Galvanic Corrosion between Alloy 690 and Magnetite in Alkaline Aqueous Solutions
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Material Preparation
| Element (wt. %) | C | Cr | Fe | Si | Mn | Ti | Al | Ni |
|---|---|---|---|---|---|---|---|---|
| Alloy 690 | 0.02 | 28.0 | 10.2 | 0.1 | 0.3 | 0.1 | 0.1 | Bal. |
2.2. Deposition of Magnetite Films
2.3. Electrochemical Tests
3. Results and Discussion
3.1. Electrodeposition of Magnetite
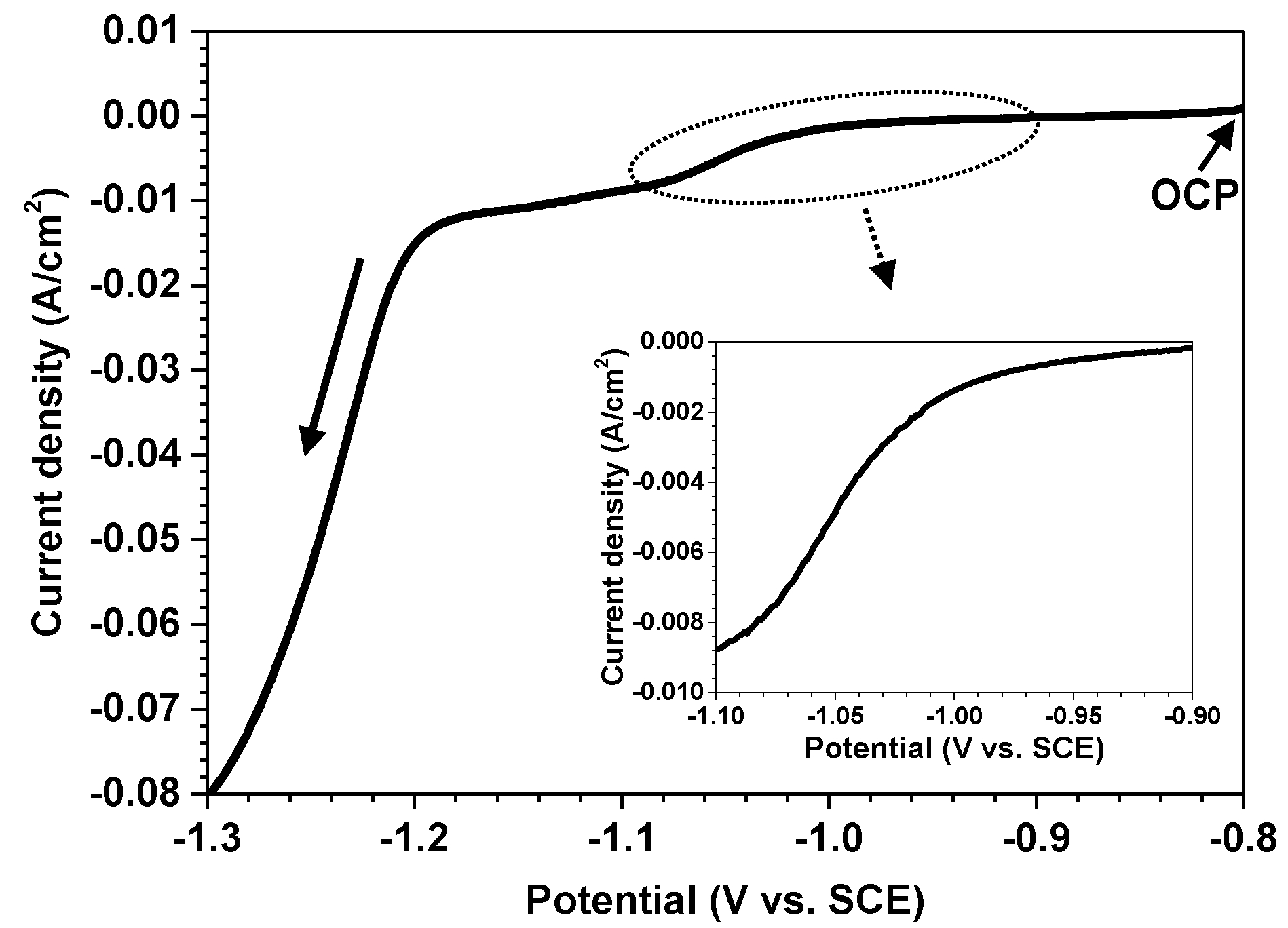
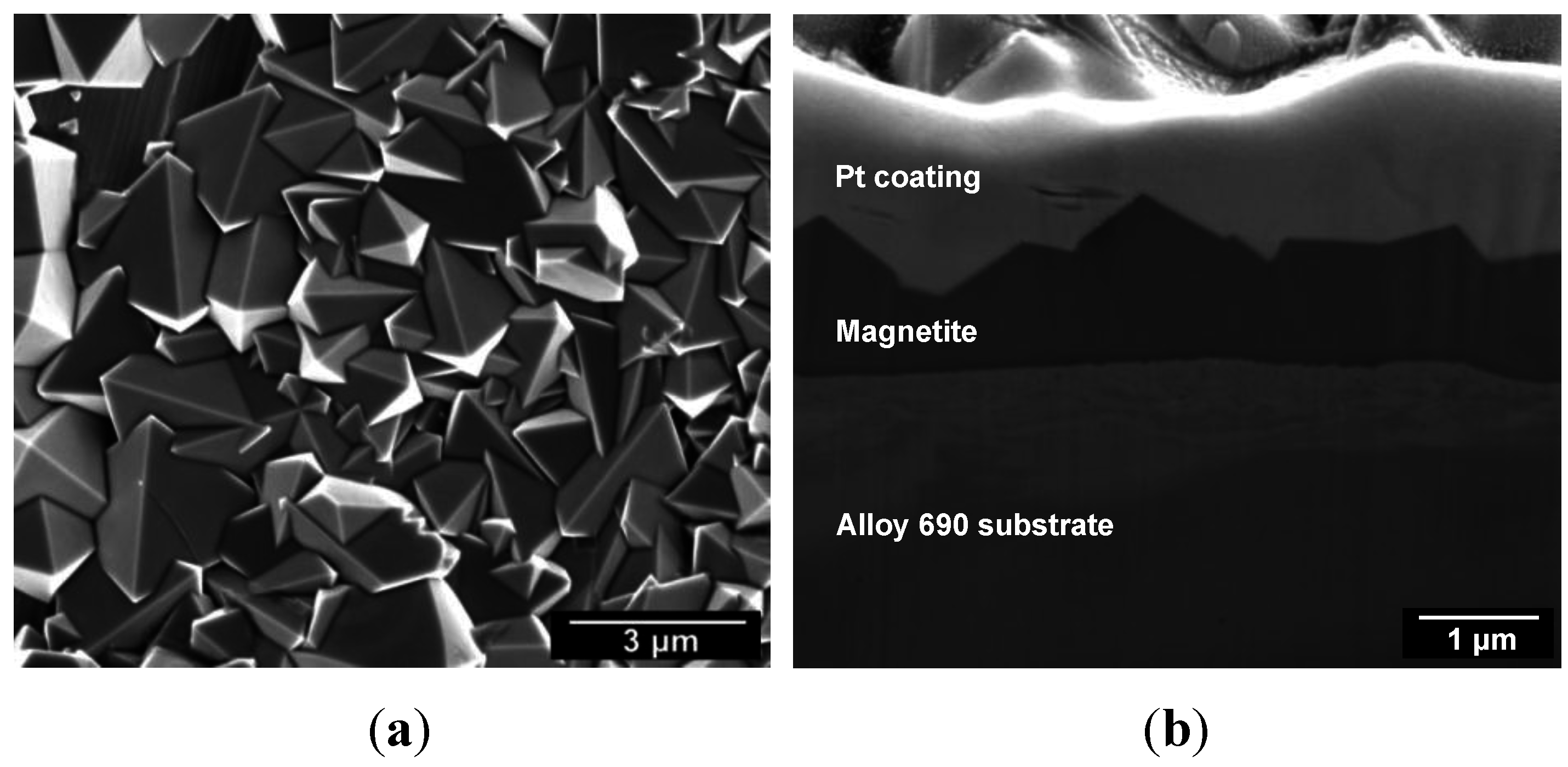

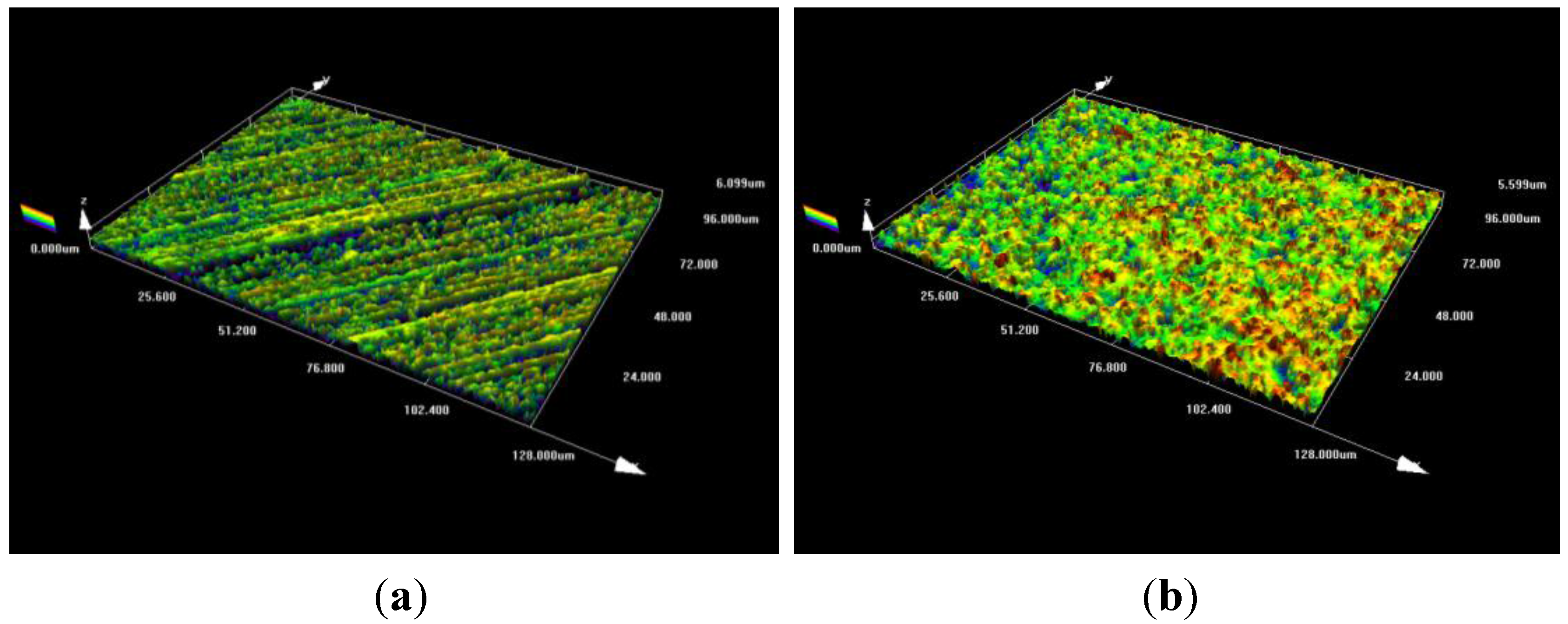
3.2. Electrochemical Behavior of Alloy 690 and Magnetite
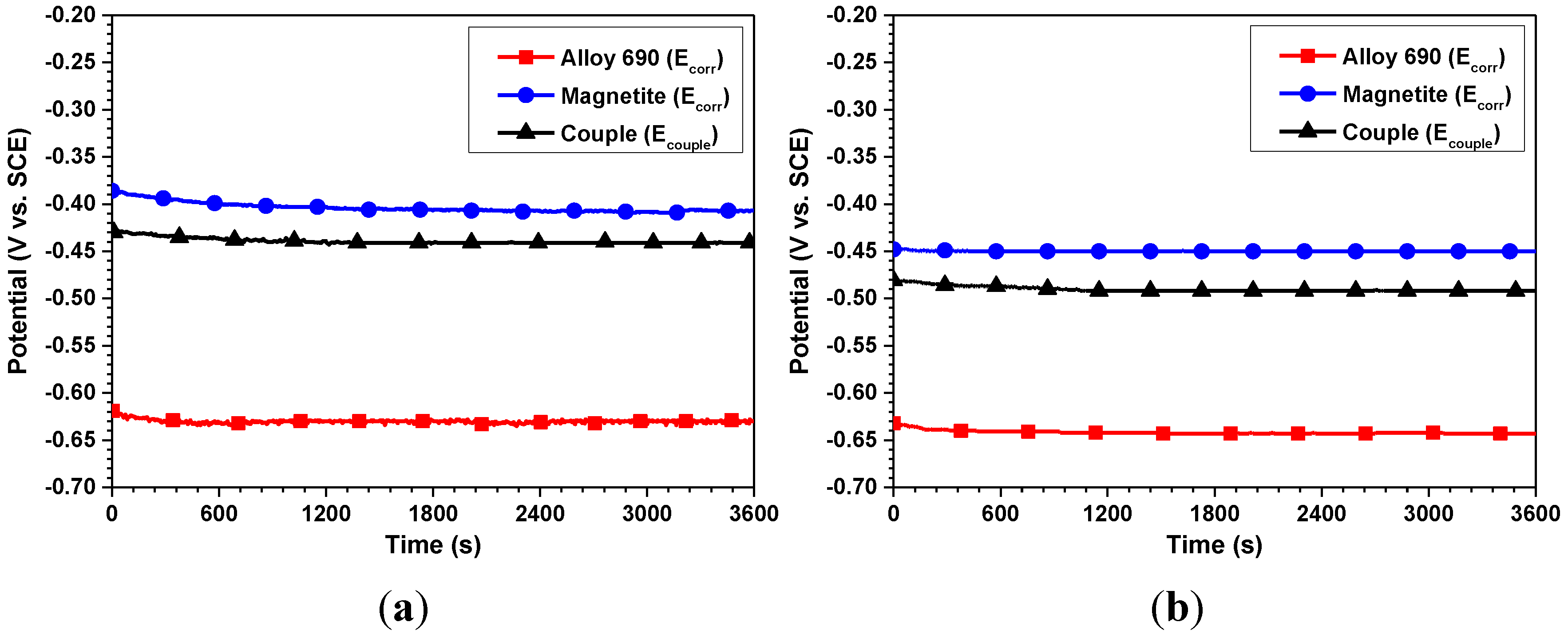
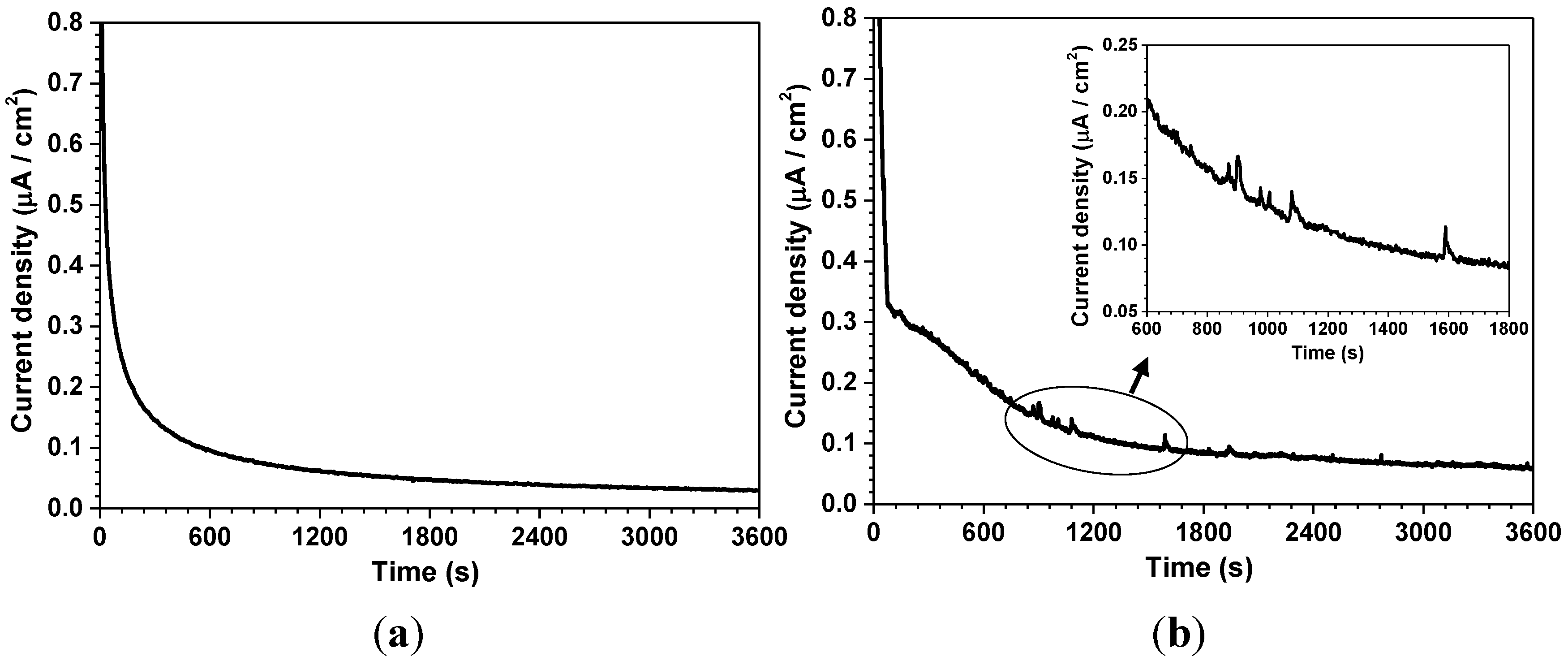
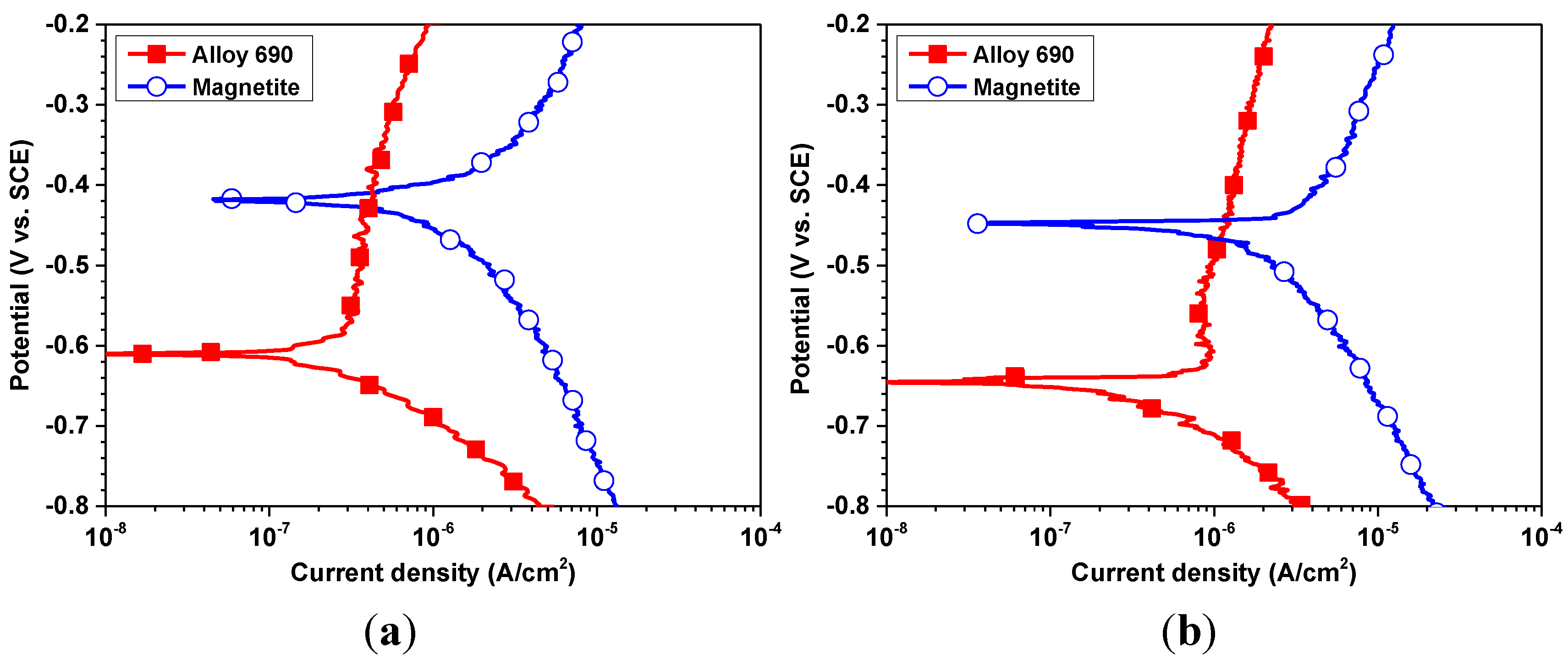
| Corrosion parameters | 30 °C | 60 °C | ||
|---|---|---|---|---|
| Alloy 690 | Magnetite | Alloy 690 | Magnetite | |
| Ecorr (VSCE) | −0.610 ± 0.012 | −0.418 ± 0.008 | −0.647 ± 0.014 | −0.445 ± 0.004 |
| Ecouple (VSCE) | −0.432 ± 0.005 | −0.469 ± 0.010 | ||
| icorr (μA/cm2) | 0.21 ± 0.02 | 0.79 ± 0.08 | 0.41 ± 0.05 | 1.31 ± 0.11 |
| icouple (μA/cm2) | 0.39 ± 0.10 | 1.08 ± 0.17 | ||
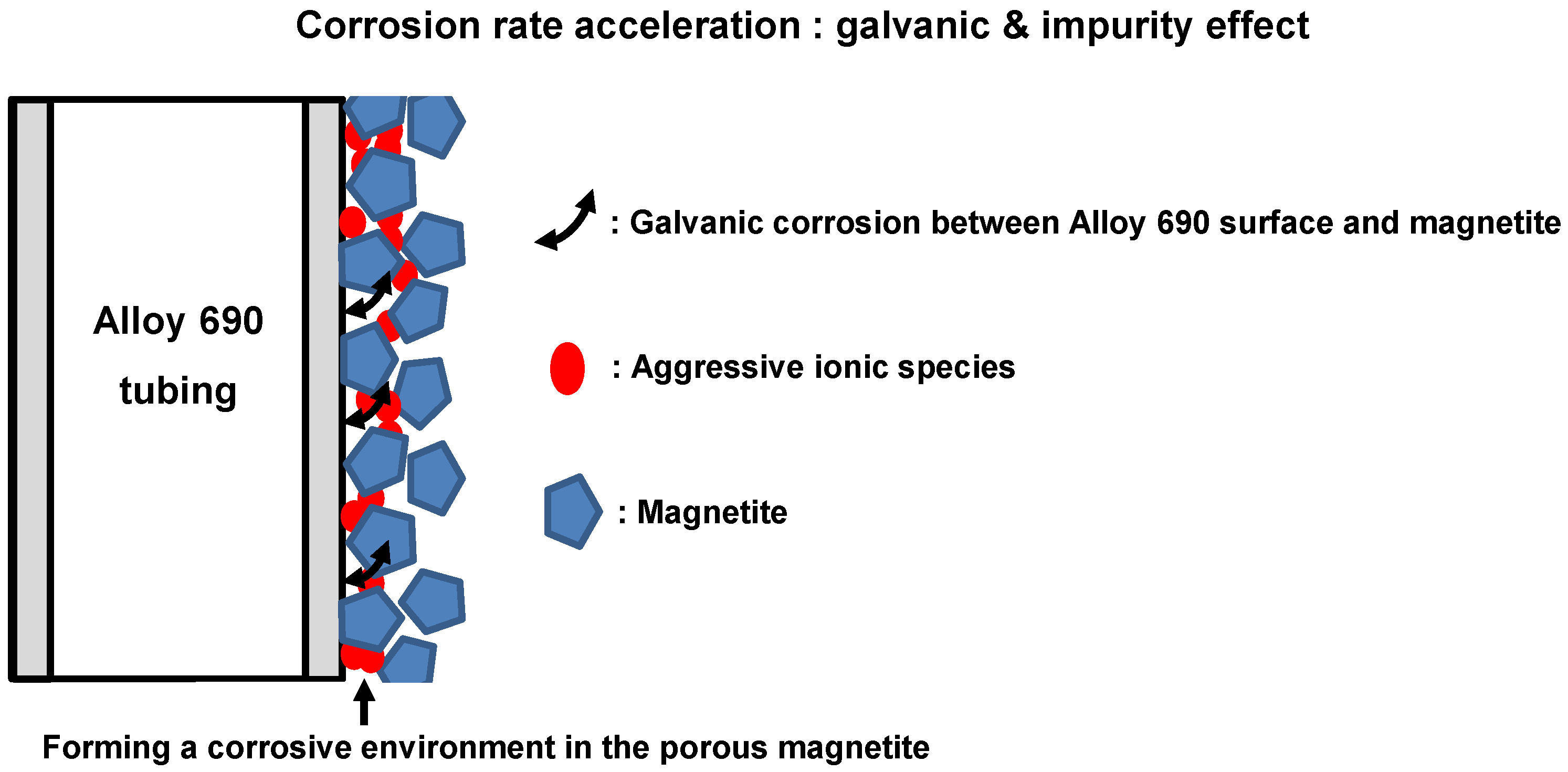
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dutta, R.S. Corrosion aspects of Ni-Cr-Fe based and Ni-Cu based steam generator tube materials. J. Nucl. Mater. 2009, 393, 343–349. [Google Scholar] [CrossRef]
- Ziemniak, S.E.; Guilmette, P.A.; Turcotte, R.A.; Tunison, H.M. Oxidative dissolution of nickel metal in hydrogenated hydrothermal solutions. Corros. Sci. 2008, 50, 449–462. [Google Scholar] [CrossRef]
- Zagal, J.M.; Lopez, H.F.; Flores, O.; Albarran, J.L.; Martinez, L. Microstructural effects on the hydrogen permeation of an Inconel alloy 690. Corros. Sci. 2008, 50, 3371–3377. [Google Scholar] [CrossRef]
- Pandey, M.D.; Datla, S.; Tapping, R.L.; Lu, Y.C. The estimation of lifetime distribution of Alloy 800 steam generator tubing. Nucl. Eng. Des. 2009, 239, 1862–1869. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Han, E.H.; Wu, X.Q. Corrosion behavior of Alloy 690 in aerated supercritical water. Corros. Sci. 2013, 66, 369–379. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Han, E.H.; Ke, W. Influence of dissolved oxygen on oxide films of Alloy 690TT with different surface status in simulated primary water. Corros. Sci. 2011, 53, 3623–3635. [Google Scholar] [CrossRef]
- Kuang, W.; Wu, X.; Han, E.H.; Rao, J. The mechanism of oxide film formation on Alloy 690 in oxygenated high temperature water. Corros. Sci. 2011, 53, 3853–3860. [Google Scholar] [CrossRef]
- Kuang, W.; Wu, X.; Han, E.H. Influence of dissolved oxygen concentration on the oxide film formed on Alloy 690 in high temperature water. Corros. Sci. 2013, 69, 197–204. [Google Scholar] [CrossRef]
- Huang, F.; Wang, J.; Han, E.H.; Ke, W. Microstructural characteristics of the oxide films formed on Alloy 690 TT in pure and primary water at 325 °C. Corros. Sci. 2013, 76, 52–59. [Google Scholar] [CrossRef]
- Plonski, I.H. Effect of bare metal surface on the dissolution in aqueous citrate solutions of magnetite films on carbon steel. J. Appl. Electrochem. 1997, 27, 1184–1192. [Google Scholar] [CrossRef]
- Ramesh, C.; Murugesan, N.; Prince, A.A.M.; Velmurugan, S.; Narasimhan, S.V.; Ganesan, V. Application of polymer electrolyte based hydrogen sensor to study corrosion of carbon steel in acid medium. Corros. Sci. 2001, 43, 1865–1875. [Google Scholar] [CrossRef]
- Prince, A.A.M.; Velmurugan, S.; Narasimhan, S.V.; Ramesh, C.; Murugesan, N.; Raghavan, P.S.; Gopalan, R. Dissolution behaviour of magnetite film formed over carbon steel in dilute organic acid media. Nucl. Mater. 2001, 289, 281–290. [Google Scholar]
- Gonzalez, F.; Spekkens, P. Concentration Processes under Tubesheet Sludge Piles in Nuclear Steam Generators. Nucl. J. Can. 1987, 1, 129–140. [Google Scholar]
- Manahan, M.P. Mechanical behaviour of magnetite from the Oconee-2 and TMI-1 steam generators using miniaturized specimen technology. J. Sci. 1990, 25, 3415–3423. [Google Scholar] [CrossRef]
- Hur, D.H.; Choi, M.S.; Kim, U.C.; Han, J.H. Magnetite dissolution and corrosion behavior in high temperature EDTA solvents. Nucl. Eng. Des. 2003, 220, 11–16. [Google Scholar]
- Carlier, D.; Terrier, C.; Arm, C.; Anserment, J.P. Preparation and magnetic properties of Fe3O4 nanostructures grown by electrodeposition. Electrochem. Solid State Lett. 2005, 8, 43–46. [Google Scholar] [CrossRef]
- Peulon, S.; Antony, H.; Legrand, L.; Chausse, A. Thin layers of iron corrosion products electrochemically deposited on inert substrates: Synthesis and behavior. Electrochim. Acta 2004, 49, 2891–2899. [Google Scholar] [CrossRef]
- Ho, S.Y.; Wang, K.C.; Kuo, S.L.; Wu, N.L. Investigation on capacitance mechanisms of Fe3O4 electrochemical capacitors. Electrochem. Soc. 2006, 153, A75–A80. [Google Scholar]
- Teng, C.L.; Ryan, M.P. A morphological study of nanocrystalline magnetite electrodeposited onto polycrystalline copper substrates. Electrochem. Solid State Lett. 2007, 10, 108–112. [Google Scholar] [CrossRef]
- Mitra, S.; Poizot, P.; Finke, A.; Tarascon, J.M. Growth and electrochemical characterization versus lithium of Fe3O4 electrodes made by electrodeposition. Adv. Funct. Mater. 2006, 16, 2281–2287. [Google Scholar] [CrossRef]
- Kothari, H.M.; Kulp, E.A.; Limmer, S.J.; Poizot, P.; Bohannan, E.W.; Switzer, J.A. Electrochemical deposition and characterization of Fe3O4 films produced by the reduction of Fe(III)-triethanolamine. J. Mater. Res. 2006, 21, 293–301. [Google Scholar] [CrossRef]
- Goujon, C.; Pauporté, T.; Mansour, C.; Delaunary, S.; Bretelle, J.L. Electrochemical deposition of thick iron oxide films on nickel based supperalloy substrates. Electrochim. Acta 2015, 176, 230–239. [Google Scholar]
- Kulp, E.A.; Kothari, H.M.; Limmer, S.J.; Yang, J.; Gudavarthy, R.V.; Bohannan, E.W.; Switzer, J.A. Electrodeposition of epitaxial magnetite films and ferrihydrite nanoribbons on single-crystal gold. Chem. Mater. 2009, 21, 5022–5031. [Google Scholar]
- Mohr, S.; Bechtold, T. Electrochemical behaviour of iron-complexes in presence of competitive ligands: A strategy for optimization of current density. J. Appl. Electrochem. 2001, 31, 363–368. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C.; Souto, R.M. The effect of temperature on the nucleation of corrosion pits on titanium in Ringer's physiological solution. Biomaterials 2005, 26, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Ilevbare, G.O.; Burstein, G.T. The role of alloyed molybdenum in the inhibition of pitting corrosion in stainless steels. Corros. Sci. 2001, 43, 485–513. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C. Nucleation of corrosion pits in Ringer’s solution containing bovine serum. Corros. Sci. 2007, 49, 4296–4306. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C. Depassivation current transients measured between identical twin microelectrodes in open circuit. Corros. Sci. 2008, 50, 2–7. [Google Scholar] [CrossRef]
- Jung, K.S.; Sung, K.W. Magnetite: Structure, Properties and Applications; Nova Science Publishers: New York, NY, USA, 2010; pp. 261–298. [Google Scholar]
- Steahle, R.W.; Gorman, J.A. Quantitative assessment of submodes of stress corrosion cracking on the secondary side of steam generator tubing in pressurized water reactors: Part I. Corrosion 2003, 59, 931–994. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.-H.; Song, G.-D.; Hur, D.H. Galvanic Corrosion between Alloy 690 and Magnetite in Alkaline Aqueous Solutions. Metals 2015, 5, 2372-2382. https://doi.org/10.3390/met5042372
Jeon S-H, Song G-D, Hur DH. Galvanic Corrosion between Alloy 690 and Magnetite in Alkaline Aqueous Solutions. Metals. 2015; 5(4):2372-2382. https://doi.org/10.3390/met5042372
Chicago/Turabian StyleJeon, Soon-Hyeok, Geun-Dong Song, and Do Haeng Hur. 2015. "Galvanic Corrosion between Alloy 690 and Magnetite in Alkaline Aqueous Solutions" Metals 5, no. 4: 2372-2382. https://doi.org/10.3390/met5042372
APA StyleJeon, S.-H., Song, G.-D., & Hur, D. H. (2015). Galvanic Corrosion between Alloy 690 and Magnetite in Alkaline Aqueous Solutions. Metals, 5(4), 2372-2382. https://doi.org/10.3390/met5042372








