New Approaches to Aluminum Integral Foam Production with Casting Methods
Abstract
:1. Introduction
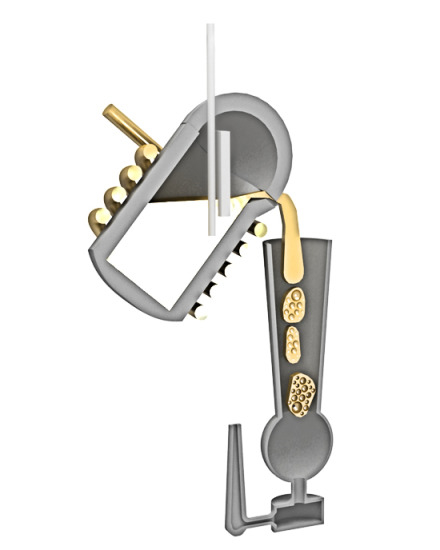
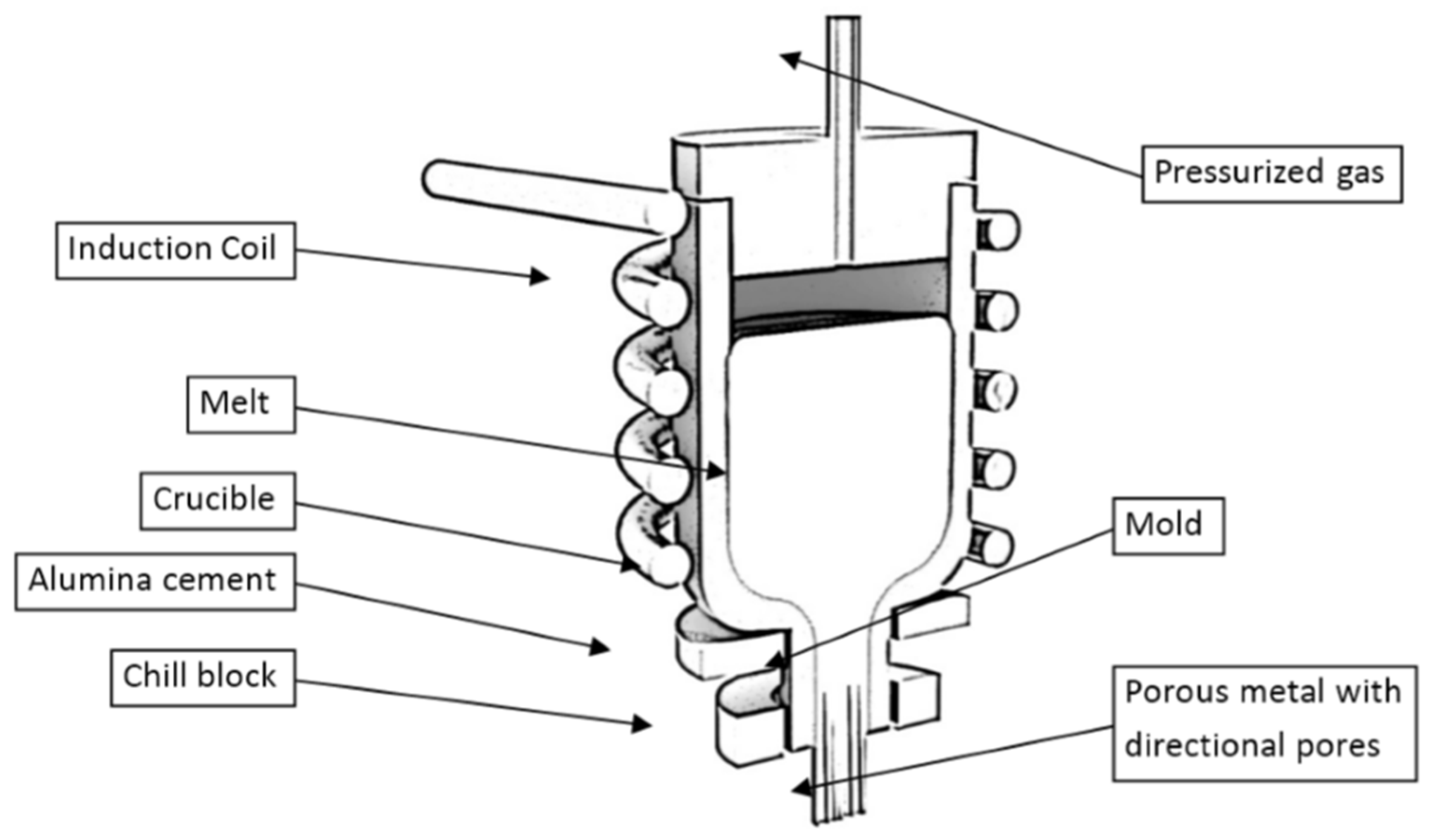
2. Aluminum Integral Foam Casting
2.1. Gas Sources of Metal Foam Production Systems
2.1.1. Gas Agents
| Equation Number | Equation | Temperature |
|---|---|---|
| 1 | TiH2→Ti + H2 | at 480 °C [48] |
| 2 | nH2O + mM→MmOn + 2nH | [49] |
| 3 | MgCO2→MgO + CO2 | at 1300 °C [46] |
| 4 | SrCO2→SrO + CO2 | at 1300 °C [46] |
| 5 | CaCO2→CaO + CO2 | at 830 °C [50] |
| 6 | 2Al(l) + 3CO2(g)→Al2O3(s) + 3CO(g) | [51] |
| 7 | 8Al(l) + 3CO2(g)→2Al2O3(s) + Al4C3(s) | [51] |
2.1.2. Precursor Gas Tablets
2.1.3. Decomposition of Moisture from Mold Surfaces
| Equation Number | Equation | Temperature Range/K |
|---|---|---|
| 1 | MFH = 4 × 10−9 × e0.006T | 675–938 |
| 2 | MFH = 4 × 10−13 × e0.320T | 938–946 |
| 3 | MFH = 1 × 10−7 × e0.005T | 946–1140 |
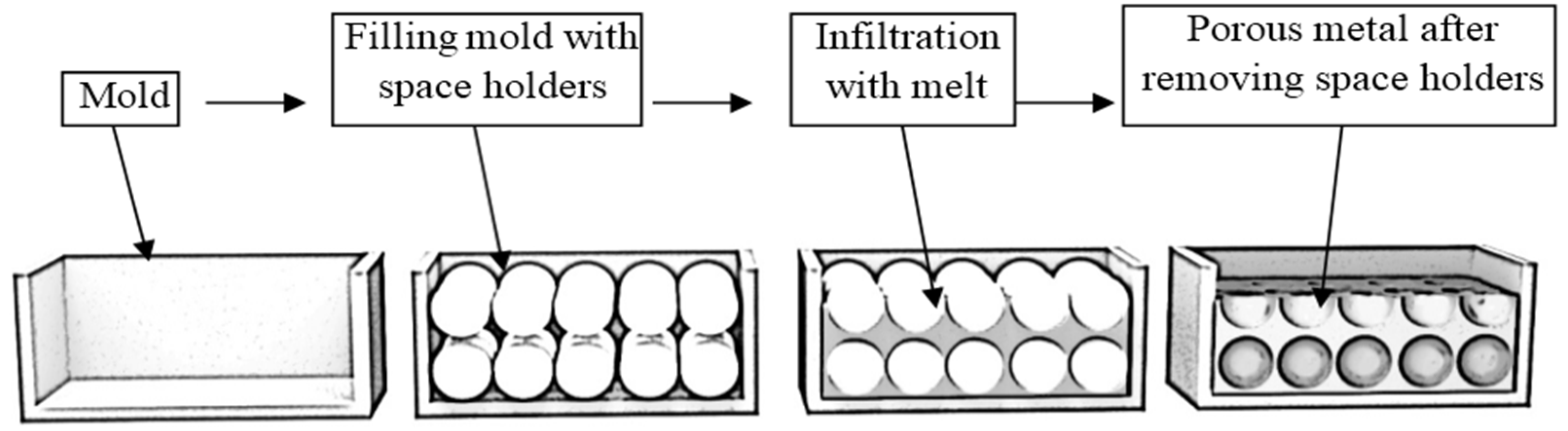
2.1.4. Direct Gas Injection
2.2. Aluminum Foam Casting Methods
2.2.1. Mold Casting
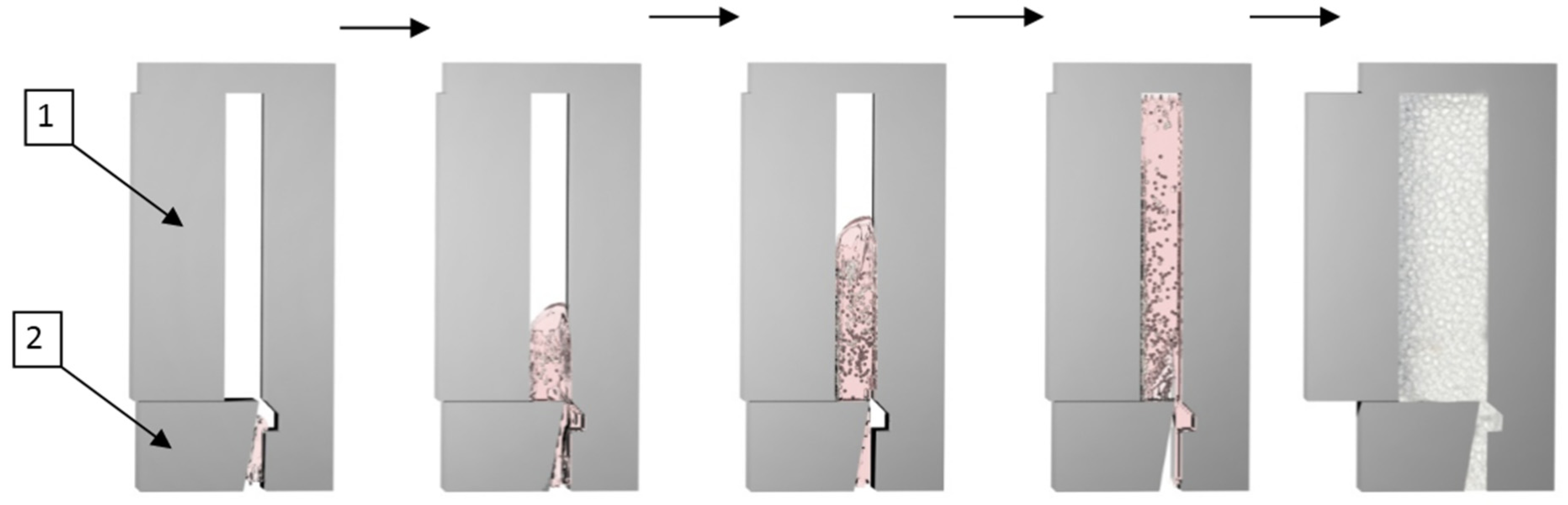
2.2.2. Continuous Casting
2.2.3. Investment Casting and Evaporative Pattern Casting
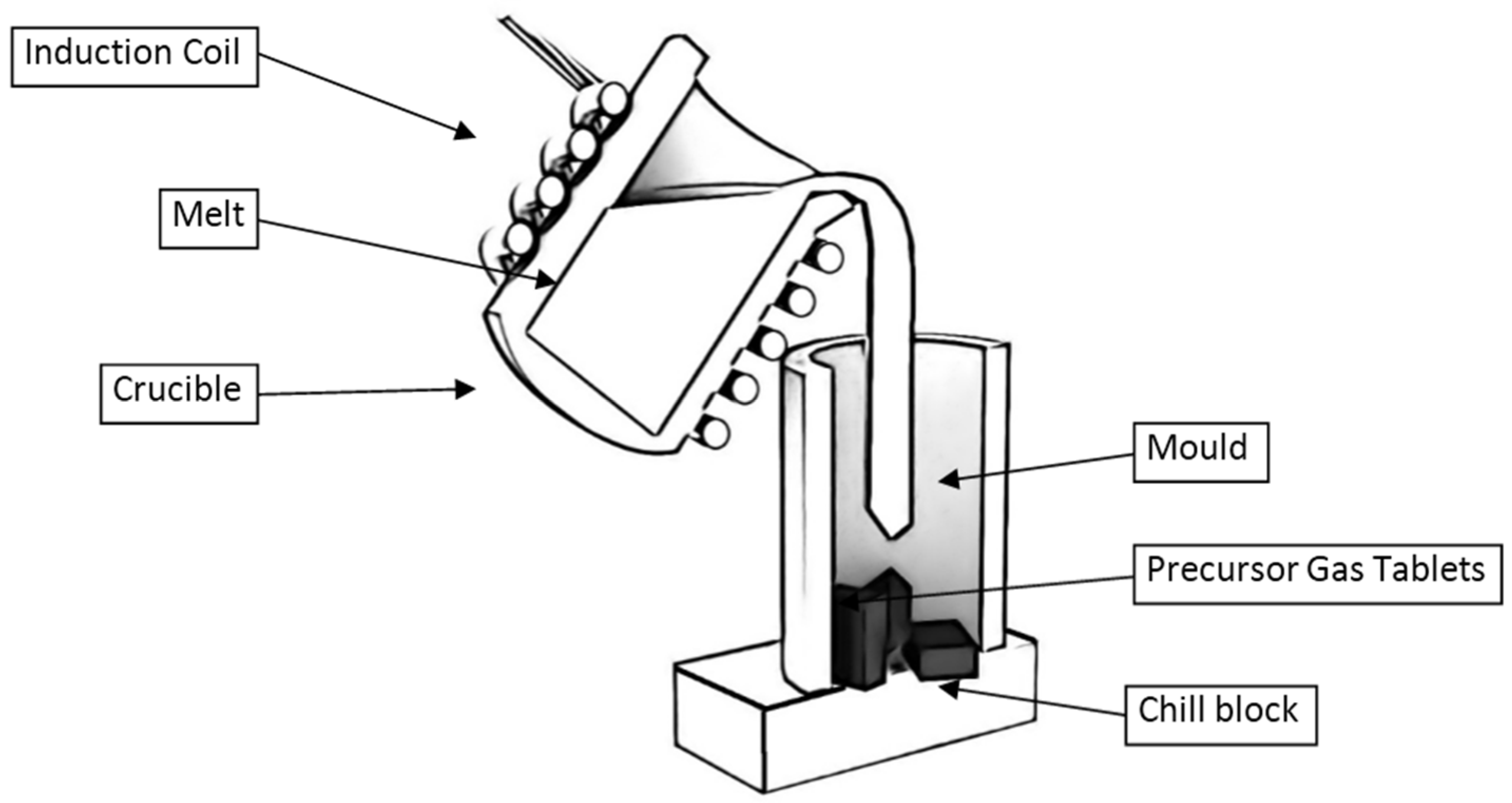
2.2.4. Casting with Precursor Gas Tablets
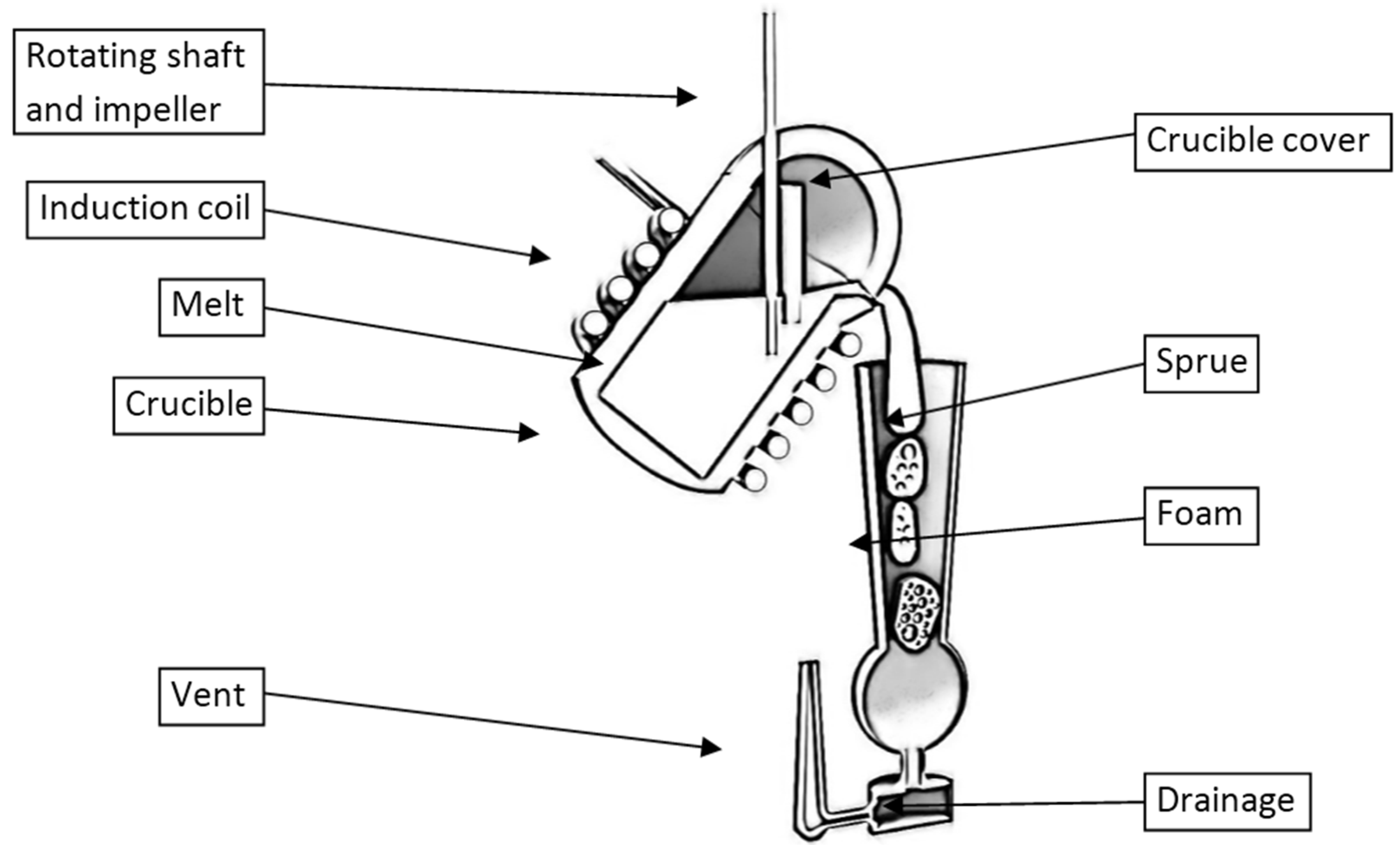
2.2.5. Direct Foaming Investment Casting
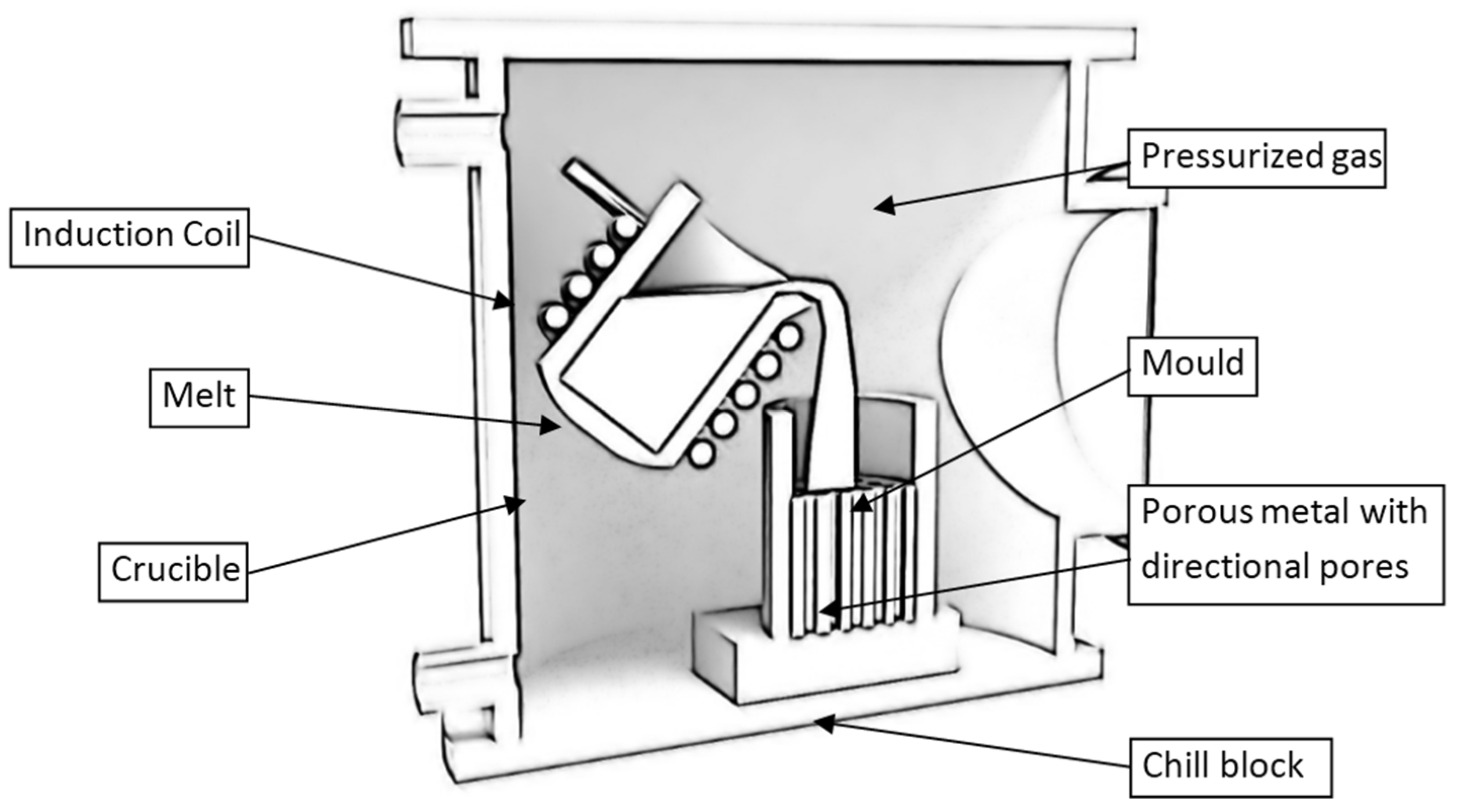
2.2.6. High Pressure Integral Foam Moulding (HP-IFM)
| Properties | Cymat, Alcan | Mepura, Alulight | Shinko Wire, Alporas | ERG, Duocel |
|---|---|---|---|---|
| Materials | Al–SiC | Al–Mg–Si | Al–5Ca–Ti | Al 6061 T6 |
| Relative density | 0.02–0.2 | 0.1–0.35 | 0.08–0.1 | 0.05–0.1 |
| Structure | Closed Cell | Closed Cell | Closed Cell | Open Cell |
| Young’s Modulus [MPa] | 0.02–2.0 | 1.7–12 | 0.4–1.0 | 0.06–0.3 |
| Poisson’s ratio, ν | 0.31–0.34 | 0.31–0.35 | 0.31–0.36 | 0.31–0.37 |
| Compression strength [MPa] | 0.04–7.0 | 1.9–14.0 | 1.3–1.7 | 0.9–3.0 |
| Tensile strength [MPa] | 0.05–8.5 | 2.2–30 | 1.6–1.9 | 1.9–3.5 |
| Fracture toughness [MPa·m1/2] | 0.03–0.5 | 0.3–1.6 | 0.1–0.9 | 0.1–0.2 |
| Thermal conductivity [W/m·K] | 0.3–10 | 3.0–3.5 | 3.5–4.5 | 6.0–11 |
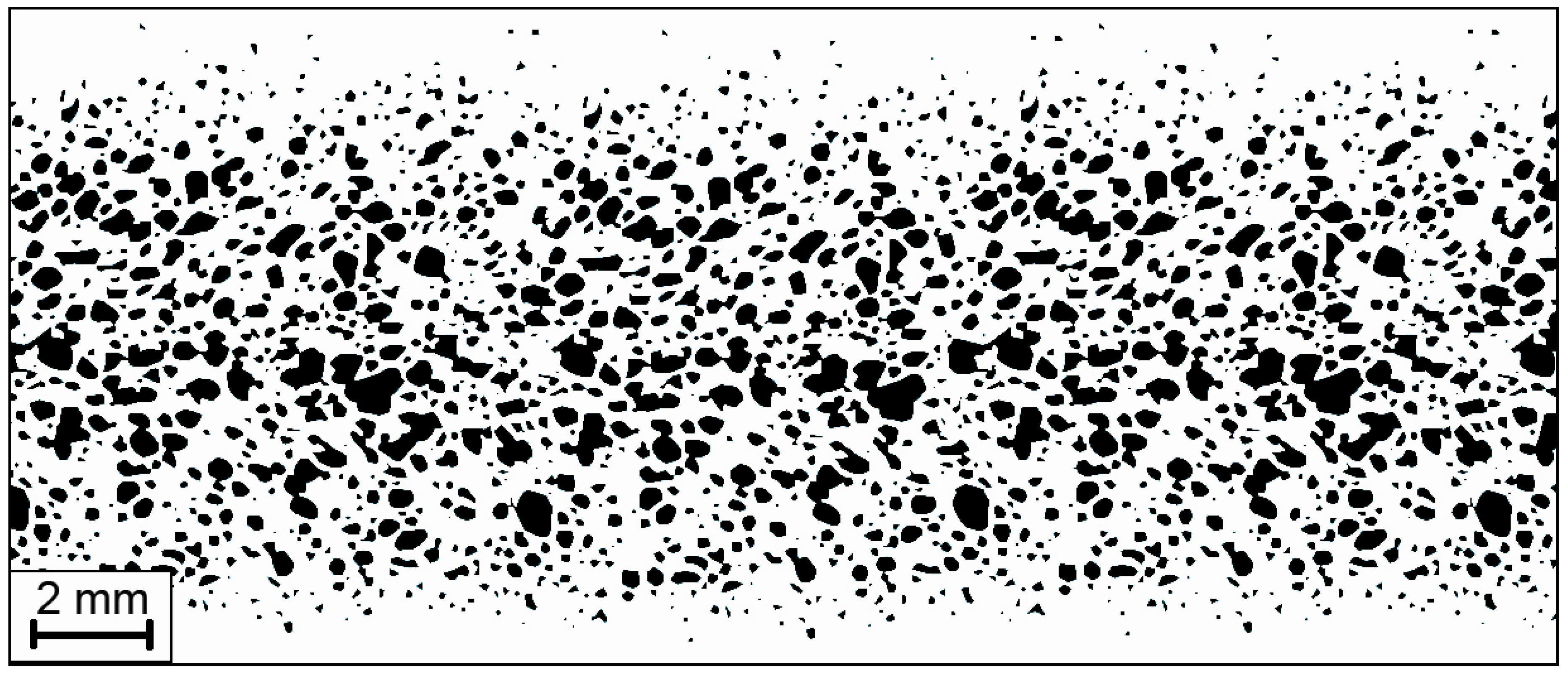
2.3. Properties of Some of the Commercial Al Foams
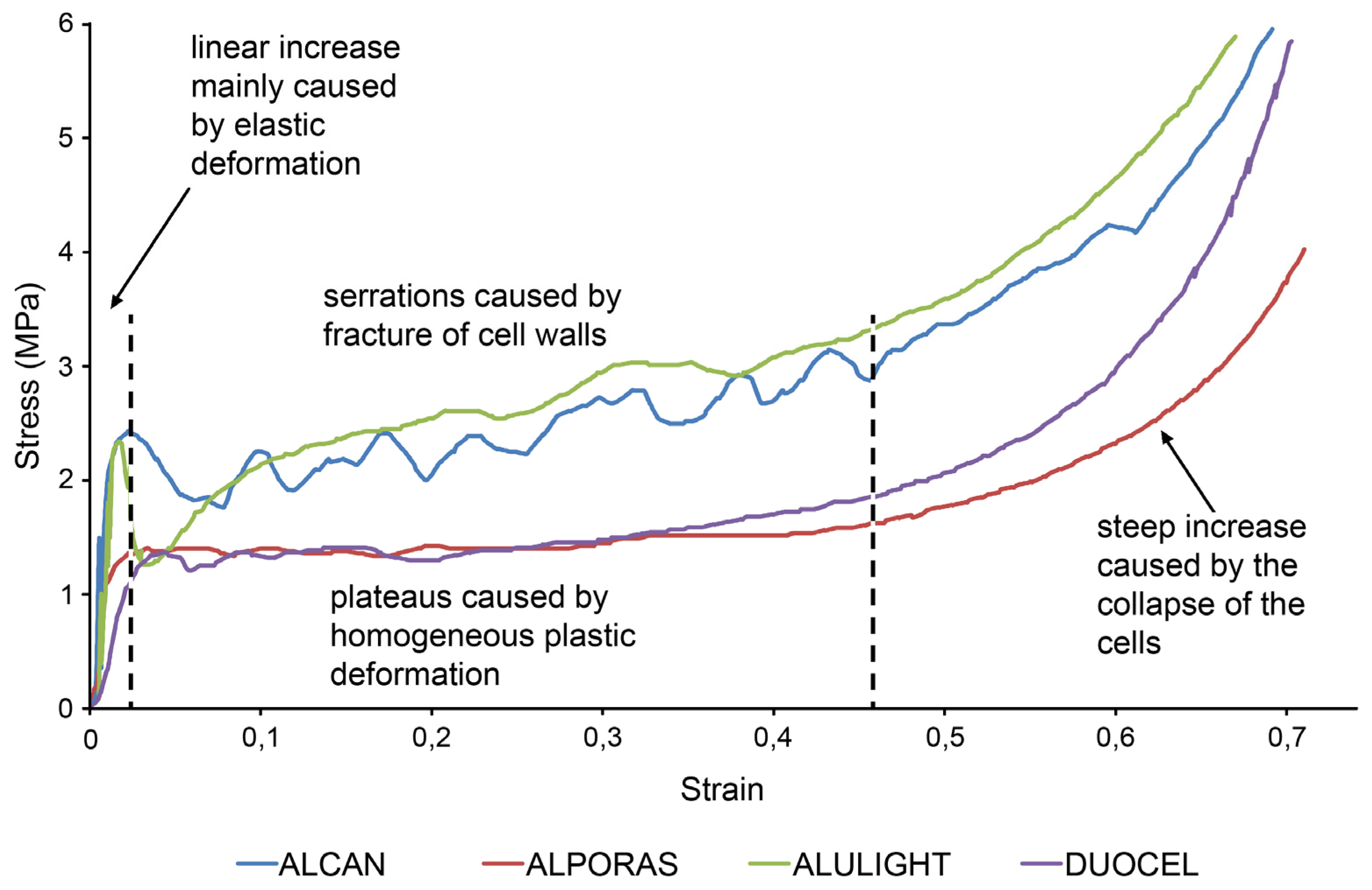
3. Results
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peroni, M.; Solomos, G.; Pizzinato, V. Impact Behaviour Testing of Aluminium Foam. Int. J. Impact Eng. 2013, 53, 74–83. [Google Scholar] [CrossRef]
- Olurin, O.B.; Fleck, N.A.; Ashby, M.F. Indentation Resistance of an Aluminium Foam. Scr. Mater. 2000, 43, 983–989. [Google Scholar] [CrossRef]
- Yang, B.; Tang, L.; Liu, Y.; Liu, Z.; Jiang, Z.; Fang, D. Localized Deformation in Aluminium Foam During Middle Speed Hopkinson Bar Impact Tests. Mater. Sci. Eng. A 2013, 560, 734–743. [Google Scholar] [CrossRef]
- McCullough, K.Y.G.; Fleck, N.A.; Ashb, M.F. Uniaxial Stress-Strain Behaviour of Aluminium Alloy Foams. Acta Mater. 1999, 47, 2331–2343. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, H.; Liu, Z. A New Prediction Model for the Effective Thermal Conductivity of High Porosity Open-Cell Metal Foams. Int. J. Therm. Sci. 2015, 97, 56–67. [Google Scholar] [CrossRef]
- Kanaun, S.; Babaii, K.S. Conductive Properties of Foam Materials with Open or Closed Cells. Int. J. Eng. Sci. 2012, 50, 124–131. [Google Scholar] [CrossRef]
- Chen, S.; Bourham, M.; Rabiei, A. Neutrons Attenuation on Composite Metal Foams and Hybrid Open-Cell Al Foam. Radiat. Phys. Chem. 2015, 109, 27–39. [Google Scholar] [CrossRef]
- Hartmann, J.; Jüchter, V. FLOW-3D News: Summer Application Note 2012, Department of Mater. Sci., Chair of Metals Science and Technology, University of Erlangen-Nuremberg. Available online: http://www.wtm.uni-erlangen.de (accessed on 24 August 2015).
- Kammer, C.; Germany, G. Training in Aluminium Application Technologies (TALAT) Lecture 1410. Available online: http://www.european-aluminium.eu/talat/lectures/1410.pdf (accessed on 24 August 2015).
- Wang, F.; Wang, L.-C.; Wu, J.-G.; You, X.-H. Sound Absorption Property of Open-Pore Aluminum Foams. China Foundry Res. Dev. 2007, 4, 31–33. [Google Scholar]
- Rivard, J.; Brailovski, V.; Dubinskiy, S.; Prokoshkin, S. Fabrication, Morphology And Mechanical Properties of Ti and Metastable Ti-Based Alloy Foams for Biomedical Applications. Mater. Sci. Eng. C 2014, 25, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.K.; Bogetti, T.A.; Gama, B.; Gillespie, J.W.; Yu, C.J.; Claar, T.D.; Eifert, H.H. Application of Aluminum Foam for Stress-Wave Management in Light Weight Composite Integral Armor; Army Research Laboratory: Adelphi, MD, USA, May 2001. [Google Scholar]
- Niebylski, L.M.; Jarema, C.P.; Immethun, P.A. Metal Foams and Process Therefor. Patent US 3794481, 26 February 1974. [Google Scholar]
- Ruch, W.; Kirkevag, B. A process of manufacturing particle reinforced metal foam and produtct thereof. Patent WO 9101387, 2 February 1991. [Google Scholar]
- Lloyd, D.J.; Mcleod, A.D.; Morris, P.L.; Jin, I. Melt process for the production of metal matrix composite materials with enhanced particle/matrix wetting. PCT Patent WO 91/19823, 26 December 1991. [Google Scholar]
- Jin, I.; Kenny, L.D.; Sang, H. Method of producing lightweight foamed metal. US Patent 4973358, 27 November 1990. [Google Scholar]
- Jin, I.; Kenny, L.D.; Sang, H. Lightweight foamed metal and its production. Patent WO 91/03578, 21 March 1991. [Google Scholar]
- Sang, H.; Kenny, L.D.; Jin, I. Process and apparatus for producing shaped slabs of particle stabilized foamed metal. PCT Patent WO 92/21457, 10 December 1992. [Google Scholar]
- Shapovalov, V. Method for manufacturing porous articles. US Patent 5181549, 26 January 1993. [Google Scholar]
- Thomas, M.; Kenny, L.D. Production of particle-stabilized metal foams. PCT Patent WO 94/017218, 21 January 1994. [Google Scholar]
- Kenny, L.D.; Thomas, M. Process and apparatus for shape casting of particle stabilized metal foam. PCT Patent WO 94/09931, 11 May 1994. [Google Scholar]
- Simone, A.E.; Gibson, L.J. Aluminum Foams Produced by Liquid-State Processes. Acta Mater. 1998, 46, 3109–3123. [Google Scholar] [CrossRef]
- Simone, A.E.; Gibson, L.J. Effects of Solid Distribution on the Stiffness and Strength of Metallic Foams. Acta Mater. 1998, 46, 2139–2150. [Google Scholar] [CrossRef]
- Banhart, J.; Seeliger, H. Aluminium Foam Sandwich Panels: Manufacture, Metallurgy and Applications. Adv. Eng. Mater. 2008, 10, 793–802. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Sahoo, K.L. Processing, Stabilization and Applications of Metallic Foams. Art of Science. Mater. Sci. Pol. 2007, 25, 733–753. [Google Scholar]
- Körner, C.; Hirschmann, M.; Wiehler, H. Integral Foam Moulding of Light Metals. Mater. Trans. 2006, 47, 2188–2194. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, A.G.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H.N.G. Making Metal Foams. In Metal Foams: A Design Guide; Butterworth-Heinemann: Woburn, MA, USA, 2000; pp. 6–23. [Google Scholar]
- Simančík, F.; Florek, R.; Nosko, M.; Tobolka, P.; Jerz, J.; Kováčik, J. New Trends in Manufacturing of PM Aluminium Foams. In Proceedings of the Materials And Technologies For Lightweight Design, Workshop on Recent Developments in the World of Engineering Materials, Smolenice, Slovakia, 13–14 Decmber 2011.
- Miyoshi, T.; Itoh, M.; Akiyama, S.; Kitahara, A. “ALPORAS” Aluminium Foam: Production Process, Properties and Application. Adv. Eng. Mater. 2000, 2, 179–183. [Google Scholar] [CrossRef]
- Simančík, F.; Rajner, W.; Laag, R. Alulight—Aluminum Foam for Lightweight Construction. SAE Tech. Pap. Ser. 2000, 1, 0337. [Google Scholar]
- Zhang, B.; Kim, T.; Lu, T.J. Analytical Solution for Solidification of Close-Celled Metal Foams. Int. J. Heat Mass Transf. 2009, 52, 133–141. [Google Scholar] [CrossRef]
- Mukherjee, M.; Garcia-Moreno, F.; Banhart, J. Solidification of Metal Foams. Acta Mater. 2010, 58, 6358–6370. [Google Scholar] [CrossRef]
- Jamshidi-Alashti, R.; Roudini, G. Producing replicated Open Cell Aluminium Foams by a novel method of melt squizing method. Mater. Lett. 2012, 76, 233–236. [Google Scholar] [CrossRef]
- Jamshidi-Alashti, R.; Kaskani, M.; Niroumand, B. Semisolid Melt Squeezing Procedure for Production of Open-Cell Al–Si Foams. Mater. Des. 2014, 56, 325–333. [Google Scholar] [CrossRef]
- Babcsan, N.; Garcia-Moreno, F.; Banhart, J. Metal foams—High temperature colloids Part II: In Situ Analysis of Metal Foams. Coll. Surf. A 2007, 309, 254–263. [Google Scholar] [CrossRef]
- Suematsu, Y.; Hyun, S.; Nakajima, H. Fabrikation of Lotus-Type Porous Nickel Using Moisture in Mould. J. Jpn. Inst. Met. 2004, 68, 257–261. [Google Scholar] [CrossRef]
- Yang, C.C.; Nakae, H. The Effects of Viscosity and Cooling Conditions on the Foamability of Aluminum Alloy. J. Mater. Process. Technol. 2003, 141, 202–206. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, Characterisation and Application of Cellular Metals and Metal Foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Deqing, W.; Ziyuan, S. Effect of Ceramic Particles on Cell Size and Wall Thickness of Aluminum Foam. Mater. Sci. Eng. A 2003, 361, 45–49. [Google Scholar] [CrossRef]
- Goodall, R.; Mortensen, A. 24-Porous Metals. In Physical Metallurgy, 5th ed.; Laughlin, D.E., Hono, K., Eds.; Elsevier: Oxford, UK, 2014; Volume 3, pp. 2399–2595. [Google Scholar]
- Ferguson, J.B.; Maria, J.A.S.; Schultz, B.F.; Rohatgi, P.K. Al–Al2O3 Syntactic Foams-Part II: Predicting Mechanical Properties of Metal Matrix Syntactic Foams Reinforced with Ceramic Spheres. Mater. Sci. Eng. A 2013, 582, 423–432. [Google Scholar] [CrossRef]
- Xia, X.; Chen, X.; Zhang, Z.; Chen, X.; Zhao, W.M.; Liao, B.; Hur, B. Effects of Porosity and Pore Size on the Compressive Properties of Closed-Cell Mg Alloy Foam. J. Magnes. Alloys 2013, 1, 330–335. [Google Scholar] [CrossRef]
- Babcsan, N.; Leitlmeier, D.; Banhart, J. Metal Foams—High Temperature Colloids Part I. Ex situ Analysis of Metal Foams. Coll. Surf. A 2005, 261, 123–130. [Google Scholar] [CrossRef]
- Duarte, I.; Banhart, J. A Study of Aluminium Foam Formation—Kinetics and Microstructure. Acta Mater. 2000, 48, 2349–2362. [Google Scholar] [CrossRef]
- Von Zeppelin, F.; Hirscher, M.; Stanzick, H.; Banhart, J. Desorption of Hydrogen From Blowing Agents Used for Foaming Metals. Compos. Sci. Technol. 2003, 63, 2293–2300. [Google Scholar] [CrossRef]
- Park, C.; Nutt, S.R. PM Synthesis and Properties of Steel Foams. Mater. Sci. Eng. A 2000, 288, 111–118. [Google Scholar] [CrossRef]
- Irretier, A.; Banhart, J. Lead and Lead Alloy Foams. Acta Mater. 2005, 53, 4903–4917. [Google Scholar] [CrossRef]
- Reglero, J.A.; Solórzano, E.; Rodríguez-Pérez, M.A.; de Saja, J.A.; Porras, E. Design and Testing of an Energy Absorber Prototype Based on Aluminium Foams. Mater. Des. 2010, 31, 3568–3573. [Google Scholar] [CrossRef]
- Nakajima, H. Fabrication, Properties and Application of Porous Metals with Directional Pores. Prog. Mater. Sci. 2007, 52, 1091–1173. [Google Scholar] [CrossRef]
- Haesche, M.; Lehmhus, D.; Weise, J.; Wichmann, M.; Mocellin, I.C.M. Carbonates as Foaming Agent in Chip-Based Aluminium Foam Precursor. J. Mater. Sci. Technol. 2010, 26, 845–850. [Google Scholar]
- Kevorkijan, V. Low Cost Aluminium Foams Made by CaCO3 Particulates. Assoc. Metall. Eng. Serb. 2010, 16, 205–219. [Google Scholar]
- Degischer, H.P. Innovative Light Metals: Metal Matrix Composites and Foamed Aluminium. Mater. Des. 1997, 18, 221–226. [Google Scholar] [CrossRef]
- Baumeister, J.; Schrader, H.D. Methonds for manufacturing foamable metal body and use the same. German Patent DE 4101630, 12 December 1991. [Google Scholar]
- Baumeister, J. Porous metal body prodn.—Involves compaction at low temp. Followed by heating to near melting point of metal. German Patent DE 4018360, 29 May 1990. [Google Scholar]
- Baumeister, J.; Schrader, H. Methods for manufacturing foamable metal bodies. US Patent 5151246, 29 September 1992. [Google Scholar]
- Schrader, H.; Baumeister, J. Process for making foamed metal bodies. European Patent EP 0460392A1, 11 December 1991. [Google Scholar]
- Baumeister, J.; Banhart, J.; Weber, M. Metallic composite material and a method for its production. German Patent DE 4426627, 2 February 1995. [Google Scholar]
- Baumgärtner, F.; Duarte, I.; Banhart, J. Industrialization of powder compact foaming. Adv. Eng. Mater. 2000, 2, 168–174. [Google Scholar] [CrossRef]
- Banhart, J. Metal Foams—From Fundamental Research to Applications. In Frontiers in the Design of Materials; Raj, B., Ranganathan, S., Bhanusankararao, K., Matthew, M.D., Shankar, P., Eds.; Universities Press Limited: Telangana, India, 2007; pp. 279–289. [Google Scholar]
- Stanzick, H.; Wichmann, M.; Weise, J.; Banhart, J.; Helfen, L.; Baumbach, T. Process control in aluminium foam production using real-time X-ray radioscopy. Adv. Eng. Mater. 2000, 4, 814–823. [Google Scholar] [CrossRef]
- Bum, K.T.; Tane, M.; Suzuki, S.; Utsunomiyab, H.; Ide, T.; Nakajima, H. Strength and Pore Morphology of Porous Aluminum and Porous Copper with Directional Pores Deformed By Equal Channel Angular Extrusion. Mater. Sci. Eng. A 2011, 528, 2363–2369. [Google Scholar]
- Ye, J.; Jiang, L.; Li, Z.; Liu, X.; Wang, S.; Li, X. Numerical Analysis of Heat and Mass Transfer During Absorption Of Hydrogen in Metal Hydride Based Hydrogen Storage Tanks. Int. J. Hydrog. Energy 2010, 35, 8216–8224. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Vafai, K. Development and Investigation of Three-Phase Model of the Mushy Zone For Analysis of Porosity Formation In Solidifying Castings. Int. J. Heat Mass Transf. 1995, 38, 2557–2567. [Google Scholar] [CrossRef]
- Pinto, P.; Peixinho, N.; Silva, F.; Soares, D. Compressive Properties and Energy Absorption of Aluminum Foams with Modified Cellular Geometry. J. Mater. Process. Technol. 2014, 214, 571–577. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, P.; Shan, H.S. Effect of evaporative Pattern Casting Process Parameters on the Surface Roughness of Al-7% Si alloy castings. J. Mater. Process. Technol. 2007, 182, 615–623. [Google Scholar] [CrossRef]
- Cingi, C.; Niini, E.; Orkas, J. Foamed aluminum parts by investment casting. Coll. Surf. A 2009, 344, 113–117. [Google Scholar] [CrossRef]
- Mukherjee, M.; Garcia-Moreno, F.; Banhart, J. Defect Generation during Solidification of Aluminium Foams. Scr. Mater. 2010, 63, 235–238. [Google Scholar] [CrossRef]
- Xia, X.; Zhao, W.; Feng, X.; Feng, H.; Zhang, X. Effect of Homogenizing Heat Treatment on the Compressive Properties of Closed-Cell Mg Alloy Foams. Mater. Des. 2013, 49, 19–24. [Google Scholar] [CrossRef]
- Jeenager, V.K.; Pancholi, V. Influence of Cell Wall Microstructure on the Energy Absorbtion Capability of Aluminium Foam. Mater. Des. 2014, 56, 454–459. [Google Scholar] [CrossRef]
- Andrews, E.; Sanders, W.; Gibson, L.J. Compressive and Tensile Behaviour of Aluminum Foams. Mater. Sci. Eng. A 1999, 270, 113–124. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güner, A.; Arıkan, M.M.; Nebioglu, M. New Approaches to Aluminum Integral Foam Production with Casting Methods. Metals 2015, 5, 1553-1565. https://doi.org/10.3390/met5031553
Güner A, Arıkan MM, Nebioglu M. New Approaches to Aluminum Integral Foam Production with Casting Methods. Metals. 2015; 5(3):1553-1565. https://doi.org/10.3390/met5031553
Chicago/Turabian StyleGüner, Ahmet, Mustafa Merih Arıkan, and Mehmet Nebioglu. 2015. "New Approaches to Aluminum Integral Foam Production with Casting Methods" Metals 5, no. 3: 1553-1565. https://doi.org/10.3390/met5031553
APA StyleGüner, A., Arıkan, M. M., & Nebioglu, M. (2015). New Approaches to Aluminum Integral Foam Production with Casting Methods. Metals, 5(3), 1553-1565. https://doi.org/10.3390/met5031553