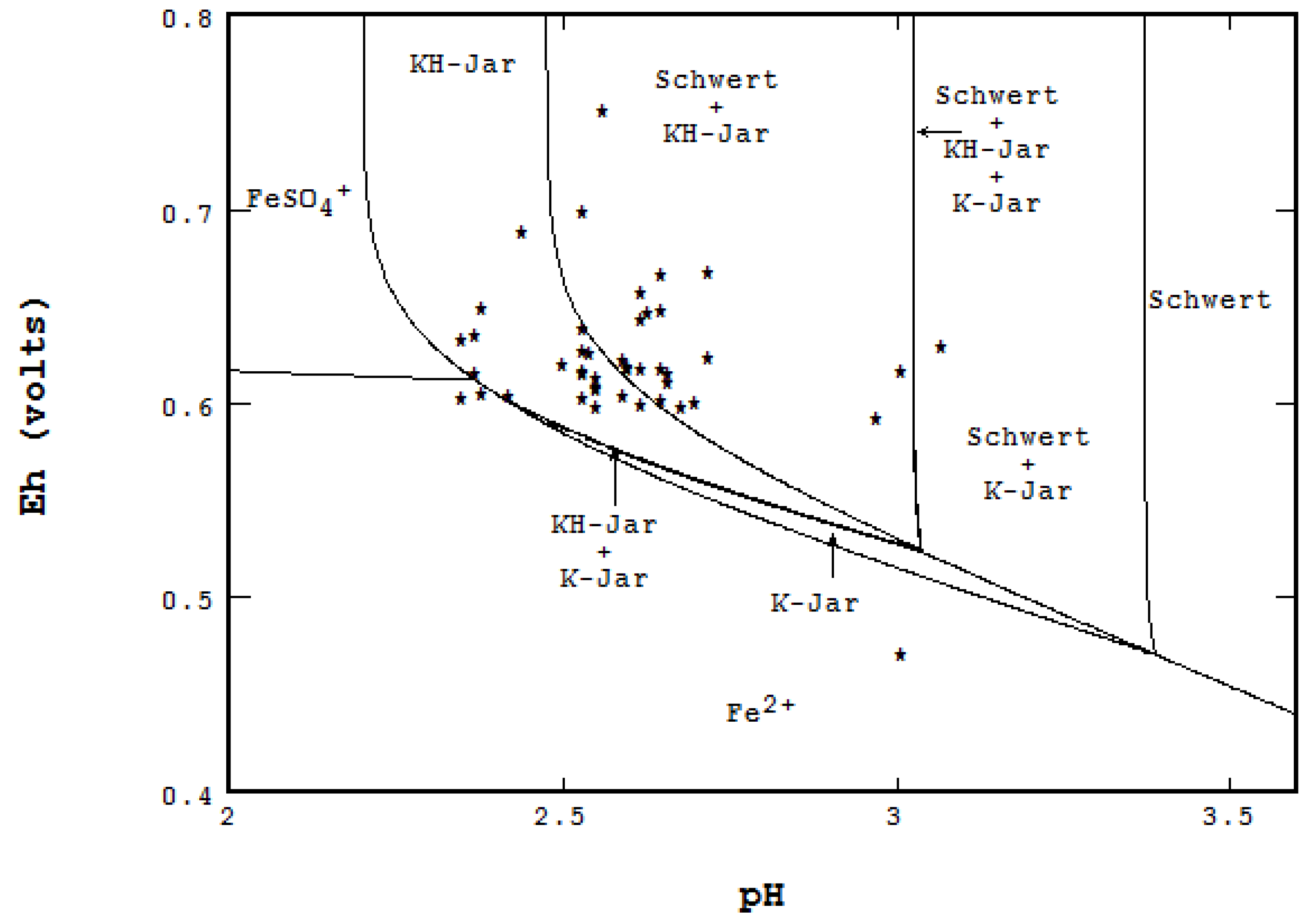
- When multiple phases of a solid need to be shown: Figure 16 was constructed by showing the coexistence of schwertmannite with various forms of jarosites in Berkeley pit water. Water samples were taken and analyzed from 1987 to 2012 by the Montana Bureau of Mines and Geology [20], and the thermodynamic data for the solids species were regression estimated by Srivastave [21].
- A diagram will most likely be mass balanced if a speciation program, such as PHREEQC (USGS) [22], was used to construct it. Results from the program were collected manually or electronically, then combined into an Eh-pH diagram.
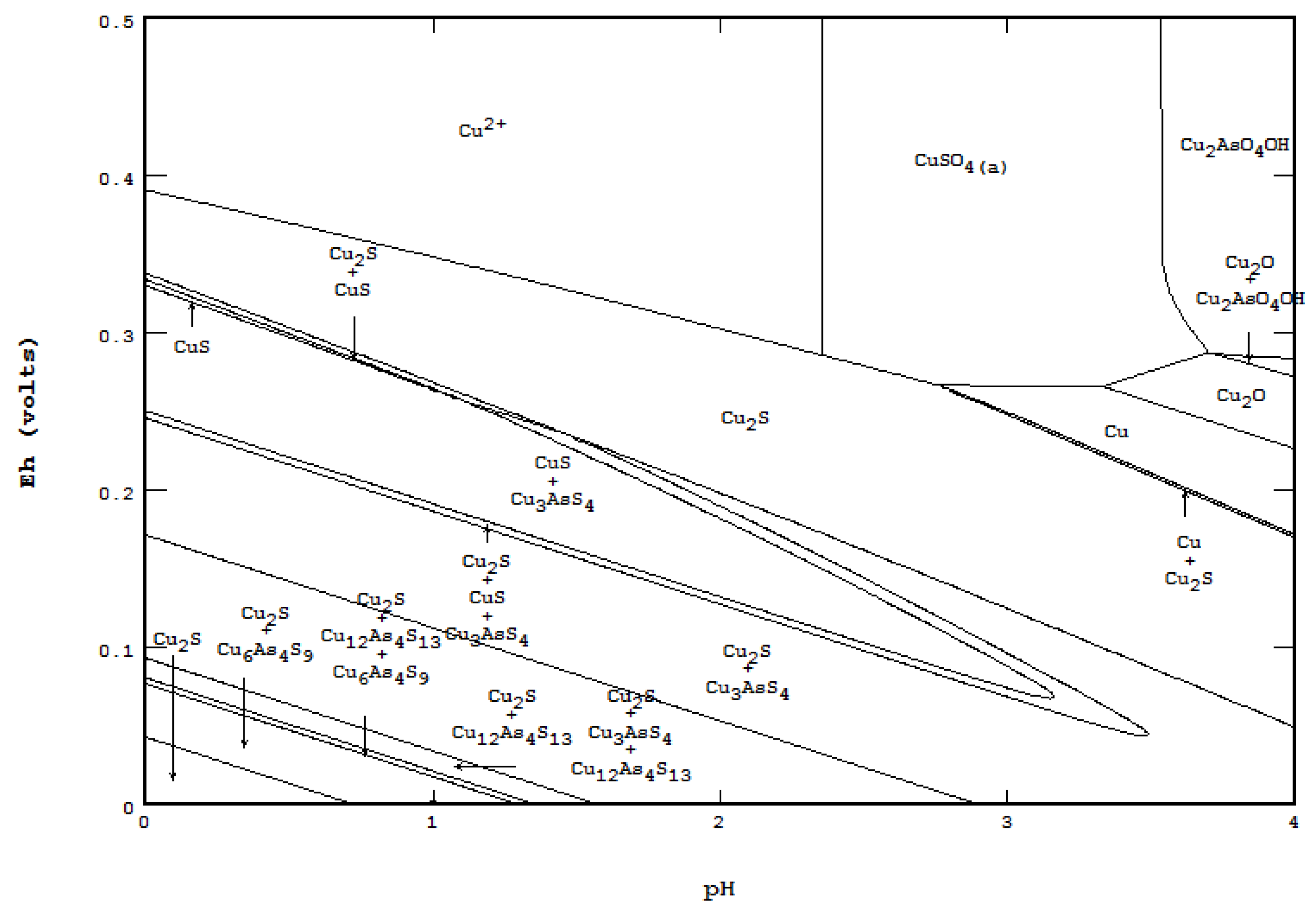
- The concentration of ligand component(s) is much greater than the main component, such as metal corrosion by sea water, and
- Gas is the only ligand, such as the Fe–CO2(g) system.
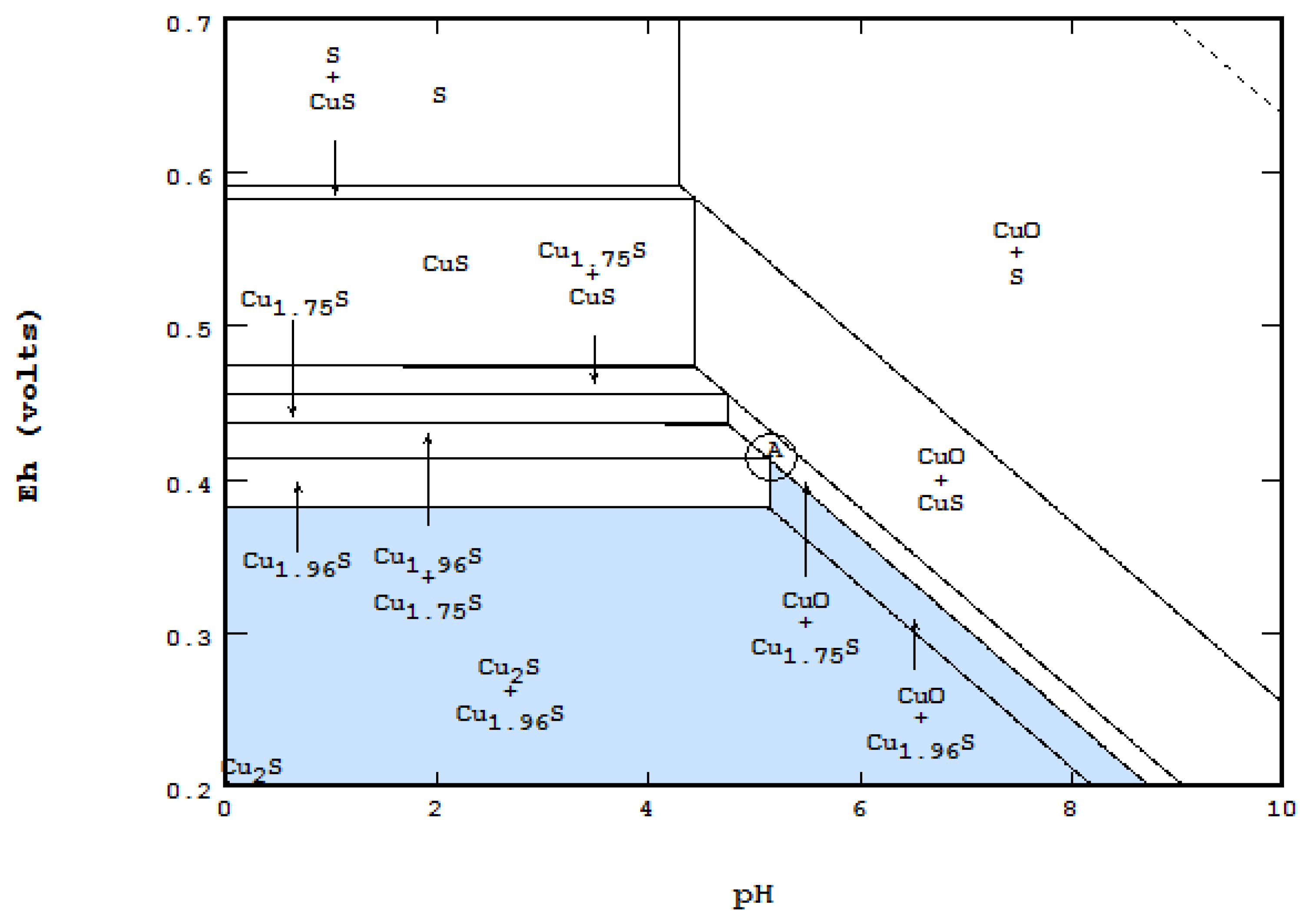
4.5. Gibbs Phase Rule Applied to an Eh-pH Diagram
- P is the total number of phases = 1 (liquid water) + 1 (gas if considered) + N (maximum number of solids/liquids),
- F is the degree of freedom on the diagram, which is two for an open area, one on a boundary line and zero on a triple point,
- C is the total number of components = 3 + EC (extra components). Three components are essential for Eh-pH calculation in an aqueous system. These are H(+1), O(−2) and e(−1). The extra components include the main component to be plotted, as well as all ligands.
- The term of +2 is for temperature and pressure variables. Since both are considered to be constant, +2 will be dropped off. If any system involved a gaseous species, +1 should be used, but it will be canceled out with one extra gaseous phase to the equation.
- 1a.
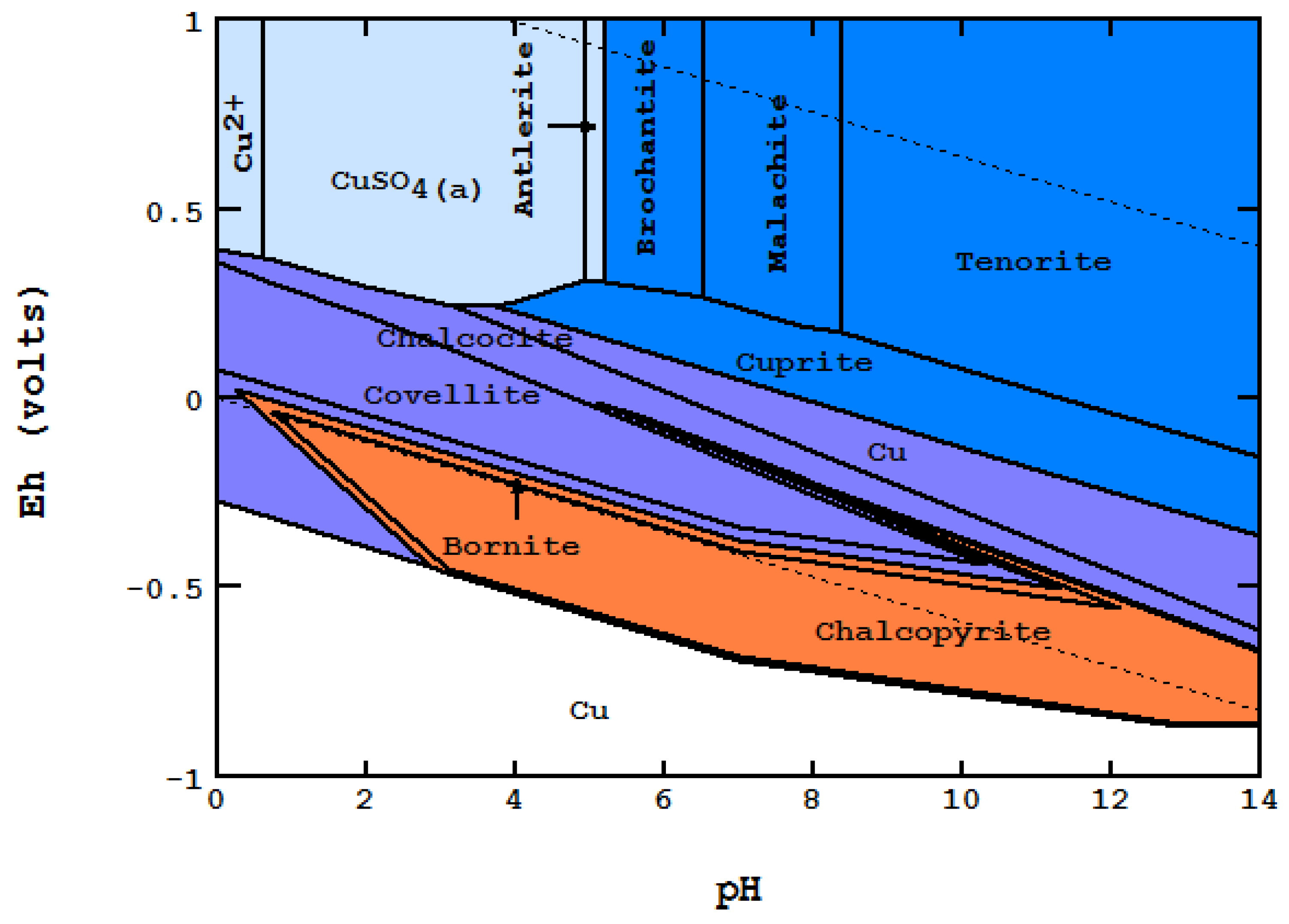
- In an open area of the diagram where F = 2, Nmaxsolid will be equal to two. The co-existence of two solids can be seen in many places on the diagram,
- 1b.
- On a boundary line where F = 1, Nmaxsolid becomes three. For instance, while each of the light blue areas contains two solids, the line between them represents the presence of three: CuO, Cu2S and Cu1.96S,
- 1c.
- On a triple point where three lines meet, F = 0, Nmaxsolid = 4. At the point labeled A, for instance, even though four areas meet, only three solids are coexistent at the point: CuO, Cu1.75S and Cu1.96S.
5. Enhancing the Eh-pH Plot by Merging Two or More Diagrams
5.1. Cyanidation of Gold and Cementation with Zn Metal
5.2. Cementation of Copper with Metallic Iron
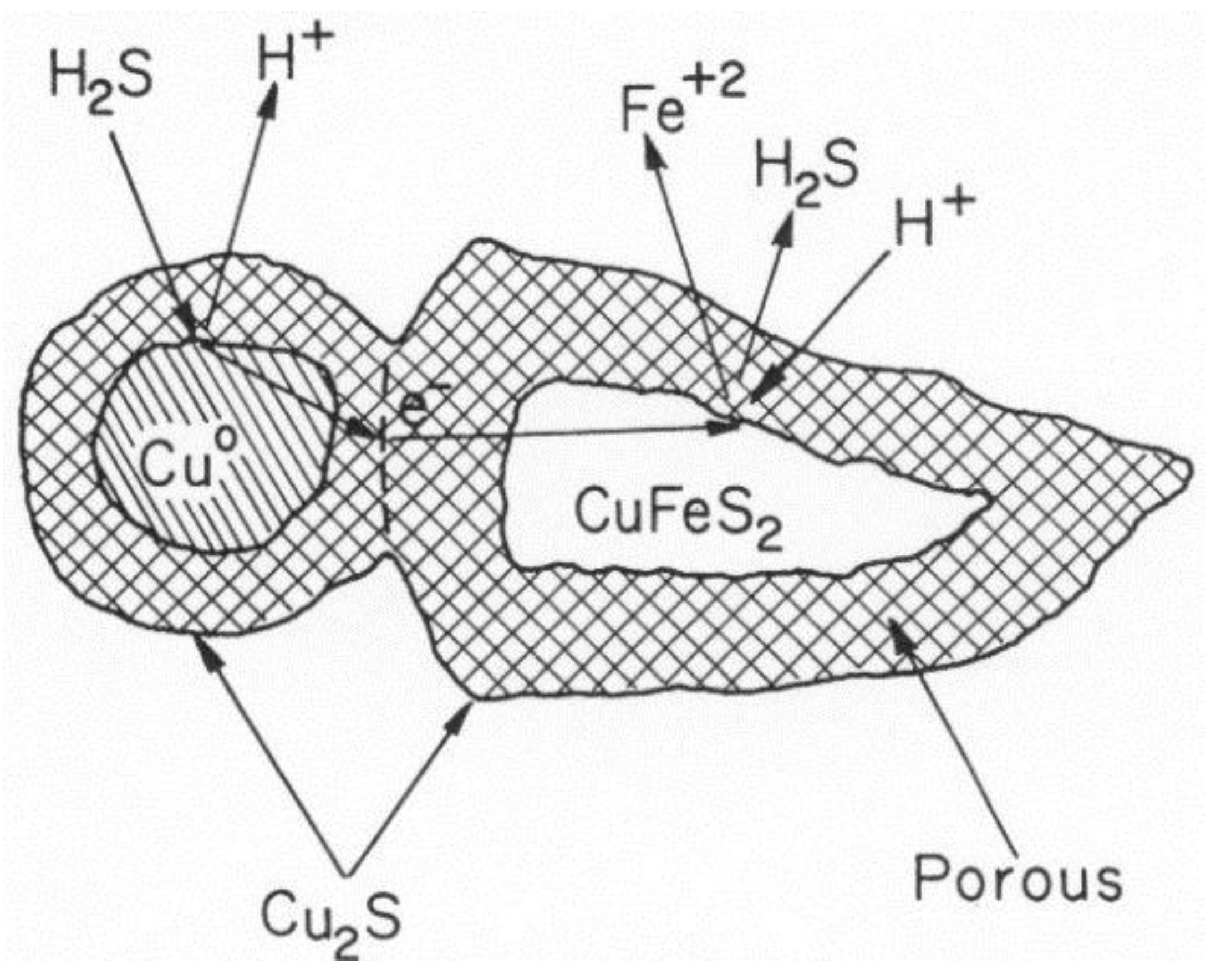
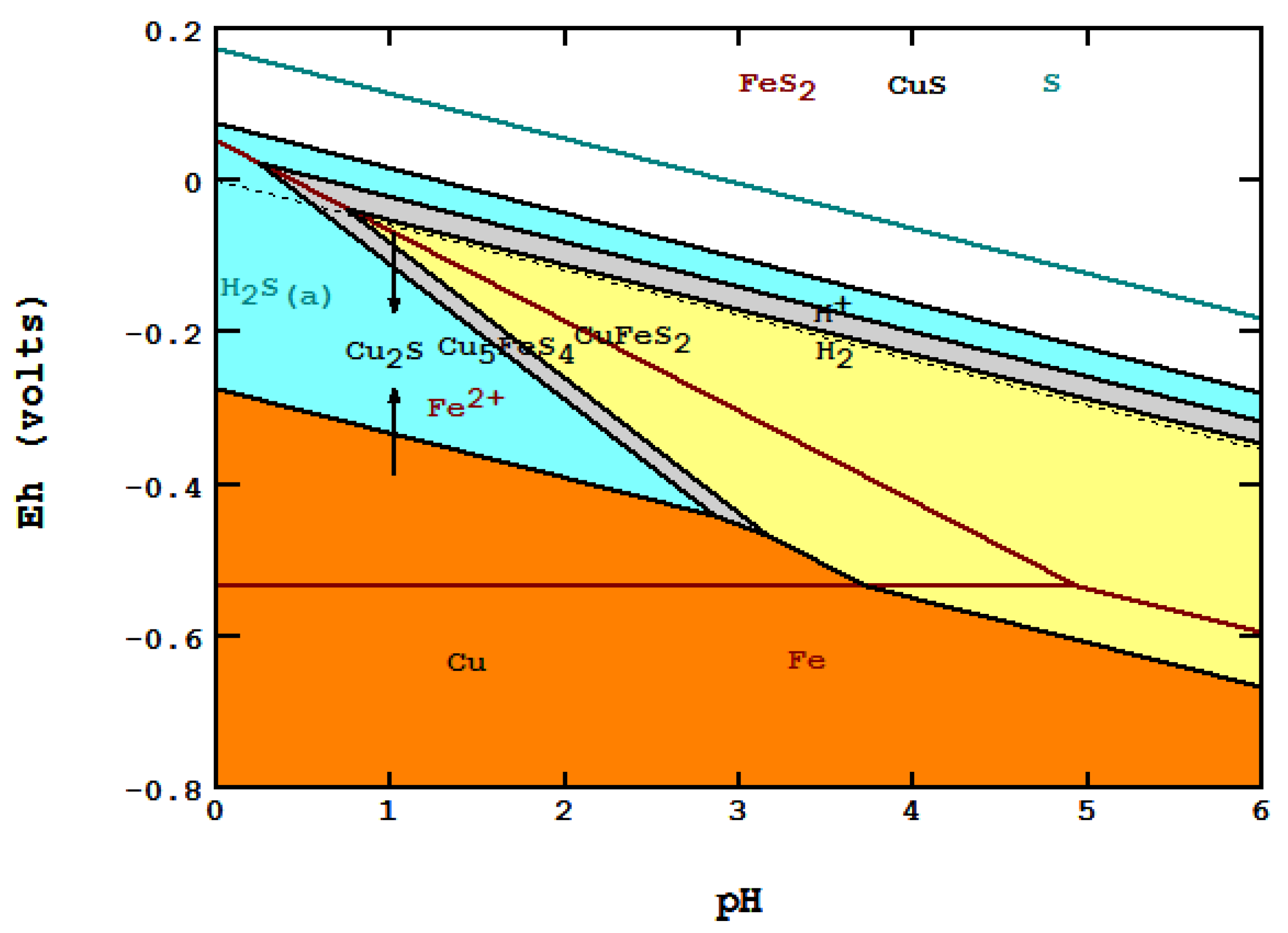
5.3. Galvanic Conversion of Chalcopyrite with Copper Metal [24]
- The three diagrams used are: S species in cyan, Fe and Fe–S in red and Cu–Fe–S in black.
- Areas of predominance are shown as: chalcopyrite in yellow, bornite in gray, chalcocite in light blue and metallic copper in orange.
- The down-arrow indicates where galvanic conversion occurs down from chalcopyrite to bornite and, finally, to Cu2S. The up-arrow indicates where the anodic reaction occurs up from metallic copper to Cu2S.
- The diagram indicates that both cathodic and anodic reactions lead to the formation of Cu2S, and other final species match what Hiskey and Wadsworth [24] described.
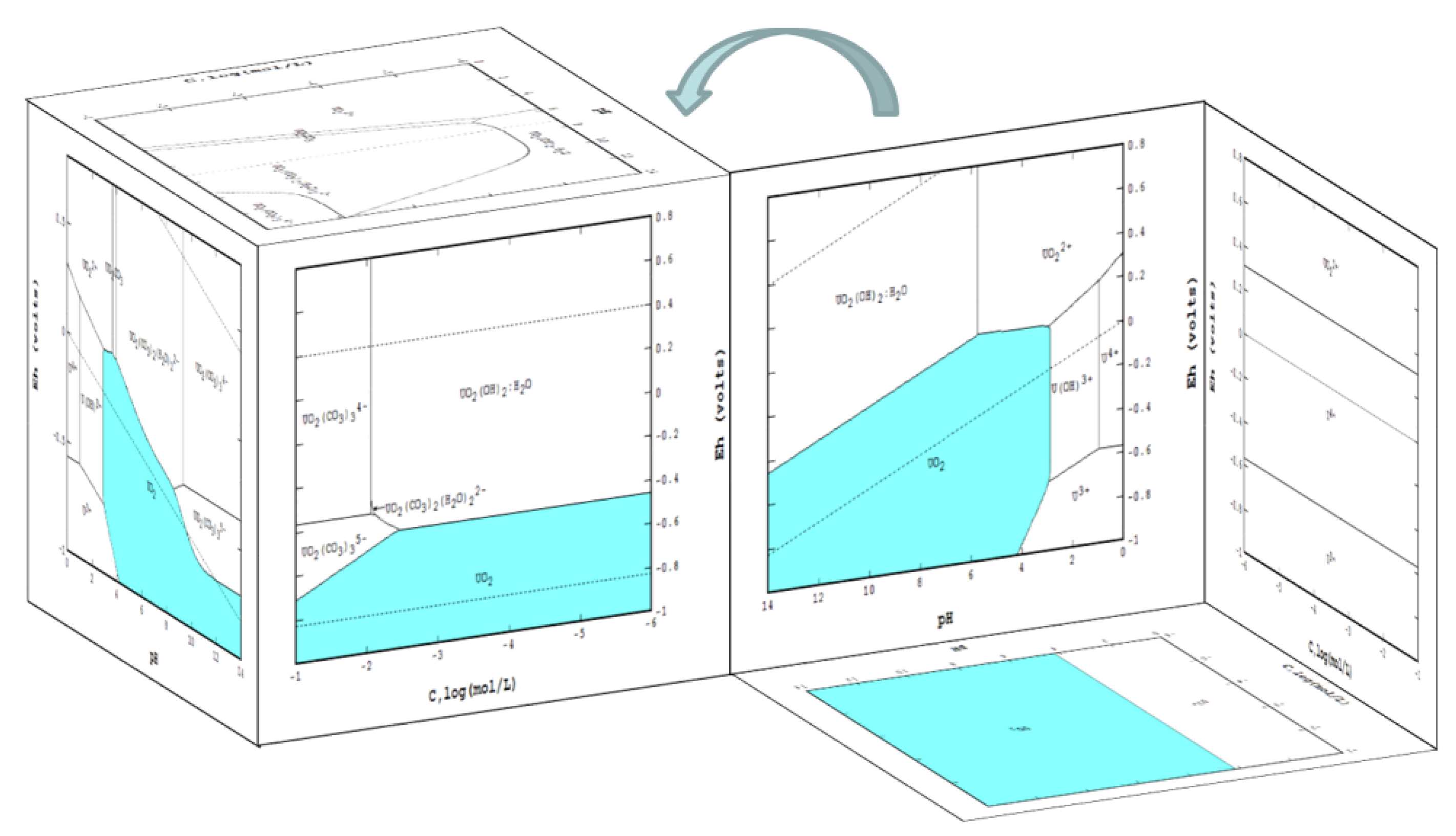
6. Third Dimension to an Eh-pH Diagram
- Eh-pH with an extra axis for ligand CO2: two wireframe volume diagrams of Eh-pH-CO2 taken from Garrels and Christ [2] are used for verifying the results; these two are:
- Figure 7.32b: in order to match the given ΣCO2 for the third axis, the mass balance method has to be used; the output of 3D and discussion for this case are presented in more detail.
- Figure 7.32a: since the third axis is given as the pressure of CO2(g), the equilibrium line method can be applied; the time required for the Eh-pH calculation was much less.
- Presentation of a system in which two or more solid phases, such as CuS and Cu2S, can coexist.
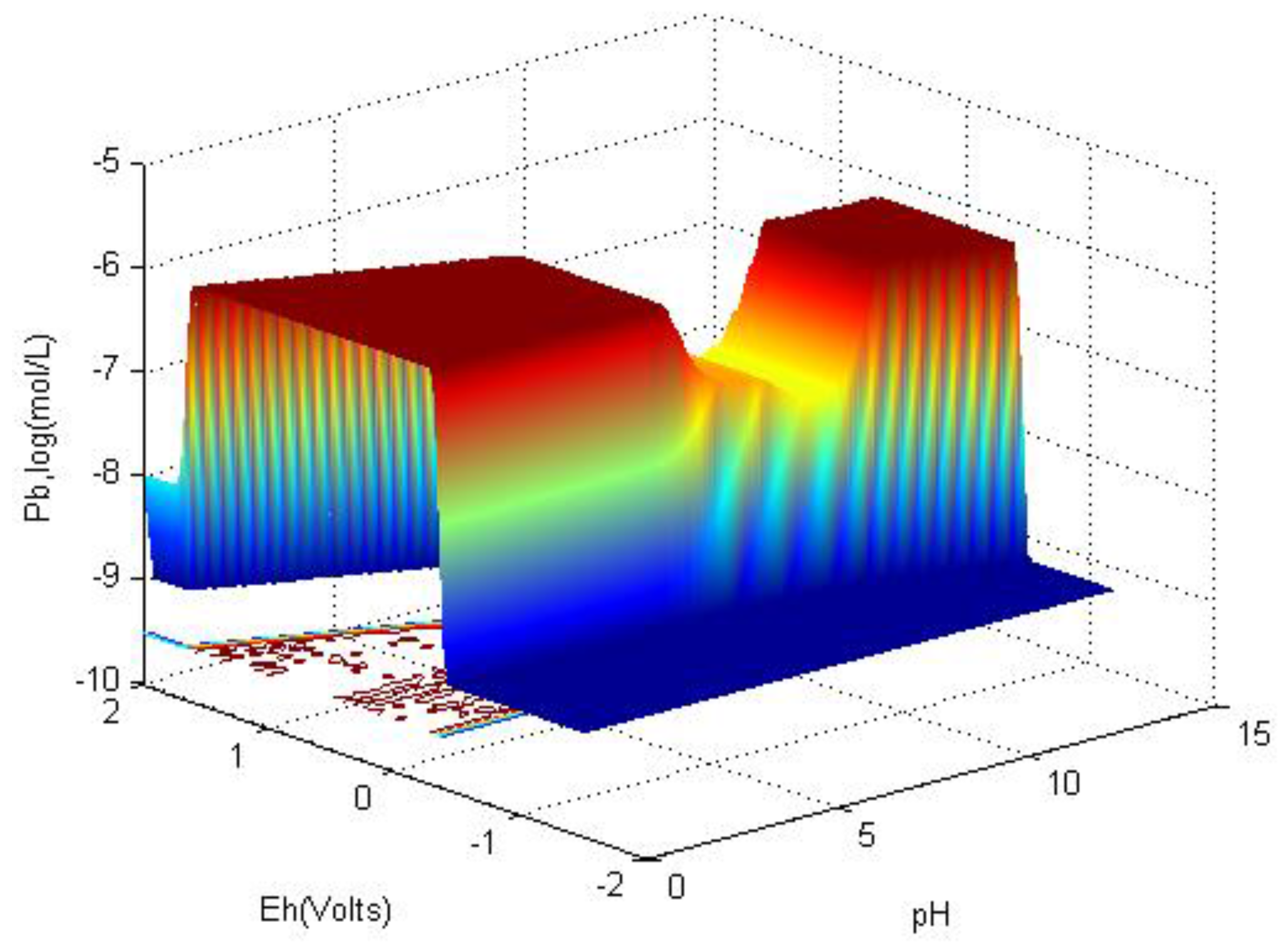
6.1. Eh-pH with Solubility
6.2. 3D Eh-pH, Uranium with ΣCO2: Using the Mass-Balance Point Method
- One species in one volume: Since total carbonate is given, assuming total CO2 means all carbonates, including dissolved, solids and complexes with U, the mass-balanced method for Eh-pH diagram is used. However, to match the Garrels and Christ plot, only one single solid in each volume was selected, with no regions of mixed solids allowed.
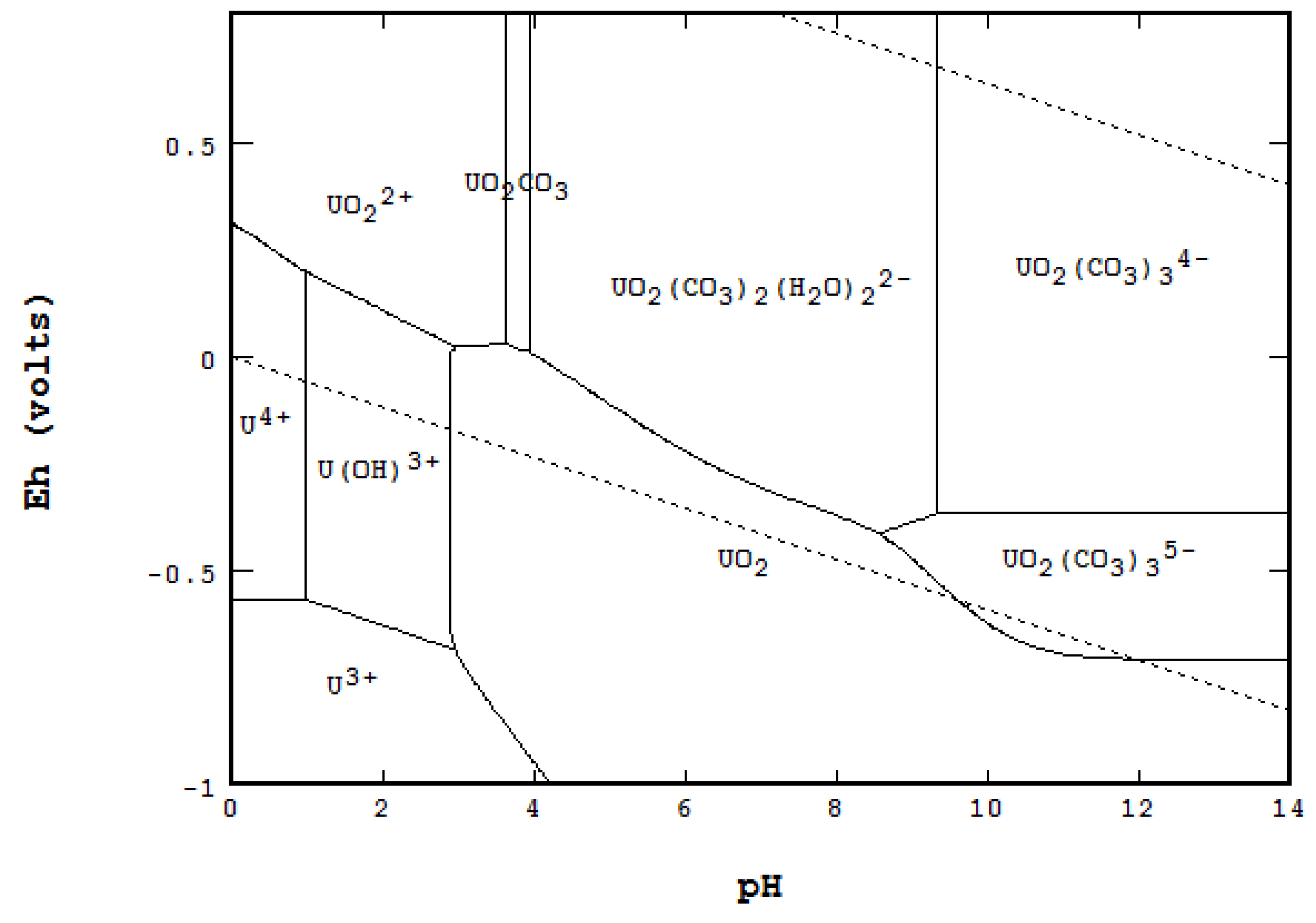
- One missing species: One species on the Garrels and Christ diagram, indicated by a red letter A in Figure 25, seems to have been mislabeled as UO2(CO3)4−. Judging from its high pH and carbonate location, and being sandwiched between U(IV) and U(VI), the species UO2(CO3)35− [28] seems to be a good fit. Figure 26 is the regenerated 2D Eh-pH diagram using logΣCO2 = −1 M.
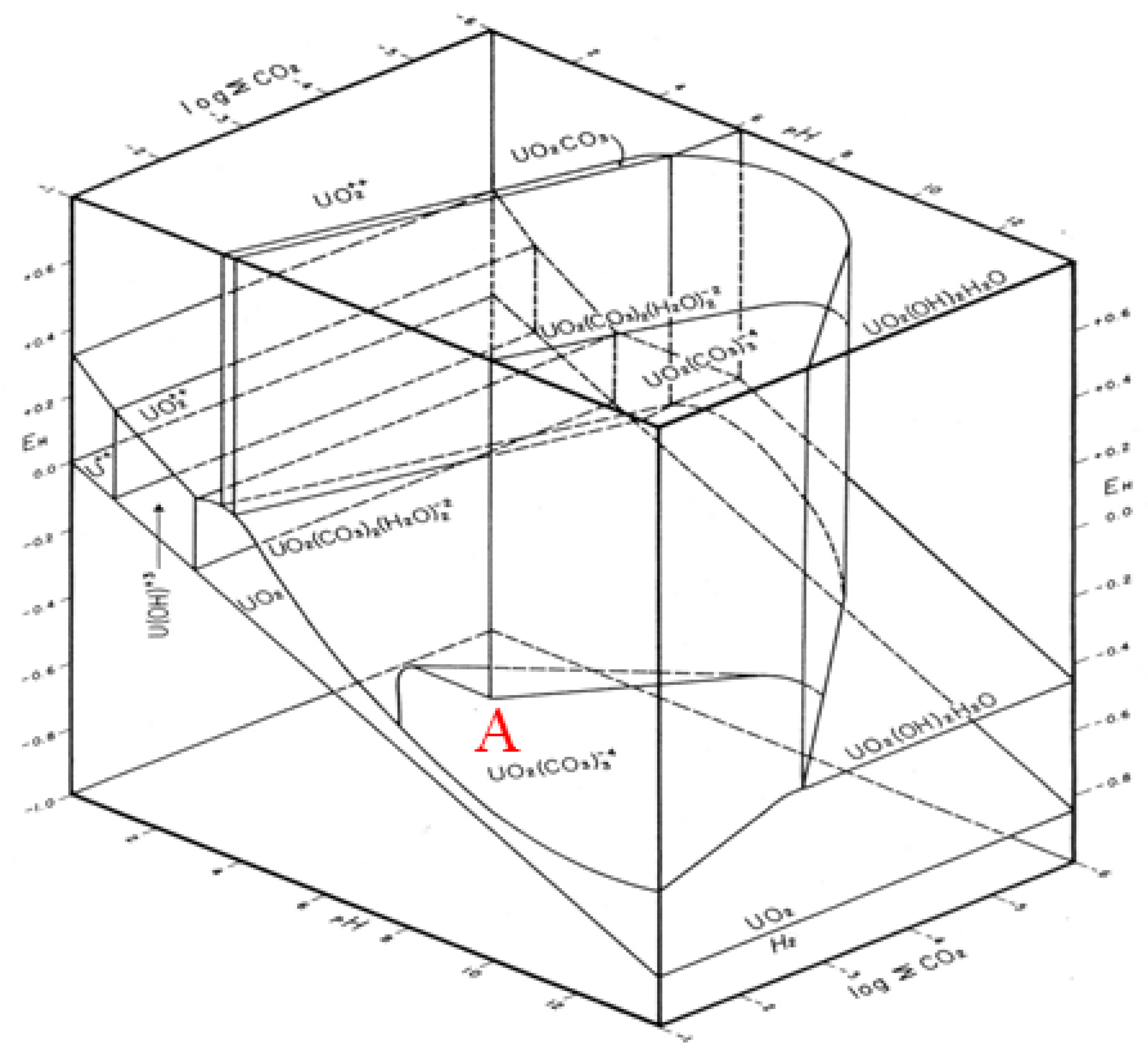
- Ranges: Eh = −1 to 0.8, pH = 0 to 14 and logΣCO2 = −6 to −1,
- Grid point: 250 × 250 × 250,
- Computer: 64 bit, 3.40 GHz, 16 G of RAM,
- Program algorithm: mass balance point method using mass action law,
- Accuracy (sum of squared residual) <1 × 10−8 and
- Time to complete the calculation: 1:36:25 (h:mm:ss) from i7 PC or 2:00:51 from i5; contrary to using the line method, shown later, which took less than 30 s.

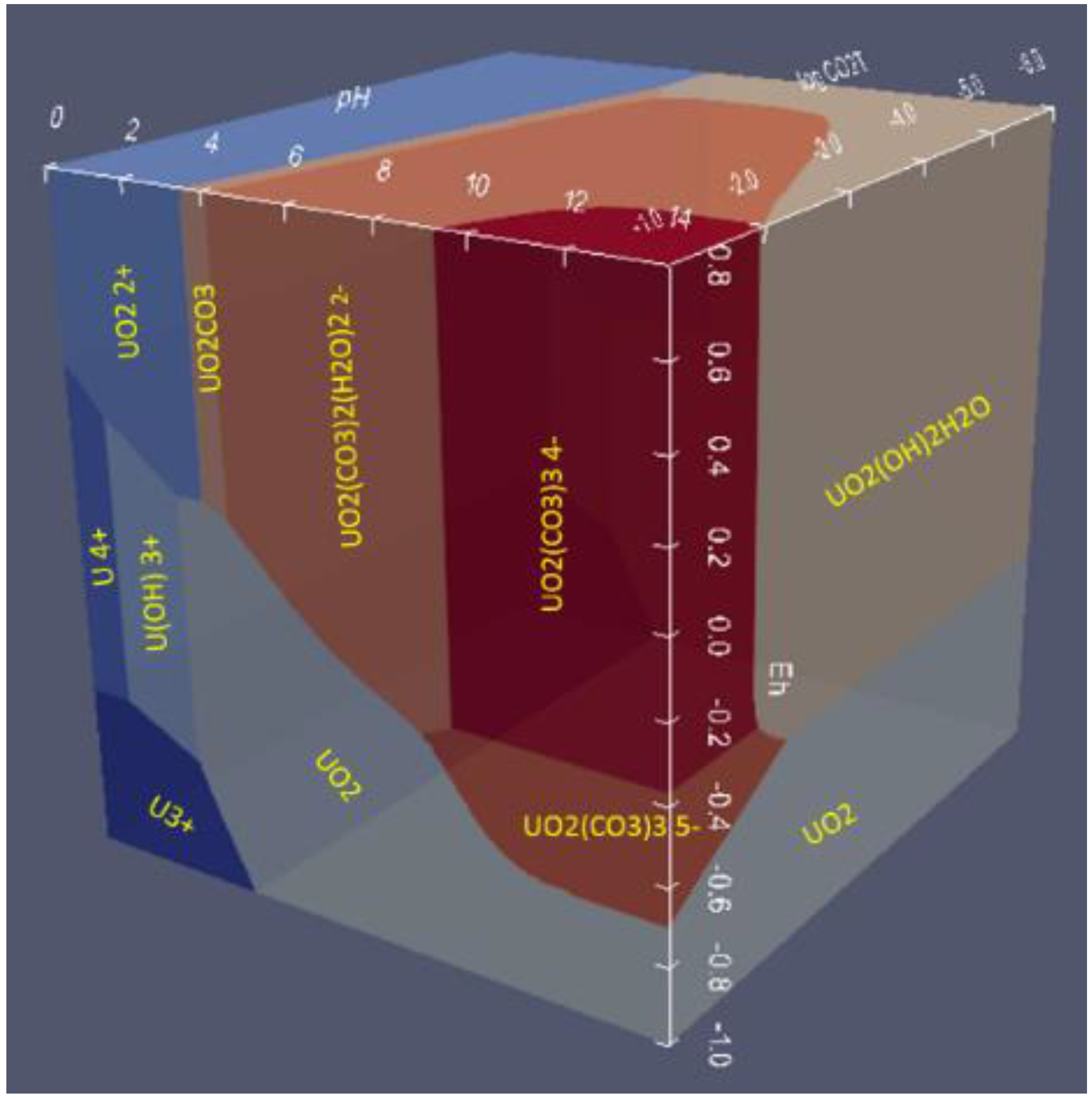
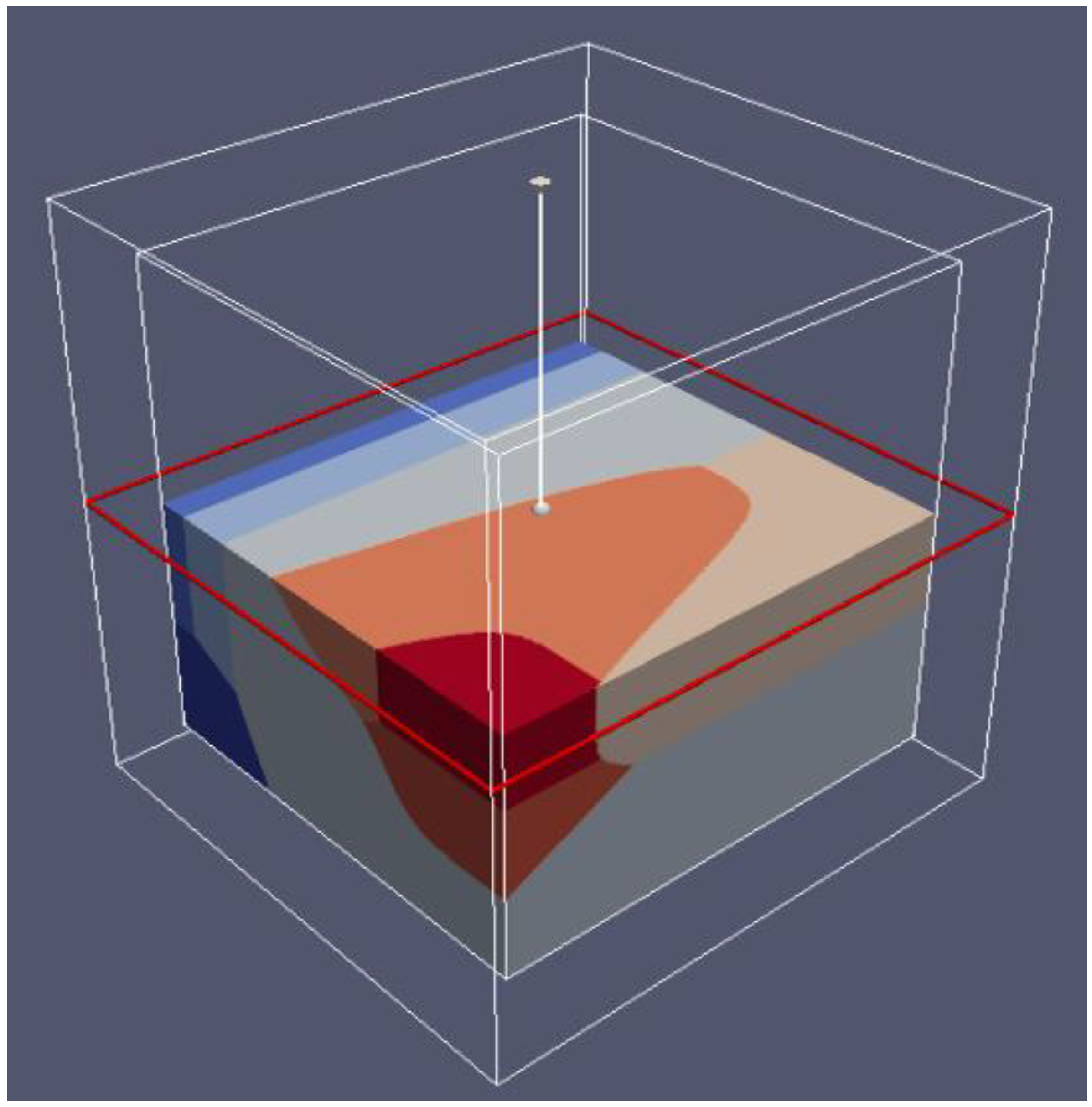
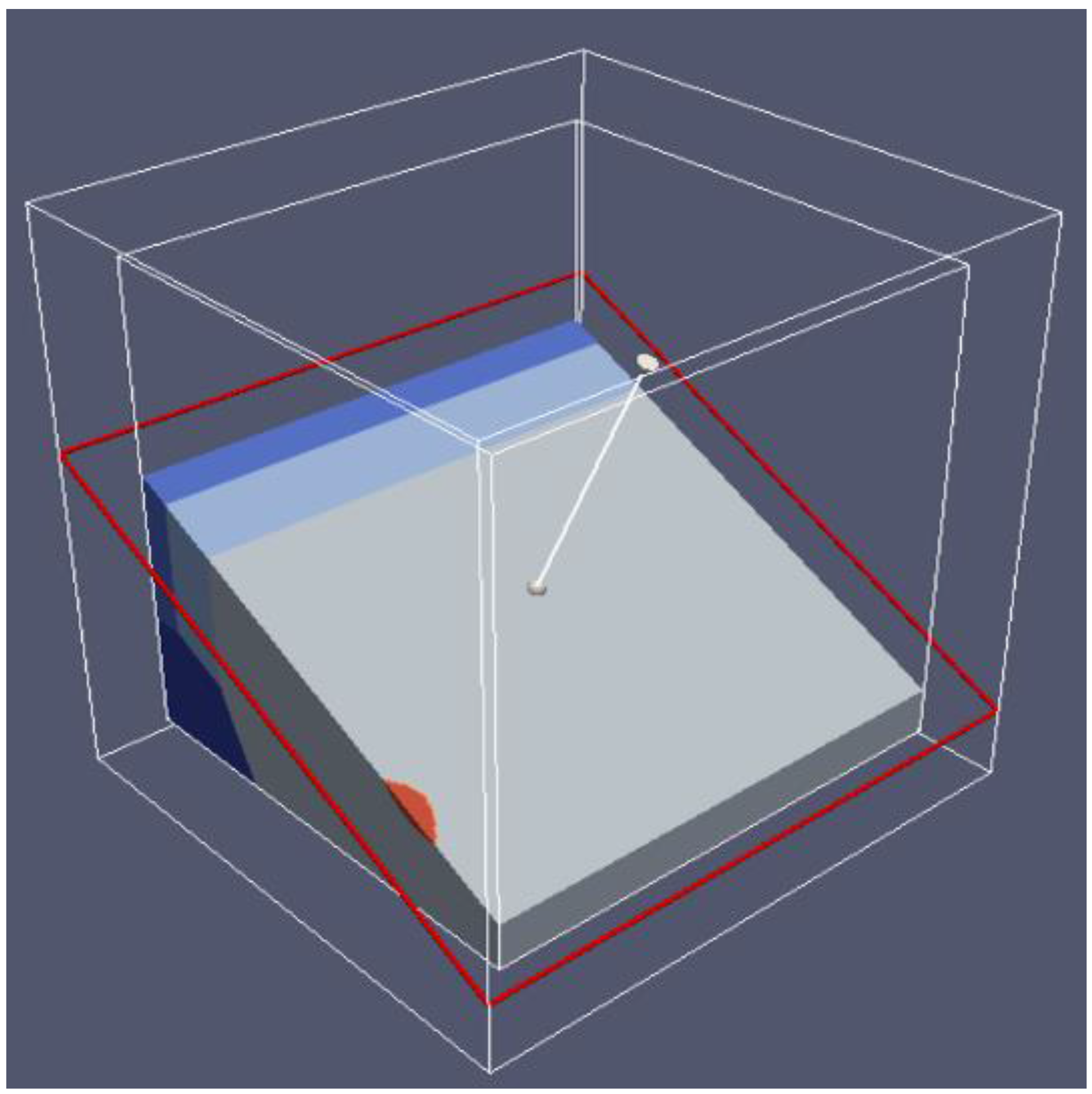
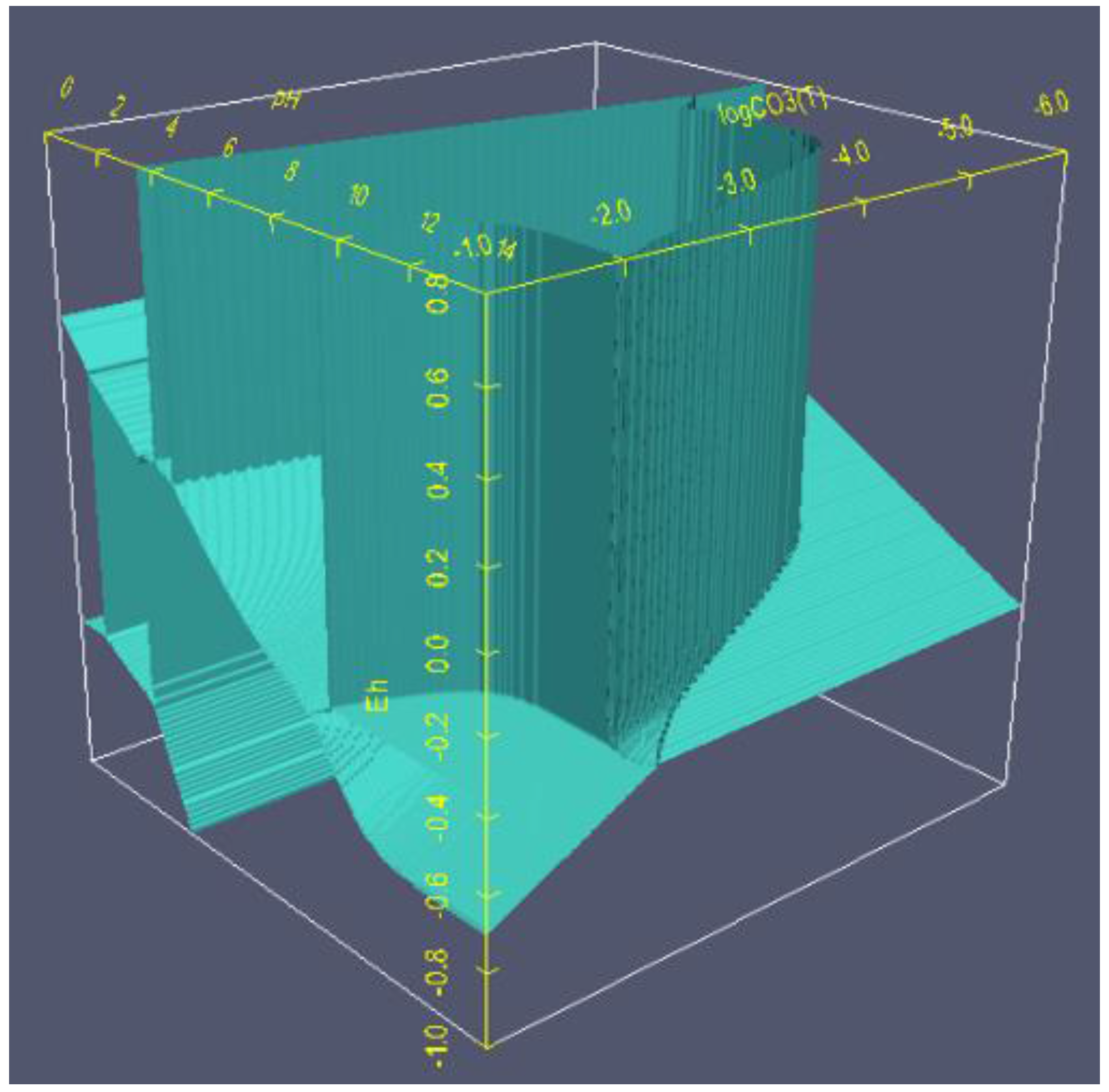
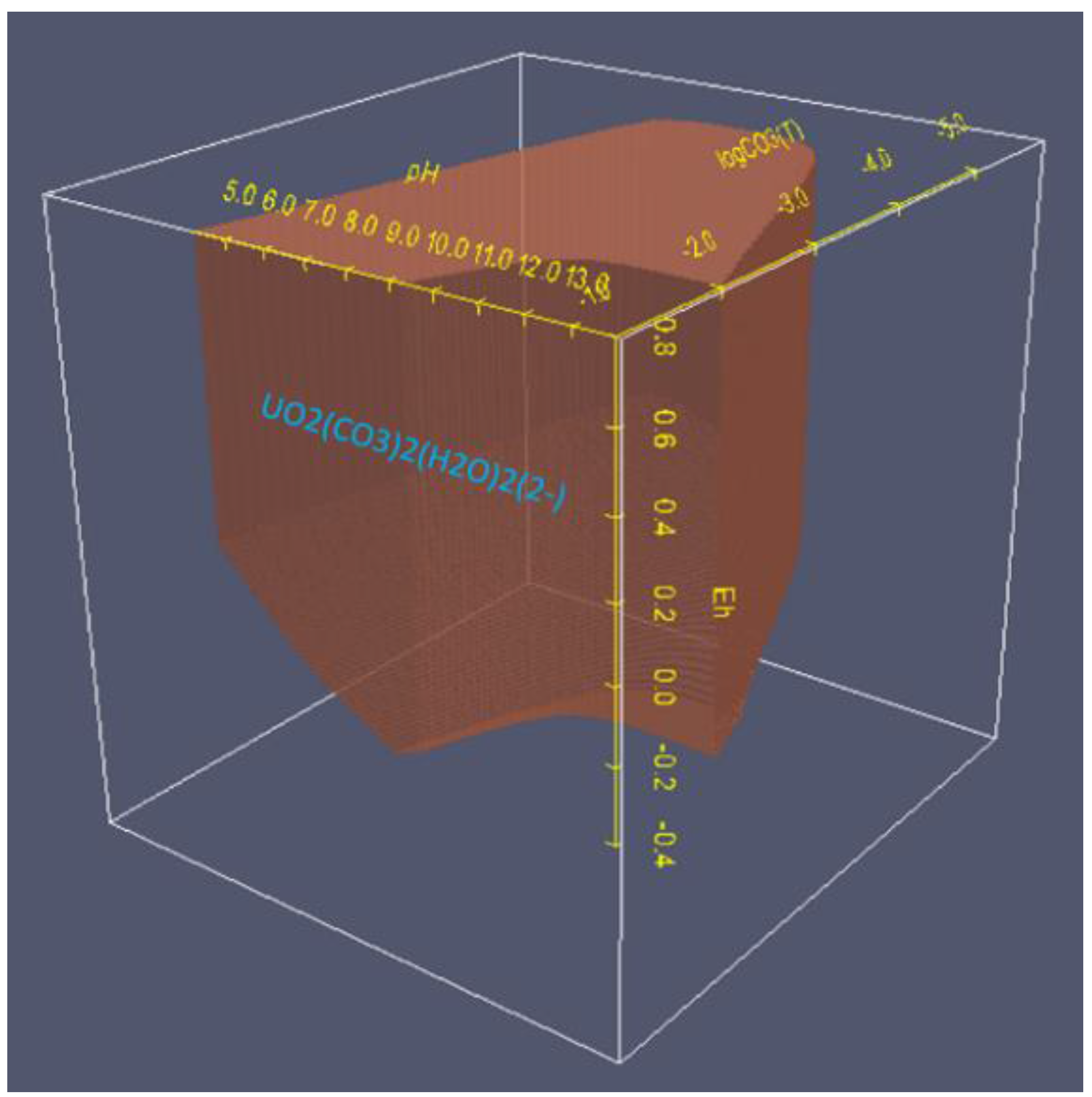
6.3. 3D Eh-pH, Uranium with Pressure CO2(g): Using the Equilibrium Line Method
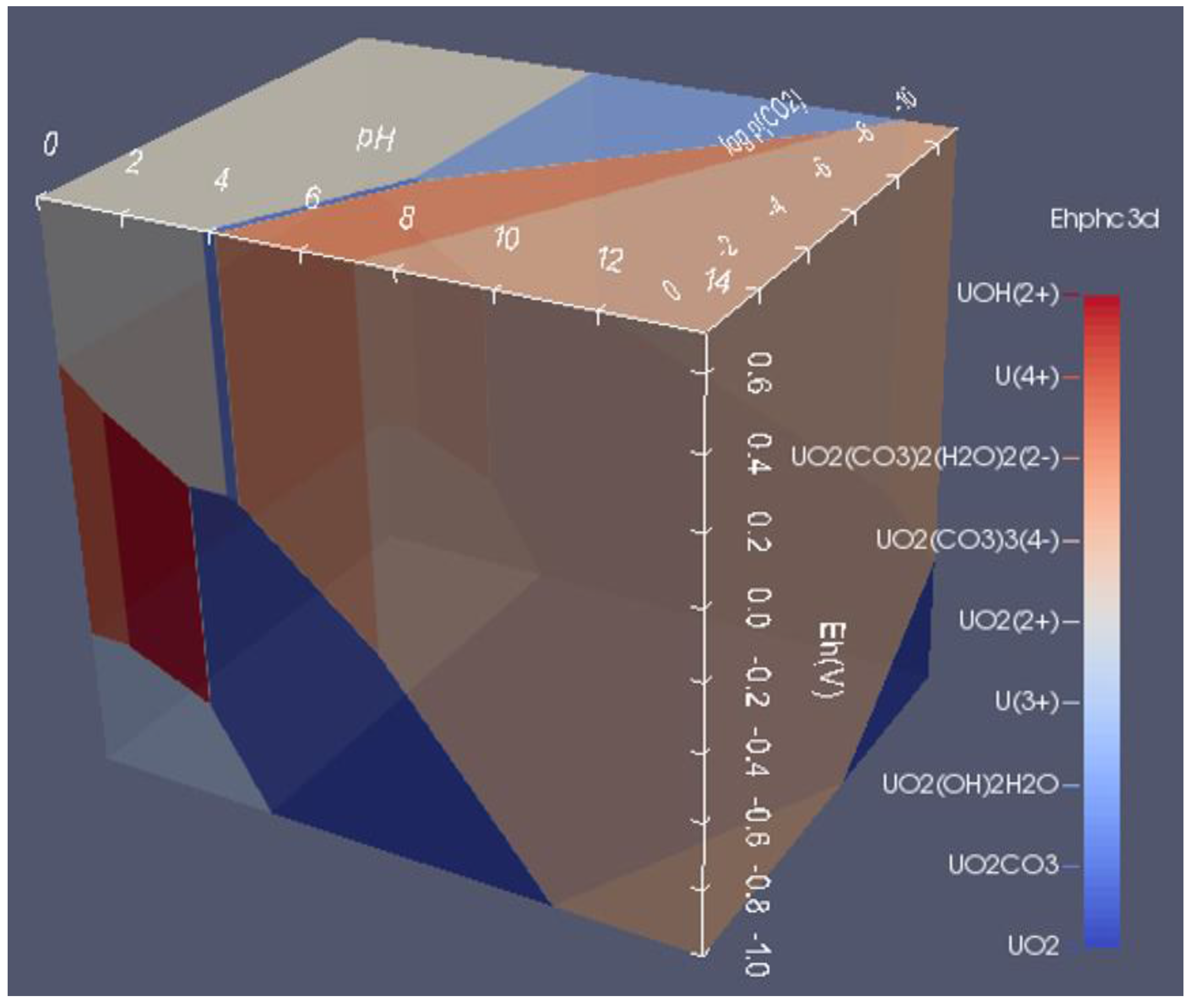
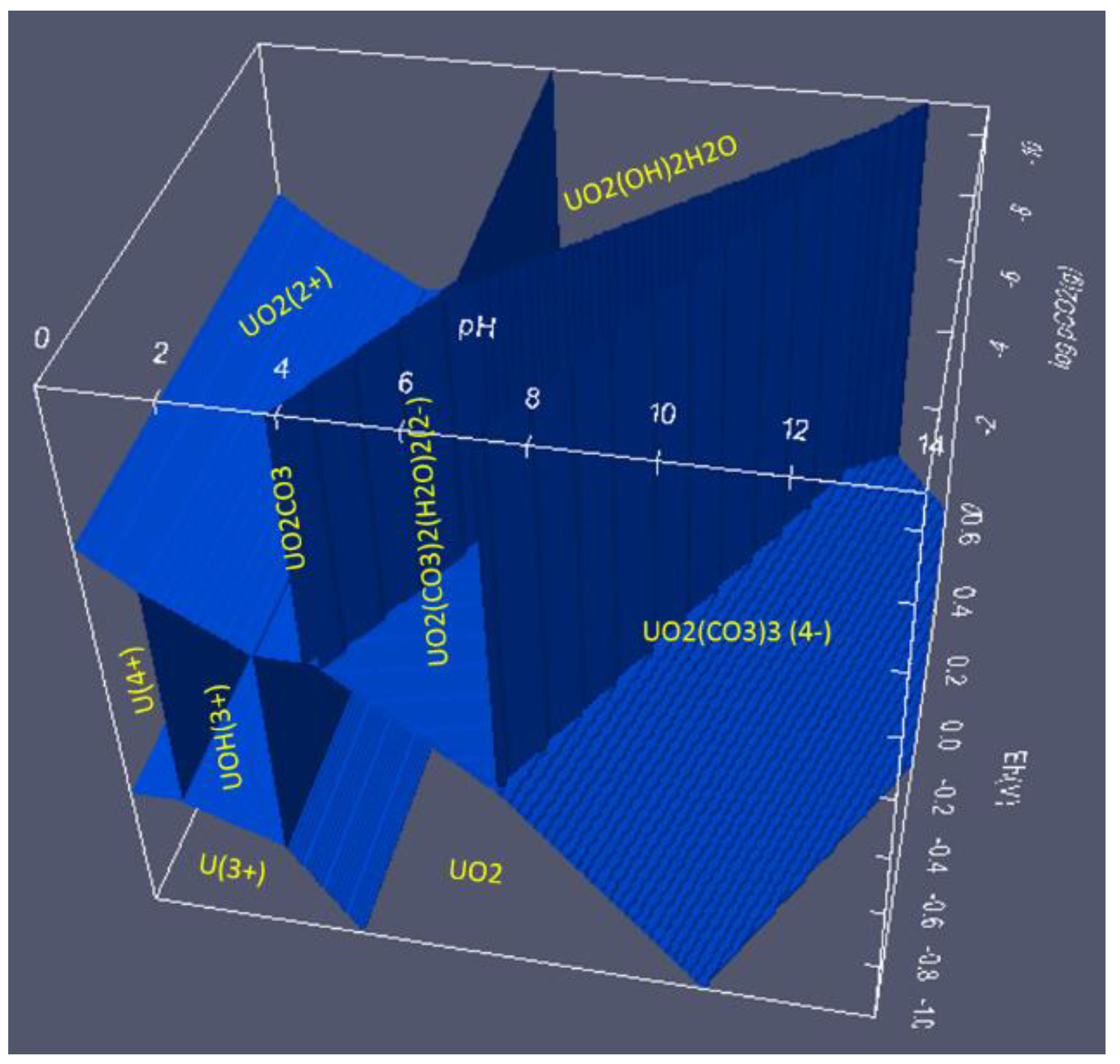
6.4. How to Show Two or More Solids in One Area
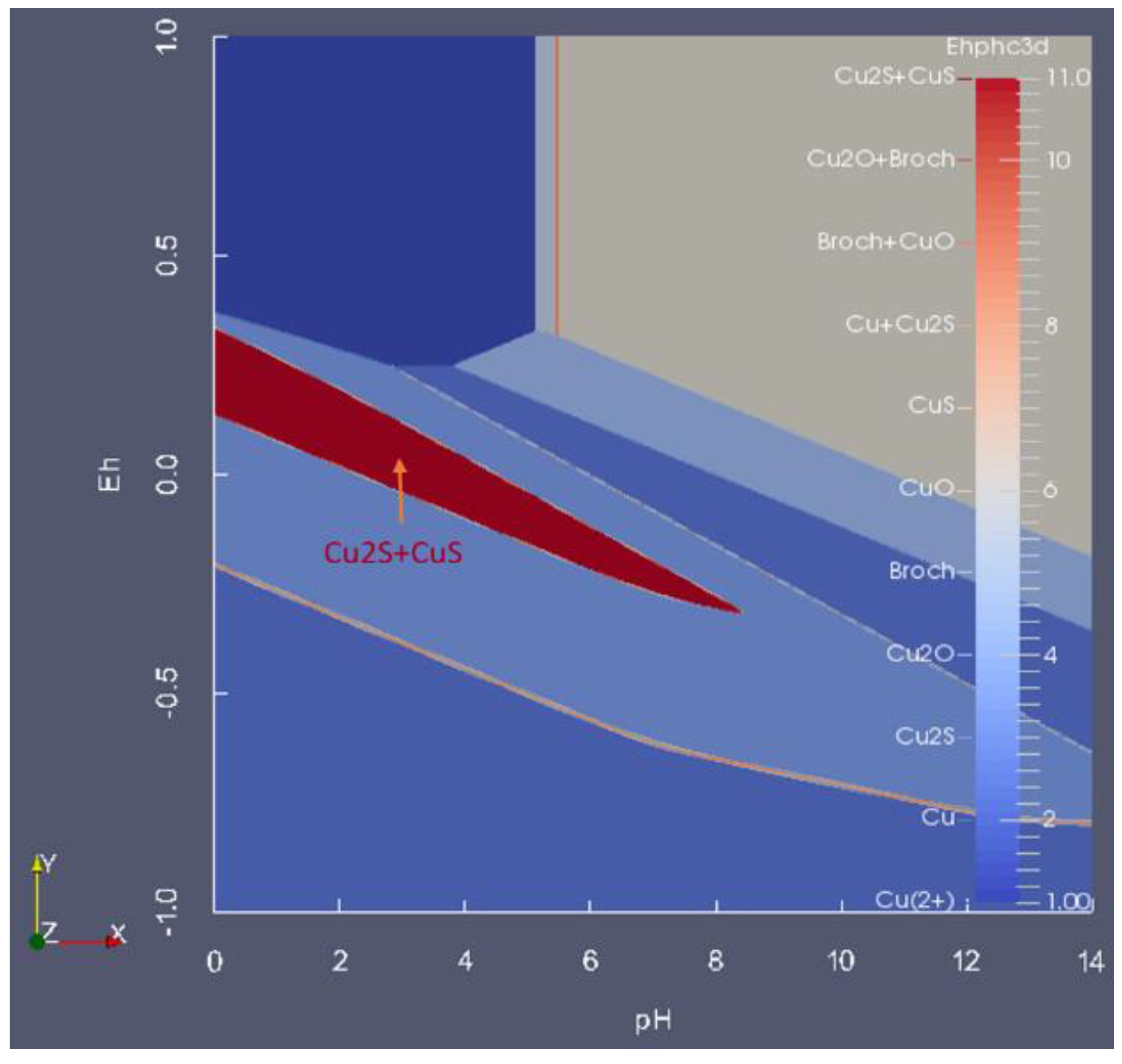
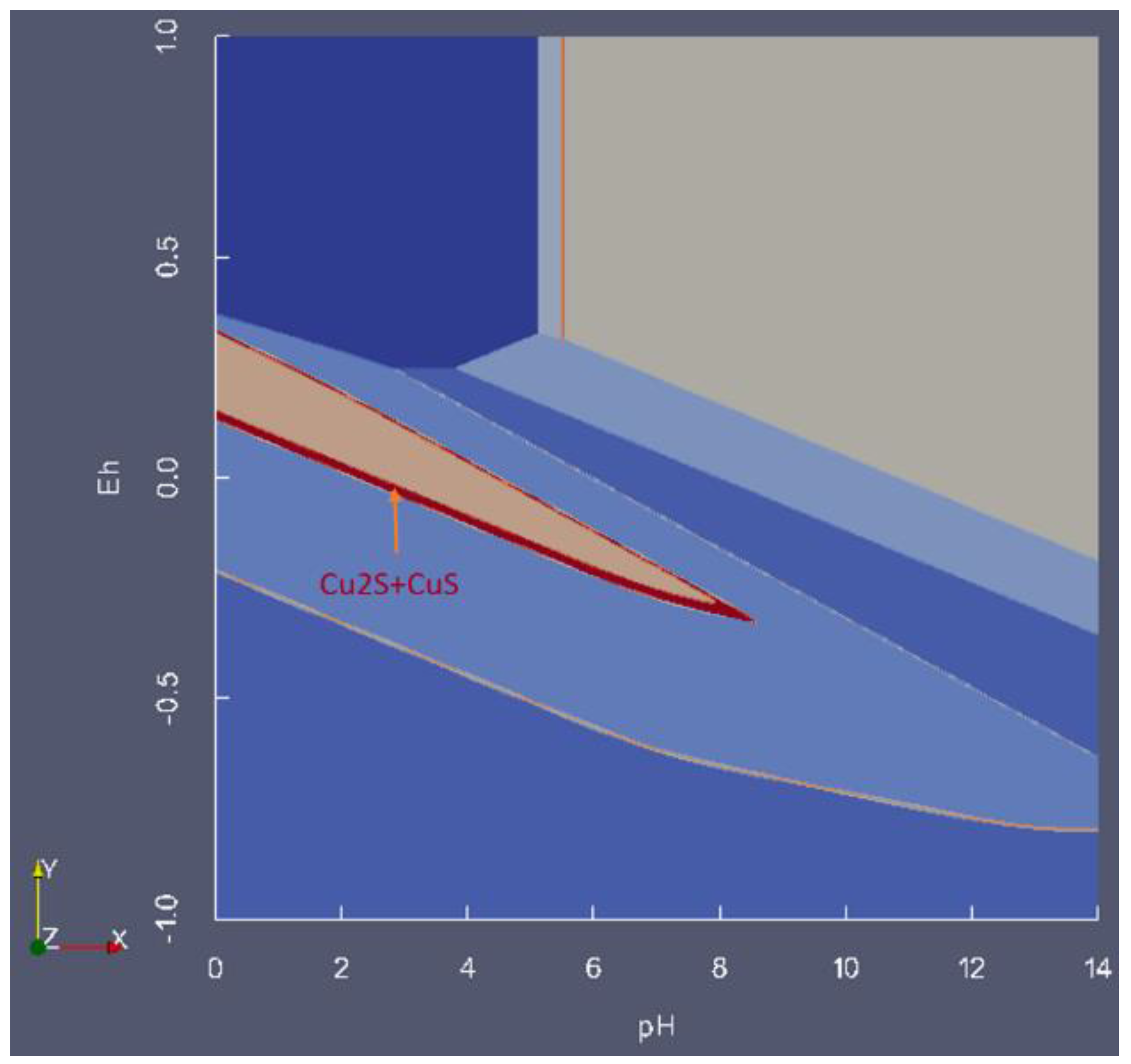
7. Summary
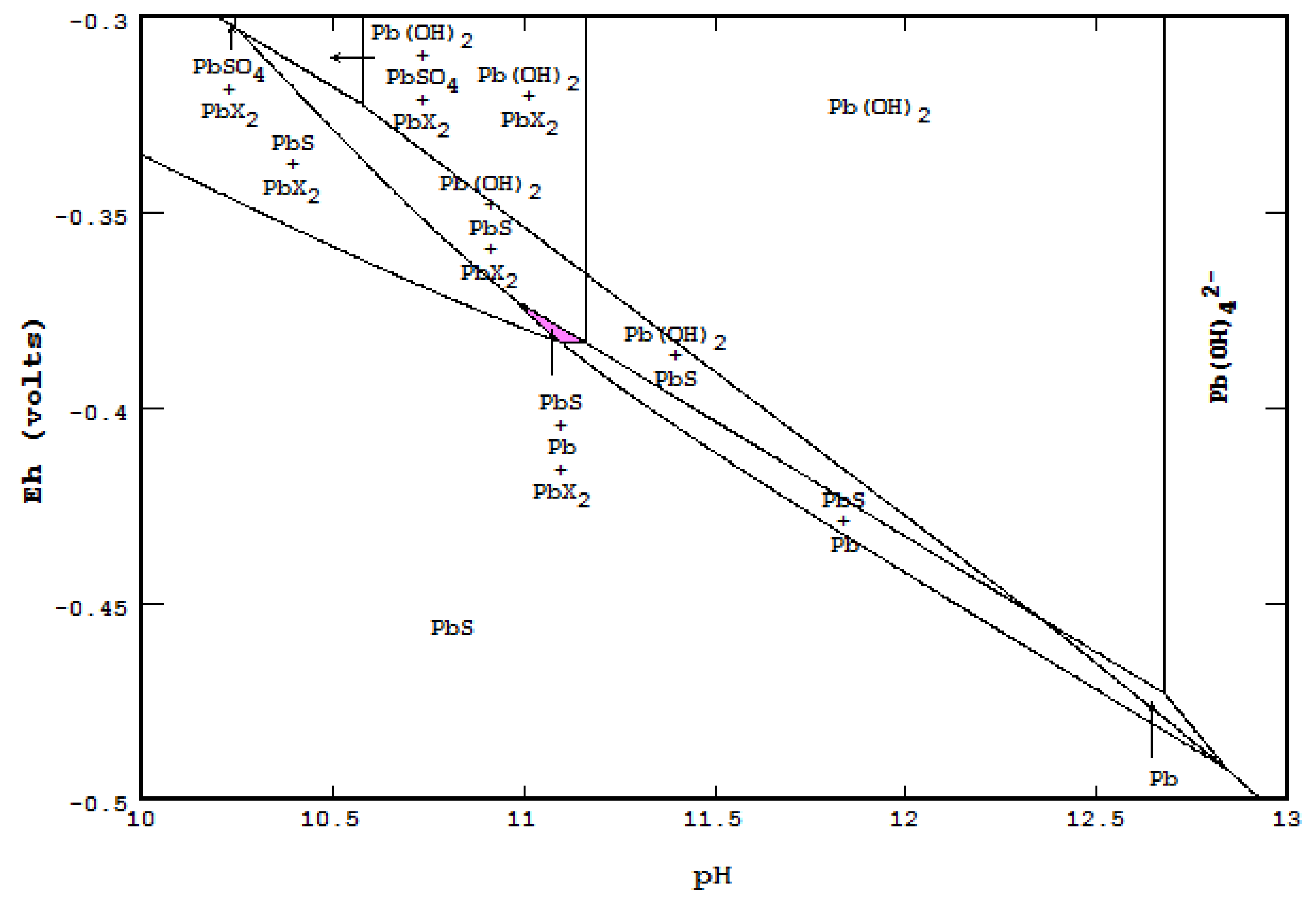
- An Eh-pH diagram that shows solubility. Examples include: lead corrosion prevention with CO2 and concentrations of arsenic adsorption by ferrihydrite; MATLAB was used for more colorful pictures.
- An Eh-pH with an independent variable; diagrams created by ParaView were illustrated for its functionality, and example are:
- The mass balance point method for the Uranium system where the extra axis is log(ΣCO3);
- The equilibrium line method for the Uranium system where the third axis is logp(CO2(g));
- The Cu–S system for showing the coexistence of two or more solids in one volume.
Acknowledgments
Conflicts of Interest
References
- Wagman, D.D.; Evans, W.H.; Parker, V.B.; Schumm, R.H.; Halow, I.; Bailey, S.M.; Churney, K.L.; Nuttall, R.L. The NBS tables of chemical thermodynamic properties. J. Phys. Chem. Ref. Data 1982. [Google Scholar] [CrossRef]
- Garrels, R.M.; Christ, C.L. Eh-pH. In Solutions, Minerals, and Equilibria; Freeman, Cooper & Company: New York, NY, USA, 1975; Chapter 7; pp. 172–277. [Google Scholar]
- STABCAL, version 2015; Stability Calculation for Aqueous and Nonaqueous System; Montana Tech: Butte, MT, USA, 2015.
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solution, 1st ed.; Pergamon Press: Bristol, UK, 1966. [Google Scholar]
- Huang, H.H.; Cuentas, L. Construction of Eh-pH and Other Stability Diagrams of Uranium in a Multicomponent system with a Microcomputer—I. Domains of Predominance Diagram. Can. Metall. Q. 1989, 28, 225–234. [Google Scholar] [CrossRef]
- Forssberg, E.; Antti, B.-M.; Palsson, B. Computer-assisted Calculations of thermodynamic equilibria in the Chalcopyrite-ethyl xanthate system. In Reagents in the Minerals Industry; Jones, M.J., Oblatt, R., Eds.; The Institution of Mining and Metallurgy: London, UK, 1984; pp. 251–264. [Google Scholar]
- Eriksson, G.A. An algorithm for the computation of aqueous multicomponent, multiphase equilibria. Anal. Chim. Acta 1979, 112, 375–383. [Google Scholar] [CrossRef]
- Woods, R.; Yoon, R.H.; Young, C.A. Eh-pH diagrams for stable and metastable phases in copper-sulfur-water system. Int. J. Miner. Process. 1987, 20, 109–120. [Google Scholar] [CrossRef]
- Huang, H.H.; Twidwell, L.G.; Young, C.A. Speciation for aqueous system—An equilibrium calculation approach. In Computational Analysis in Hydrometallurgy—35th Annual Hydrometallurgy Meeting; Dixon, D.G., Dry, M.J., Eds.; CIM: Calgary, AB, Canada, 2005; pp. 295–310. [Google Scholar]
- Dudas, L.; Maass, H.; Bhappu, R. Role of mineralogy in heap and in situ leaching of copper ores. In Solution Mining Symposium; AIME: New York, NY, USA, 1974; pp. 193–201. [Google Scholar]
- LLnL database, version V8.R6.230; Lawrence Livermore National Laboratory: Livermore, CA, USA, 2010.
- Johnson, J.; Oelkers, E.H.; Helgeson, H.C. SUPCRT92: A software package for calculating the Standard molal thermodynamic properties of mineral, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000 °C. Comput. Geosci. 1992, 18, 899–947, The database for the program has be updated as slop98.dat from Geopig group. Available online: https://www.asu.edu/sites/default/files/slop98.dat (accessed on 4 July 2006). [Google Scholar] [CrossRef]
- Kontny, A.; Friedrich, A.; Behr, H.J.; de Wall, H.; Horn, E.E. Formation of ore minerals in metamorphic rocks of the German continental deep drilling site (KTB). J. Geophys. Res. 1997, 102, 18323–18336. [Google Scholar] [CrossRef]
- Huang, H.H. The Application of Revised HKF Model for Thermodynamically Describing Elevated Pressure and Temperature processes such as Treatment of Gold Bearing Materials in Autoclaves. In Hydrometallurgy 2008—Proceedings of the Sixth International Sixth International Symposium; Young, C.A., Taylor, P.R., Anderson, C.G., Choi, Y., Eds.; Society for Mining, Metallurgy, and Exploration (SME): Littleton, CO, USA, 2008; pp. 1066–1077. [Google Scholar]
- Pourbaix, M. Lectures on Electrochemical Corrosion; Plenum Press: New York, NY, USA, 1973; pp. 143–144. [Google Scholar]
- Wang, Q.; Nishimura, T.; Umetsu, Y. Oxidative Precipitation for Arsenic Removal in Effluent Treatment. In SME Minor Elements 2000 Processing and Environmental Aspects of As, Sb, Se, Te and Bi; Young, C.A., Ed.; SME: Littleton, CO, USA, 2000; pp. 39–52. [Google Scholar]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Huang, H.H.; Young, C.A. Mass-Balanced Calculations of Eh-pH diagrams using STABCAL. In Mineral and Metal Processing IV; Woods, R., Ed.; Electrochemical Society: Pennington, NJ, USA, 1996; pp. 227–233. [Google Scholar]
- Gow, R.N.V. Spectroelectrochemistry and Modelling of Enargite (Cu3AsS4) Reactivity under Atmospheric Conditions. Ph.D. Thesis, The University of Montana, Butte, MT, USA, June 2015. [Google Scholar]
- Duaime, T.E.; Tucci, N.J. Butte Mine Flooding Operable Unit: Water-Level Monitoring and Water-Quality Sampling 2011. Available online: http://www.pitwatch.org/download/mbmgannual/BMF-2011.pdf (accessed on 25 Marth 2014).
- Srivastave, R. Estimation and Thermodynamic Modeling of Solid Iron Species in the Berkeley Pit water. M.Sc. Thesis, The University of Montana, Butte, MT, USA, June 2015. [Google Scholar]
- Parkhust, D.L.; Appelo, C.A.A. PHREEQC Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations version 3.3.3. Available online: http://wwwbrr.cr.usgs.gov/projects/GWC_coupled/phreeqc/ (accessed on 13 March 2014).
- Pritzker, M.D.; Yoon, R.H. Thermodynamic Calculations on Sulfide Flotation System: I. Galena-Ethyl Xanthate System in the Absence of Metastable Species. Int. J. Miner. Process. 1984, 12, 95–125. [Google Scholar] [CrossRef]
- Hiskey, J.B.; Wadsworth, M.E. Galvanic Conversion of Chalcopyrite. Metall. Trans. B 1975, 6, 183–190. [Google Scholar] [CrossRef]
- ParaView version 4.3.1 64-bit. Available online: http://www.paraview.org/download/ (accessed on 11 February 2015).
- VisIt 2015 Version 2.4.2. Available online: https://wci.llnl.gov/simulation/computer-codes/visit/ (accessed on 23 April 2012).
- MATLAB; Version R2013a; Mathworks Computer program: Natick, MA, USA, 2013.
- Grossmann, K.; Arnold, T.; Lkeda-Ohno, A.; Steudtner, R.; Geipel, G.; Bernhard, G. Fluorescence properties of a uranyl(V)-carbonate species [U(V)O2(CO3)3]5− at low temperature. Spectrochim. Acta A 2009, 72, 449–453. [Google Scholar] [CrossRef] [PubMed]
- VTK Format. The VTK User’s Guide, Version 4.2, Kitware. 2010. Available online: http://www.vtk.org/wp-content/uploads/2015/04/file-formats.pdf (accessed on 19 February 2015).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-H. The Eh-pH Diagram and Its Advances. Metals 2016, 6, 23. https://doi.org/10.3390/met6010023
Huang H-H. The Eh-pH Diagram and Its Advances. Metals. 2016; 6(1):23. https://doi.org/10.3390/met6010023
Chicago/Turabian StyleHuang, Hsin-Hsiung. 2016. "The Eh-pH Diagram and Its Advances" Metals 6, no. 1: 23. https://doi.org/10.3390/met6010023
APA StyleHuang, H.-H. (2016). The Eh-pH Diagram and Its Advances. Metals, 6(1), 23. https://doi.org/10.3390/met6010023




