Optimization of Rare Earth Yield from Fluoride Roasting of Neodymium–Iron–Boron Waste Using Response Surface Methodology
Abstract
1. Introduction
2. Experimental Materials and Equipment
2.1. Materials
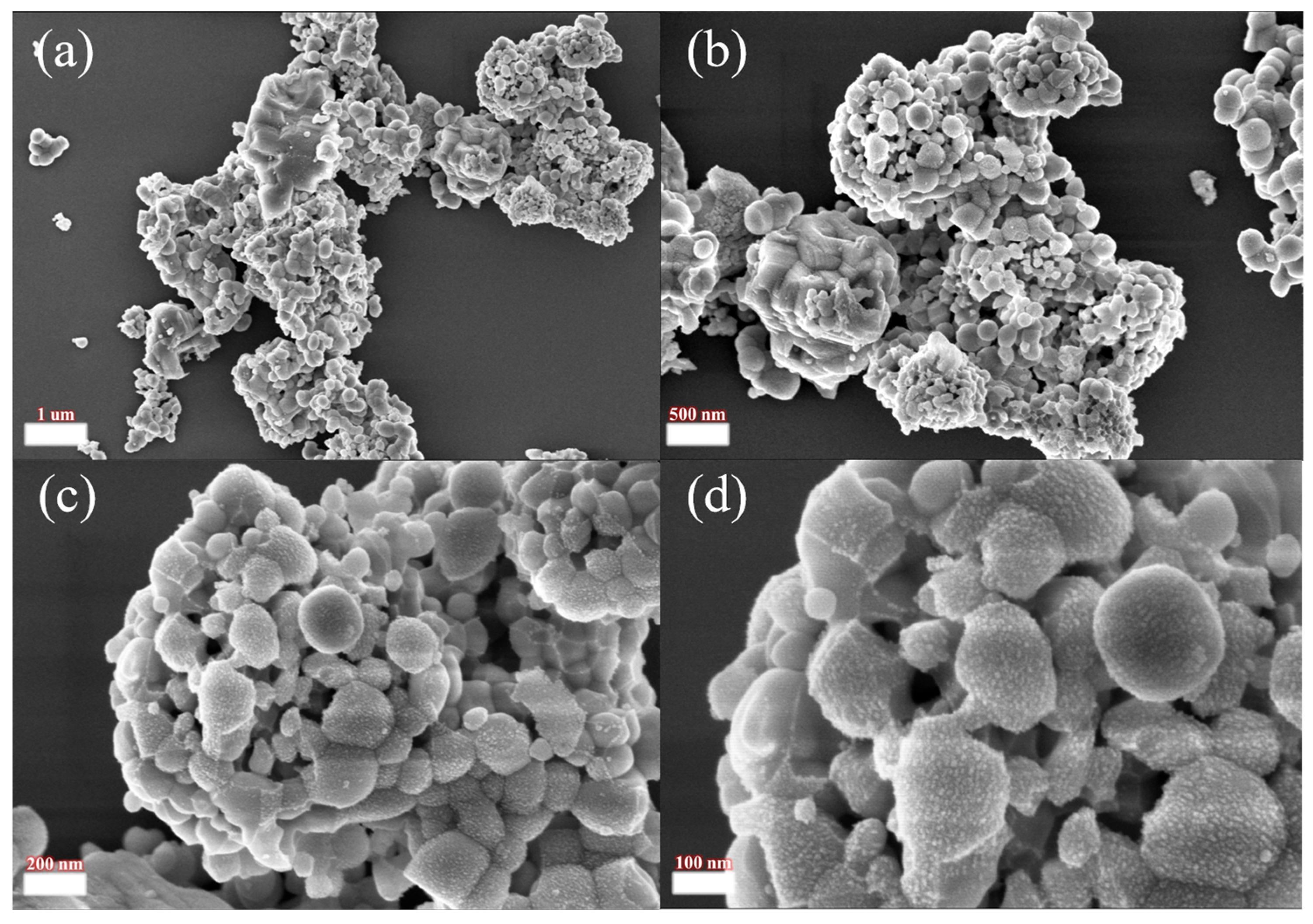
2.2. Analytical Characterization
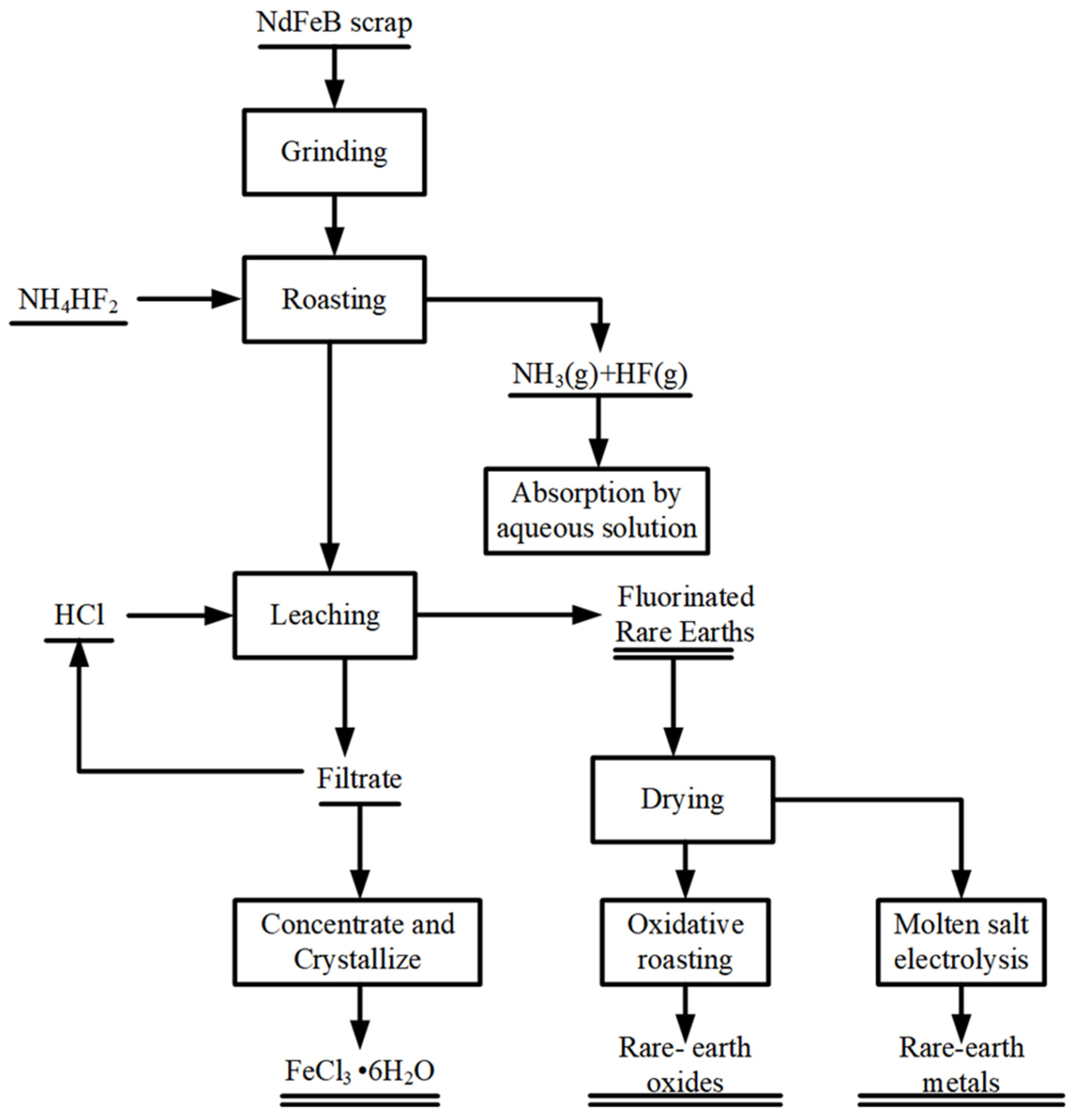
2.3. Experimental Procedures and Methods
- Xi—leaching rate of rare earth “i” (%);
- V—volume of leachate (L);
- Ci—concentration of rare earth “i” in the leach solution (g/L);
- m—weigh the mass of NdFeB scrap (g);
- ωi—content of rare earth “i” in NdFeB waste (%).
- X—total rare earth leaching rate (%);
- C—total concentration of rare earths in the leach solution (g/L);
- ω—total content of rare earths in the roasting product (%).
- η—rare earth recovery rate (%);
- X—total rare earth leaching rate (%).
3. Results and Discussion
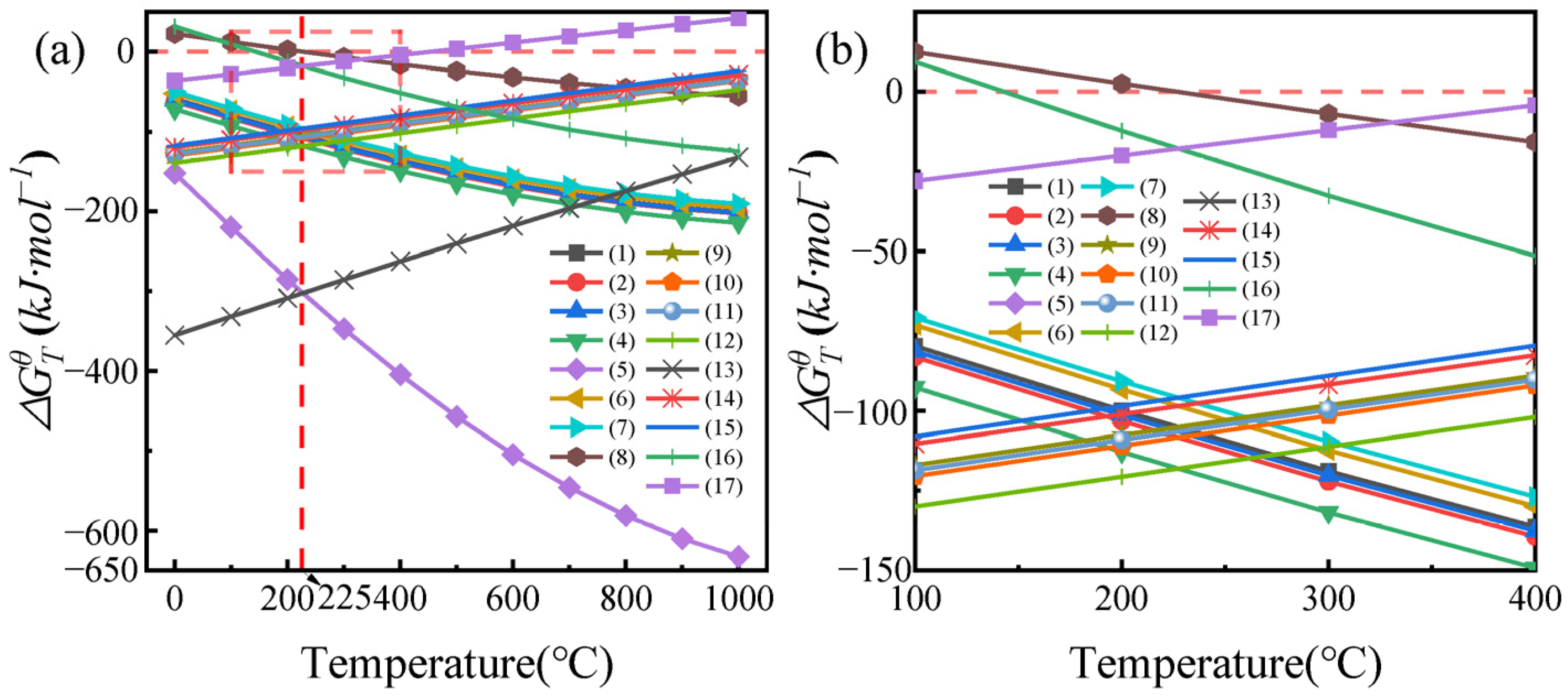
3.1. Thermodynamic Analysis of Roasting Reactions
3.2. Effect of Roasting Conditions on Rare Earth Recovery Rates
3.2.1. Effect of Roasting Temperature on Rare Earth Recovery Rates
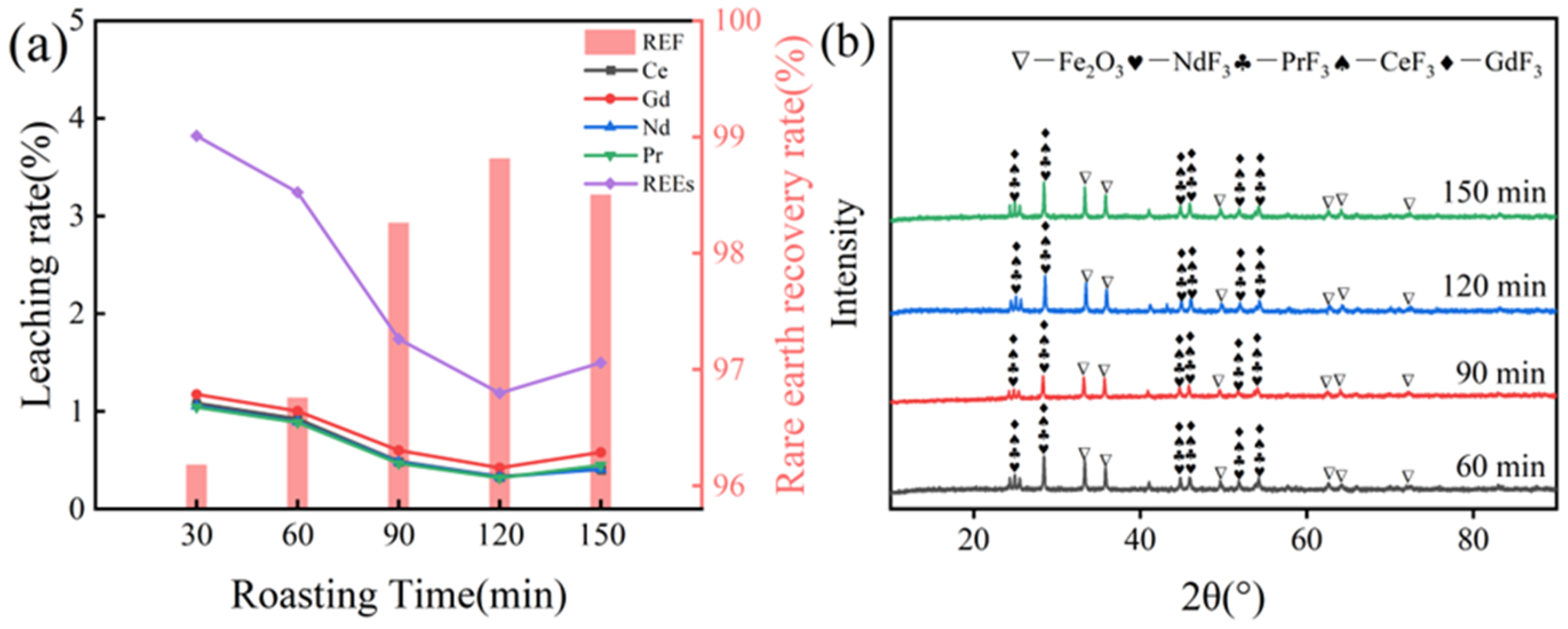
3.2.2. Effect of Roasting Time on Rare Earth Recovery Rate
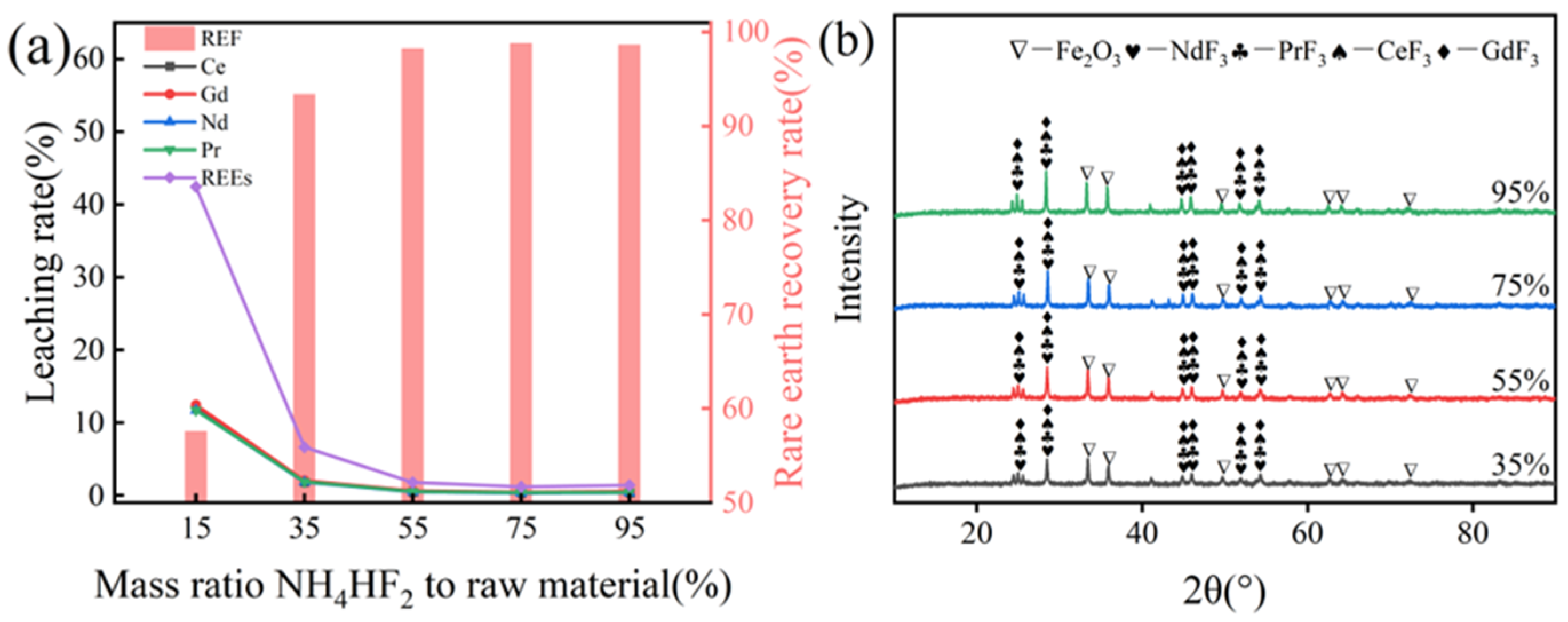
3.2.3. Effect of NH4HF2 Addition on Rare Earth Recovery Rate
3.3. Optimization of NH4HF2 Roasting Process Conditions by Response Surface Methodology (RSM)
3.3.1. Box–Behnken Experimental Design
3.3.2. BBD Experimental Results
3.3.3. Statistical Analysis and Analysis of Variance
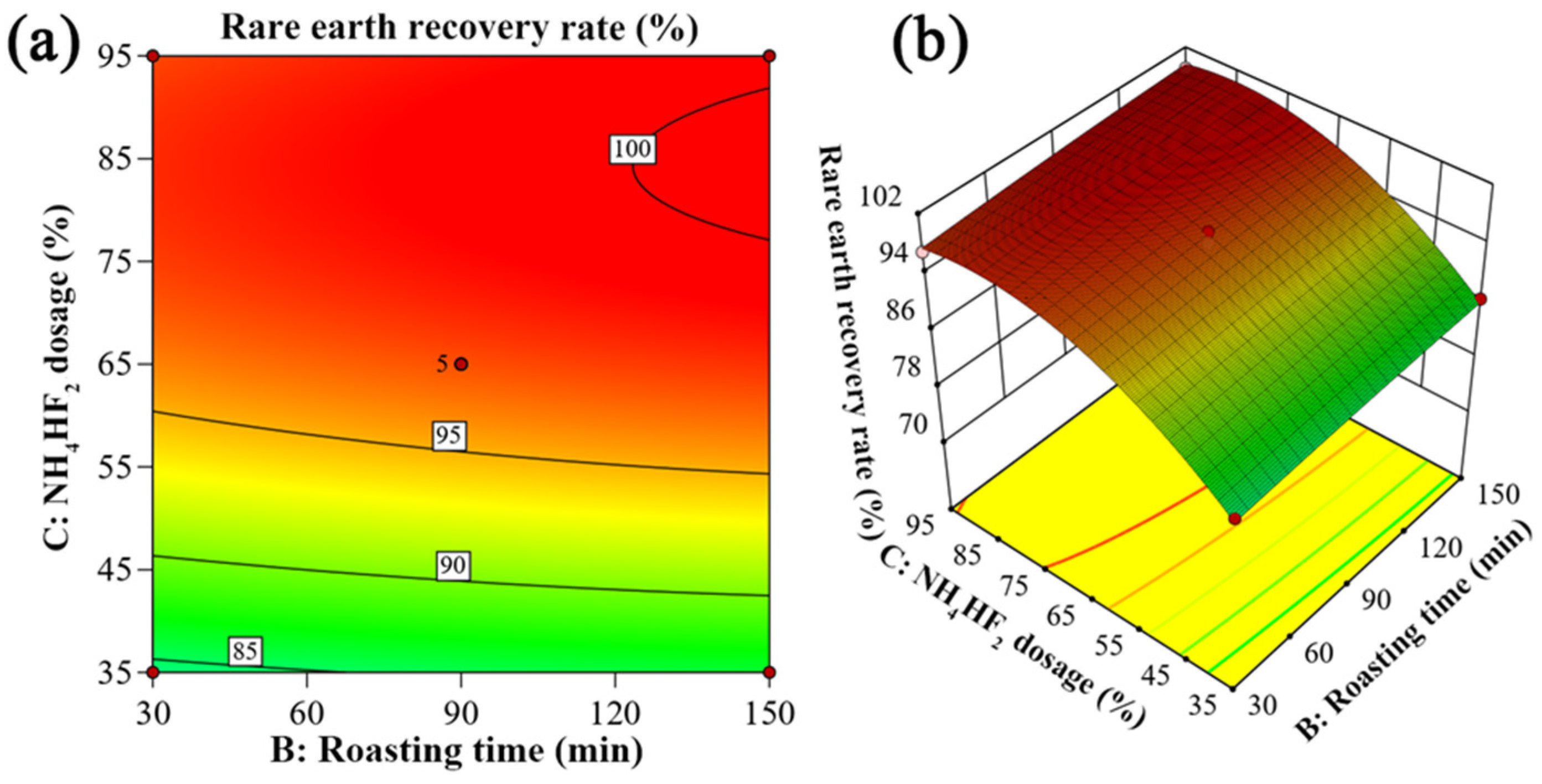
3.3.4. Response Surface Analysis
3.4. Acid Leach Analysis
3.5. Recommendations for the Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, T.; Qu, P.; Pan, W.; Liu, R.; Li, M.; Rehman, S.U.; Zhong, Z.; Xie, G. Sintered NdFeB Magnets with Tb-Dy Double-Layer Core/Shell Structure Fabricated by Double Alloy Method and Grain Boundary Diffusion. J. Alloys Compd. 2021, 856, 158191. [Google Scholar] [CrossRef]
- Kumari, A.; Sahu, S.K. A Comprehensive Review on Recycling of Critical Raw Materials from Spent Neodymium Iron Boron (NdFeB) Magnet. Sep. Purif. Technol. 2023, 317, 123527. [Google Scholar] [CrossRef]
- Yadav, J.; Sarker, S.K.; Bruckard, W.; Jegatheesan, V.; Haque, N.; Singh, N.; Pramanik, B.K. Greening the Supply Chain: Sustainable Approaches for Rare Earth Element Recovery from Neodymium Iron Boron Magnet Waste. J. Environ. Chem. Eng. 2024, 12, 113169. [Google Scholar] [CrossRef]
- Alguacil, F.J. Utilizing Deep Eutectic Solvents in the Recycle, Recovery, Purification and Miscellaneous Uses of Rare Earth Elements. Molecules 2024, 29, 1356. [Google Scholar] [CrossRef]
- Cao, Y.; Shao, P.; Chen, Y.; Zhou, X.; Yang, L.; Shi, H.; Yu, K.; Luo, X.; Luo, X. A Critical Review of the Recovery of Rare Earth Elements from Wastewater by Algae for Resources Recycling Technologies. Resour. Conserv. Recycl. 2021, 169, 105519. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J. Extraction and Separation of Heavy Rare Earth Elements: A Review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Gaustad, G.; Williams, E.; Leader, A. Rare Earth Metals from Secondary Sources: Review of Potential Supply from Waste and Byproducts. Resour. Conserv. Recycl. 2020, 167, 105213. [Google Scholar] [CrossRef]
- Kinnunen, P.H.-M.; Kaksonen, A.H. Towards Circular Economy in Mining: Opportunities and Bottlenecks for Tailings Valorization. J. Clean. Prod. 2019, 228, 153–160. [Google Scholar] [CrossRef]
- Xavier, L.H.; Giese, E.C.; Ribeiro-Duthie, A.C.; Lins, F.A.F. Sustainability and the Circular Economy: A Theoretical Approach Focused on E-Waste Urban Mining. Resour. Policy 2019, 74, 101467. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Darcy, J.W.; Apelian, D.; Emmert, M.H. Value Analysis of Neodymium Content in Shredder Feed: Toward Enabling the Feasibility of Rare Earth Magnet Recycling. Environ. Sci. Technol. 2014, 48, 6553–6560. [Google Scholar] [CrossRef]
- Reimer, M.V.; Schenk-Mathes, H.Y.; Hoffmann, M.F.; Elwert, T. Recycling Decisions in 2020, 2030, and 2040—When Can Substantial NdFeB Extraction be Expected in the EU? Metals 2018, 8, 867. [Google Scholar] [CrossRef]
- Silvestri, L.; Forcina, A.; Silvestri, C.; Traverso, M. Circularity Potential of Rare Earths for Sustainable Mobility: Recent Developments, Challenges and Future Prospects. J. Clean. Prod. 2021, 292, 126089. [Google Scholar] [CrossRef]
- Choubey, P.K.; Singh, N.; Panda, R.; Jyothi, R.K.; Yoo, K.; Park, I.; Jha, M.K. Development of Hydrometallurgical Process for Recovery of Rare Earth Metals (Nd, Pr, and Dy) from Nd-Fe-B Magnets. Metals 2021, 11, 1987. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Z.; Zhang, G. Recovering REEs from NdFeB Wastes with High Purity and Efficiency by Leaching and Selective Precipitation Process with Modified Agents. J. Rare Earths 2019, 37, 205–210. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; Van Gerven, T. Recycling of NdFeB Magnets Using Sulfation, Selective Roasting, and Water Leaching. J. Sustain. Metall. 2015, 1, 199–215. [Google Scholar] [CrossRef]
- Liu, F.; Chen, F.; Wang, L.; Ma, S.; Wan, X.; Wang, J. Selective Separation of Rare Earths from Spent Nd-Fe-B Magnets Using Two-Stage Ammonium Sulfate Roasting Followed by Water Leaching. Hydrometallurgy 2021, 203, 105626. [Google Scholar] [CrossRef]
- Shirayama, S.; Okabe, T.H. Selective Extraction and Recovery of Nd and Dy from Nd-Fe-B Magnet Scrap by Utilizing Molten MgCl2. Metall. Mater. Trans. B 2018, 49, 1067–1077. [Google Scholar] [CrossRef]
- Abbasalizadeh, A.; Malfliet, A.; Seetharaman, S.; Sietsma, J.; Yang, Y. Electrochemical Recovery of Rare Earth Elements from Magnets: Conversion of Rare Earth Based Metals into Rare Earth Fluorides in Molten Salts. Mater. Trans. 2017, 58, 400–405. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, N.; Li, Y.; Wu, P.; Dang, Z.; Ke, Y. Efficient Recovery of Rare Earth Elements from Discarded NdFeB Magnets. Process Saf. Environ. Prot. 2019, 124, 317–325. [Google Scholar] [CrossRef]
- Gergoric, M.; Ravaux, C.; Steenari, B.-M.; Espegren, F.; Retegan, T. Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids. Metals 2018, 8, 721. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M.; Van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Hua, Z.; Wang, J.; Wang, L.; Zhao, Z.; Li, X.; Xiao, Y.; Yang, Y. Selective Extraction of Rare Earth Elements from NdFeB Scrap by Molten Chlorides. ACS Sustain. Chem. Eng. 2014, 2, 2536–2543. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Lei, X.; Lin, S.; Zhu, X.; Wang, J. Mechanism and Experimental Study on the Recovery of Rare Earth Elements from Neodymium Iron Boron Waste by NaBF4 Fluorination Method. Int. J. Refract. Met. Hard Mater. 2025, 126, 106943. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Y.; Lei, X.; Wang, J. Mechanism and Experimental Study on the Recovery of Rare Earth Elements from Neodymium Iron Boron Waste Using the ZnF2 Fluorination Method. Materials 2024, 17, 5807. [Google Scholar] [CrossRef] [PubMed]
- Botla, G.; Barmavatu, P.; Pohorely, M.; Jeremias, M.; Sikarwar, V.S. Optimization of Value-Added Products Using Response Surface Methodology from the HDPE Waste Plastic by Thermal Cracking. Therm. Sci. Eng. Prog. 2024, 50, 102514. [Google Scholar] [CrossRef]
- Anaklı, D.; Erşan, M. Optimization of Reduced Graphene Oxide Yield Using Response Surface Methodology. Diamond Relat. Mater. 2024, 148, 111524. [Google Scholar] [CrossRef]
- Battestini-Vives, M.; Xiao, X.; Lipnizki, F.; Rudolph-Schöpping, G. Response Surface Methodology to Optimize Membrane Cleaning in Nanofiltration of Kraft Black Liquor. Sep. Purif. Technol. 2025, 354, 128626. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Q.; Xu, C.; Gao, Y.; Yu, X. Response Surface Optimization for Deep Eutectic Solvents Extraction of Bamboo Shoots Proteins. Adv. Bamboo Sci. 2024, 7, 100080. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Ma, B.; Wang, C.; Chen, Y. Strengthening Extraction of Lithium and Rubidium from Activated α-Spodumene Concentrate via Sodium Carbonate Roasting. J. Ind. Eng. Chem. 2023, 123, 248–259. [Google Scholar] [CrossRef]
- Mwanat, H.-M.M.; Kasongo, K.B. Cobalt Dissolution from Concentrate in Sulfuric Acid-Ferrous Sulfate System: Process Parameters Optimization by Response Surface Methodology (RSM). J. Sustain. Metall. 2021, 7, 1838–1851. [Google Scholar] [CrossRef]
- Abeng, D.; Sutaryo, S.; Purnomoadi, A.; Susanto, S.; Purbowati, E.; Adiwinarti, R.; Purwasih, R.; Widiharih, T. Optimization of Methane Production from Dairy Cow Manure and Germinated Papaya Seeds Using Response Surface Methodology. Case Stud. Chem. Environ. Eng. 2024, 10, 100927. [Google Scholar] [CrossRef]
- Ai, F.; Hu, Y.; Kang, K.; Lam, S.S.; Foong, S.Y.; Yong, C.; Li, Y.; Zhang, Q.; Zhang, Y.; Zhu, S.; et al. Optimization of Photo-Fermentation Bio-Hydrogen Production from Corncob via Genetic Algorithm Optimized Neural Network and Response Surface Method Model. Int. J. Hydrogen Energy 2025, 138, 1293–1302. [Google Scholar] [CrossRef]
- OuYang, J.; He, Q.; Liao, X.; Deng, Q.; Bai, J.; Zhang, Y.; Dong, F.; Shi, L.; Jiang, J. Optimizing High-Water-Resistance Cementitious Materials Using Modified Phosphogypsum Synergy Multiple Solid Wastes with Response Surface Methodology. J. Build. Eng. 2025, 111, 113443. [Google Scholar] [CrossRef]
- Putra, V.G.V.; Mohamad, J.N.; Abdullah, F.; Paramahasti, M. Artificial Neural Networks (ANNs) and Response Surface Methodology (RSM) for Optimizing Wetting and Anti-Bacterial Properties of Woven Fabric. J. Text. Inst. 2025, 116, 1078–1094. [Google Scholar] [CrossRef]
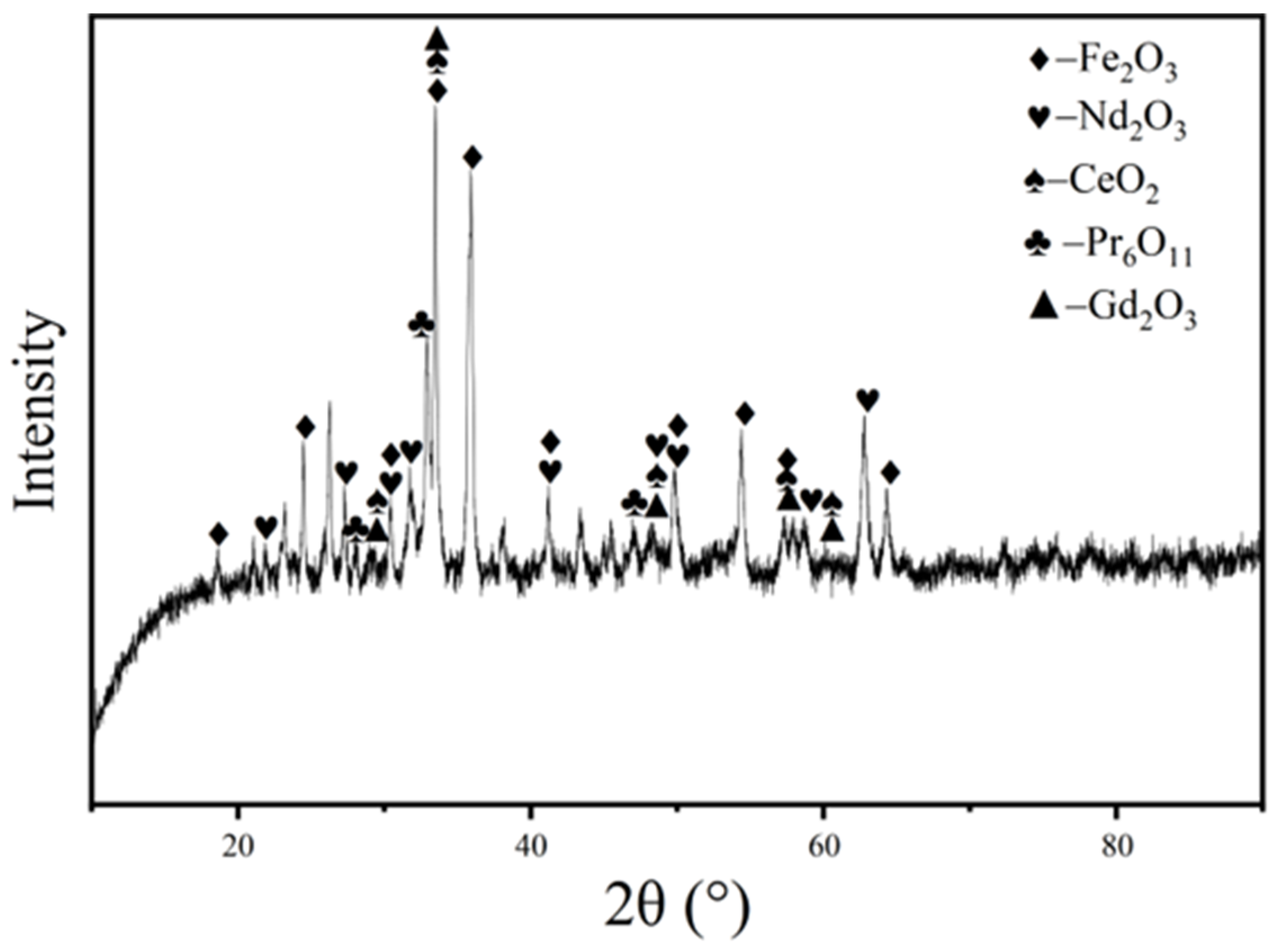
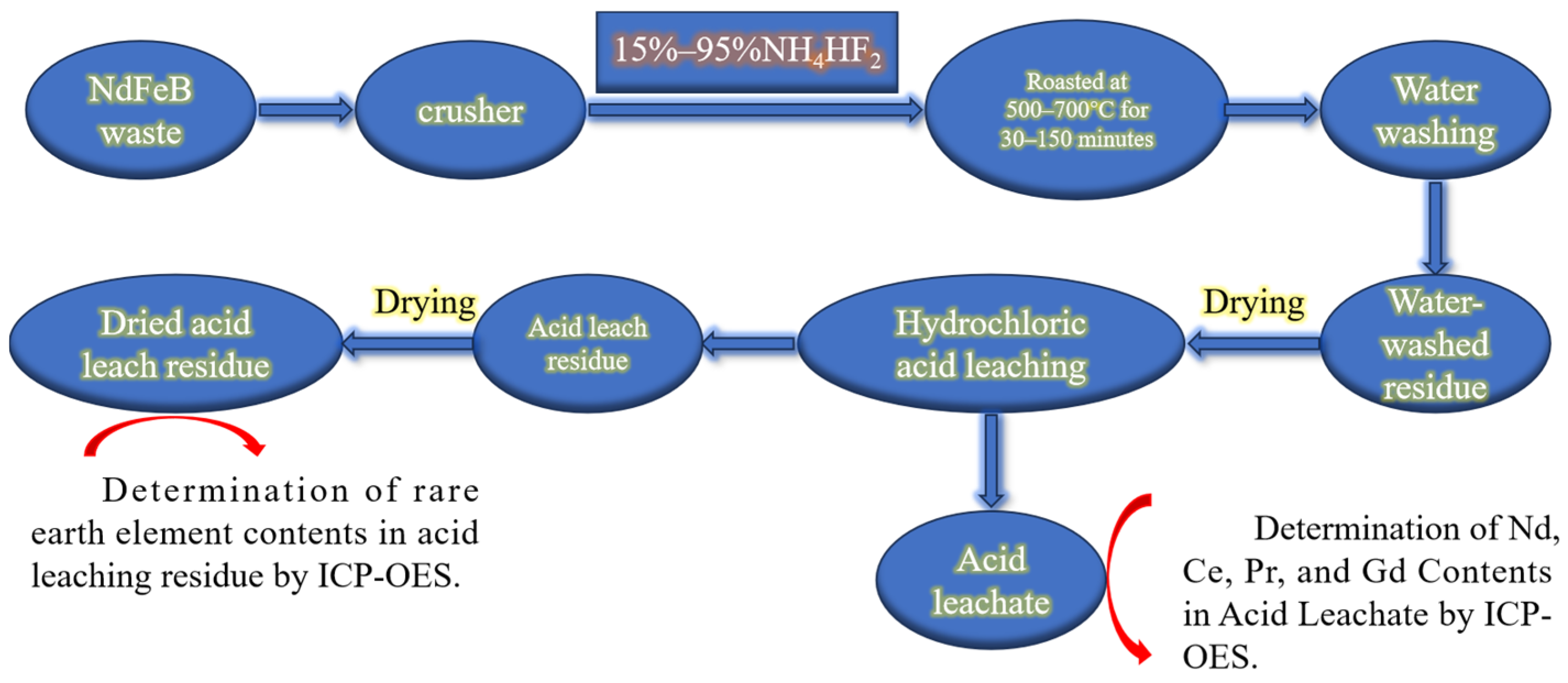

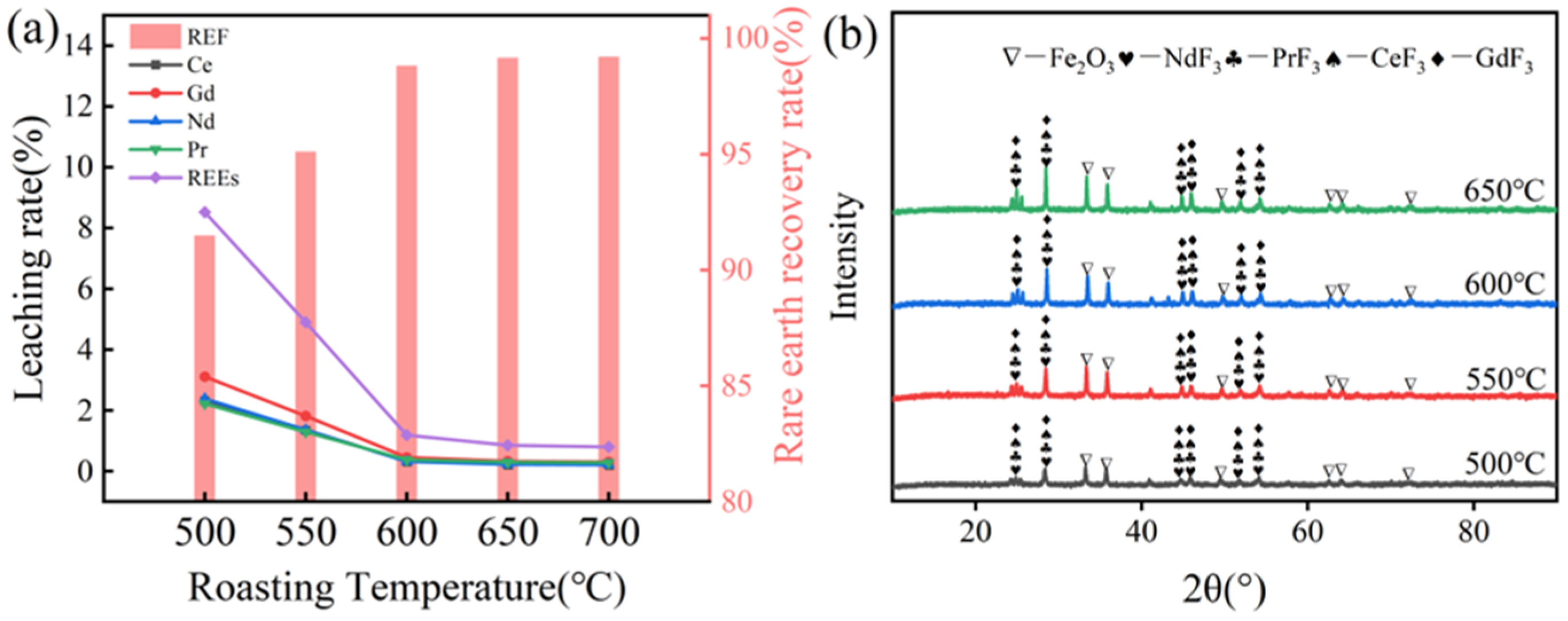




| Component | REO | Fe2O3 * | Al2O3 | CoO | K2O | ZnO | CuO | NiO | Na2O | MgO | B2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 26.98 | 68.94 | 1.81 | 0.50 | 0.49 | 0.27 | 0.23 | 0.19 | 0.18 | 0.2 | 0.29 |
| Component | CeO2 | Pr6O11 | Nd2O3 | Sm2O3 | Gd2O3 | Dy2O3 | Ho2O3 | La2O3 |
|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 28.82 | 10.90 | 51.57 | 1.91 | 3.53 | 2.29 | 0.79 | 0.18 |
| No. | Reactions |
|---|---|
| 1 | 3NH4HF2 + Nd2O3 = 2NdF3 + 3NH3(g) + 3H2O(g) |
| 2 | 3NH4HF2 + Ce2O3 = 2CeF3 + 3NH3(g) + 3H2O(g) |
| 3 | 3NH4HF2 + Gd2O3 = 2GdF3 + 3NH3(g) + 3H2O(g) |
| 4 | 3NH4HF2 + La2O3 = 2LaF3 + 3NH3(g) + 3H2O(g) |
| 5 | 9NH4HF2 + Pr6O11 = 6PrF3 + 9NH3(g) + 9H2O(g) + O2(g) |
| 6 | 3NH4HF2 + Dy2O3 = 2DyF3 + 3NH3(g) + 3H2O(g) |
| 7 | 3NH4HF2 + Ho2O3 = 2HoF3 + 3NH3(g) + 3H2O(g) |
| 8 | NH4HF2 = NH3(g) + 2HF(g) |
| 9 | 6HF(g) + Nd2O3 = 2NdF3 + 3H2O(g) |
| 10 | 6HF(g) + Ce2O3 = 2CeF3 + 3H2O(g) |
| 11 | 6HF(g) + Gd2O3 = 2GdF3 + 3H2O(g) |
| 12 | 6HF(g) + La2O3 = 2LaF3 + 3H2O(g) |
| 13 | 18HF(g) + Pr6O11 = 6PrF3 + 9H2O(g) + O2(g) |
| 14 | 6HF(g) + Dy2O3 = 2DyF3 + 3H2O(g) |
| 15 | 6HF(g) + Ho2O3 = 2HoF3 + 3H2O(g) |
| 16 | 3NH4HF2 + Fe2O3 = 2FeF3 + 3NH3(g) + 3H2O(g) |
| 17 | 6HF(g) + Fe2O3 = 2FeF3 + 3H2O(g) |
| Levels | Factors | ||
|---|---|---|---|
| Roasting Temperature (°C) | Roasting Time (min) | NH4HF2 Dosage(%) | |
| −1 | 500 | 30 | 35 |
| 0 | 600 | 90 | 65 |
| 1 | 700 | 150 | 95 |
| Run | Roasting Temperature (°C) | Roasting Time (min) | NH4HF2 Dosage (%) | Rare Earth Recovery Rate (%) |
|---|---|---|---|---|
| 1 | 700 | 90 | 35 | 88.07 |
| 2 | 600 | 90 | 65 | 97.80 |
| 3 | 600 | 90 | 65 | 97.76 |
| 4 | 600 | 90 | 65 | 96.19 |
| 5 | 500 | 30 | 65 | 87.16 |
| 6 | 600 | 150 | 35 | 86.50 |
| 7 | 500 | 90 | 35 | 74.98 |
| 8 | 700 | 150 | 65 | 97.01 |
| 9 | 600 | 150 | 95 | 99.14 |
| 10 | 700 | 90 | 95 | 96.88 |
| 11 | 700 | 30 | 65 | 98.44 |
| 12 | 600 | 90 | 65 | 97.57 |
| 13 | 600 | 30 | 95 | 96.97 |
| 14 | 600 | 30 | 35 | 84.80 |
| 15 | 500 | 150 | 65 | 92.59 |
| 16 | 600 | 90 | 65 | 97.33 |
| 17 | 500 | 90 | 95 | 95.11 |
| Sum of | Mean | F | p-Value | |||
|---|---|---|---|---|---|---|
| Source | Squares | df | Square | Value | Prob > F | significant |
| Model | 702.34 | 9 | 78.04 | 136.94 | <0.0001 | |
| A-Roasting temperature (°C) | 116.62 | 1 | 116.62 | 204.65 | <0.0001 | |
| B-Roasting time (min) | 7.74 | 1 | 7.74 | 13.58 | 0.0078 | |
| C-Mass ratio of NH4HF2 to raw material (%) | 361.18 | 1 | 361.18 | 6351 | <0.0001 | |
| AB | 11.80 | 1 | 11.80 | 20.70 | 0.0026 | |
| AC | 32.04 | 1 | 32.04 | 56.23 | 0.0001 | |
| BC | 0.0558 | 1 | 0.0558 | 0.0980 | 0.7634 | |
| A2 | 46.22 | 1 | 46.22 | 81.11 | <0.0001 | |
| B2 | 0.1989 | 1 | 0.1989 | 0.3490 | 0.5732 | |
| C2 | 116.45 | 1 | 116.45 | 204.35 | <0.0001 | |
| Residual | 69 | 7 | 0.5699 | |||
| Lack of Fit | 2.22 | 3 | 0.7416 | 1.68 | 0.3072 | not significant |
| Pure Error | 1.76 | 4 | 0.4411 | |||
| Cor Total | 706.33 | 16 | ||||
| Fit Statistics | ||||||
| Std. Dev. | 0.7549 | R2 | 0.9944 | |||
| Mean | 93.20 | Adjusted R2 | 0.9871 | |||
| C.V. | 0.8100 | Predicted R2 | 0.9457 | |||
| PRESS | 38.35 | Adeq Precision | 41.9437 |
| REF | CeF4 | PrF3 | NdF3 | SmF3 | GdF3 | DyF3 | HoF3 | LaF3 | EuF3 | TbF3 | ErF3 | YF3 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 32.22 | 10.33 | 48.77 | 1.75 | 3.20 | 2.04 | 0.71 | 0.18 | 0.08 | 0.12 | 0.02 | 0.03 | 99.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, D.; Lei, X.; Wang, J.; Xiao, Y. Optimization of Rare Earth Yield from Fluoride Roasting of Neodymium–Iron–Boron Waste Using Response Surface Methodology. Metals 2025, 15, 942. https://doi.org/10.3390/met15090942
Liu Y, Li D, Lei X, Wang J, Xiao Y. Optimization of Rare Earth Yield from Fluoride Roasting of Neodymium–Iron–Boron Waste Using Response Surface Methodology. Metals. 2025; 15(9):942. https://doi.org/10.3390/met15090942
Chicago/Turabian StyleLiu, Youwei, Dewei Li, Xiang Lei, Jinliang Wang, and Yanfei Xiao. 2025. "Optimization of Rare Earth Yield from Fluoride Roasting of Neodymium–Iron–Boron Waste Using Response Surface Methodology" Metals 15, no. 9: 942. https://doi.org/10.3390/met15090942
APA StyleLiu, Y., Li, D., Lei, X., Wang, J., & Xiao, Y. (2025). Optimization of Rare Earth Yield from Fluoride Roasting of Neodymium–Iron–Boron Waste Using Response Surface Methodology. Metals, 15(9), 942. https://doi.org/10.3390/met15090942






