The Effect of Electrolytic Temperature on the Purity of Electrolytic Pure Iron
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Electrode
2.2. Electrolytic Refining Process
2.3. Material Characterization
3. Experimental Results
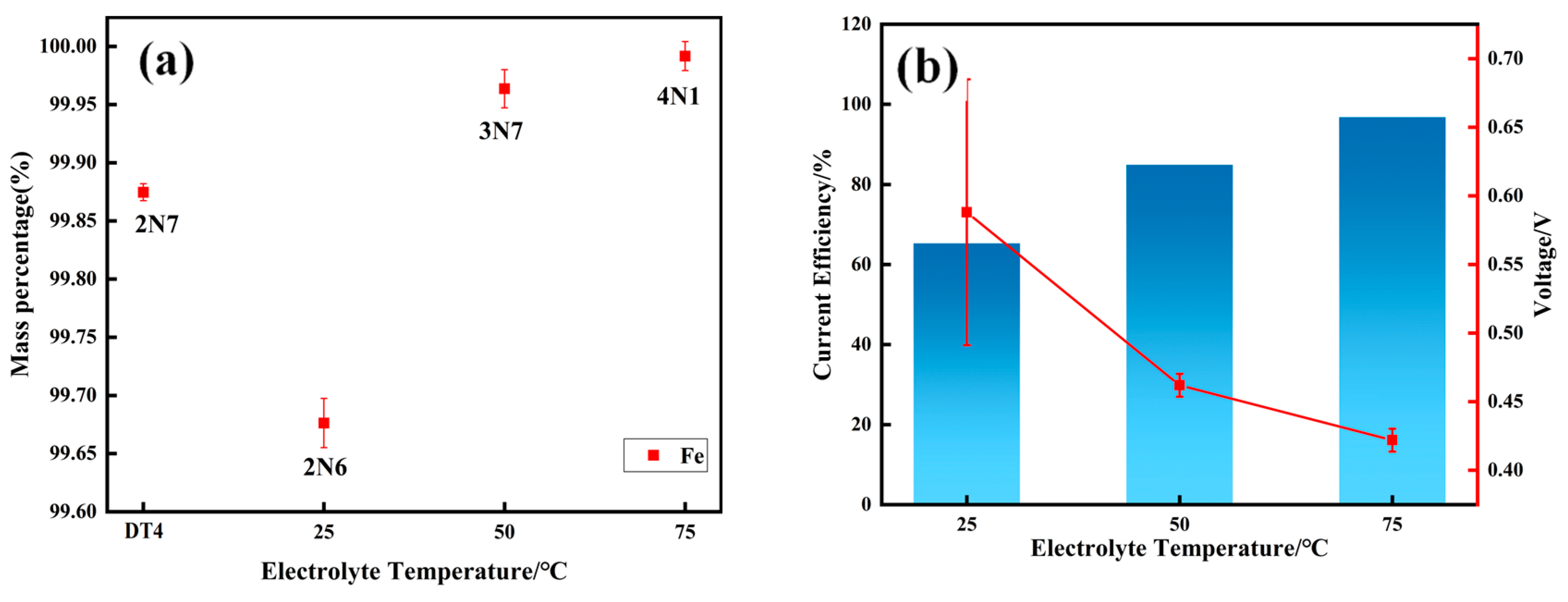
3.1. Purity Grade of Electrolytic Pure Iron
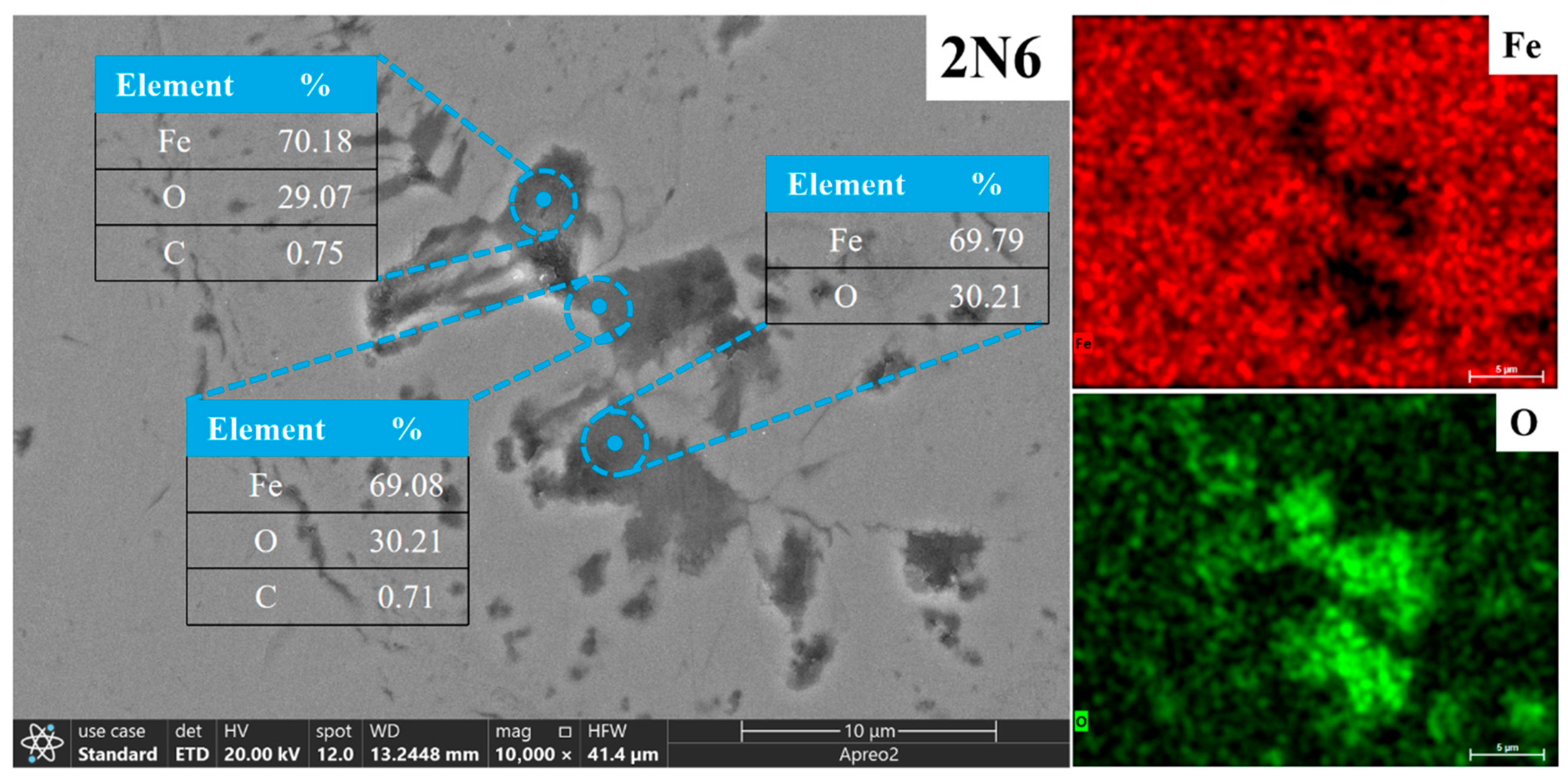
3.2. Microstructure of Electrolytic Pure Iron
4. Discussion on the Principle of Electrolytic Purification
4.1. Reaction Principle
- (1)
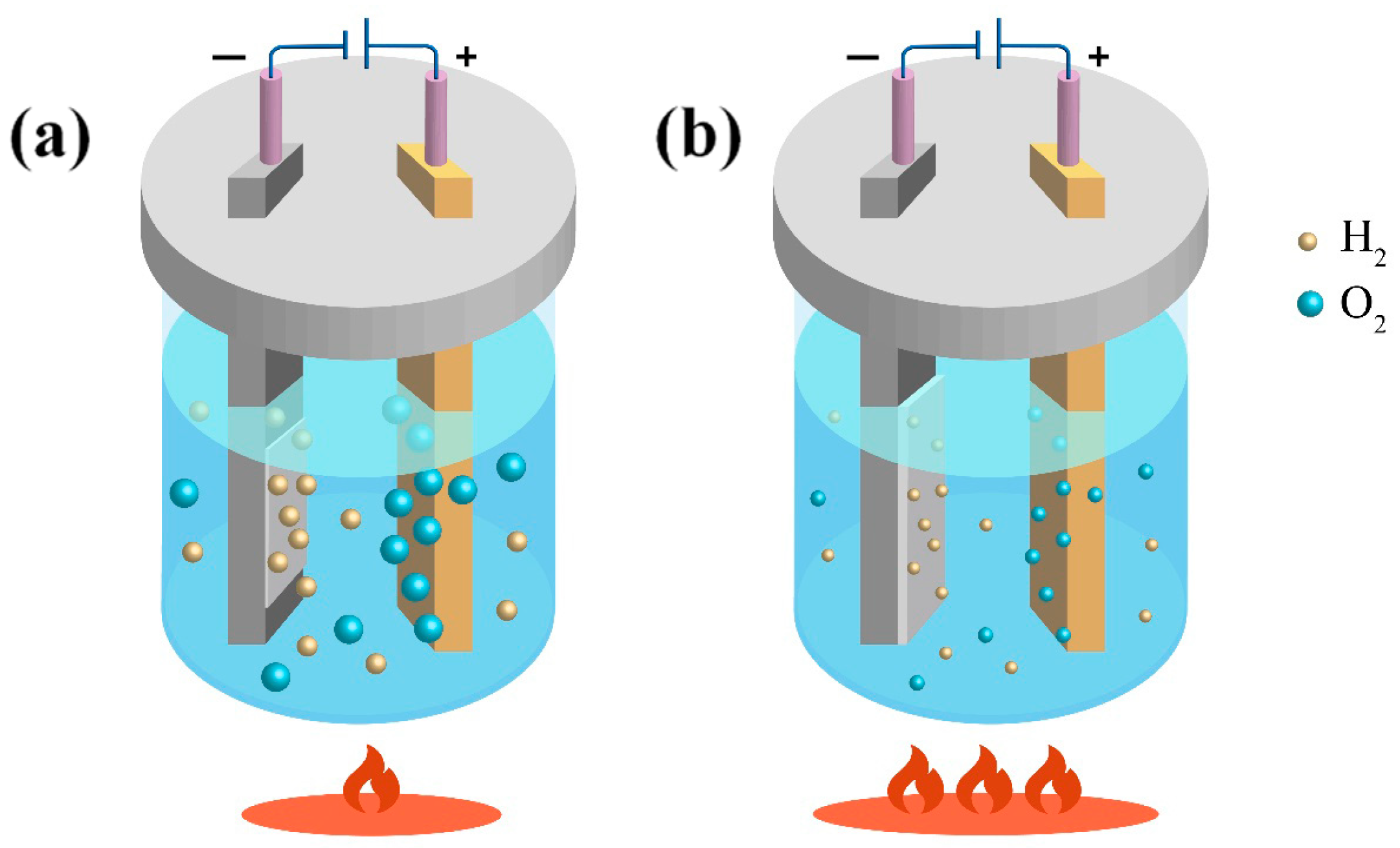
- Cathodic Fe deposition and H2 evolution.
- (2)
- Anode Fe oxidation and O2 evolution.
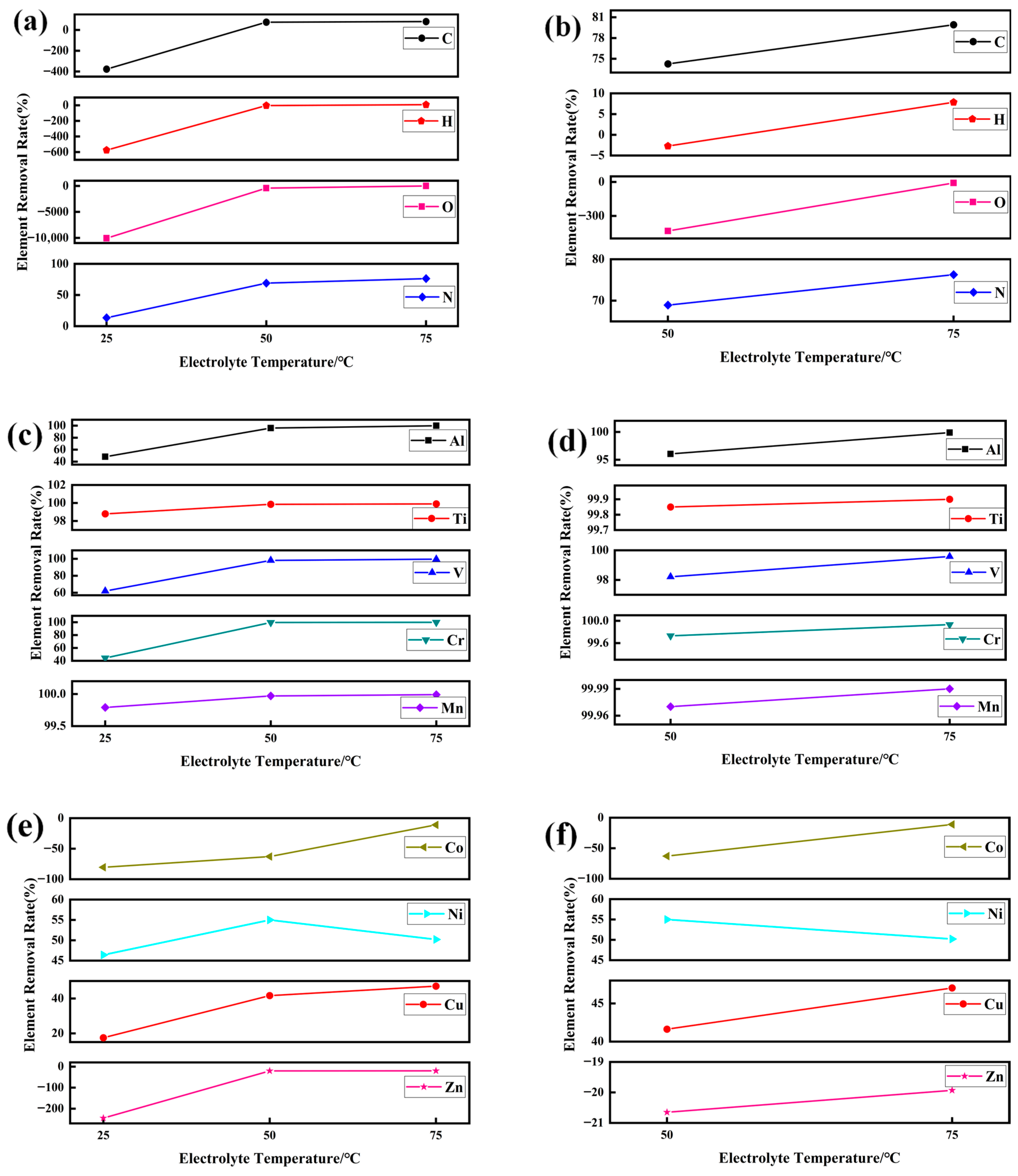
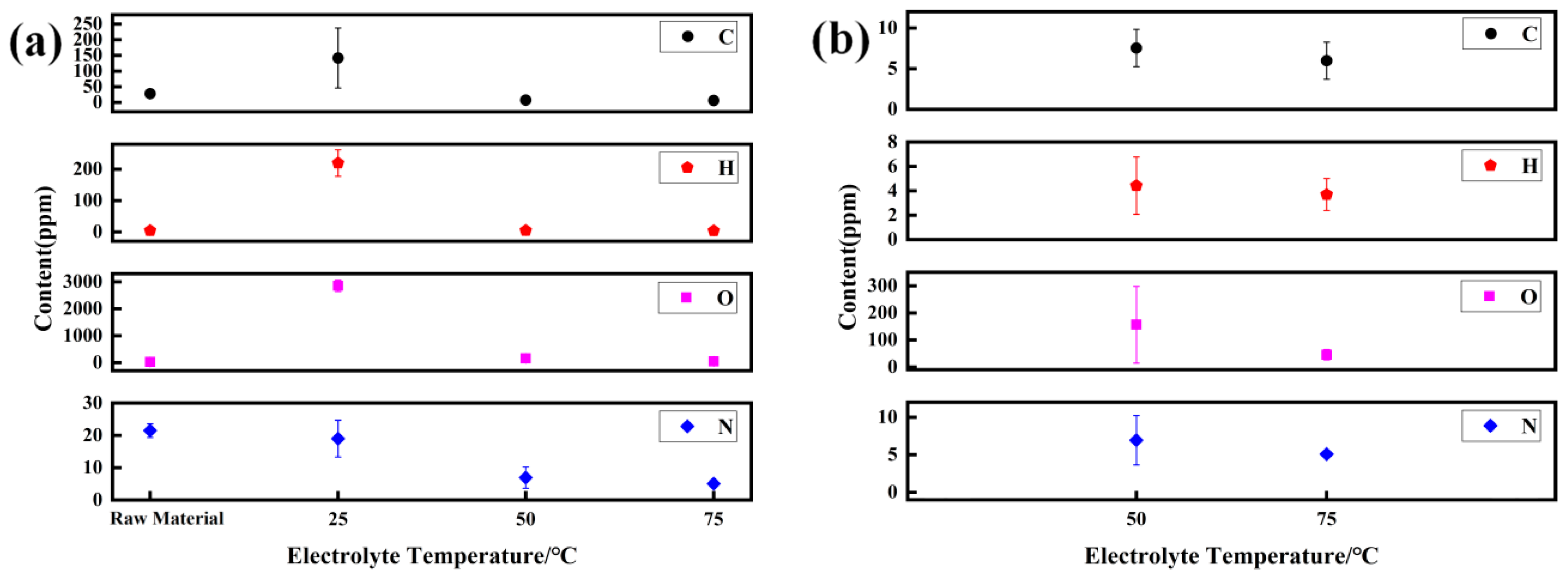
4.2. Purification Principle of Gas Element
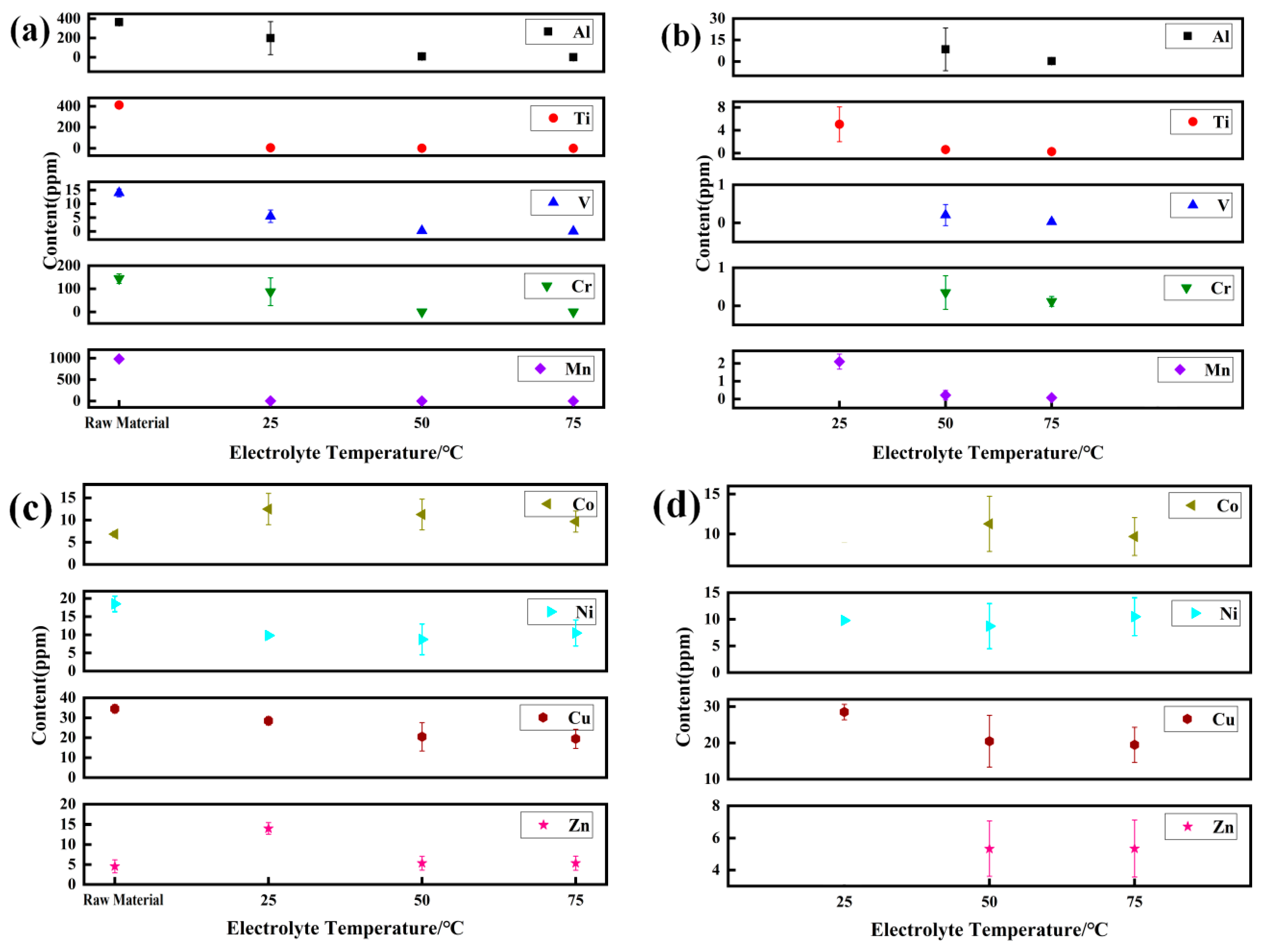
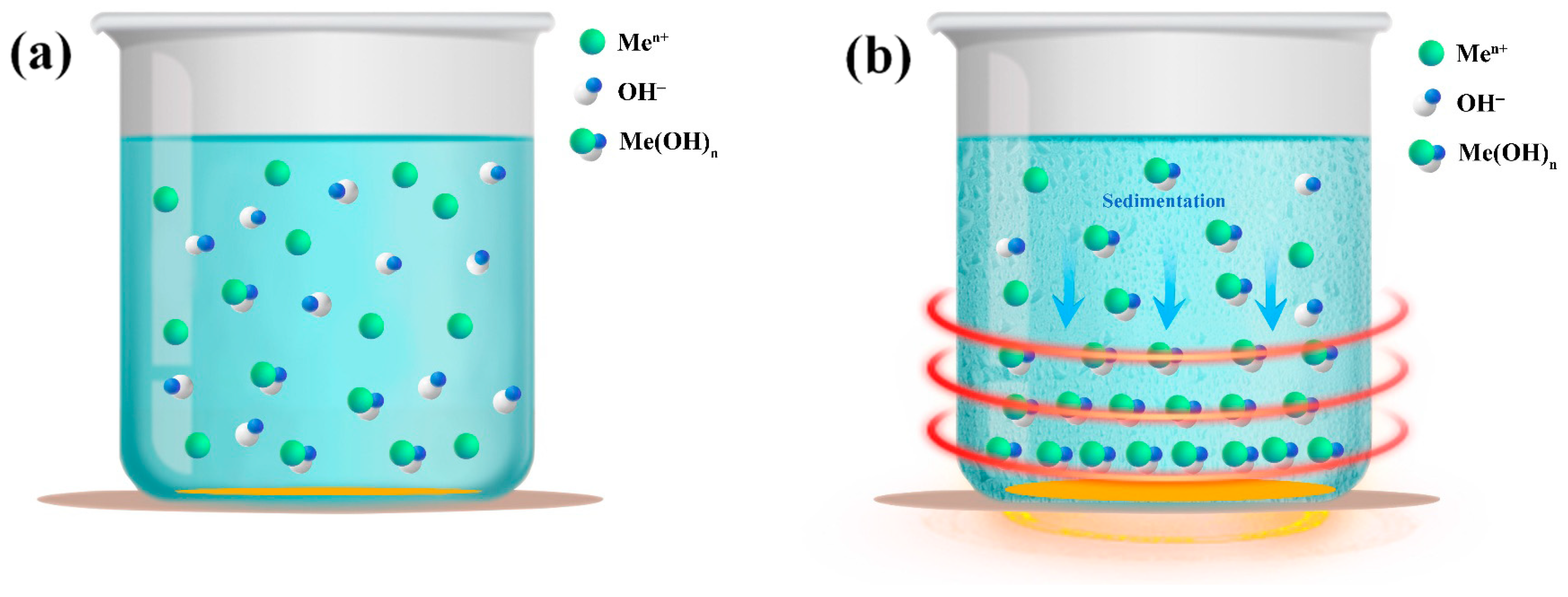
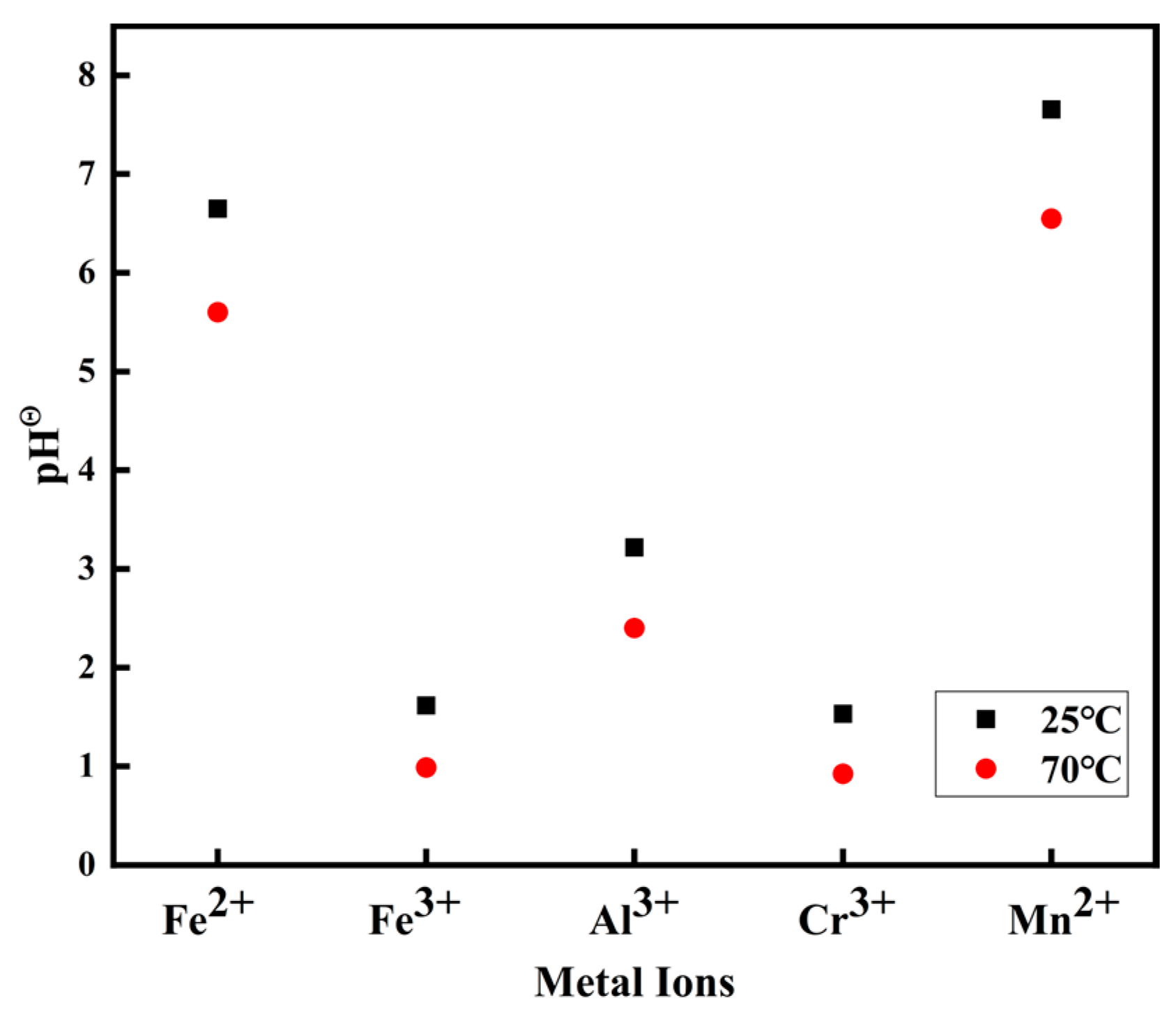
4.3. Purification Principle of Metal Elements
5. Conclusions
- (1)
- When electrolysis was performed at 75 °C, the purity of the electrolytic pure iron reached a maximum of 4N1.
- (2)
- At 75 °C, it was found that the total gas element content was the lowest at only 46.07 ppm. The hydrogen evolution potential was found to shift negatively at high temperatures, and a decrease in hydrogen evolution was observed. The hydrogen content in the prepared electrolytic pure iron was relatively low, reducing the possibility of hydrogen embrittlement. Except for metals like Co, Ni, Cu, and Zn, which are difficult to remove, the removal rate of other metal elements was above 99.9%.
- (3)
- As the electrolysis temperature increased, the macroscopic morphology transitioned from severe cracking to a smooth and intact form. The microstructure of the surface and cross-section evolved from pores to a typical irregular polygonal nucleus structure with banded features.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| Term | Definition |
| Electrolytic Pure Iron | High-purity iron of 99.9% purity and above was obtained by electrolytic deposition. |
| Purity | The proportion of iron elements in high-purity electrolytic iron is characterized. It is usually represented by “N”. For instance, 4N denotes a purity of 99.99%, while 5N signifies a purity of 99.999%. |
| Impurity Elements | Elements other than iron exist in high-purity electrolytic iron. |
| Gas Impurities | Including carbon, hydrogen, oxygen, nitrogen, and other elements. |
| part per million (ppm) | One millionth, commonly used to precisely indicate the extremely low content of impurity elements in high-purity electrolytic iron. |
| Electrolytic Cell | The core equipment for conducting the electrolysis reaction. It is primarily composed of an anode, cathode, and electrolyte. |
| Anode | The electrode through which the current flows in. Industrial pure iron is generally used. |
| Cathode | The electrode through which the current flows out. Pure titanium plates are generally used. |
| Electrolyte | The liquid in the electrolytic cell that conducts current and participates in electrochemical reactions. |
| Electrolytic Refining | The method of purifying crude metals based on the principle of electrolysis in the preparation of high-purity electrolytic iron based on the differences in the redox reaction activities of different elements on the electrodes. Iron in crude iron preferentially dissolves at the anode and enters the electrolyte in the form of ions. Most of the impurities remain in the anode mud, and high-purity iron is ultimately obtained at the cathode. |
| Electrolysis Temperature | The temperature of the electrolyte during electrolysis. |
| Current Density | The current intensity passing through the electrode per unit area. It is an important parameter in the electrolysis process. |
| pH value of the electrolyte | The indicator for assessing the acidity or alkalinity of the electrolyte. It plays a significant role in the electrolysis process. |
| Removal efficiency | A key indicator for measuring the extent to which impurity elements are removed or reduced. The calculation formula is as follows: (Initial Content − Final Content)/Initial Content × 100% |
References
- Jonathan, W.; Xu, H.; Wang, X.F.; Liao, X.C. China Accelerates Towards Carbon Neutrality: Steel Industry Carbon Reduction Pathways. 22 November 2024. Available online: https://www.mckinsey.com.cn/ (accessed on 13 July 2025).
- Wang, X.; Yang, H.T.; Yu, X.H.; Hu, J.C.; Cheng, J.X.; Jing, H.L. Research progress in the preparation of iron by electrochemical reduction route without CO2 emissions. J. Appl. Electrochem. 2023, 53, 1521–1536. [Google Scholar] [CrossRef]
- Abiko, K. Why do we study ultra-high purity base metals. Mater. Trans. JIM 2000, 41, 233–237. [Google Scholar] [CrossRef]
- Luo, Z.J.; Xu, J.Y.; Weng, W.; Tan, W.; Chi, X.P.; Zhong, S.P. Study on Arsenic Removal Process by Electrodeposition and Effect of Additives on Electrodeposition. Nonferrous Met. (Extr. Metall.) 2023, 7–16. [Google Scholar]
- Wang, F.Q.; Lu, M.H.; Wang, S.J.; Wang, F.; Xia, X.W.; Geng, S.H.; Zou, X.L.; Lu, X.G. Application advances of ionic liquids in hydrometallurgical leaching and electrodeposition. J. Shanghai Univ. (Nat. Sci. Ed.) 2023, 29, 569–589. [Google Scholar]
- Wang, H.; Cao, X.Z.; Xue, X.X. Research progress on electrodeposition of tin and tin alloys coatings in eutectic solvents. Plat. Finish. 2023, 45, 56–61. [Google Scholar]
- Zhang, Z.K. Preparation of Purity Iron by Low-Carbon Steel Electrolysis; Dalian Maritime University: Dalian, China, 2022. [Google Scholar]
- Zhang, D.; Wang, B.; Cao, X.; Bai, J.X.; Liu, T.S.; Dong, H. Electrolytic preparation of pure iron in aqueous solutions state-of-the-art. J. Lron Steel Res. 2025, 37, 708–727. [Google Scholar]
- Zhou, J.; Li, X.Y.; Tian, M.G.; Yan, W.; Zhang, D.M.; Xu, H. Research Progress on Treatment and Recovery of Metals in Wastewater by Electrodeposition. Chin. J. Anal. Chem. 2023, 51, 1735–1746. [Google Scholar]
- Fu, F.E.; Xu, C.Y.; Li, J.R.; Wang, Z.X. Research Progress of Chromium Electrodeposition from Deep Eutectic Solvents. Mater. Prot. 2024, 57, 155–167. [Google Scholar]
- Yu, P.P.; Zhou, X.J.; Mukhtar, A.; Wang, S.S.; Huang, R.Q.; Wu, K.M. Research status and prospect of high purity iron preparation. China Metall. 2023, 33, 9–20. [Google Scholar]
- Hang, T.; Xue, Q.; Li, M. A Review on Metal Micro-Nanostructured Array Materials Routed by Template-Free Electrodeposition. Acta Metall. Sin. 2022, 58, 486–502. [Google Scholar]
- Xu, Z.H.; Liu, A.M.; Liu, F.G.; Lu, X.Q.; Liu, Y.B.; Shi, Z.N. Research progress on the preparation of rare earth metals and their alloys by molten salt electrolysis. China Nonferrous Metall. 2025, 54, 9–24. [Google Scholar]
- Liu, L.; Cai, C.C.; Dong, X.B. Study on the mechanism of enhanced Ga electrodeposition on three dimensional porous electrodes. Chin. J. Process Eng. 2023, 23, 136–143. [Google Scholar]
- Lie, J.Z.; Huang, Z.X.; Jie, X.H.; Mai, Y.J. Electrodeposition of gradient nanostructured copper coating and its tribological behavior. Electroplat. Finish. 2023, 42, 1–7. [Google Scholar]
- Li, X.H.; Deng, R.R.; Li, Y.; Xu, C.Y.; Hua, Y.X.; Zhang, Q.B. Application of additives for the electrodeposition of metals and alloys from ionic liquids. Chin. J. Eng. 2024, 46, 657–675. [Google Scholar]
- Showa Denko. Preparation of Electrolytic Iron. JP1997111488, 28 April 1997. [Google Scholar]
- Toho Zinc Co., Ltd. Preparation of Electrolytic Pure Iron. JP2005105334, 21 April 2005. [Google Scholar]
- Wang, S.P.; Xue, Y.P.; Fan, L.; Pan, Y.Z.; Cheng, J.; Cui, B.J. Effects of Janus Green B Leveler on Nucleation Mechanism of Cobalt Electrodeposition in Chip Interconnection. Surf. Technol. 2024, 53, 178–187. [Google Scholar]
- Lin, M.P.; Jiao, H.D.; Yuan, R.; Li, Y.Y.; Wang, L.L.; Sun, R.Y.; Tian, D.H.; Jiao, S.Q. Recent progress on electrodeposition of metal/alloy films or coatings in deep eutectic solvents. Trans. Nonferrous Met. Soc. China 2014, 1–36. Available online: https://link.cnki.net/urlid/43.1239.tg.20241022.1624.004 (accessed on 22 July 2025).
- Chu, X.P.; Li, Y.X.; Liu, J.X.; Liu, N.H.; Lai, X.Q.; Luo, Y.B.; Li, W.L. Research progress of electroplating technology in ionic liquid system. Plat. Finish. 2025, 47, 89–99. [Google Scholar]
- Wang, H.; Cao, X.Z.; Xue, X.X. Study on Electrodeposition of Antimony in Choline Chloride-Ethylene Glycol Eutectic Solvent. J. Electrochem. 2022, 28, 62–70. [Google Scholar]
- Liu, J.R.; Wei, Y.S.; Ou, Y.Y.B.; Li, R.Y.; Li, T.F.; Hou, J.H.; Liu, H.Q.; Qiu, R. Preparation of superhydrophobic coating and its corrosion resistance on aluminum surface by one-step electrodeposition. Plat. Finish. 2025, 47, 29–36+73. [Google Scholar]
- Maryam, M.; Amira, S.; Prima, F.; Rahem, A.; Fiset, M.; Mantovani, D. Effect of electrodeposition current density on the microstructure and the degradation of electroformed iron for degradable stents. Mater. Sci. Eng. B 2011, 176, 1812–1822. [Google Scholar]
- Abbar, A.H.; Abdul-Ridha, J.Y.; Kareem, S.H. Electrolytic preparation of Iron powder with particle Size Less than 106 μm. Iraqi J. Chem. Pet. Eng. 2007, 8, 51–57. [Google Scholar] [CrossRef]
- Ye, C.M.; Huang, J.; Li, W.B.; Zhang, Y.; Hu, Z.T.; Ren, K.M. Effects of Ammonium Sulfite on Electrolytic Manganese. Hydrometall. China 2022, 41, 351–354. [Google Scholar]
- Ai, L.T.; Wang, Y.Z. Manufacture of high quality electrolytic pure iron powder from waste steel scraps. China Resour. Compr. Util. 1994, 13, 17–20. [Google Scholar]
- Ai, L.T.; Song, F.L.; Wang, J.C.; Lu, Q.K.; Guo, J.B. Preparation, performance and use of electrolytic pure iron. J. Process Eng. 1993, 14, 76–78. [Google Scholar]
- Abd El Meguid, E.A.; Abd El Rehim, S.S.; Moustafa, E.M. Electroplating of iron from alkaline gluconate baths. Thin Solid Film. 2003, 443, 53–59. [Google Scholar] [CrossRef]
- Jartych, E.; Jałochowski, M.; Budzyński, M. Influence of the electrodeposition parameters on surface morphology and local magnetic properties of thin iron layers. Appl. Surf. Sci. 2002, 193, 210–216. [Google Scholar] [CrossRef]
- Liu, B.; Song, X.S.; Song, X.C.; Yue, Z.L.; Fan, J.S. Single-Membrane Double Chamber Electrodeposition Process of Manganese Metal. Nonferrous Met. (Extr. Metall.) 2024, 1, 11–20. [Google Scholar] [CrossRef]
- Guan, P.P.; Liu, A.M.; Zhang, X.; Kang, H.G.; Su, K.J.; Meng, Q.L.; Liu, Z.S.; Shi, Z.N. Electrodeposition of copper in 1, 3-dimethyl-2-imidazolidinone-AlCl3-Cu2O ionic liquid. Chin. J. Nonferrous Met. 2024, 34, 268–278. [Google Scholar]
- Ye, C.M.; Li, W.B.; Huang, J.; Zhang, Y.; Hu, Z.T.; Wu, Z.X. Effects of 1-butyl-3-methylimidazole on Electrodeposition of Manganese. Nonferrous Met. (Extr. Metall.) 2022, 12, 29–33. [Google Scholar] [CrossRef]
- Zhang, X.X.; Gao, Y.; Liu, Y.H.; Sun, X.J. Preparation of Co/W catalyst by electrodeposition and study on its performance in electrocatalytic hydrogen evolution. Mod. Chem. Ind. 2025, 45, 184–187. [Google Scholar]
- Rudnik, E. Effect of pH-dependent bath speciation on cobalt electrodeposition from sulfate−gluconate solutions. Trans. Nonferrous Met. Soc. China 2025, 35, 2399–2420. [Google Scholar] [CrossRef]
- Liu, C.Q.; Peng, Q.C.; Xue, Z.L.; Huang, H.B.; Qiu, W.T. Preparation of electrolytic pure iron powder and its compression sintering properties. J. Iron Steel Res. 2019, 31, 822–829. [Google Scholar]
- Cao, D.W.; Song, W.L.; Xu, Y.X. Exploration of production process conditions of electrolytic pure iron. J. Fuzhou Univ. (Nat. Sci. Ed.) 1961, 107–111. [Google Scholar]
- Qing, Y.; Tan, D.S.; Wan, S.B.; Ding, W.Z. Treatment of Ferrous Chloride Solution by Ion Exchange Membrane Electrolysis Process. Hydrometall. China 2016, 35, 503–506. [Google Scholar]
- Chen, D.M.; Gu, L.; Chen, P.Y.; Gao, J.T.; Wang, Y.G.; Li, S.Q. Pre-Experiment on Electrolyzing Pure Iron in Aqueous Solution by Clean Energy. J. Iron Steel Res. 2011, 23, 4. [Google Scholar]
- Dong, H.B.; Hou, M.S.; Liu, C.; Liu, R.Z.; Li, S.Q. Electrolytic preparation of iron from aqueous solution using solar energy. Electroplat. Finish. 2012, 31, 3. [Google Scholar]
- Cao, W.M.; Yin, R.H.; Yan, H.G.; Ye, Z.Q. Pure Iron Electrolyzed from Scrap Iron Chips and its Corrosion Characteristics. Electrochemistry 2003, 9, 93–97. [Google Scholar] [CrossRef]
- Lu, W.C.; Xu, Y.L.; Liu, P.A.; Wang, Z.L. Electroforming of Iron Foil. Chin. J. Process Eng. 1988, 9, 7–13. [Google Scholar]
- Showa Denko. Manufacturing Method of High Purity Electrolytic and Electrolytic Cell. JP1989212788, 25 August 1989. [Google Scholar]
- Huang, H.B.; Peng, Q.C.; Qiu, W.T. Preparation of high purity iron by industrial pure iron electrolysis. J. Chongqing Univ. 2017, 40, 59–70. [Google Scholar]
- Wei, C.; Li, C.X. Zinc Extraction Metallurgy; Metallurgical Industry Press: Beijing, China, 2013; pp. 81–82. [Google Scholar]
- Xie, Z. Nonlinear Dynamic Behaviors and Regulation Law of Electrode Reaction of Manganese Electrodeposition; Chongqing University: Chongqing, China, 2021. [Google Scholar]
- Xu, Q.; Li, A.Q.; Sun, C.T.; Cheng, H.W.; Zou, X.L.; Lu, X.G. Progress of Research on Fabrication of Iron by Electrochemical Process. Shanghai Met. 2021, 43, 93–99. [Google Scholar]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.I.; Fahidy, T.Z. Morphological characteristics of copper deposition on stainless steel cathodes. J. Appl. Electrochem. 1981, 11, 543–549. [Google Scholar] [CrossRef]
- Chen, X.H. The Relationship between Gas Solubility and Temperature. Univ. Chem. 1993, 8, 54–56. [Google Scholar]

| T/°C | Cathode Material | Anode Material | CFe2+/(mol·L−1) | Current Density/(A·m−2) | pH | Electrolyzing Time/h | Number of Experiments |
|---|---|---|---|---|---|---|---|
| 25 | Titanium metal sheets | Industrial pure iron | 3.5 | 100 | 2.5 | 72 | 5 |
| 50 | |||||||
| 75 |
| Component | Sulfate | Fe(Ⅲ) | Cu | Zn | As | Pb | Alkali Metals and Alkaline Earth Metals |
|---|---|---|---|---|---|---|---|
| Content | ≤200 | ≤100 | ≤150 | ≤20 | ≤2 | ≤50 | ≤400 |
| Materials and Equipment | Version | Manufacturer |
|---|---|---|
| FeCl2·4H2O | http://www.tmreagent.com/ | Xian Tianmao Chemical Co., Ltd. (Xi’an, China) |
| C2H5OH | http://www.qschem.com/ | Jiangsu Qiangsheng Functional Chemical Co., Ltd. (Changshu, China) |
| HCl | https://www.ksjingke.com/ | Kunshan Jingke Microelectronics Materials Co., Ltd. (Kunshan, China) |
| High-purity titanium plate | 700 mm × 700 mm × 2 mm | Hefei Wenghe Metal Materials Co., Ltd. (Hefei, China) |
| DT4 | 760 mm × 820 mm × 2 mm | Taiyuan Iron & Steel (group) Co., Ltd. (Taiyuan, China) |
| High-frequency switch power supply | 10000A8V | Yangjiang Jianxing Changsheng Electromechanical Equipment Co., Ltd. (Yangjiang, China) |
| Portable pH meter | https://www.smartsensor.cn/ | Dongguan Wanchuang Electronic Products Co., Ltd. (Dongguan, China) |
| Electric heating hot water boiler | CWDR-72KW-D | Henan Hengxin Boiler Manufacturing Co., Ltd. (Zhoukou, China) |
| Filter machine | TSB-2018-3-P | Kunshan Meibao Environmental Protection Equipment Co., Ltd. (Kunshan, China) |
| PVC electrolytic cell | / | Yangjiang Jianxing Changsheng Electromechanical Equipment Co., Ltd. (Yangjiang, China) |
| Electrolyte | Anode | Cathode | Process Parameters | Characteristic | Reference | ||
|---|---|---|---|---|---|---|---|
| pH | T (°C) | Current Density (A/m2) | |||||
| Chlorate salt | Iron filings + Graphite plate | Lead–tin alloy | 2~3 | 75 | 400 | Split | [37] |
| 500 | / | ||||||
| 600 | Rough | ||||||
| 80 | 300 | With bumps Poor toughness | |||||
| 500 | With bumps | ||||||
| 600 | Rough | ||||||
| 85 | 300 | With bumps | |||||
| 400 | / | ||||||
| 500 | Few bumps | ||||||
| 600 | / | ||||||
| 90 | 400 | Tightly smooth | |||||
| 500 | / | ||||||
| 600 | / | ||||||
| Graphite rod | Low-carbon steel | 2.0 | 75 | 800 | Current Efficiency is 50.2% | [39] | |
| Industrial pure iron | Passivated metal | 2.5 | 79 | 1000~2000 | CE is 92.13% | [42] | |
| 95 | 3N CE is 98.67% | ||||||
| Platinum-coated titanium plate | Stainless steel | 1.5~5 | 50~70 | 50~200 | Heavy metal impurities <0.05 ppm | [43] | |
| Industrial pure iron | Stainless steel | 4.0~5.5 | 40~60 | 70~150 | Co, Cu <0.01 ppm | [18] | |
| Industrial pure iron | Titanium plate | 2.5 | 25 | 100 | 2N6 CE is 65.3% | Self-made | |
| 50 | 3N6 CE is 84.9% | ||||||
| 75 | 4N1 Smooth CE is 96.8% | ||||||
| Element | C | H | O | N |
|---|---|---|---|---|
| DT4 | 28 ± 2.83 | 4 ± 1.41 | 28 ± 1.42 | 21.5 ± 2.12 |
| 25 °C | 142 ± 96.17 | 220 ± 42.43 | 2850 ± 212.13 | 19 ± 5.66 |
| 50 °C | 7.55 ± 2.29 | 4.43 ± 2.36 | 156.22 ± 141.83 | 6.94 ± 3.28 |
| 75 °C | 5.98 ± 2.27 | 3.69 ± 1.31 | 31.31 ± 19.08 | 5.09 ± 0.42 |
| Sample | O |
|---|---|
| Before annealing | 2850 ± 212.13 |
| After annealing | 25 ± 22.54 |
| Element | Al | Ti | V | Cr | Mn | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|
| DT4 | 365.0 ± 21.21 | 412.5 ± 17.67 | 14.0 ± 1.41 | 144.5 ± 20.51 | 982.5 ± 17.67 | 6.8 ± 0.63 | 18.5 ± 2.12 | 34.5 ± 2.12 | 4.5 ± 1.63 |
| 25 °C | 198.5 ± 171.83 | 5.1 ± 3.04 | 5.5 ± 2.26 | 87.5 ± 60.11 | 2.1 ± 0.42 | 12.5 ± 3.54 | 9.8 ± 0.14 | 28.5 ± 2.13 | 14.0 ± 1.41 |
| 50 °C | 8.5 ± 14.94 | 0.63 ± 0.45 | 0.21 ± 0.27 | 0.35 ± 0.44 | 0.21 ± 0.27 | 11.3 ± 3.45 | 8.7 ± 4.23 | 20.5 ± 7.12 | 5.34 ± 1.72 |
| 75 °C | 0.25 ± 0.48 | 0.26 ± 0.42 | 0.02 ± 0.06 | 0.09 ± 0.11 | 0.07 ± 0.05 | 7.7 ± 2.38 | 9.5 ± 3.57 | 18.5 ± 4.83 | 5.33 ± 1.77 |
| Electrode Notations | Electrode Reaction | E/V | Potential Difference with Iron/V |
|---|---|---|---|
| Fe2+/Fe | Fe2+ + 2e− = Fe | −0.440 | 0 |
| Co2+/Co | Co2+ + 2e− = Co | −0.277 | 0.163 |
| Ni2+/Ni | Ni2+ + 2e− = Ni | −0.250 | 0.190 |
| Cu2+/Cu | Cu2+ + 2e− = Cu | +0.337 | 0.777 |
| Zn2+/Zn | Zn2+ + 2e− = Zn | −0.763 | 0.323 |
| T/°C | Cathode with Deposit | Surface | Cross-Section |
|---|---|---|---|
| 25 |  | 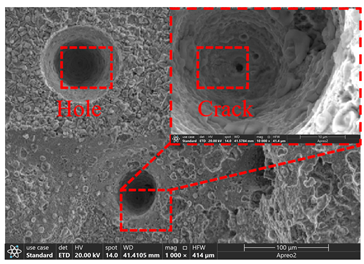 | 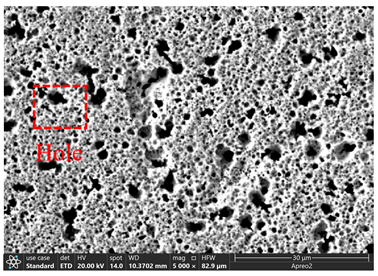 |
| 50 | 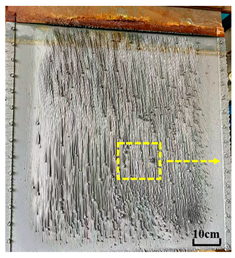 | 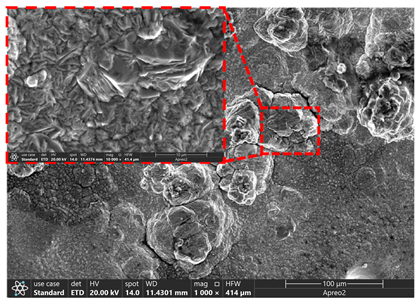 | 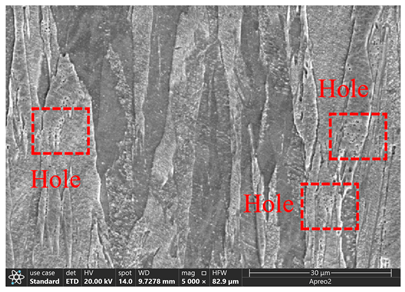 |
| 75 |  | 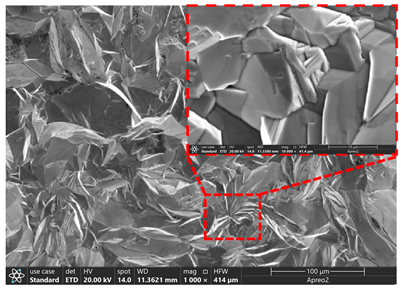 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Liu, T.; Xie, G.; Wang, B.; Cao, X.; Bai, J.; Zhong, M.; Dong, H. The Effect of Electrolytic Temperature on the Purity of Electrolytic Pure Iron. Metals 2025, 15, 1055. https://doi.org/10.3390/met15091055
Zhang D, Liu T, Xie G, Wang B, Cao X, Bai J, Zhong M, Dong H. The Effect of Electrolytic Temperature on the Purity of Electrolytic Pure Iron. Metals. 2025; 15(9):1055. https://doi.org/10.3390/met15091055
Chicago/Turabian StyleZhang, Di, Tengshi Liu, Gangsheng Xie, Bo Wang, Xin Cao, Jiaxin Bai, Mingyue Zhong, and Han Dong. 2025. "The Effect of Electrolytic Temperature on the Purity of Electrolytic Pure Iron" Metals 15, no. 9: 1055. https://doi.org/10.3390/met15091055
APA StyleZhang, D., Liu, T., Xie, G., Wang, B., Cao, X., Bai, J., Zhong, M., & Dong, H. (2025). The Effect of Electrolytic Temperature on the Purity of Electrolytic Pure Iron. Metals, 15(9), 1055. https://doi.org/10.3390/met15091055






