Evaluation of the Resistance of APS-Developed Woka-Diamalloy Carbide Coatings to High-Temperature Damage
Abstract
1. Introduction
2. Materials and Method
2.1. Substrate and Coatings Preparation
2.2. Investigations on Oxidation and Hot Corrosion
3. Results and Discussion
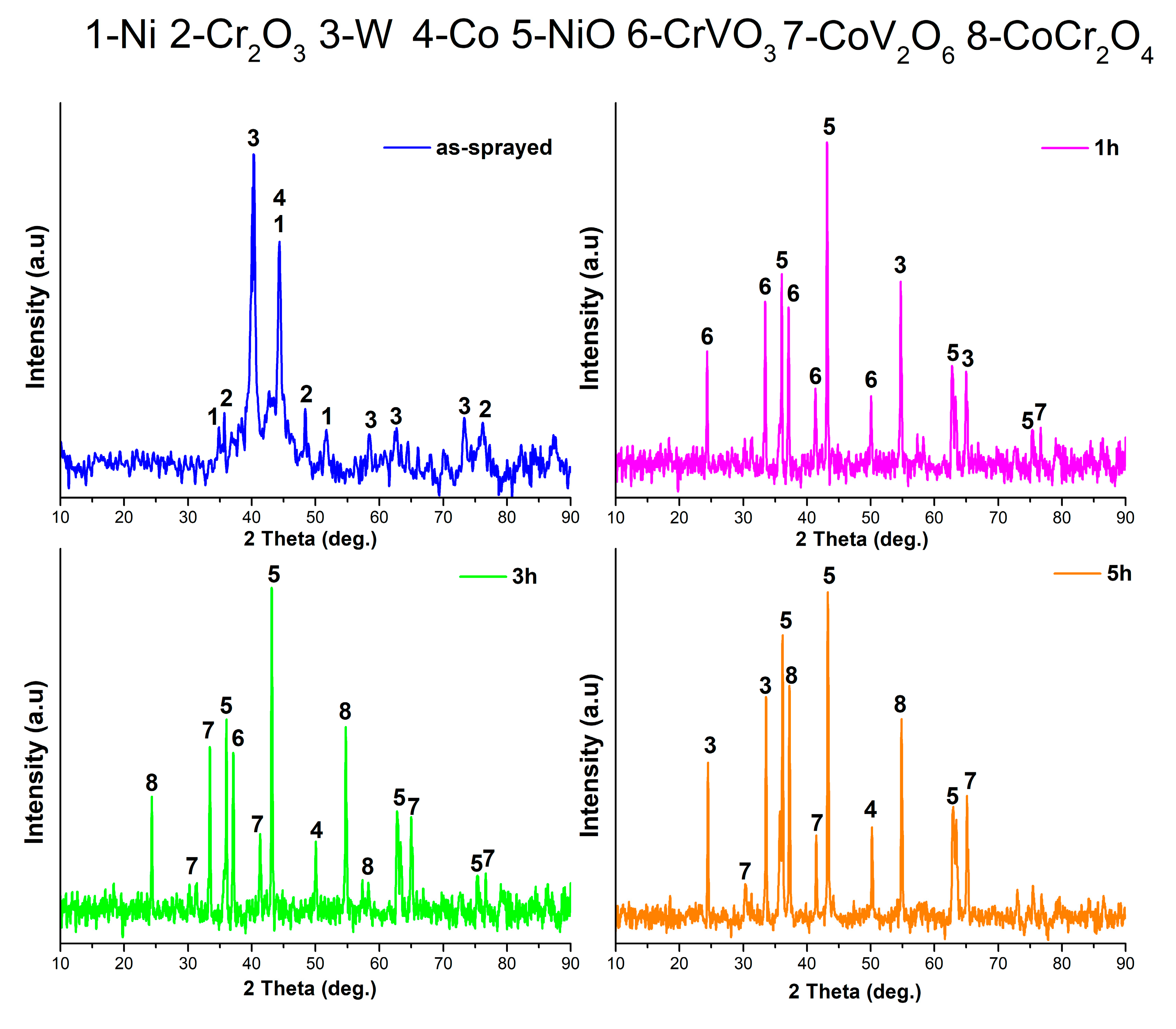
3.1. Characterization of As-Sprayed Coating System
3.2. Oxidation Effect on Coating Systems
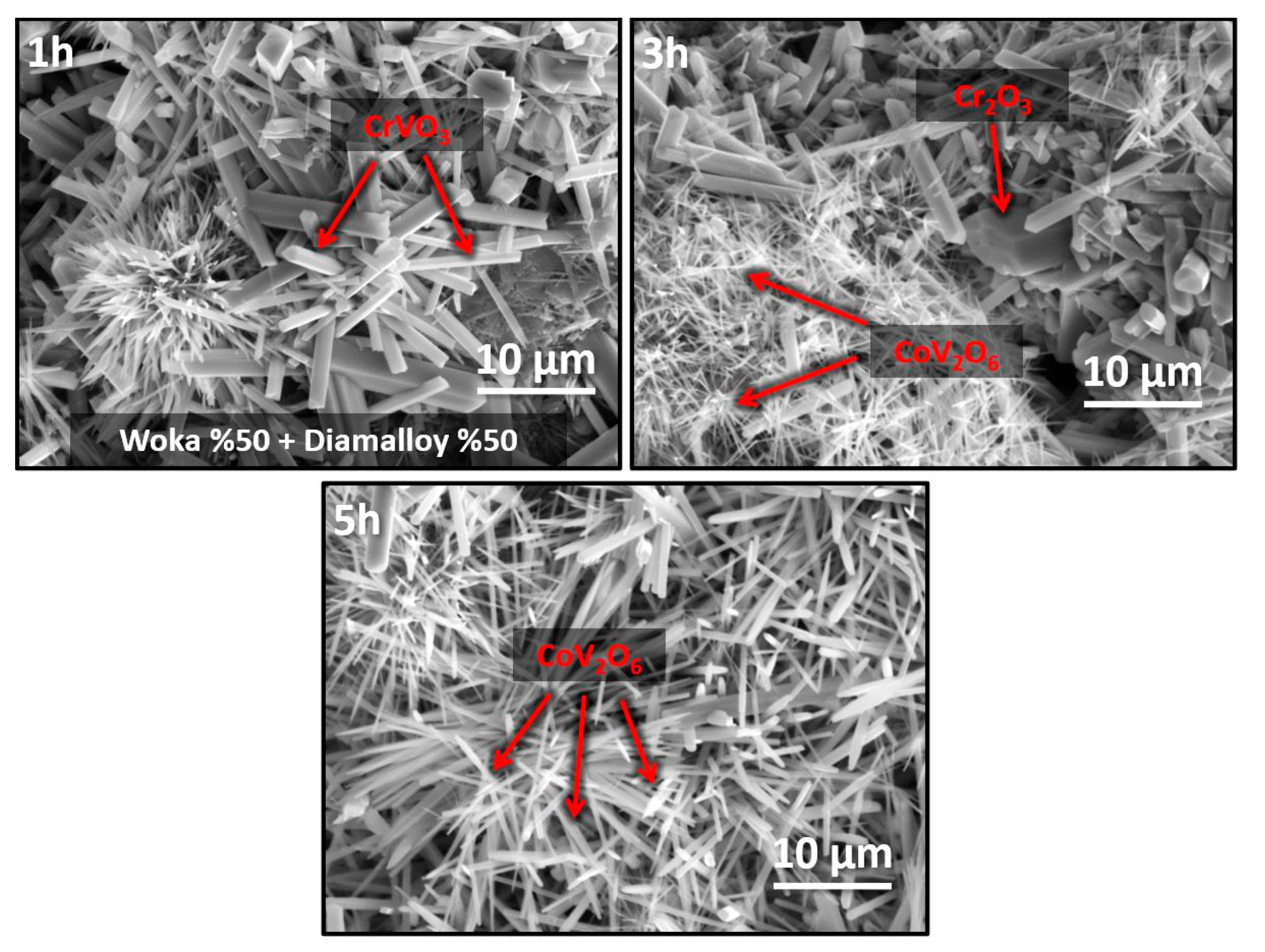
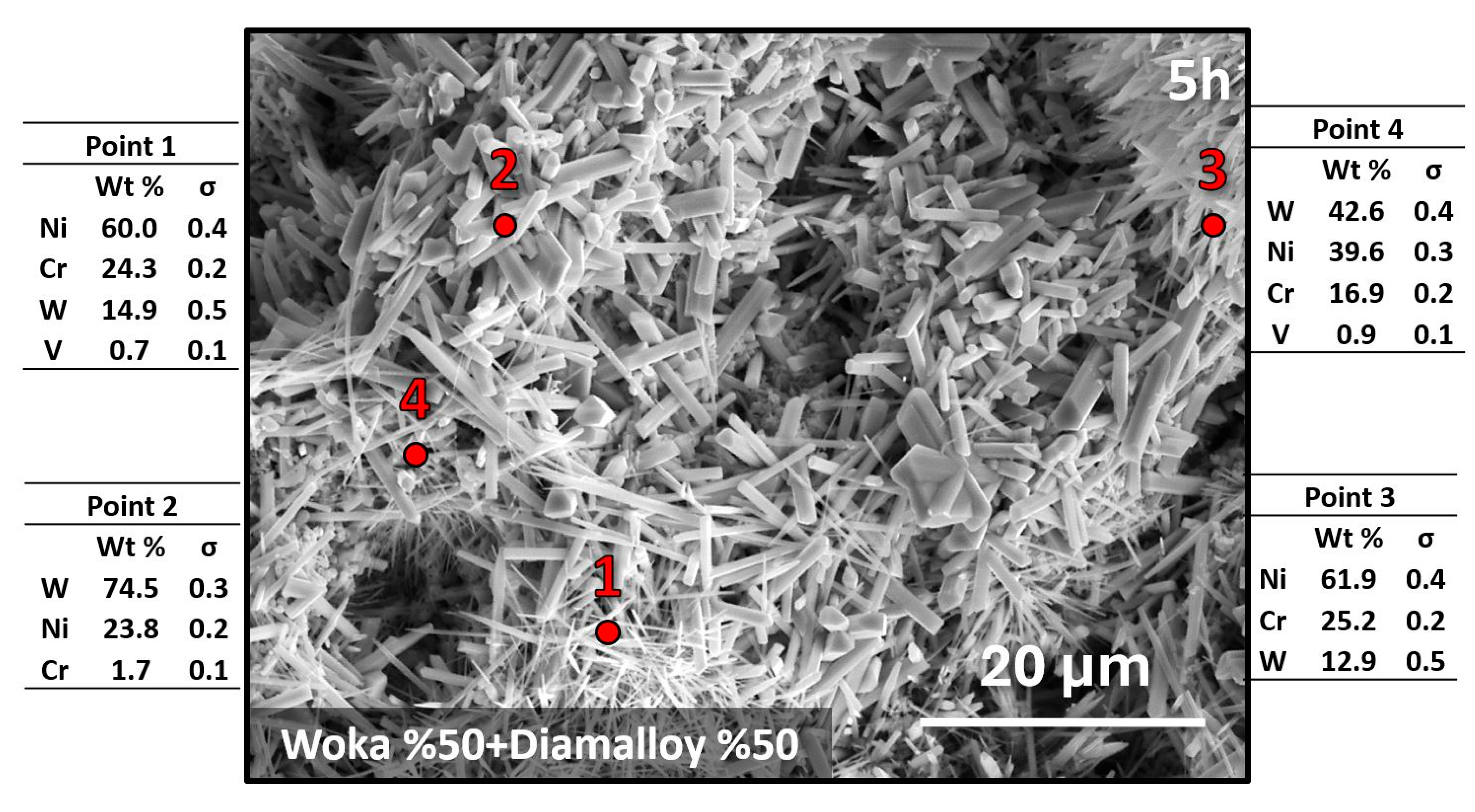
3.3. Hot Corrosion Effect on Coating System
4. Conclusions
- Although the 50% Woka 7202–50% Diamalloy 2002 coatings obtained with APS provide sufficient integrity under high temperature conditions, porosity and oxide contents can limit mechanical strength and wear resistance. This suggests that process parameters and densification methods should be optimized in coating design.
- Oxidation tests at 900 °C show that oxide phases (NiO, NiWO4, Cr2O3, Cr2NiO4) are formed in coatings with increasing oxygen penetration. This reveals that the coatings provide chemical stability by forming protective oxide layers at high temperature, but excessive oxidation may limit the mechanical strength.
- Hot corrosion tests at 900 °C show that corrosive salts leach out of the coating surface over time, leading to oxide formation and cracking. This finding suggests that the mechanical integrity and corrosion resistance of coatings under long-term service conditions may be limited and additional protective measures may be required in design.
- The formation of Cr2O3, CrVO3, CoCr2O4 and CoV2O6 phases in the coating during hot corrosion indicates that the coating provides chemical stability in corrosive environments. However, needle and rod-like brittle structures can lead to localized stress accumulation and crack formation, limiting mechanical strength, which may affect long-term service performance.
- The coating system maintained its structural integrity at high temperature and showed no flaking or lifting, indicating that it can be used as a reliable protective surface solution in high temperature applications such as turbines and power generation equipment.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, K.; Chen, C.; Xu, G.; Jiang, Z.; Zhang, S.; Liu, X. HVOF sprayed Ni–Mo coatings improved by annealing treatment: Microstructure characterization, corrosion resistance to HCl and corrosion mechanisms. J. Mater. Res. Technol. 2022, 19, 1906–1921. [Google Scholar] [CrossRef]
- Sidhu, H.S.; Sidhu, B.S.; Prakash, S. The role of HVOF coatings in improving hot corrosion resistance of ASTM-SA210 GrA1 steel in the presence of Na2SO4-V2O5 salt deposits. Surf. Coatings Technol. 2006, 200, 5386–5394. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, B.; He, L.; Xu, Z.; Xu, J.; Mu, R.; Cao, X. Hot corrosion behaviour of La2(Zr0.7Ce0.3)2O7 thermal barrier coating ceramics exposed to molten calcium magnesium aluminosilicate at different temperatures. Corros. Sci. 2015, 100, 566–578. [Google Scholar] [CrossRef]
- Zou, Z.; Donoghue, J.; Curry, N.; Yang, L.; Guo, F.; Nylén, P.; Zhao, X.; Xiao, P. A comparative study on the performance of suspension plasma sprayed thermal barrier coatings with different bond coat systems. Surf. Coatings Technol. 2015, 275, 276–282. [Google Scholar] [CrossRef]
- Lee, C.; Han, J.; Yoon, J.; Shin, M.; Kwun, S. A study on powder mixing for high fracture toughness and wear resistance of WC-Co-Cr coatings sprayed by HVOF. Surf. Coatings Technol. 2010, 204, 2223–2229. [Google Scholar] [CrossRef]
- Kalush, A.; Texier, D.; Ecochard, M.; Sirvin, Q.; Choquet, K.; Gheno, T.; Vanderesse, N.; Jomaa, W.; Bocher, P. Size effects on high temperature oxidation of MCrAlY coatings processed via APS and HVOF depositions. Surf. Coatings Technol. 2022, 440, 128483. [Google Scholar] [CrossRef]
- Ghadami, F.; Sohi, M.H.; Ghadami, S. Effect of bond coat and post-heat treatment on the adhesion of air plasma sprayed WC-Co coatings. Surf. Coatings Technol. 2015, 261, 289–294. [Google Scholar] [CrossRef]
- López Cantera, E.; Mellor, B.G. Fracture toughness and crack morphologies in eroded WC-Co-Cr thermally sprayed coatings. Mater. Lett. 1998, 37, 201–210. [Google Scholar] [CrossRef]
- Zhou, L.; Mukherjee, S.; Huang, K.; Park, Y.W.; Sohn, Y. Failure characteristics and mechanisms of EB-PVD TBCs with Pt-modified NiAl bond coats. Mater. Sci. Eng. A 2015, 637, 98–106. [Google Scholar] [CrossRef]
- Sundararajan, T.; Kuroda, S.; Abe, F. Steam oxidation resistance of two-layered Ni-Cr and Al APS coating for USC boiler applications. Corros. Sci. 2005, 47, 1129–1147. [Google Scholar] [CrossRef]
- Liu, X.; Hu, K.; Zhang, S.; Xu, T.; Chen, L.; Byon, E.; Liu, D. Study of KCl-induced hot corrosion behavior of high velocity oxy-fuel sprayed NiCrAlY and NiCrBSi coatings deposited on 12CrMoV boiler steel at 700 °C. Corros. Sci. 2022, 203, 110351. [Google Scholar] [CrossRef]
- Méndez-Medrano, K.O.; Martínez-González, C.J.; Alvarado-Hernández, F.; Jiménez, O.; Baltazar-Hernández, V.H.; Ruiz-Luna, H. Microstructure and Properties Characterization of WC-Co-Cr Thermal Spray Coatings. J. Miner. Mater. Charact. Eng. 2018, 6, 482–497. [Google Scholar] [CrossRef][Green Version]
- Ozgurluk, Y.; Doleker, K.M.; Karaoglanli, A.C. Hot corrosion behavior of YSZ, Gd2Zr2O7 and YSZ/Gd2Zr2O7 thermal barrier coatings exposed to molten sulfate and vanadate salt. Appl. Surf. Sci. 2018, 438, 96–113. [Google Scholar] [CrossRef]
- Karaoglanli, A.C.; Ozgurluk, Y.; Doleker, K.M. Comparison of microstructure and oxidation behavior of CoNiCrAlY coatings produced by APS, SSAPS, D-gun, HVOF and CGDS techniques. Vacuum 2020, 180, 109609. [Google Scholar] [CrossRef]
- EBakan, E.; Mack, D.E.; Mauer, G.; Vaßen, R. Gadolinium Zirconate/YSZ Thermal Barrier Coatings: Plasma Spraying, Microstructure, and Thermal Cycling Behavior. J. Am. Ceram. Soc. 2014, 97, 4045–4051. [Google Scholar] [CrossRef]
- Habibi, M.H. Hot Corrosion Behaviour of New Candidates for Thermal Barrier Coatings Application in Turbine Simulated Environments. Ph.D. Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2014. [Google Scholar] [CrossRef]
- Ahmadi-Pidani, R.; Shoja-Razavi, R.; Mozafarinia, R.; Jamali, H. Evaluation of hot corrosion behavior of plasma sprayed ceria and yttria stabilized zirconia thermal barrier coatings in the presence of Na2SO4+V2O5 molten salt. Ceram. Int. 2012, 38, 6613–6620. [Google Scholar] [CrossRef]
- Ke, P.L.; Wu, Y.N.; Wang, Q.M.; Gong, J.; Sun, C.; Wen, L.S. Study on thermal barrier coatings deposited by detonation gun spraying. Surf. Coatings Technol. 2005, 200, 2271–2276. [Google Scholar] [CrossRef]
- Doleker, K.M.; Ozgurluk, Y.; Karaoglanli, A.C. Isothermal oxidation and thermal cyclic behaviors of YSZ and double-layered YSZ/La2Zr2O7 thermal barrier coatings (TBCs). Surf. Coatings Technol. 2018, 351, 78–88. [Google Scholar] [CrossRef]
- Ozgurluk, Y. Investigation of oxidation and hot corrosion behavior of molybdenum coatings produced by high-velocity oxy-fuel coating method. Surf. Coatings Technol. 2022, 444, 128641. [Google Scholar] [CrossRef]
- Ozgurluk, Y.; Doleker, K.M.; Ozkan, D.; Ahlatci, H.; Karaoglanli, A.C. Cyclic hot corrosion failure behaviors of EB-PVD TBC systems in the presence of sulfate and vanadate molten salts. Coatings 2019, 9, 166. [Google Scholar] [CrossRef]
- McCoy, H.E., Jr.; Stephenson, R.L.; Weir, J.R., Jr. Mechanical Properties of Some Refractory Metals and Their Alloys; Oak Ridge National Laboratory Metals and Ceramics Division: Oak Ridge, TN, USA, 1964. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Lei, S.; Mo, Q.; Ling, K.; Lv, X.; Zhao, X.; Li, W. Formation and hot corrosion behavior of MoSi2/NbSi2 composite coating on Nb-based alloys by combined electrodeposition and HAPC processes. J. Alloys Compd. 2021, 887, 161365. [Google Scholar] [CrossRef]
- Shields, J.A. Applications of Molybdenum Metal and Its Alloys. 2013. Available online: https://www.imoa.info (accessed on 20 December 2021).
- Wang, W.; Kweon, S.H.; Yang, S.H. A study on roughness of the micro-end-milled surface produced by a miniatured machine tool. J. Mater. Process. Technol. 2005, 162–163, 702–708. [Google Scholar] [CrossRef]
- Tailor, S.; Modi, A.; Modi, S.C. High-Performance Molybdenum Coating by Wire–HVOF Thermal Spray Process. J. Therm. Spray Technol. 2018, 27, 757–768. [Google Scholar] [CrossRef]
- Guseva, O.; Brunner, S.; Richner, P. Service life prediction for aircraft coatings. Polym. Degrad. Stab. 2003, 82, 1–13. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, H.; Prakash, S. High-temperature corrosion studies of HVOF-sprayed Cr3C2-NiCr coating on SAE-347h boiler steel. J. Therm. Spray Technol. 2009, 18, 619–632. [Google Scholar] [CrossRef]
- Song, B.; Murray, J.W.; Wellman, R.G.; Pala, Z.; Hussain, T. Dry sliding wear behaviour of HVOF thermal sprayed WC-Co-Cr and WC-CrxCy-Ni coatings. Wear 2020, 442–443, 203114. [Google Scholar] [CrossRef]
- Katranidis, V.; Gu, S.; Allcock, B.; Kamnis, S. Experimental study of high velocity oxy-fuel sprayed WC-17Co coatings applied on complex geometries. Part A: Influence of kinematic spray parameters on thickness, porosity, residual stresses and microhardness. Surf. Coatings Technol. 2017, 311, 206–215. [Google Scholar] [CrossRef]
- Kaushal, G.; Singh, H.; Prakash, S. High-temperature erosion-corrosion performance of high-velocity oxy-fuel sprayed Ni-20 Cr coating in actual boiler environment. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 42, 1836–1846. [Google Scholar] [CrossRef]
- Gu, S.; Kamnis, S. Bonding mechanism from the impact of thermally sprayed solid particles. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2009, 40, 2664–2674. [Google Scholar] [CrossRef]
- Lih, W.C.; Yang, S.H.; Su, C.Y.; Huang, S.C.; Hsu, I.C.; Leu, M.S. Effects of process parameters on molten particle speed and surface temperature and the properties of HVOF CrC/NiCr coatings. Surf. Coatings Technol. 2000, 133–134, 54–60. [Google Scholar] [CrossRef]
- Ma, L. Effect of Hf on microstructure and creep properties of a hot corrosion resistant nickel-based single crystal superalloy. China Foundry 2025, 22, 173–181. [Google Scholar] [CrossRef]
- Demir, E. Behavior of Fe-based alloys in a liquid lead-bismuth environment under simultaneous proton irradiation and corrosion. Acta Mater. 2024, 284, 120578. [Google Scholar] [CrossRef]
- Liu, D.P. Microstructure, Mechanical Properties, and Corrosion Resistance of Ti-Cu Alloys Prepared by Electroless Copper Plating and Hot Pressing Sintering. J. Mater. Eng. Perform. 2025, 1–10. [Google Scholar] [CrossRef]
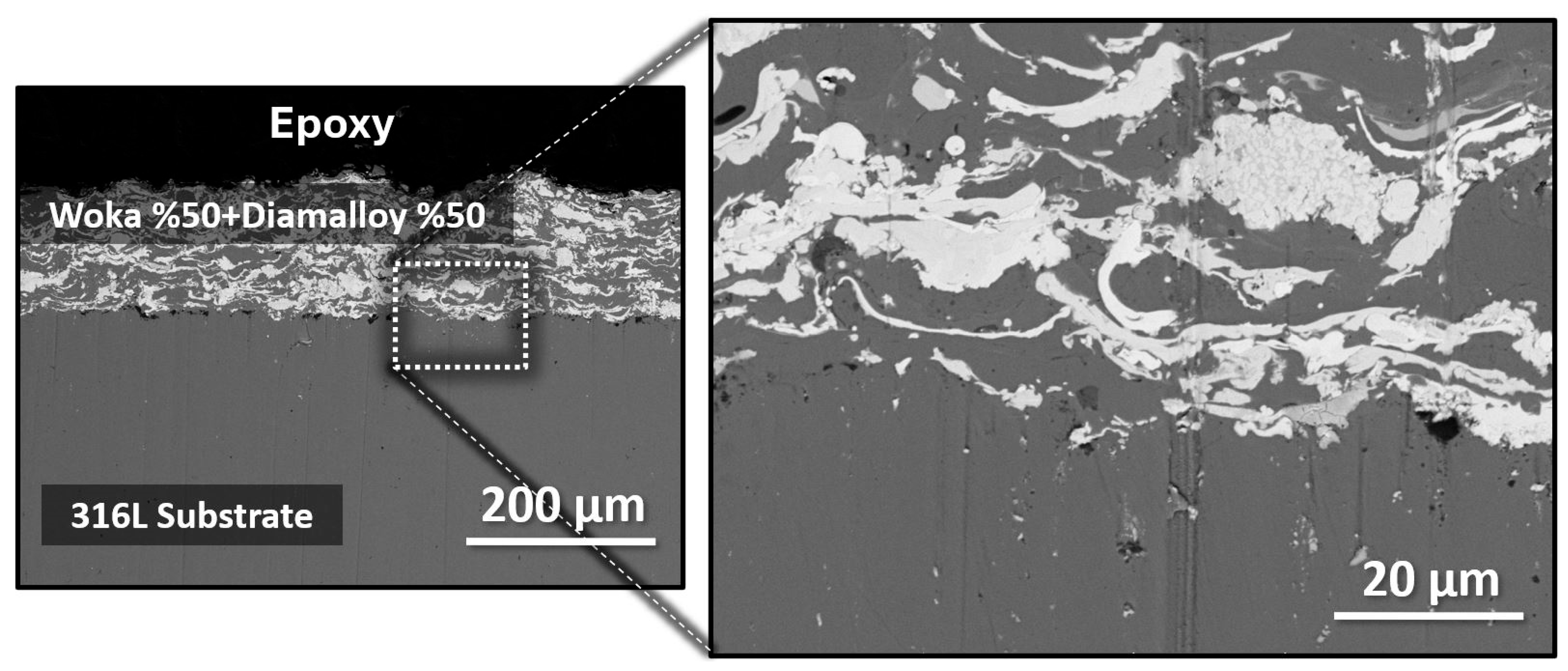

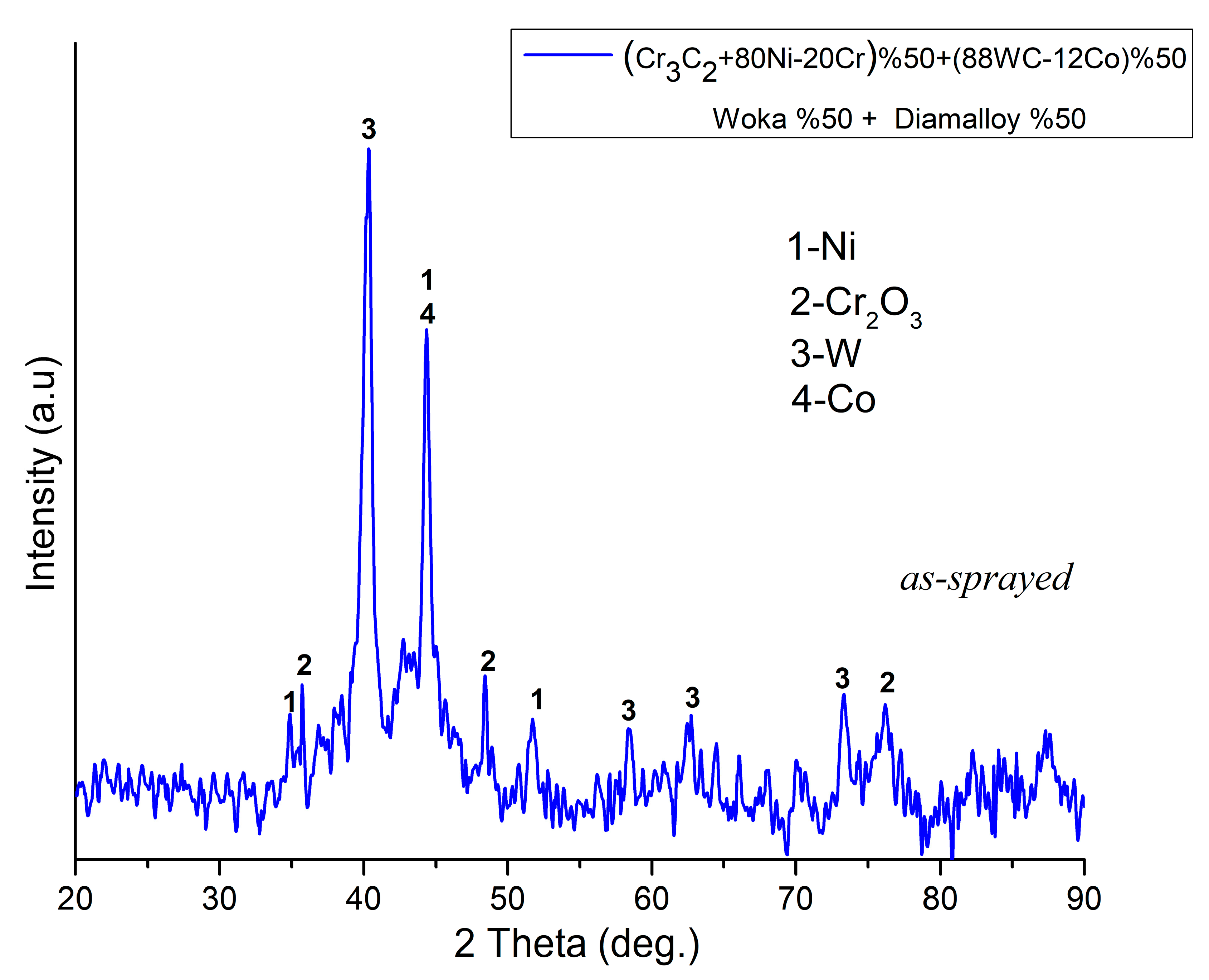
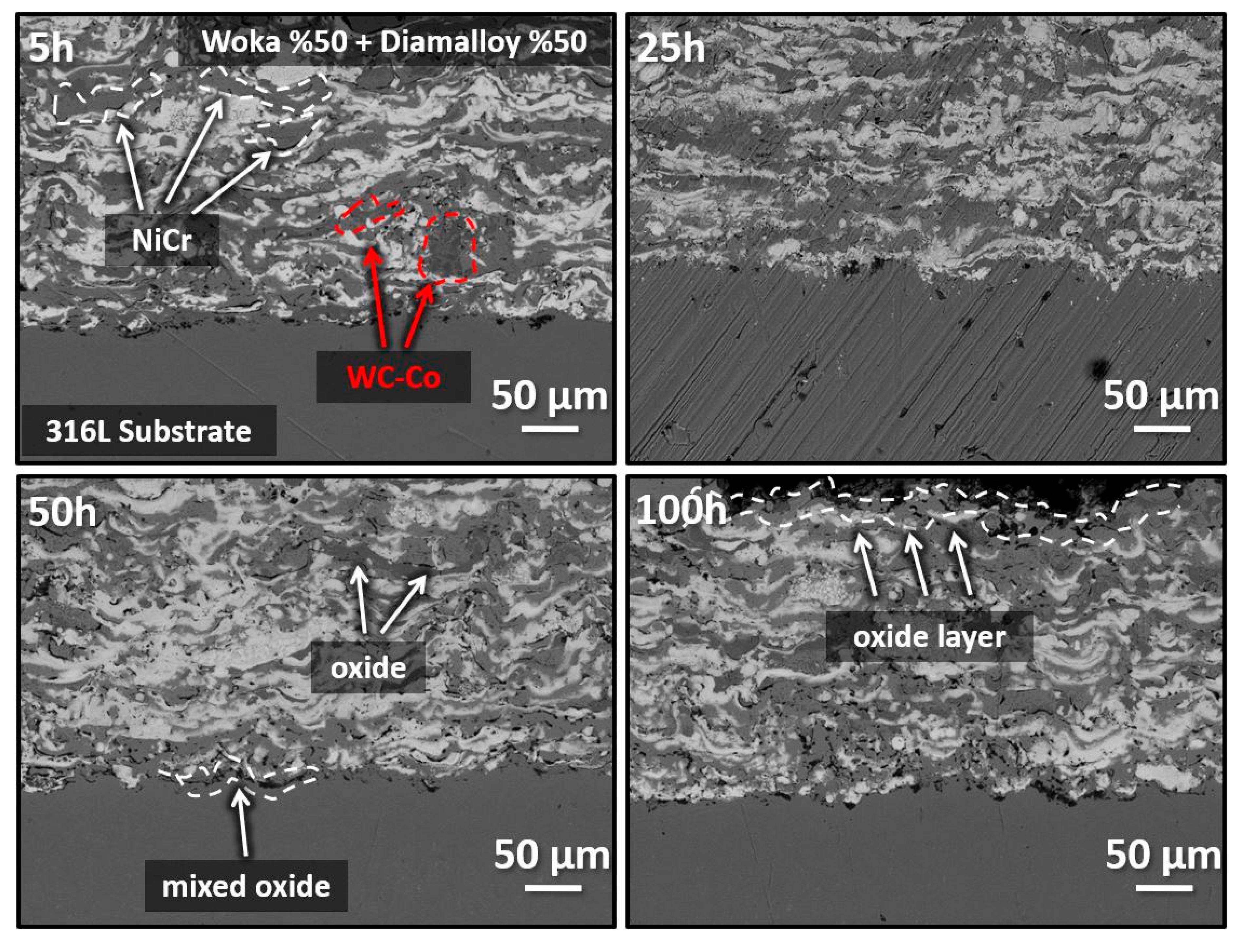


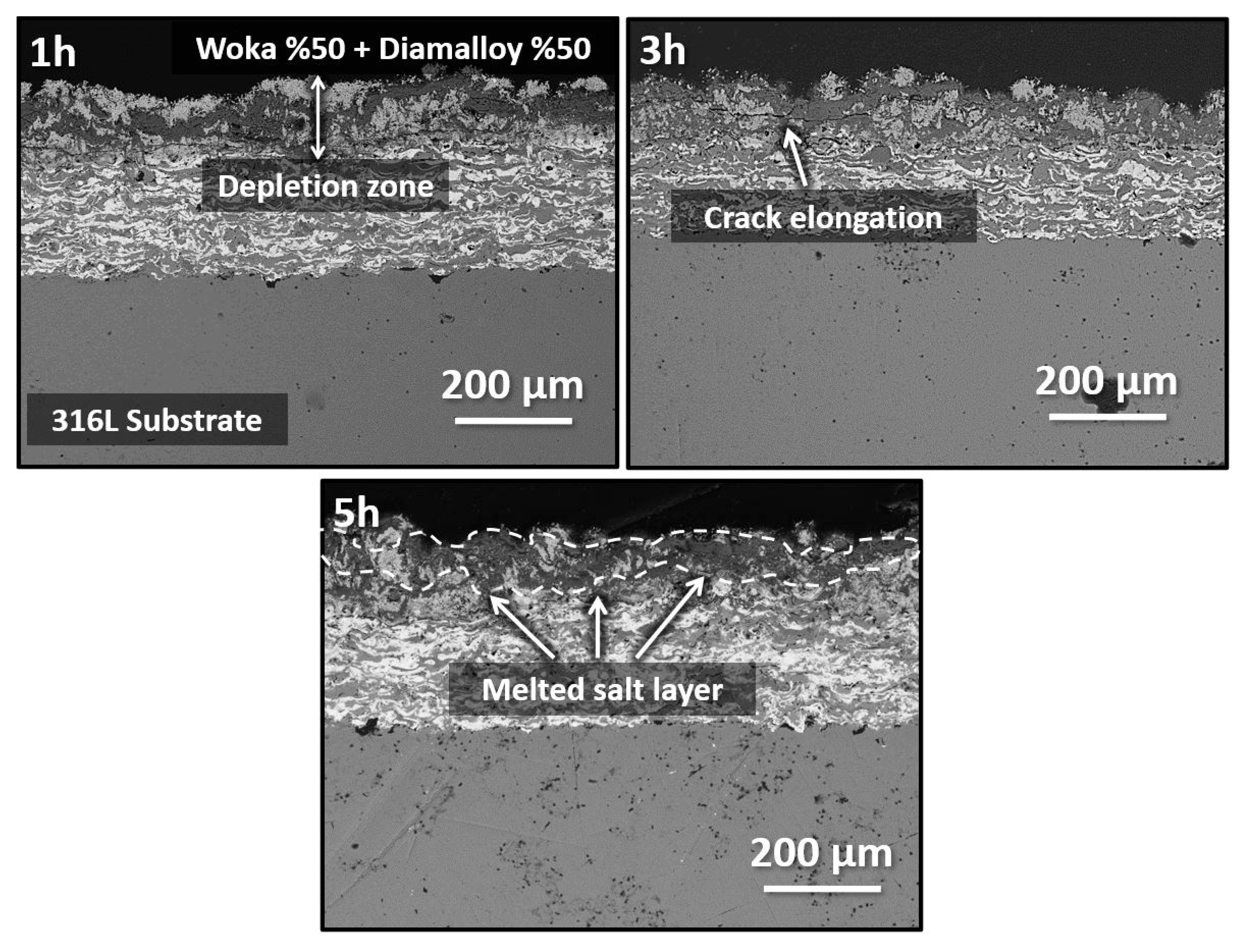

| Current | 500 A |
|---|---|
| Voltage | 65 V |
| Ar flow | 55 (L·min−1) |
| H2 flow | 8 (L·min−1) |
| Powder feed rate | 25 g/min |
| Stand-off distance | 200 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozbek, Y.Y.; Odabas, O.; Ozgurluk, Y.; Karaoglanli, A.C. Evaluation of the Resistance of APS-Developed Woka-Diamalloy Carbide Coatings to High-Temperature Damage. Metals 2025, 15, 1054. https://doi.org/10.3390/met15091054
Ozbek YY, Odabas O, Ozgurluk Y, Karaoglanli AC. Evaluation of the Resistance of APS-Developed Woka-Diamalloy Carbide Coatings to High-Temperature Damage. Metals. 2025; 15(9):1054. https://doi.org/10.3390/met15091054
Chicago/Turabian StyleOzbek, Yildiz Yarali, Okan Odabas, Yasin Ozgurluk, and Abdullah Cahit Karaoglanli. 2025. "Evaluation of the Resistance of APS-Developed Woka-Diamalloy Carbide Coatings to High-Temperature Damage" Metals 15, no. 9: 1054. https://doi.org/10.3390/met15091054
APA StyleOzbek, Y. Y., Odabas, O., Ozgurluk, Y., & Karaoglanli, A. C. (2025). Evaluation of the Resistance of APS-Developed Woka-Diamalloy Carbide Coatings to High-Temperature Damage. Metals, 15(9), 1054. https://doi.org/10.3390/met15091054






