Dehydrogenation Kinetics and Modeling Studies of MgH2 Enhanced by Transition Metal Oxide Catalysts Using Constant Pressure Thermodynamic Driving Forces
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Temperature Programmed Desorption Measurements
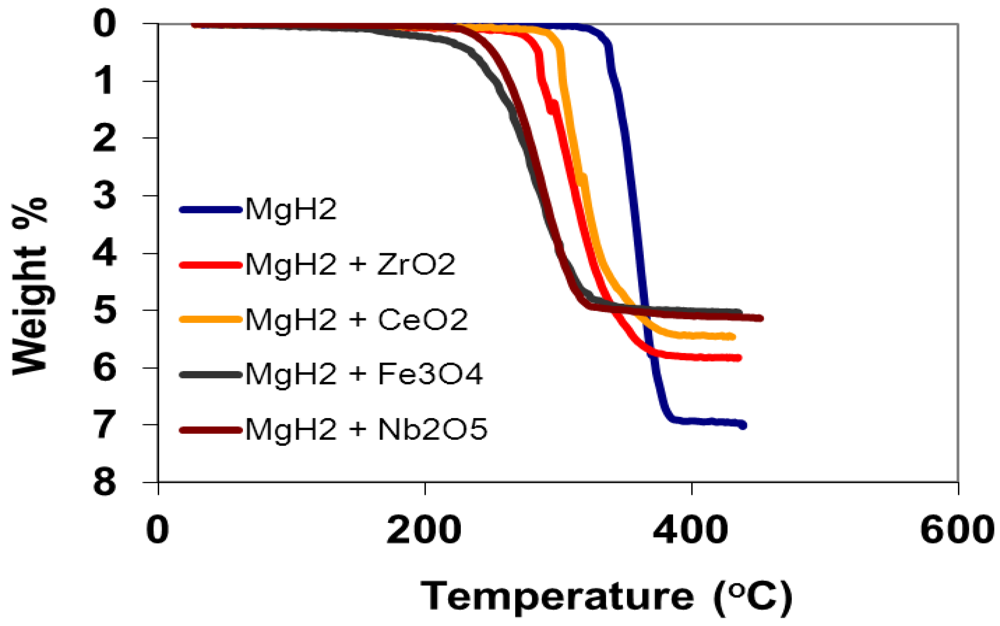
| Sample | Onset Temperature/°C | ∆H/(kJ/mol) | T90/min | Ea/(kJ/mol) |
|---|---|---|---|---|
| MgH2 | 310 | 78.8 | 32 | 174 |
| MgH2 + 4 mol % ZrO2 | 260 | 75.2 | 21 | 140 |
| MgH2 + 4 mol% CeO2 | 270 | 74.7 | 19 | 113 |
| MgH2 + 4 mol% Fe3O4 | 200 | 72.4 | 17 | 108 |
| MgH2 + 4 mol% Nb2O5 | 205 | 70.2 | 16 | 95 |
3.2. Programmed Composition Isotherm Measurements
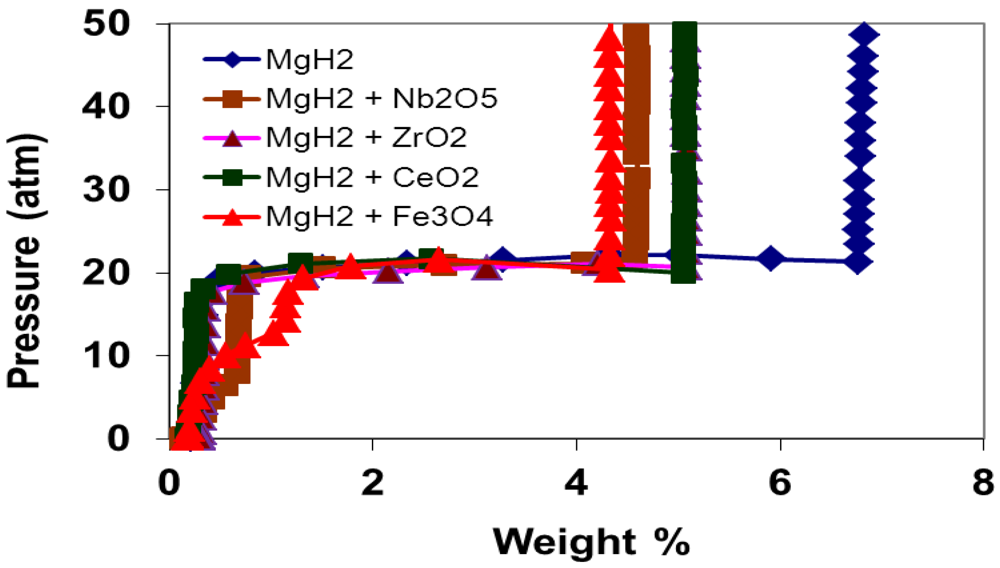
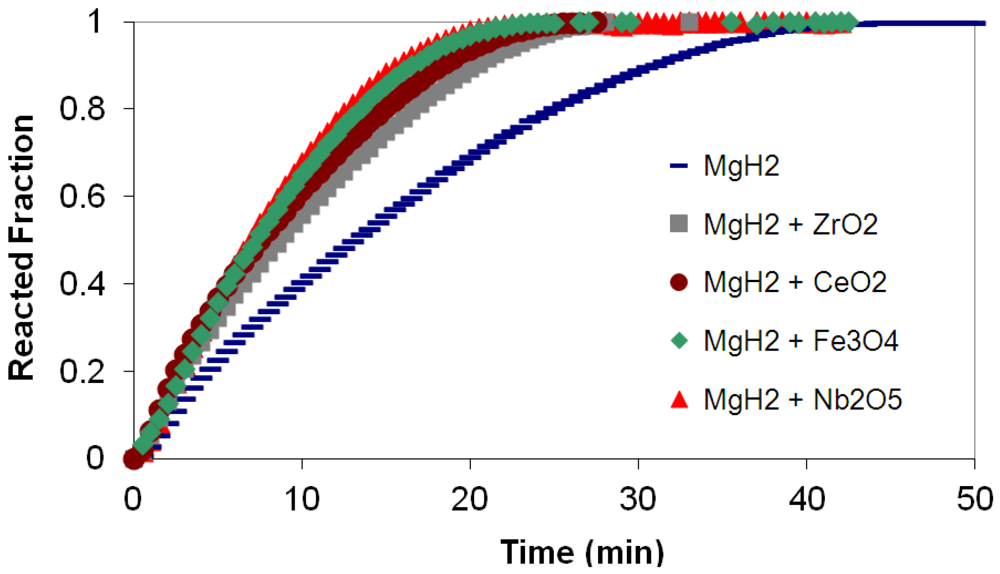
3.3. Kinetics Measurements
3.4. Kinetics Modeling Studies
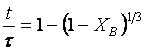 (1)
(1)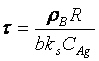
 (2)
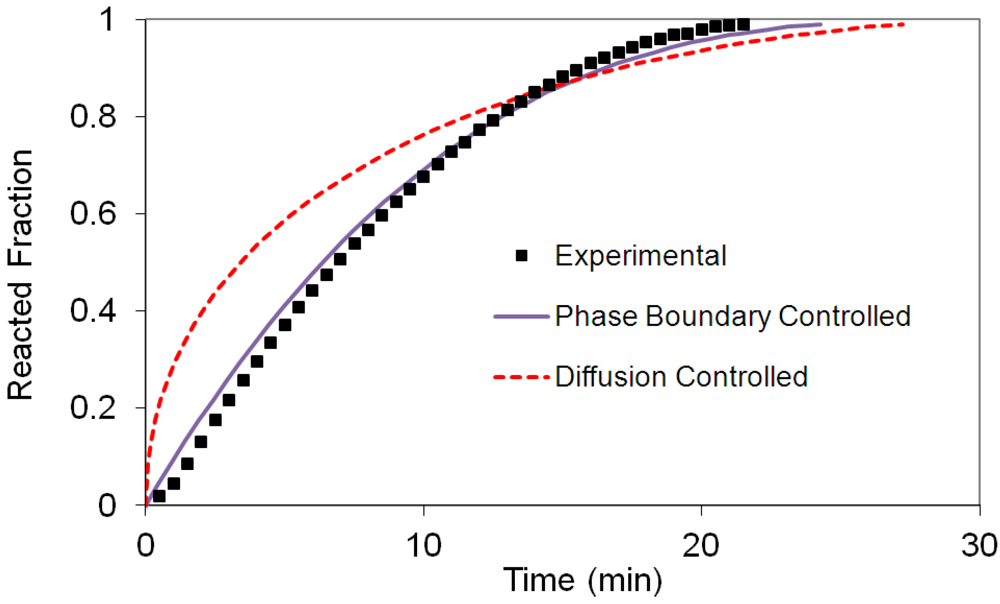
(2) t is the time at a specific point in the reaction, XB is the fraction of the metal reacted. R is the initial radius of the hydride particles, b is a stoichiometric coefficient of the metal, CAg is the gas phase concentration of reactant, De is the effective diffusivity of hydrogen atoms in the hydride, ρB is the density of the metal hydride and ks is a rate constant. It was found that a model based on Equation (1) will have chemical reaction at the phase boundary controlling the reaction rate. This is called the shrinking particle model (SPM). A model based on Equation (2) is one in which the overall reaction rate is controlled by diffusion. Both models were applied to the current study to determine which kinetic model best describes the reactions. Equations (1) and (2) were fitted to the kinetic data for each of the reaction sample mixtures. Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 each contain three curves. One is an experimental curve taken from the desorption kinetics curve shown in Figure 4, a second curve was calculated from the SCM with diffusion controlling the overall reaction and a third curve was calculated with chemical reaction at the phase boundary controlling the rate. In order to determine the theoretical curves, it was first necessary to determine a value for τ. It was not necessary to know the values of all the physical parameters in Equations (1) and (2) in order to do this. The determination of τ was accomplished through a series of statistical data analyses in which the value of τ necessary to minimize the standard deviation between the experimental and theoretical data was calculated. Thus τ was a fitting parameter in these analyses As shown in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, data generated from the SPM with chemical reaction at the phase boundary controlling the overall rate fits the experimental data better than the data generated from the SCM with diffusion controlling the overall reaction rate. Therefore we can say that chemical reaction at the phase boundary is the most likely mechanism for all the reactions in this study.
t is the time at a specific point in the reaction, XB is the fraction of the metal reacted. R is the initial radius of the hydride particles, b is a stoichiometric coefficient of the metal, CAg is the gas phase concentration of reactant, De is the effective diffusivity of hydrogen atoms in the hydride, ρB is the density of the metal hydride and ks is a rate constant. It was found that a model based on Equation (1) will have chemical reaction at the phase boundary controlling the reaction rate. This is called the shrinking particle model (SPM). A model based on Equation (2) is one in which the overall reaction rate is controlled by diffusion. Both models were applied to the current study to determine which kinetic model best describes the reactions. Equations (1) and (2) were fitted to the kinetic data for each of the reaction sample mixtures. Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 each contain three curves. One is an experimental curve taken from the desorption kinetics curve shown in Figure 4, a second curve was calculated from the SCM with diffusion controlling the overall reaction and a third curve was calculated with chemical reaction at the phase boundary controlling the rate. In order to determine the theoretical curves, it was first necessary to determine a value for τ. It was not necessary to know the values of all the physical parameters in Equations (1) and (2) in order to do this. The determination of τ was accomplished through a series of statistical data analyses in which the value of τ necessary to minimize the standard deviation between the experimental and theoretical data was calculated. Thus τ was a fitting parameter in these analyses As shown in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, data generated from the SPM with chemical reaction at the phase boundary controlling the overall rate fits the experimental data better than the data generated from the SCM with diffusion controlling the overall reaction rate. Therefore we can say that chemical reaction at the phase boundary is the most likely mechanism for all the reactions in this study.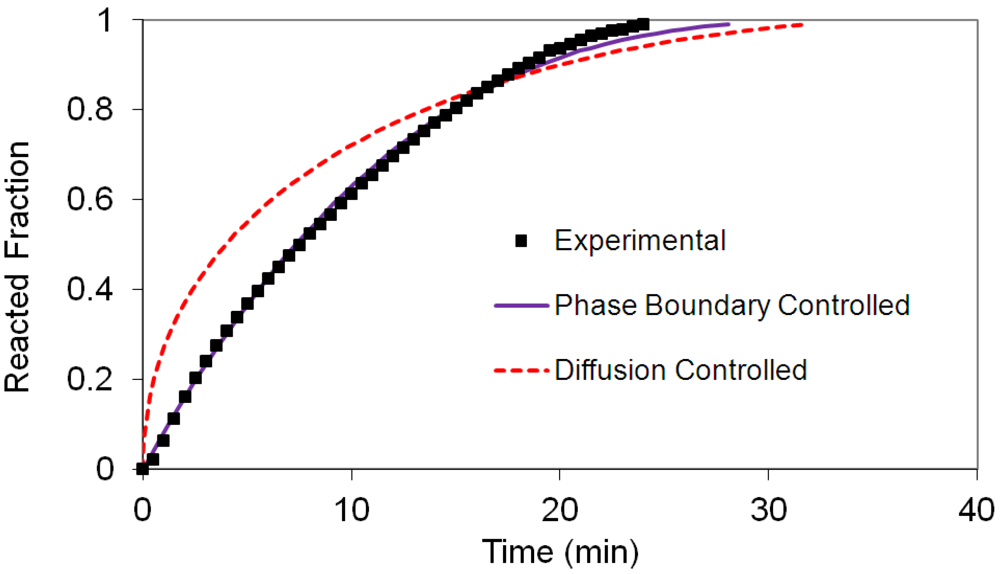
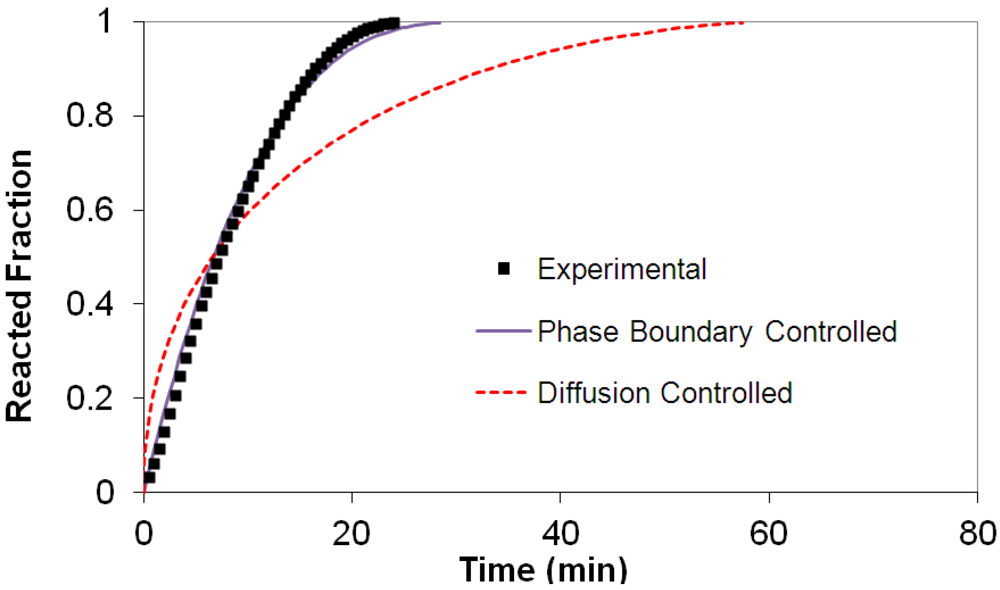
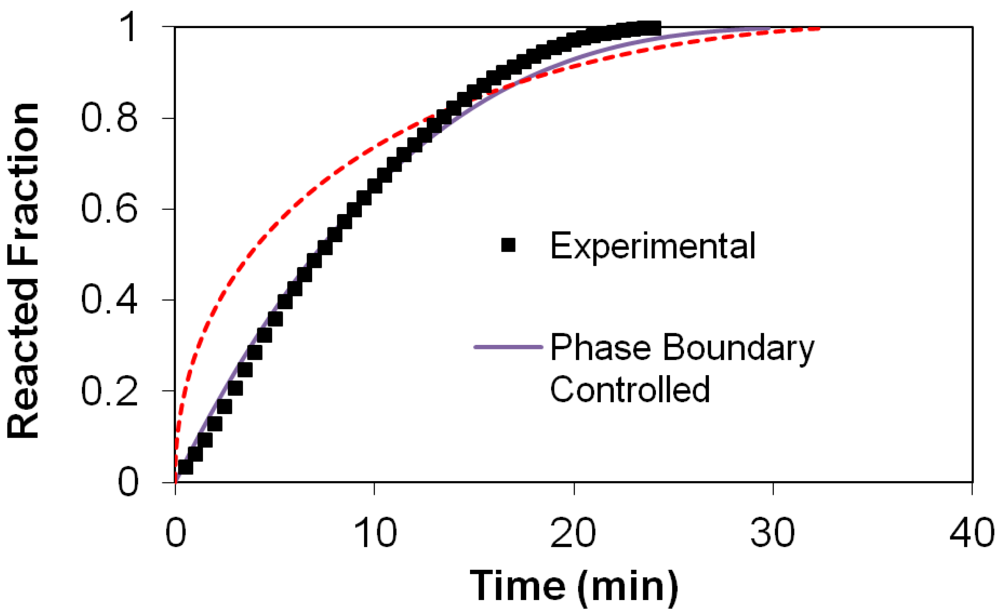
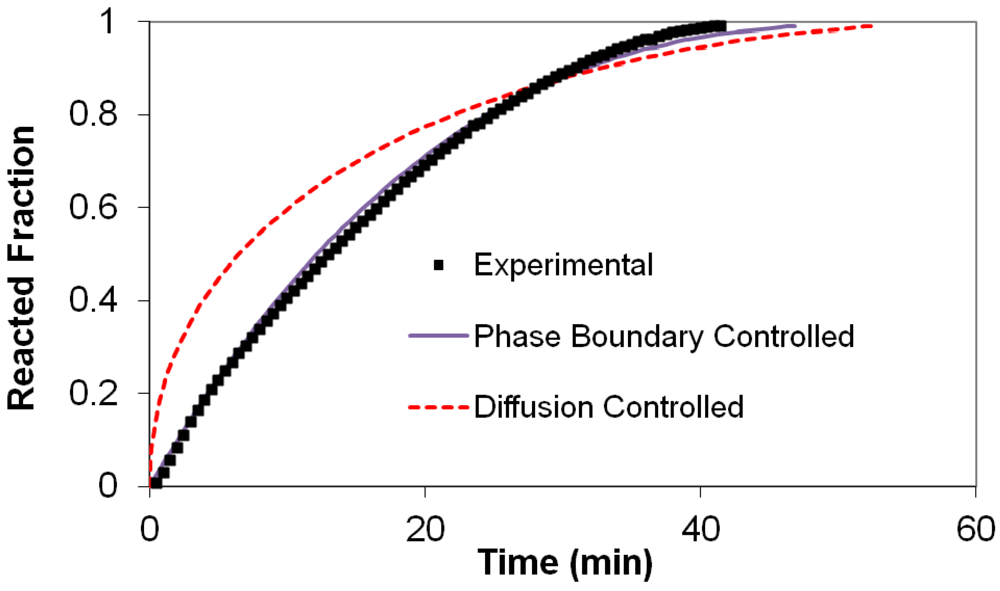
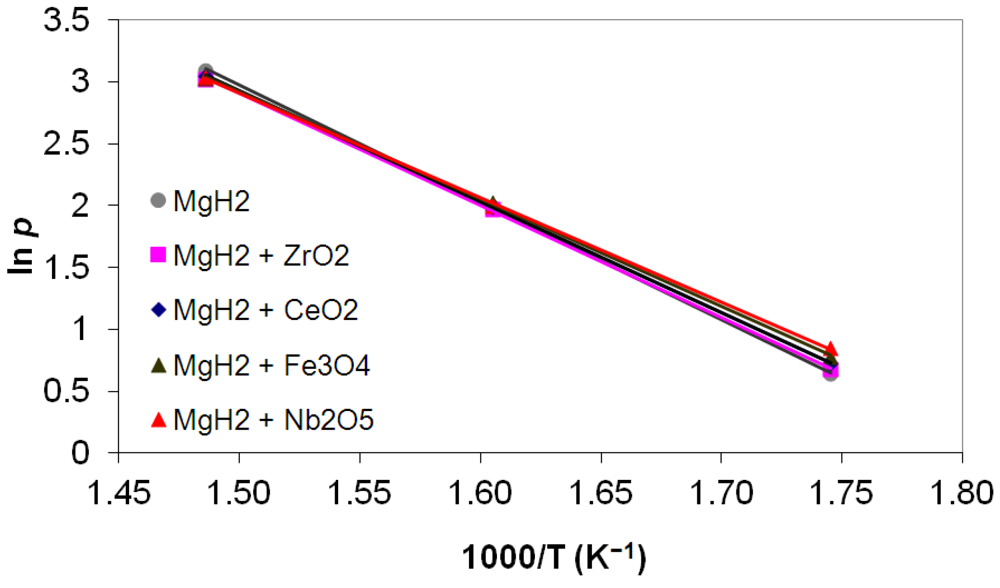
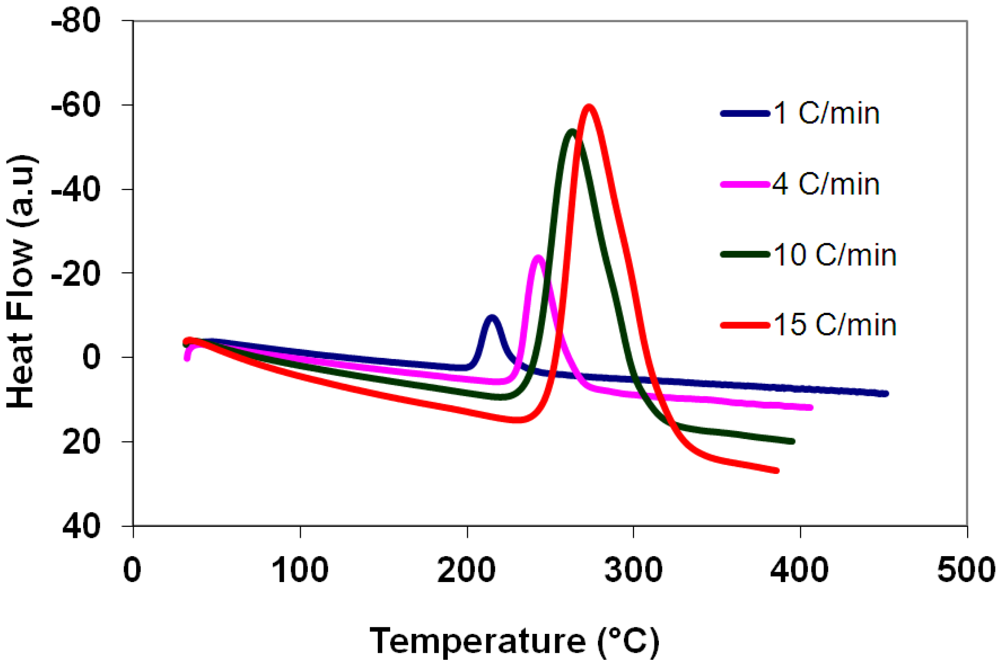
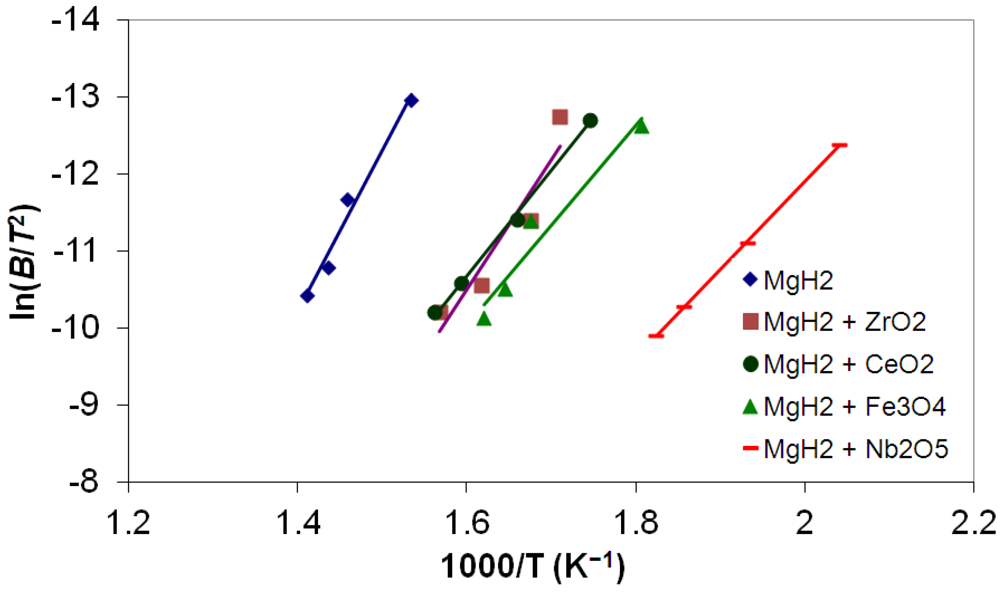
3.5. Differential Thermal Analysis and Kissinger Plots
 (3)
(3)
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Sabitu, S.T.; Gallo, G.; Goudy, A.J. Effect of TiH2 and Mg2Ni additives on the hydrogen storage properties of magnesium hydride. J. Alloys Comp. 2010, 499, 35–38. [Google Scholar] [CrossRef]
- Sabitu, S.T.; Fagbami, O.; Goudy, A.J. Kinetics and modeling study of magnesium hydride with various additives at constant pressure thermodynamic forces. J. Alloys Comp. 2011, 509, S588–S591. [Google Scholar]
- Yang, W.N.; Shang, C.X.; Guo, Z.X. Site density effect of Ni particles on hydrogen desorption of MgH2. Int. J. Hydrogen Energy 2010, 35, 4534–4542. [Google Scholar]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of Ni nano-particle and Nb oxide on H-desorption properties in MgH2 prepared by ball milling. J. Alloys Comp. 2005, 404-406, 716–719. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Ares-Fernandez, J.R.; Klassen, T.; Bormann, R. Effect of Nb2O5 on MgH2 properties during mechanical milling. Int. J. Hydrogen Energy 2007, 32, 2400–2407. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Hino, S.; Fujii, H. Remarkable improvement of hydrogen sorption kinetics in magnesium catalyzed by Nb2O5. J. Alloys Comp. 2006, 420, 46–49. [Google Scholar] [CrossRef]
- Evard, E.; Gabis, I.; Yartys, V.A. Kinetics of hydrogen evolution from MgH2: Experimental studies, mechanism and modeling. Int. J. Hydrogen Energy 2010, 35, 9060–9069. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, P.; Ma, L.; Cheng, H. Hydrogen sorption kinetics of MgH2 catalyzed with NbF5. J. Alloys Comp. 2008, 453, 138–142. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. J. Alloys Comp. 2004, 364, 242–246. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Emami, S. Kinetics of dehydrogenation of the Mg-Ti-H hydrogen storage system. Int. J. Hydrogen Energy 2011, 36, 8344–8350. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloys Comp. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Koh, J.T.; Goudy, A.J.; Huang, P.; Zhou, J. A comparison of the hydriding and dehydriding kinetics of LaNi5 hydride. J. Less-Common Metals 1989, 153, 89–100. [Google Scholar] [CrossRef]
- Zhang, W.; Cimato, J.; Goudy, A.J. The hydriding and dehydring kinetics of some LaNi5-xAlx alloys. J. Alloys Comp. 1993, 201, 175–179. [Google Scholar] [CrossRef]
- Smith, G.; Goudy, A.J. Thermodynamics, kinetics and modeling studies of LaNi5-xCox hydride system. J. Alloys Comp. 2001, 316, 93–98. [Google Scholar] [CrossRef]
- Yang, H.; Ojo, A.; Ogaro, P.; Goudy, A.J. Hydriding and dehydriding kinetics of sodium alanate at constant pressure thermodynamic driving forces. J. Phys. Chem. C 2009, 113, 14512–14517. [Google Scholar] [CrossRef]
- Ibikunle, A.; Goudy, A.J.; Yang, H. Hydrogen storage in a CaH2/LiBH4 destabilized metal hydride system. J. Alloys Comp. 2009, 475, 110–115. [Google Scholar] [CrossRef]
- Durojaiye, T.; Goudy, A.J. Desorption kinetics of lithium amide/magnesium hydride systems at constant pressure thermodynamic driving forces. Int. J. Hydrogen Energy 2012, 37, 3298–3304. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sabitu, S.T.; Goudy, A.J. Dehydrogenation Kinetics and Modeling Studies of MgH2 Enhanced by Transition Metal Oxide Catalysts Using Constant Pressure Thermodynamic Driving Forces. Metals 2012, 2, 219-228. https://doi.org/10.3390/met2030219
Sabitu ST, Goudy AJ. Dehydrogenation Kinetics and Modeling Studies of MgH2 Enhanced by Transition Metal Oxide Catalysts Using Constant Pressure Thermodynamic Driving Forces. Metals. 2012; 2(3):219-228. https://doi.org/10.3390/met2030219
Chicago/Turabian StyleSabitu, Saidi Temitope, and Andrew J. Goudy. 2012. "Dehydrogenation Kinetics and Modeling Studies of MgH2 Enhanced by Transition Metal Oxide Catalysts Using Constant Pressure Thermodynamic Driving Forces" Metals 2, no. 3: 219-228. https://doi.org/10.3390/met2030219
APA StyleSabitu, S. T., & Goudy, A. J. (2012). Dehydrogenation Kinetics and Modeling Studies of MgH2 Enhanced by Transition Metal Oxide Catalysts Using Constant Pressure Thermodynamic Driving Forces. Metals, 2(3), 219-228. https://doi.org/10.3390/met2030219




