Resistance of Hydrogenated Titanium-Doped Diamond-Like Carbon Film to Hyperthermal Atomic Oxygen
Abstract
:1. Introduction
2. Experimental Section
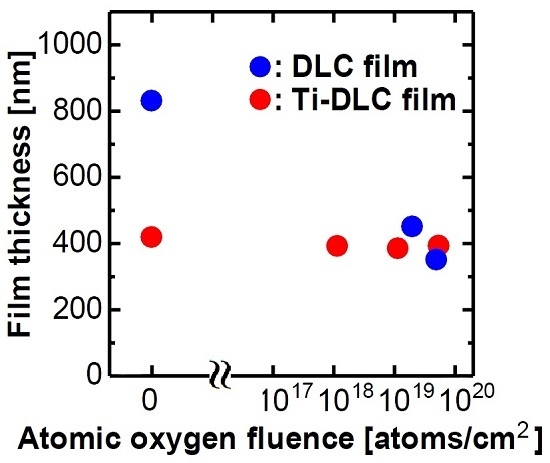
3. Results and Discussion

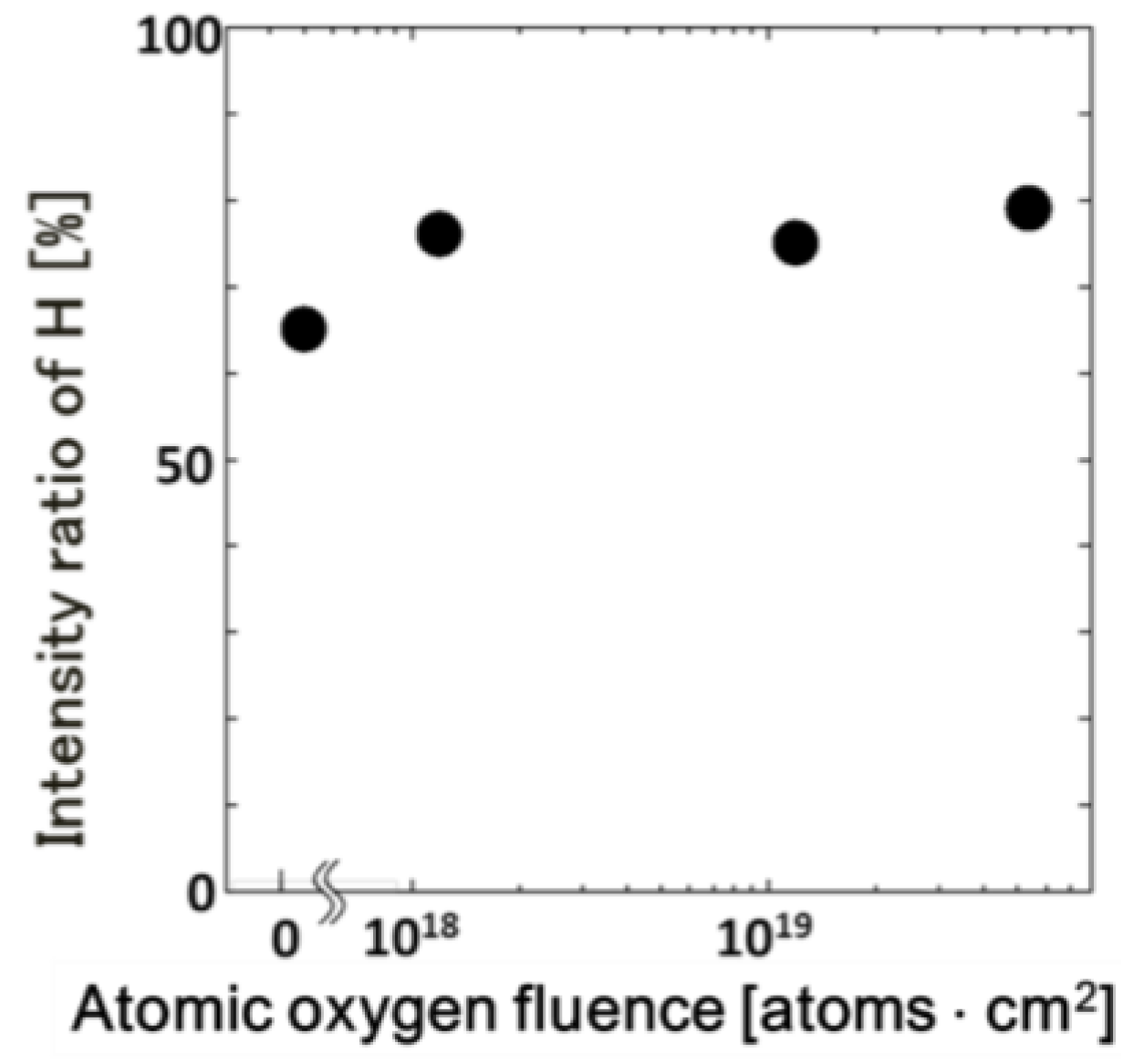
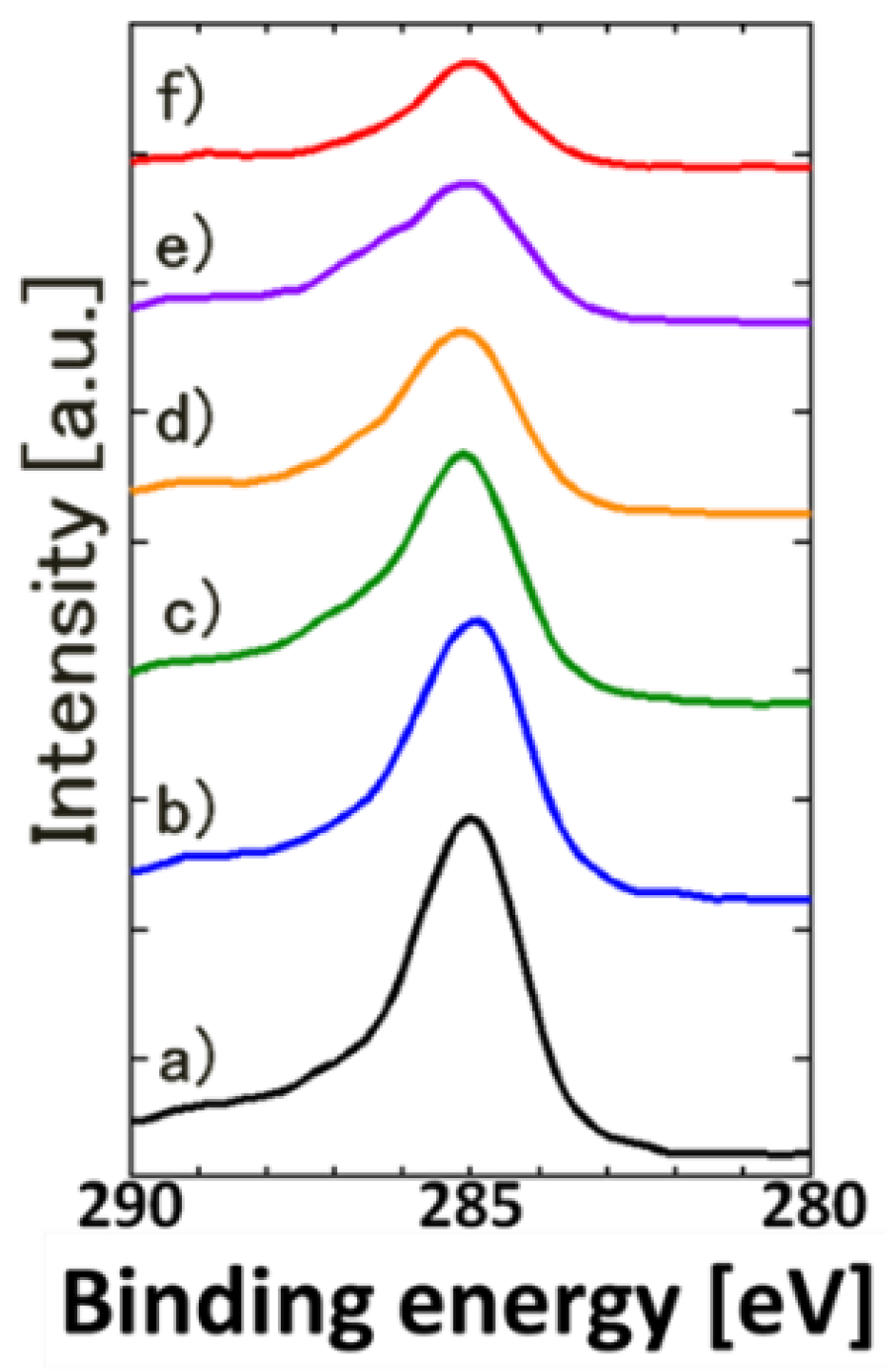
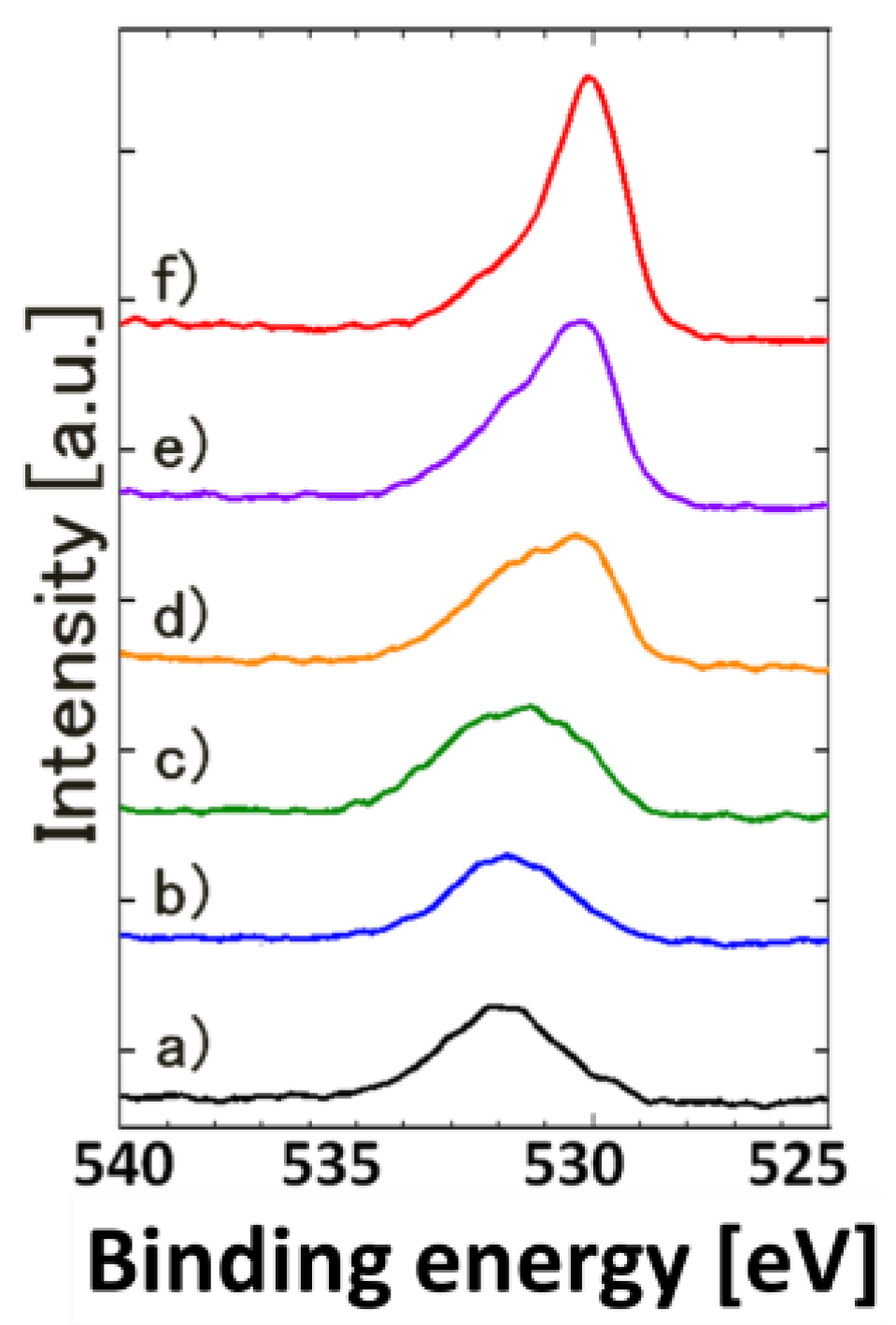
| Fluence | C | O | Ti |
|---|---|---|---|
| atoms·cm−2 | at.% | ||
| 0 | 79 | 18 | 3 |
| 1.16 × 1017 | 77 | 19 | 4 |
| 1.16 × 1018 | 68 | 25 | 7 |
| 3.52 × 1018 | 59 | 29 | 13 |
| 1.16 × 1019 | 44 | 38 | 18 |
| 2.74 × 1019 | 37 | 36 | 27 |
| 5.47 × 1019 | 35 | 35 | 30 |
| 8.3 × 1019 | 22 | 40 | 38 |
| 1.85 × 1020 | 24 | 41 | 35 |


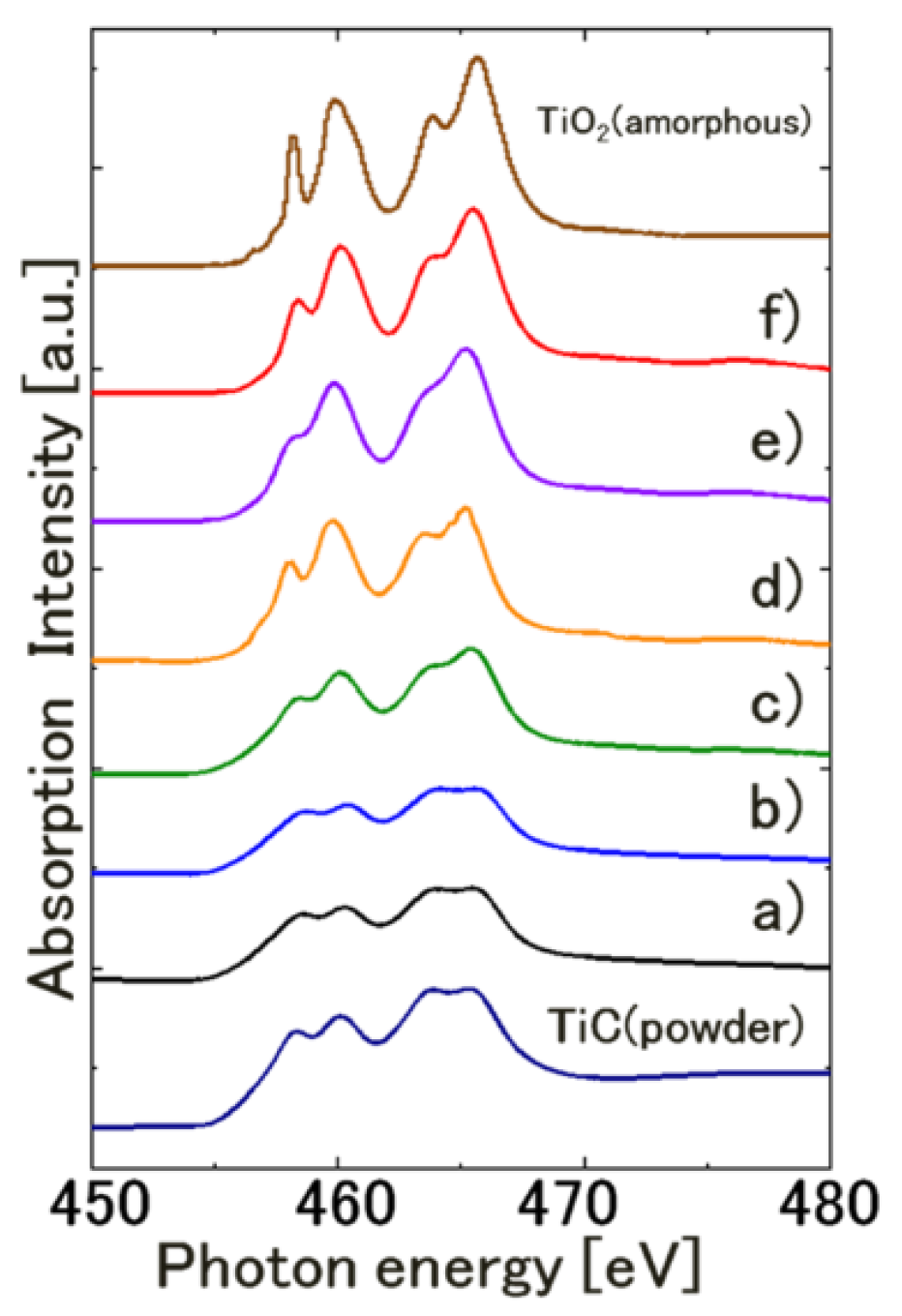
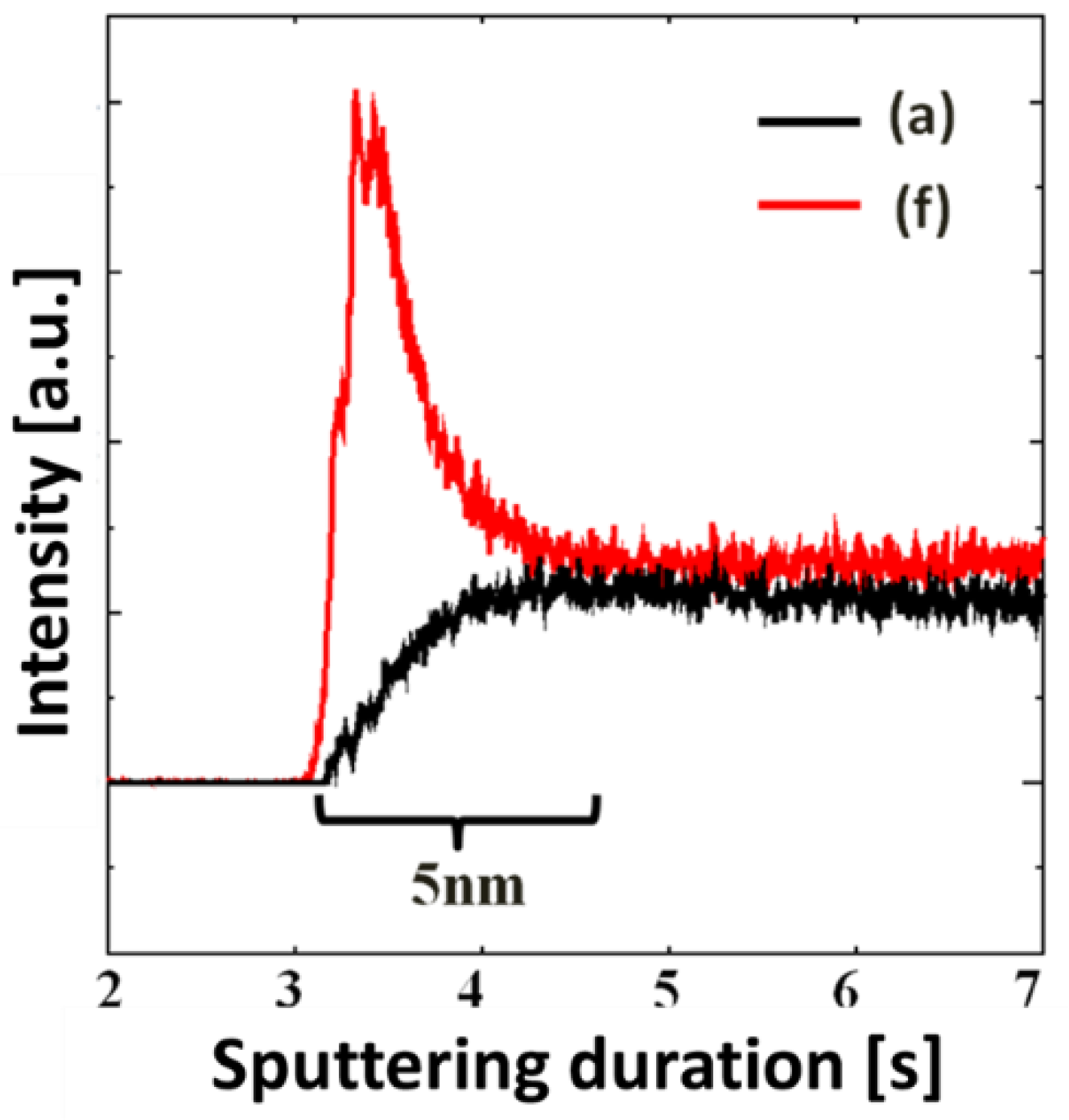

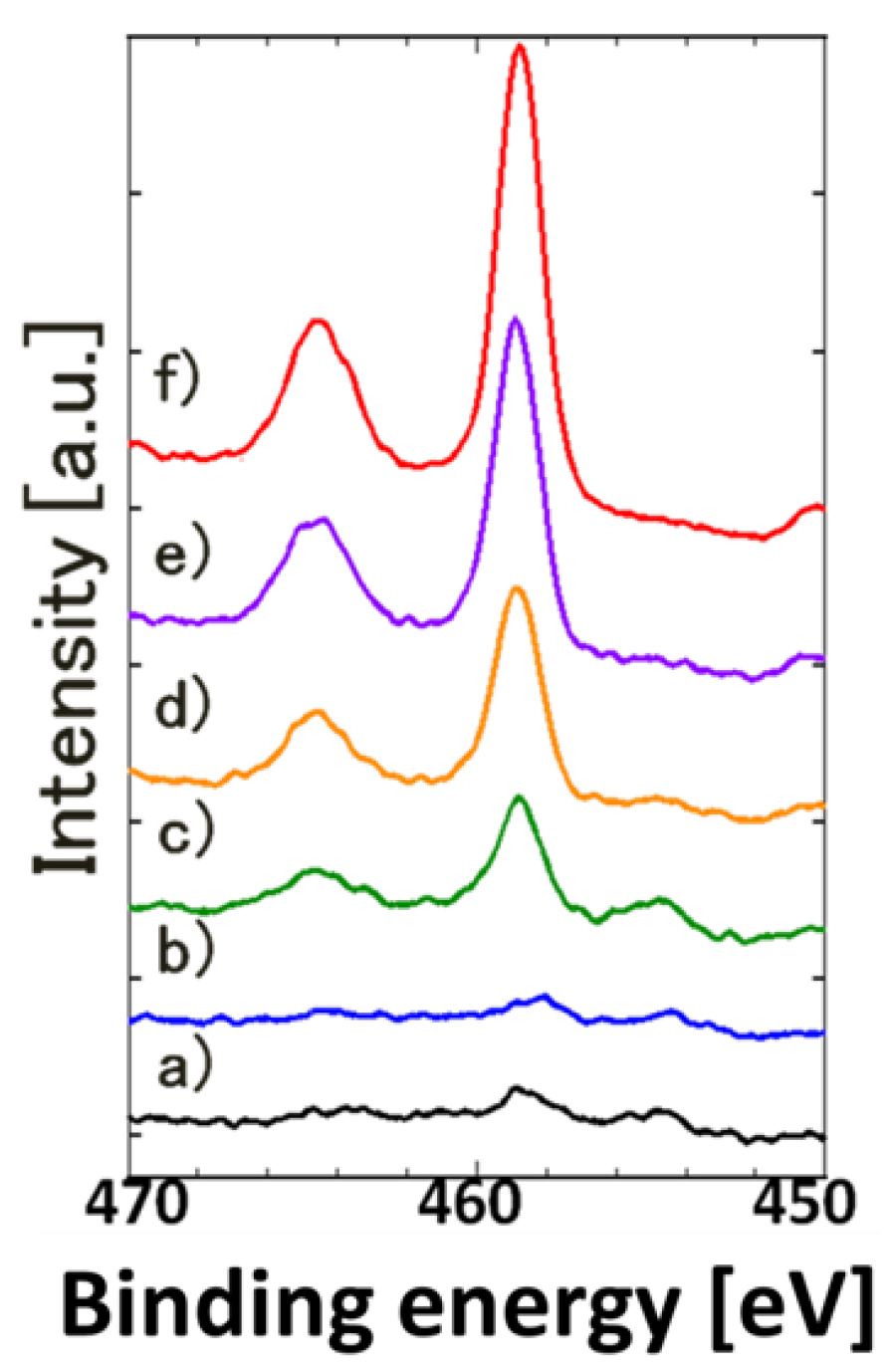
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crutcher, E.R.; Nishimura, L.S.; Warner, K.J.; Wascher, W.W. Silver Teflon Blanket: LDEF Tray C-08. In Proceedings of LDEF–69 Months in Space: First Post-Retrieval Symposium, Kissimmee, FL, USA, 2–8 June 1991; pp. 861–874.
- Banks, B.A.; de Groh, K.K.; Miller, S.K. Low Earth Orbital Atomic Oxygen Interactions with Spacecraft Materials. In Proceedings of the 2004 Fall Meeting, Boston, MA, USA, November 29–December 3, 2014; pp. 1–12.
- Han, J.H.; Kim, C.G. Low earth orbit space environment simulation and its effects on graphite/epoxy composites. Compos. Struct. 2006, 72, 218–226. [Google Scholar] [CrossRef]
- Samwel, S.W. Low Earth Orbit Atomic Oxygen Erosion Effect on Spacecraft Materials. Space Res. J. 2014, 7, 1–13. [Google Scholar]
- Aisenberg, S.; Chabot, S. Deposition of carbon films with diamond properties. Carbon 1972, 10, 356. [Google Scholar] [CrossRef]
- Robertson, J. Properties of diamond-like carbon. Surf. Coat. Technol. 1992, 50, 185–203. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Podogornik, B.; Vizintin, J. Tribological reaction between oil additives and DLC coatings for automotive applications. Surf. Coat. Technol. 2005, 200, 1982–1989. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films-from first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Luo, J.K.; Fu, Y.Q.; Le, H.R.; Williams, J.A.; Spearing, S.M.; Milne, W.I. Diamond and daiamond-like carbon MEMS. J. Micromech. Microeng. 2007, 17, S147–S163. [Google Scholar] [CrossRef]
- Donnet, C.; Belin, M.; Auge, J.C.; Martin, J.M.; Grill, A.; Patel, V. Tribochemistry of diamond-like carbon coatings in various environments. Surf. Coat. Technol. 1994, 68–69, 626–631. [Google Scholar] [CrossRef]
- Tagawa, M.; Kumiko, K.; Kitamura, A.; Matsumoto, K.; Yoshigoe, A.; Teraoka, Y.; Kanda, K.; Niibe, M. Synchrotron radiation photoelectron spectroscopy and near-edge X-ray absorption fine structure study on oxidative etching of diamond-like carbon films by hyperthermal atomic oxygen. Appl. Surf. Sci. 2010, 256, 7678–7683. [Google Scholar] [CrossRef]
- Oguri, K.; Arai, T. Tribological properties and characterization of diamond-like carbon coatings with silicon prepared by plasma-assisted chemical vapor deposition. Surf. Coat. Technol. 1991, 47, 710–721. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Rebholz, C.; Matthews, A. Comparative tribology studies of hard ceramic and composite metal-DLC coatings in sliding fraction conitions. Tribol. Trans. 1995, 38, 829–836. [Google Scholar] [CrossRef]
- Silva, S.R.P.; Robertson, J.; Amaratunga, G.A.J. Nitrogen modification of hydrogenated amorphous carbon films. J. Appl. Phys. 1997, 81, 2626–2634. [Google Scholar] [CrossRef]
- Kim, M.; Lee, K.; Eun, K. Tribological behavior of silicon-incorporated diamond-like carbon films. Surf. Coat. Technol. 1999, 112, 204–209. [Google Scholar] [CrossRef]
- Varma, A.; Palshin, V.; Meletis, E.I. Structure-property relationship of Si-DLC films. Surf. Coat. Technol. 2001, 148, 305–314. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R. Deposition and characterization of Ti- and W-containing diamonod-like carbon films by plasma source ion implantation. Surf. Coat. Technol. 2003, 169, 287–290. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Chen, W.; Mu, Z.; Zhang, C.; Wang, L. The study of doped DLC films by Ti ion implantation. Thin Solid Films. 2005, 475, 279–282. [Google Scholar]
- Ouyang, J.H.; Sasaki, S. Friction and wear characteristics of a Ti-containing diamond-like carbon coating with an SRV tester high contact load and elevated temperature. Surf. Coat. Technol. 2005, 195, 234–244. [Google Scholar] [CrossRef]
- Iseki, T.; Mori, H.; Hasegawa, H.; Tachikawa, H.; Nakanishi, K. Structural analysis of Si-containing diamond-like carbon. Diam. Relat. Mater. 2006, 15, 1004–1010. [Google Scholar] [CrossRef]
- Zhao, F.; Li, H.; Jo, L.; Wang, Y.; Zhou, H.; Chen, J. Ti-DLC films with superior friction performance. Diam. Relat. Mater. 2010, 19, 342–349. [Google Scholar] [CrossRef]
- Caschera, D.; Federici, F.; Pandolfi, L.; Kaciulis, S.; Sebastiani, M.; Bemporad, E.; Padeletti, G. Effect of composition on mechanical behaviour of diamond-like carbon coatings modified with titanium. Thin Solid Films 2011, 519, 3061–3067. [Google Scholar] [CrossRef]
- Hofmann, D.; Kunkel, S.; Bewilogua, K.; Wittorf, R. From DLC to Si-DLC layer systems with properties for tribological applications. Surf. Coat. Technol. 2013, 215, 357–363. [Google Scholar] [CrossRef]
- Amanov, A.; Watave, T.; Tsuboi, R.; Sakaki, S. Improvement in the tribological characteristics of Si-DLC coating by laser surface texturing under oil-lubricated point contacts at various temperatures. Suf. Coat. Technol. 2013, 232, 549–560. [Google Scholar] [CrossRef]
- Lubwama, M.; Corcoran, B.; McDonnell, K.A.; Dowling, D.; Kirabira, J.B.; Sebbit, A.; Sayers, K. Flexibility and frictional behaviour of DLC and Si-DLC films deposited on nitrile rubber. Surf. Coat. Technol. 2014, 239, 84–94. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Liao, Z.; Wang, Q.; Tyagi, R.; Liu, W. Tribological behaviour of titanium alloy modified by carbon–DLC composite film. Surf. Eng. 2015. [Google Scholar] [CrossRef]
- Kanda, K.; Fukuda, K.; Kidena, K.; Imai, R.; Niibe, M.; Fujimoto, S.; Yokota, K.; Tagawa, M. Hyperthermal atomic oxygen beam irradiation effect on the Ti-containing DLC film. Diam. Relat. Mater. 2014, 41, 49–52. [Google Scholar] [CrossRef]
- Nakahigashi, T.; Tanaka, Y.; Miyake, K.; Oohara, H. Properties of flexible DLC film deposited by amplitude-modulated RE P-CVD. Triol. Int. 2004, 37, 907–912. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K.; Ohmae, N.; Kinoshita, H.; Umeno, M. Oxidation Properties of Hydrogen-Terminated Si(001) Surfaces Following Use of a Hyperthermal Broad Atomic Oxygen Beam at Law Temperatures. Jpn. J. Appl. Phys. 2001, 40, 6152–6156. [Google Scholar] [CrossRef]
- Yokota, K.; Tagawa, M.; Ohmae, N. Temperature Dependence in Erosion Rates of Polyimide under Hyerthermal Atomic Oxygen Exposures. J. Spacecr. Rockets 2003, 40, 143. [Google Scholar] [CrossRef]
- Kitamura, A.; Tamai, T.; Taniike, A.; Furuyama, Y.; Maeda, T.; Ogiwara, N.; Saidoh, M. Simulation of ERD spectra for a surface with a periodic roughness. Nucl. Instrum. Methods Phys. Res. Sect. B 1998, 134, 98–106. [Google Scholar]
- Niibe, M.; Mukai, M.; Miyamoto, S.; Shoji, Y.; Hashimoto, S.; Ando, A.; Tanaka, T.; Miyai, M.; Kitamura, H. Characterization of light radiated from 11 m long undulator. AIP Conf. Proc. 2004, 705, 576–579. [Google Scholar]
- Niibe, M.; Mukai, M.; Kimura, H.; Shoji, Y. Polarization property measurement of the long undulator radiation using Cr/C multilayer polarization elements. AIP Conf. Proc. 2004, 705, 243–246. [Google Scholar]
- Saikubo, A.; Kanda, K.; Niibe, M.; Matsui, S. Near-edge X-ray absorption fine-structure characterization of diamond-like-carbon thin films formed by various method. New Diam. Front. Carbon Technol. 2006, 16, 235–244. [Google Scholar]
- Saikubo, A.; Yamada, N.; Kanda, K.; Matsui, S.; Suzuki, T.; Niihara, K.; Saitoh, H. Comprehensive classification of DLC films formed by various methods using NEXAFS measurement. Diam. Relat. Mater. 2008, 17, 1743–1745. [Google Scholar] [CrossRef]
- Nam, S.H.; Cho, S.J.; Jung, C.K.; Boo, J.H.; Šícha, J.; Heřman, D.; Musil, J.; Vlček, J. Comparison of hydrophilic properties of TiO2 thin films prepared by sol-gel method and reactive magnetron sputtering system. Thin Solid Films 2011, 519, 6944–6950. [Google Scholar] [CrossRef]
- Kanda, K.; Niibe, M.; Wada, A.; Ito, H.; Suzuki, T.; Ohana, T.; Ohtake, N.; Saitoh, H. Comprehensive Classification of Near-Edge X-ray Absorption Fine Structure Spectra of Si-Containing Diamond-Like Carbon thin Films. Jpn. J. Appl. Phys. 2013, 52, 095504. [Google Scholar] [CrossRef]
- Magnuson, M.; Lewin, E.; Hultman, L.; Jansson, U. Electronic structure and chemical bonding of nanocrystalline-TiC/amorphous-C nanocomposites. Phys. Rev. B 2009, 80, 235108. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Buuren, T.V.; Baumann, T.F.; Satcher, J.H., Jr.; Willey, T.M.; Meulenberg, R.W.; Felter, T.E.; Poco, J.F.; Gammon, S.A.; Terminello, L.J. Electronic structure of titania aerogels: Soft X-ray absorption study. Phys. Rev. B 2004, 69, 245102. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K.; Ochi, K.; Akiyama, M.; Matsumoto, K.; Suzuki, M. Comparison of Macro and Microtribological Property of Molybdenum Disulfide Film Exposed to LEO Space Environment. Tribol. Lett. 2012, 45, 349–356. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kidena, K.; Endo, M.; Takamatsu, H.; Niibe, M.; Tagawa, M.; Yokota, K.; Furuyama, Y.; Komatsu, K.; Saitoh, H.; Kanda, K. Resistance of Hydrogenated Titanium-Doped Diamond-Like Carbon Film to Hyperthermal Atomic Oxygen. Metals 2015, 5, 1957-1970. https://doi.org/10.3390/met5041957
Kidena K, Endo M, Takamatsu H, Niibe M, Tagawa M, Yokota K, Furuyama Y, Komatsu K, Saitoh H, Kanda K. Resistance of Hydrogenated Titanium-Doped Diamond-Like Carbon Film to Hyperthermal Atomic Oxygen. Metals. 2015; 5(4):1957-1970. https://doi.org/10.3390/met5041957
Chicago/Turabian StyleKidena, Kengo, Minami Endo, Hiroki Takamatsu, Masahito Niibe, Masahito Tagawa, Kumiko Yokota, Yuichi Furuyama, Keiji Komatsu, Hidetoshi Saitoh, and Kazuhiro Kanda. 2015. "Resistance of Hydrogenated Titanium-Doped Diamond-Like Carbon Film to Hyperthermal Atomic Oxygen" Metals 5, no. 4: 1957-1970. https://doi.org/10.3390/met5041957
APA StyleKidena, K., Endo, M., Takamatsu, H., Niibe, M., Tagawa, M., Yokota, K., Furuyama, Y., Komatsu, K., Saitoh, H., & Kanda, K. (2015). Resistance of Hydrogenated Titanium-Doped Diamond-Like Carbon Film to Hyperthermal Atomic Oxygen. Metals, 5(4), 1957-1970. https://doi.org/10.3390/met5041957