Removal of Zn from Contaminated Sediment by FeCl3 in HCl Solution
Abstract
:1. Introduction
2. Experiments
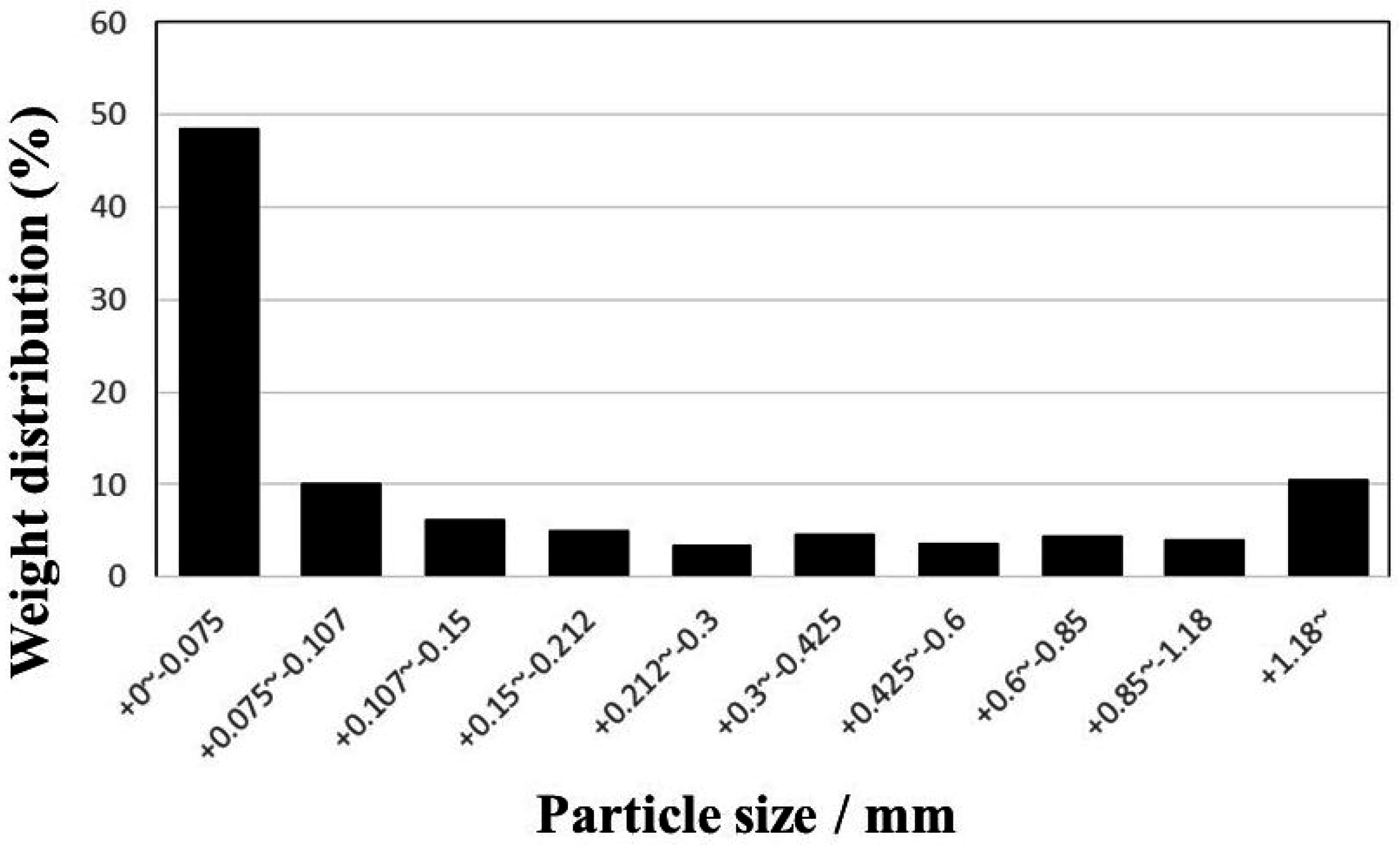
2.1. Materials
2.2. Leaching Procedures and Analytical Methods
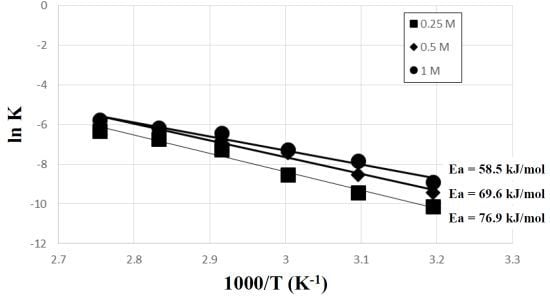
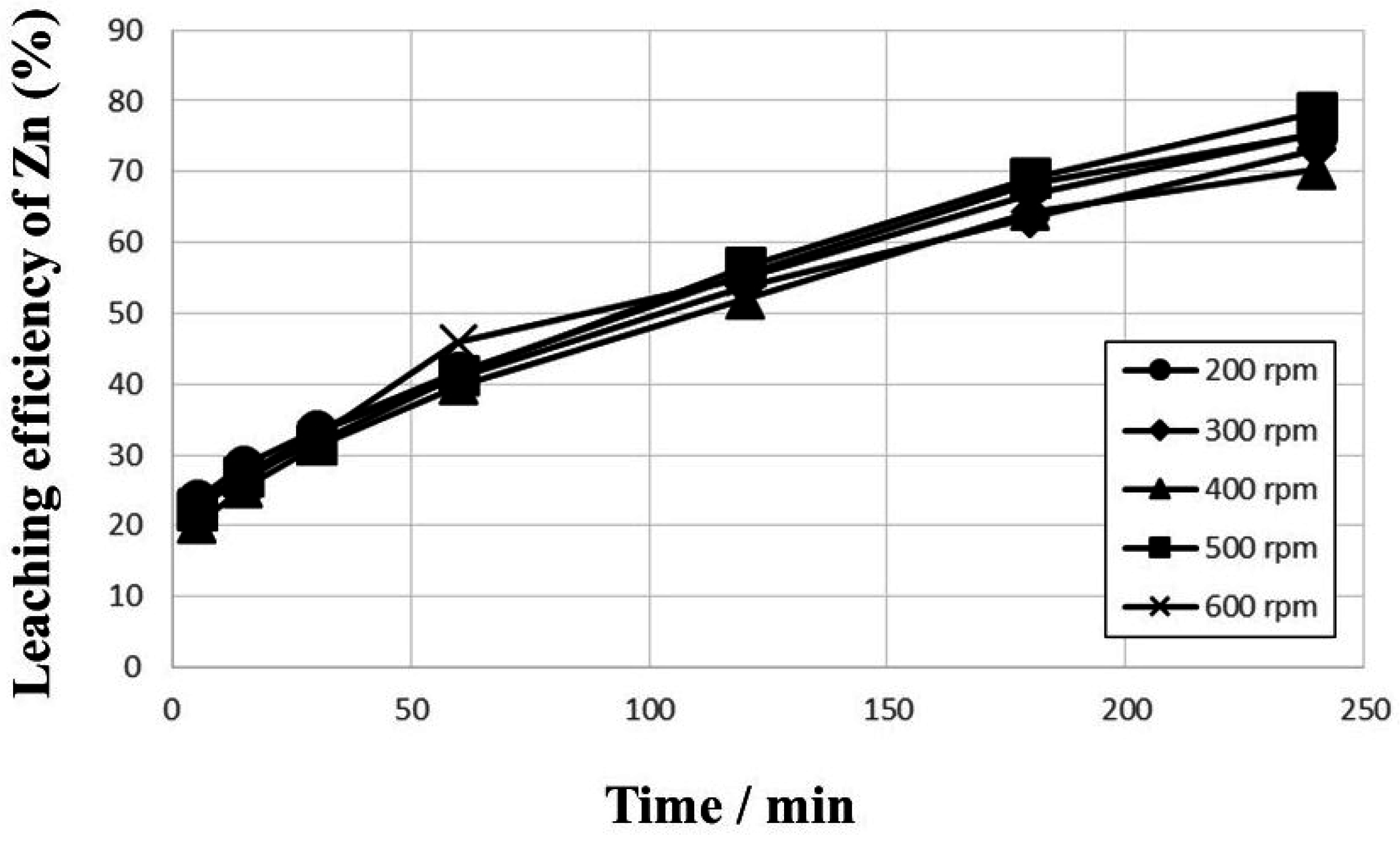
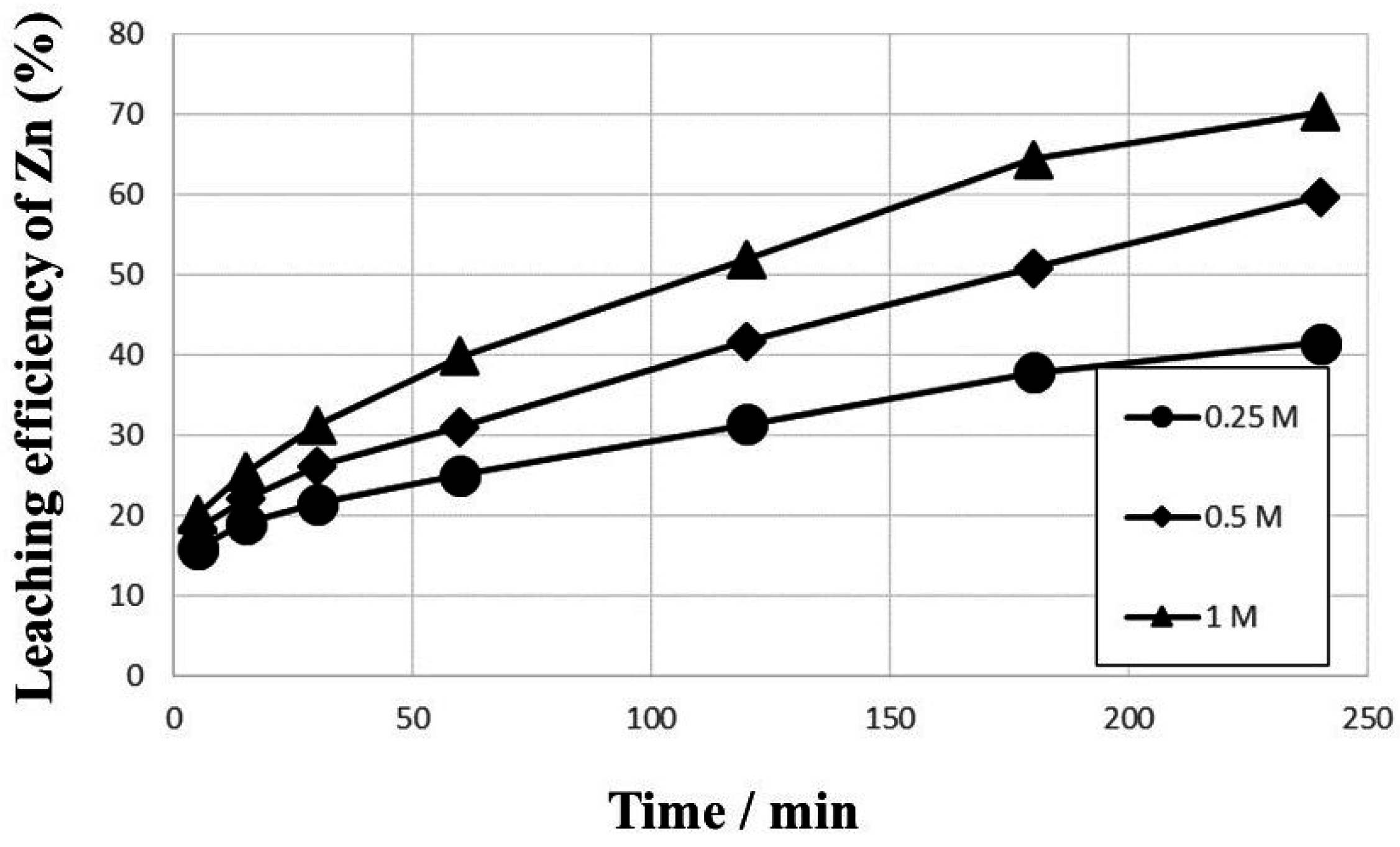
3. Results and Discussion
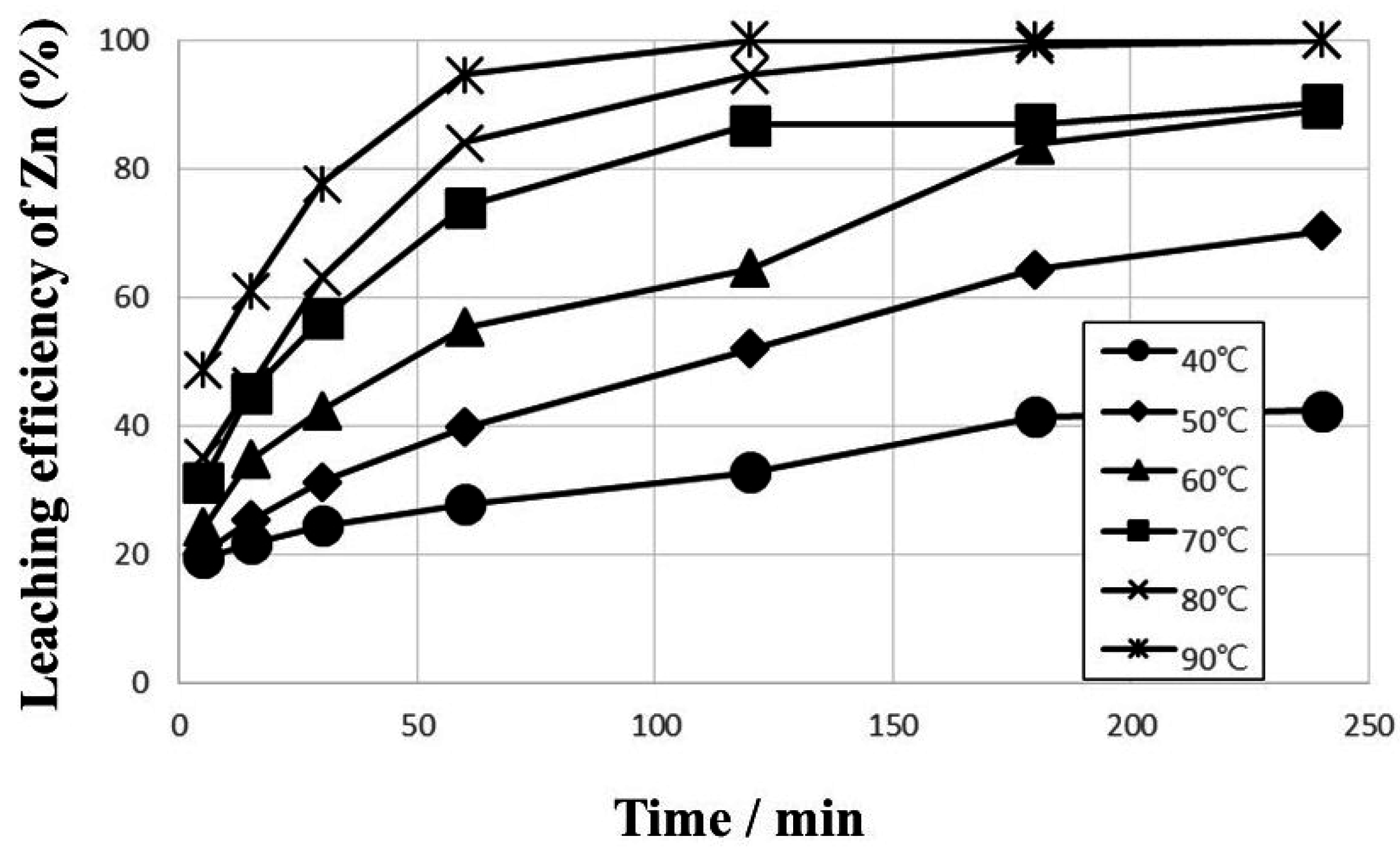
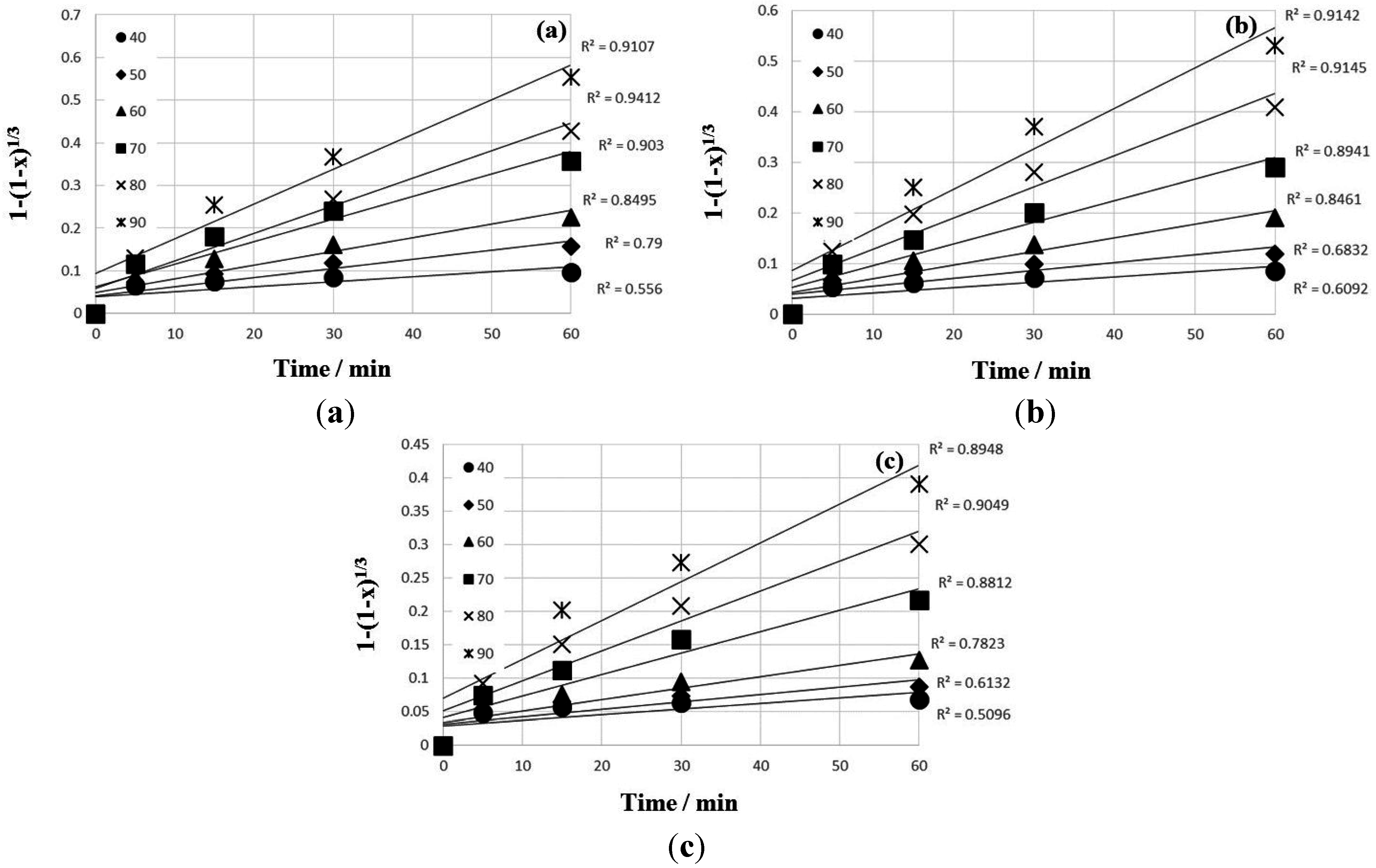
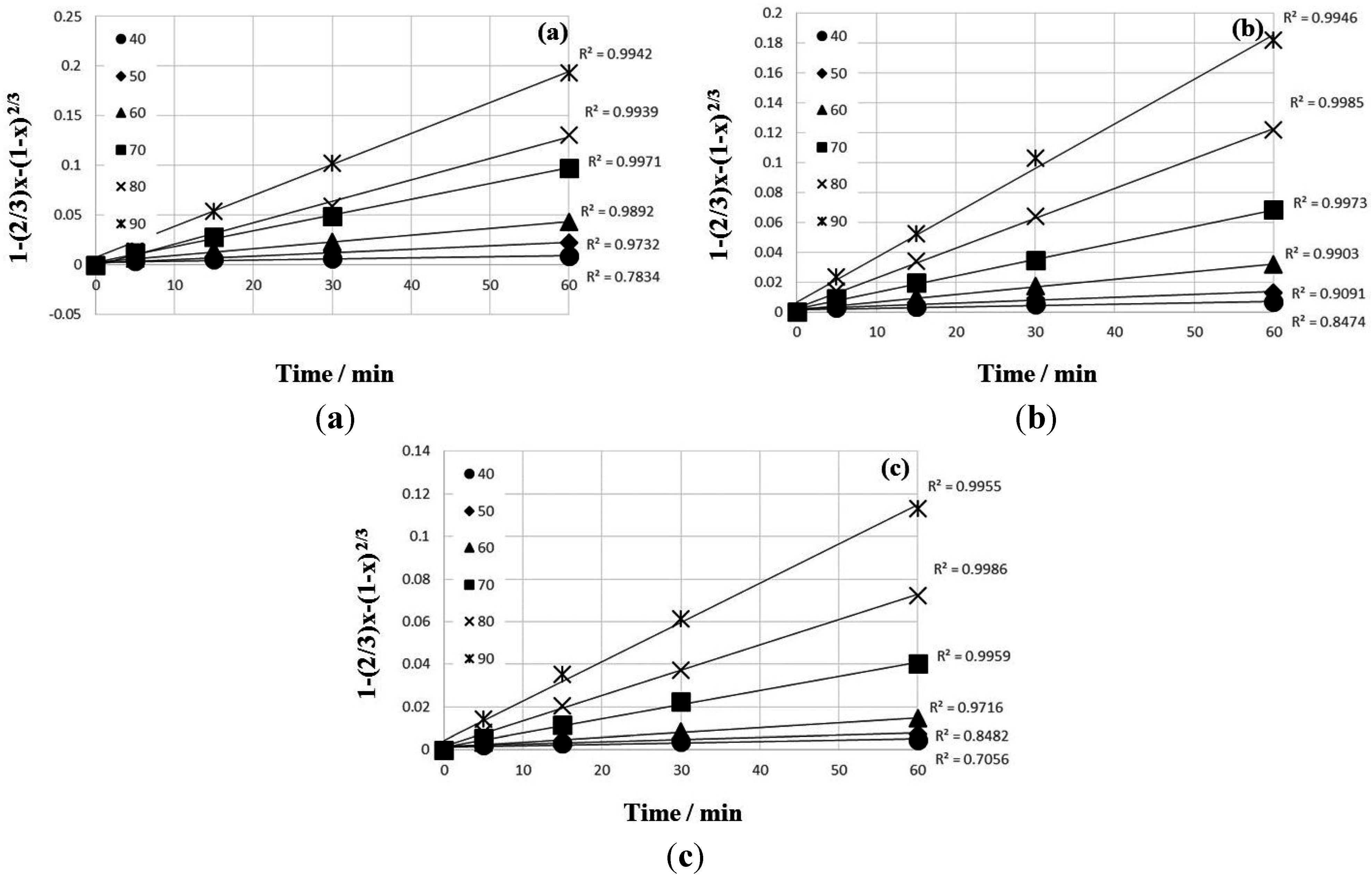
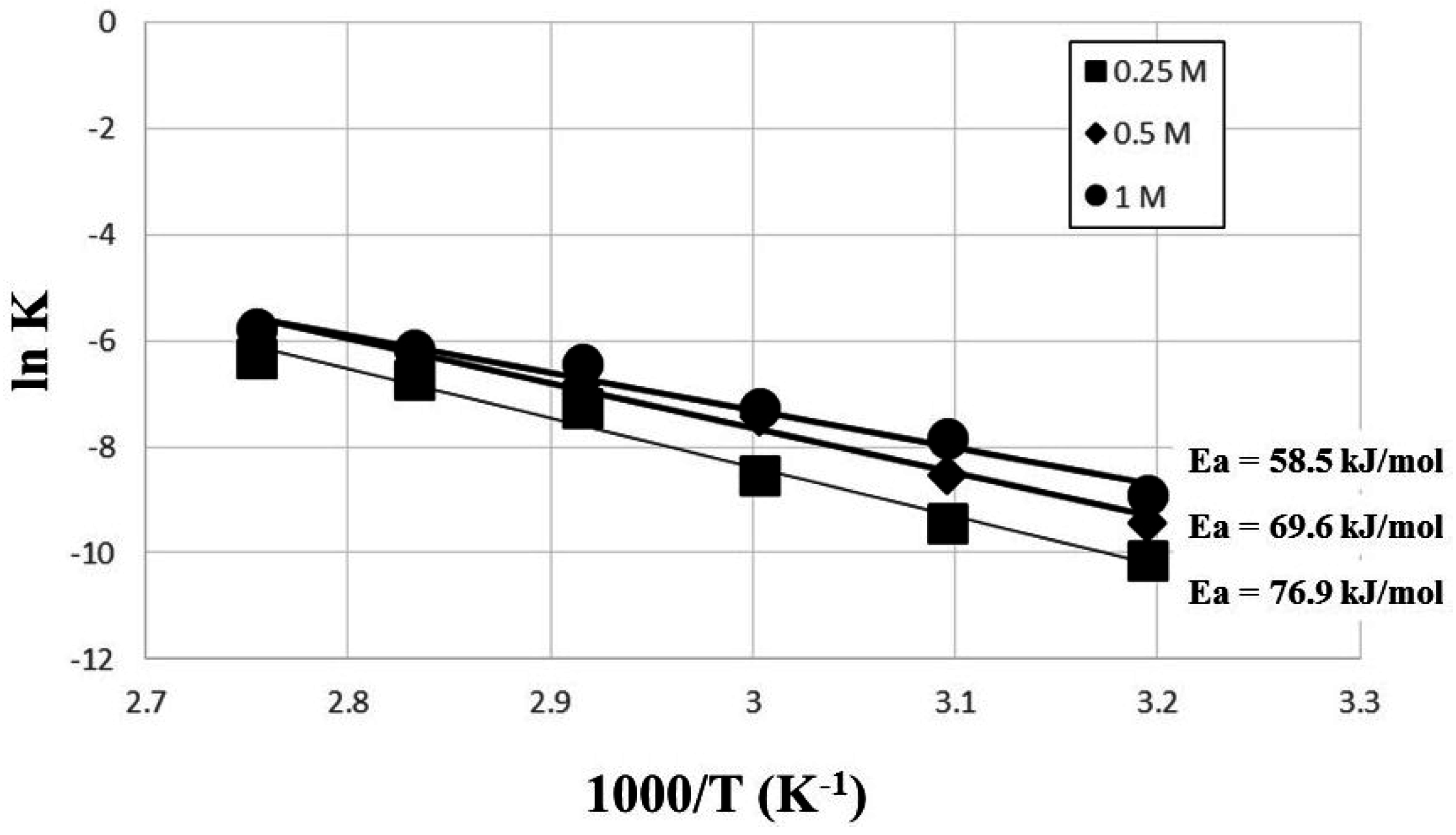
4. Conclusions
- The removal of Zn was achieved by ferric chloride leaching in HCl solution. The removal ratio increased to more than 99% within 120 min under the following leaching conditions; 1 kmol·m−3 Fe3+; 1 kmol·m−3 HCl; 400 rpm agitation speed; 90 °C leaching temperature; 10% pulp density. More importantly, the results obtained meet the Korean environmental standards for Zn of 200 mg/kg.
- About 17.7% of Zn in the sediment was associated with the soluble carbonate and oxide phases as determined by the sequential extraction procedure. The leaching efficiency of Zn increased quite rapidly in the first 5 min and then gradually in the succeeding treatment time. The rapid increase can be attributed to the dissolution of Zn in carbonate and oxide fractions. These results confirm the importance of evaluating the different metal phase associations through sequential extraction to understand the leaching behavior.
- The kinetic data indicated that the leaching behavior followed the diffusion-controlled model well rather than the surface chemical reaction model. As ferric ion concentration increases, the calculated activation energy decreases. The results also suggest that higher ferric ion concentration and temperature could enhance the leaching efficiency of Zn.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Furukawa, M.; Tokunaga, S. Extraction of heavy metals from a contaminated soil using citrate enhancing extraction by pH control and ultrasound application. J. Environ. Sci. Health 2004, 39, 627–638. [Google Scholar] [CrossRef]
- Park, H.; Jung, K.; Alorro, R.D.; Yoo, K. Leaching behavior of copper, zinc and lead from contaminated soil with citric acid. Mater. Trans. 2013, 54, 1220–1223. [Google Scholar] [CrossRef]
- Santos, S.M.C.; Machado, R.M.; Correia, M.J.N.; Reis, M.T.A.; Ismael, M.R.C.; Carvalho, J.M.R. Ferric sulhate/chloride leaching of zinc and minor elements from a sphalerite concentrate. Miner. Eng. 2010, 23, 606–615. [Google Scholar] [CrossRef]
- Souza, A.D.; Pina, P.S.; Leão, V.A.; Silva, C.A.; Siqueira, P.F. The leaching kinetics of a zinc sulphide concentrate in acid ferric sulphate. Hydrometallurgy 2007, 89, 72–81. [Google Scholar] [CrossRef]
- Aydogan, S.; Aras, A.; Canbazoglu, M. Dissolution kinetics of sphalerite in acidic ferric chloride leaching. Chem. Eng. J. 2005, 114, 67–72. [Google Scholar]
- Peng, P.; Xie, H.; Lu, L. Leaching of a sphalerite concentrate with H2SO4–HNO3 solutions in the presence of C2Cl4. Hydrometallurgy 2005, 80, 265–271. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–850. [Google Scholar] [CrossRef]
- Li, X.; Coles, B.J.; Ramsey, M.H.; Thornton, I. Sequential extraction of soils for multielement analysis by ICP-AES. Chem. Geol. 1995, 124, 109–123. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Macdonald, R.J.C. The dissolution of sphalerite in ferric chloride solutions. Metall. Trans. B 1978, 9B, 543–551. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S. The influence of microwaves on the leaching of sphalerite in ferric chloride. Chem. Eng. Prog. 2008, 47, 1246–1251. [Google Scholar] [CrossRef]
- Dehghan, R.; Noaparast, M.; Kolahdoozan, M. Leaching and kinetic modelling of low-grade calcareous sphalerite in acidic ferric chloride solution. Hydrometallurgy 2009, 96, 275–282. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: New York, NY, USA, 1999; pp. 566–588. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-h.; Kwon, O.; Yoo, K.; Alorro, R.D. Removal of Zn from Contaminated Sediment by FeCl3 in HCl Solution. Metals 2015, 5, 1812-1820. https://doi.org/10.3390/met5041812
Lee S-h, Kwon O, Yoo K, Alorro RD. Removal of Zn from Contaminated Sediment by FeCl3 in HCl Solution. Metals. 2015; 5(4):1812-1820. https://doi.org/10.3390/met5041812
Chicago/Turabian StyleLee, Sang-hun, Ohhyeok Kwon, Kyoungkeun Yoo, and Richard Diaz Alorro. 2015. "Removal of Zn from Contaminated Sediment by FeCl3 in HCl Solution" Metals 5, no. 4: 1812-1820. https://doi.org/10.3390/met5041812
APA StyleLee, S.-h., Kwon, O., Yoo, K., & Alorro, R. D. (2015). Removal of Zn from Contaminated Sediment by FeCl3 in HCl Solution. Metals, 5(4), 1812-1820. https://doi.org/10.3390/met5041812