Influence of Tramp Elements on Phase Transformations, Microstructure and Hardness of a 0.3 wt.%C Low-Alloyed Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition of Steel Alloys
2.2. Microstructural Characterization
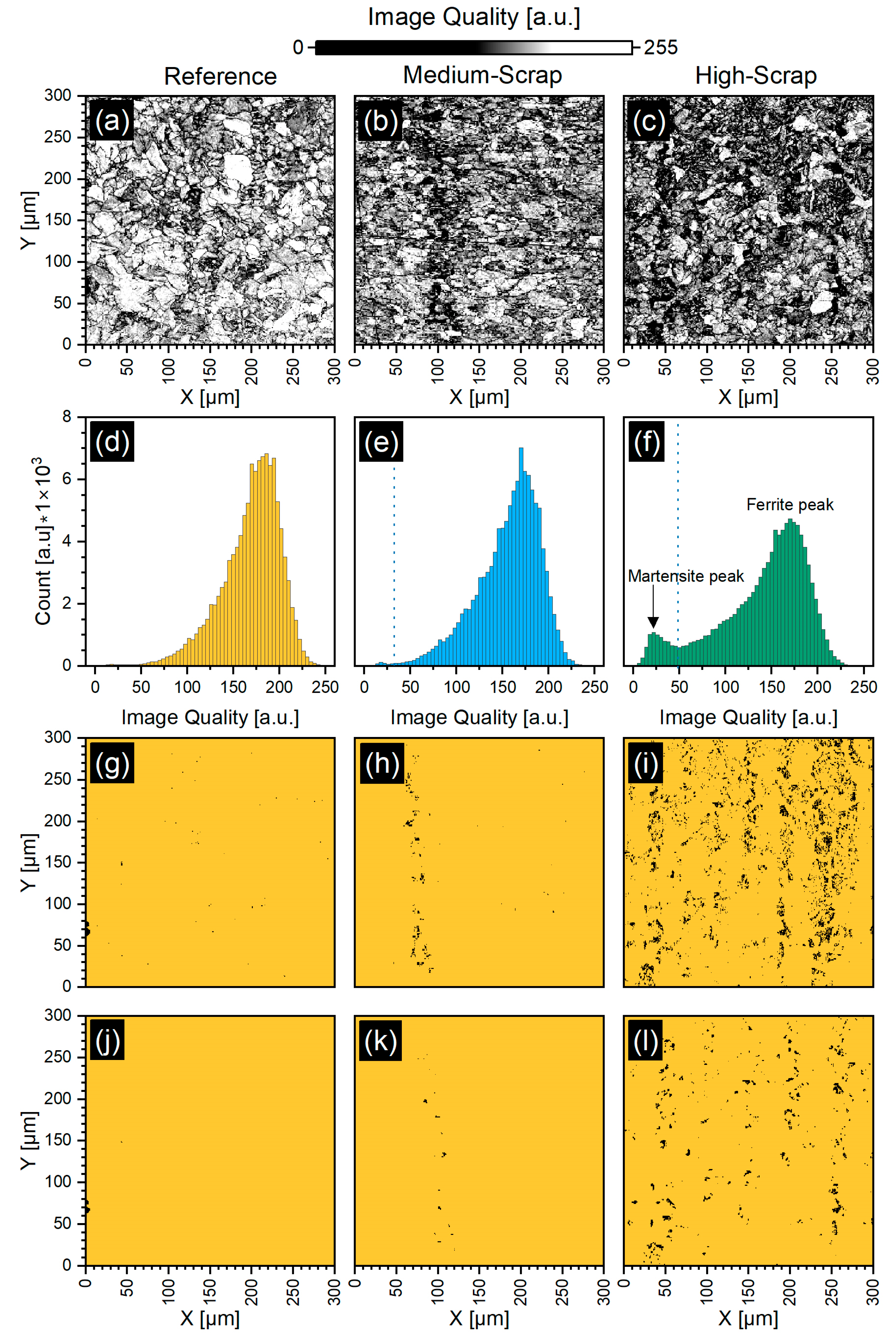
Martensite Phase Assessment
2.3. In Situ HE-XRD Analysis
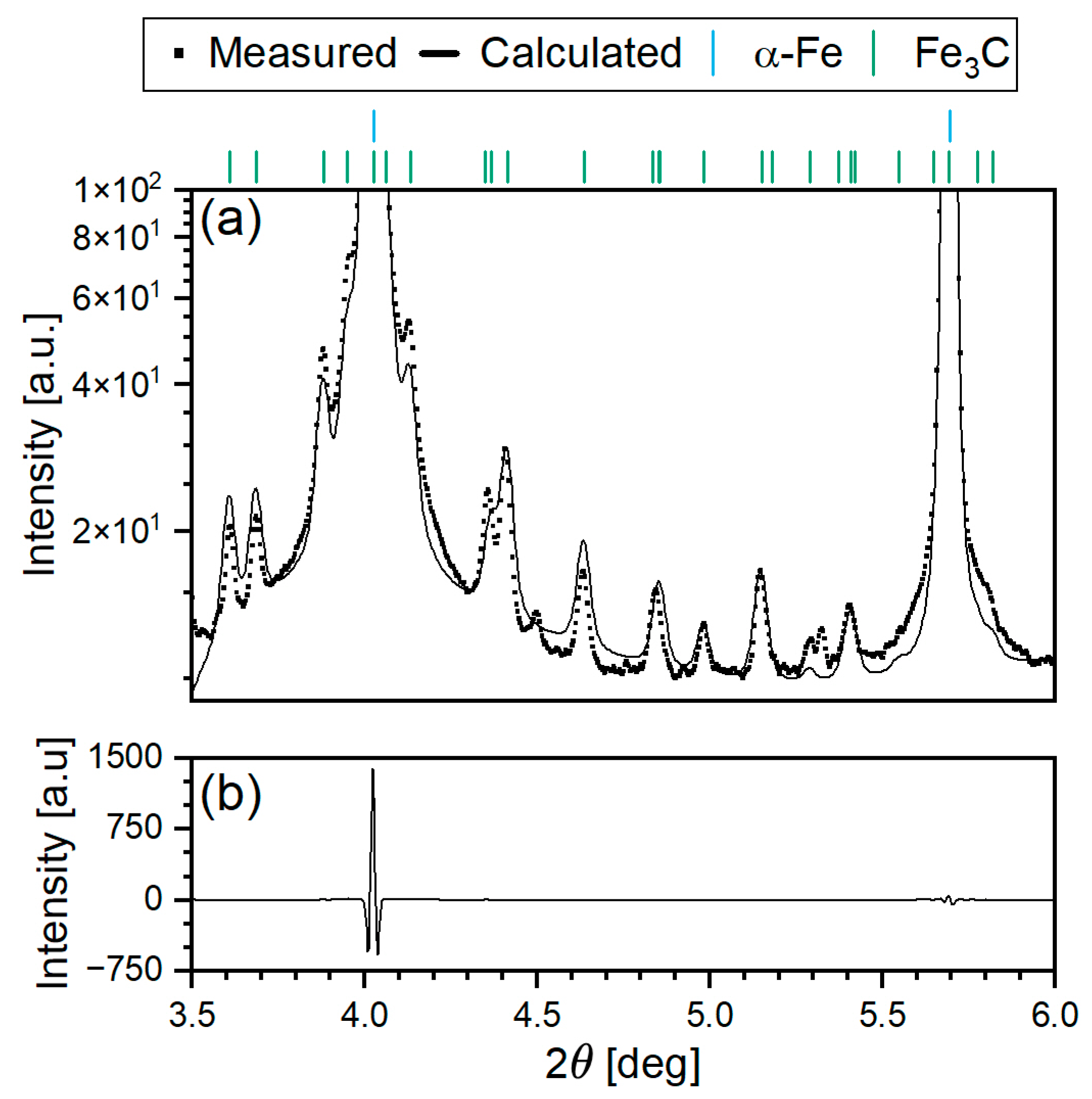
2.4. Rietveld Refinement
2.5. Hardness Testing
3. Results
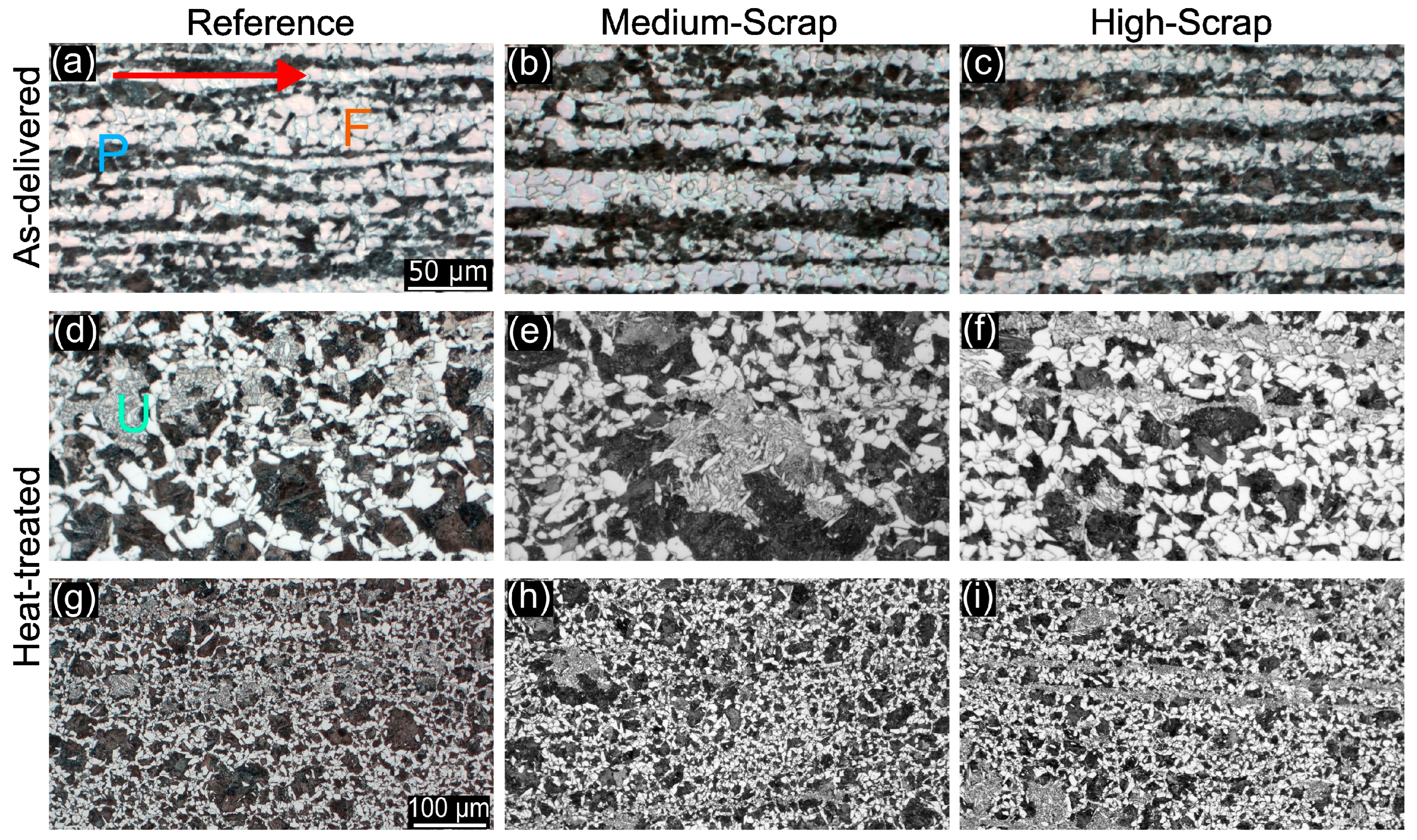
3.1. Cross-Sectional Grain Morphology
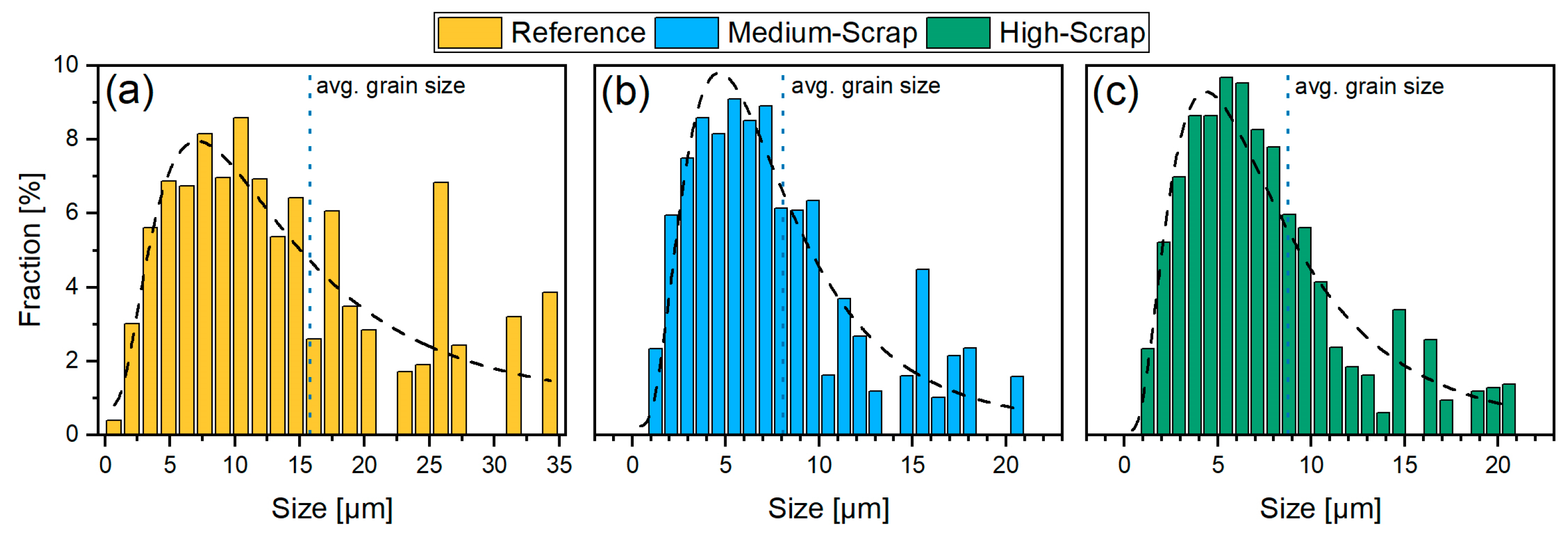
3.2. Grain Morphology and Quantitative Martensite Characterization via EBSD
3.3. In situ Evaluation of Phase Transformation Behavior as a Function of Trace and Tramp Elements
3.3.1. Evaluation of Phase Transformation Temperatures by Dilatometry
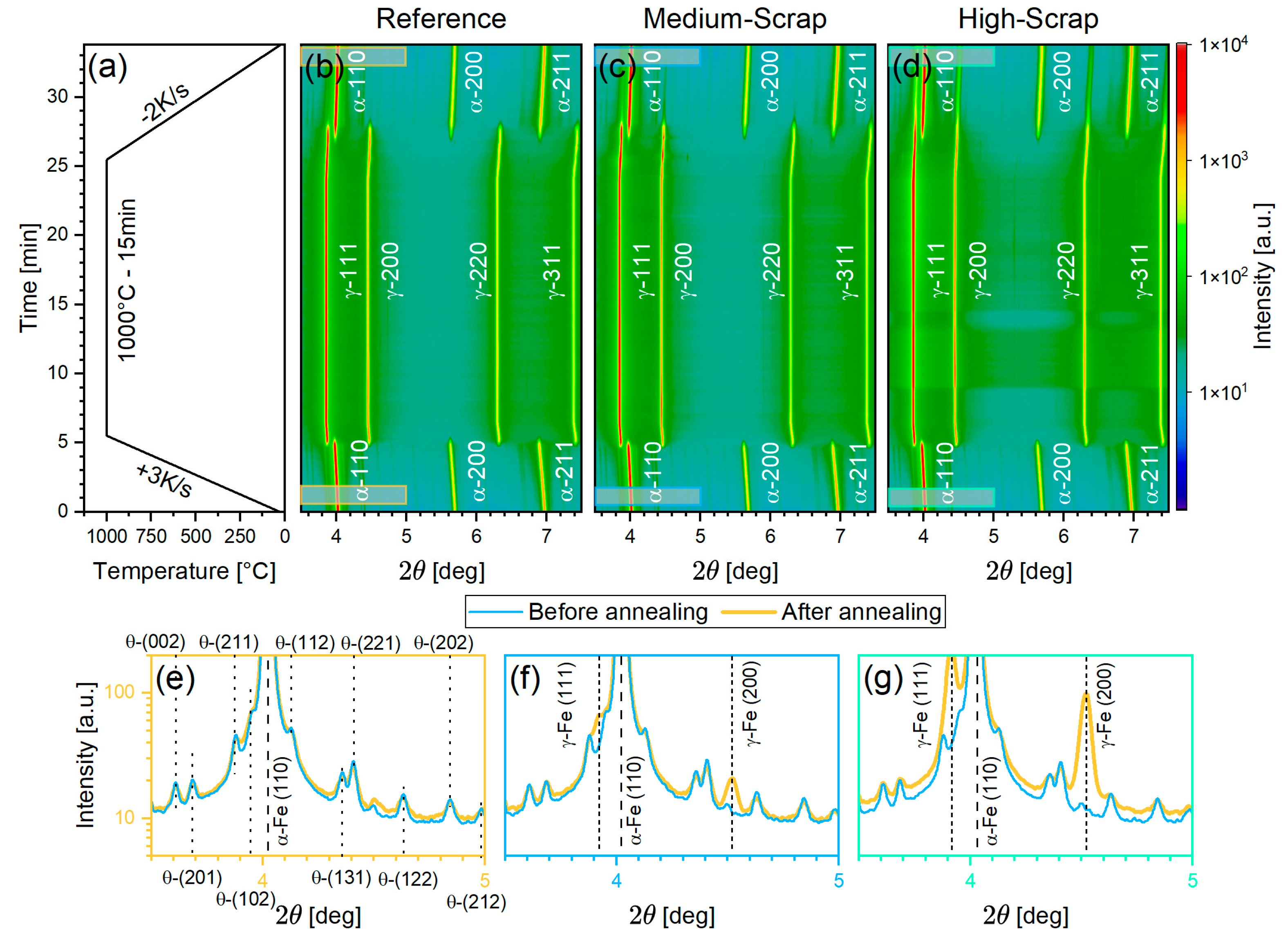
3.3.2. Qualitative Phase and Microstructure Analysis by HE-XRD
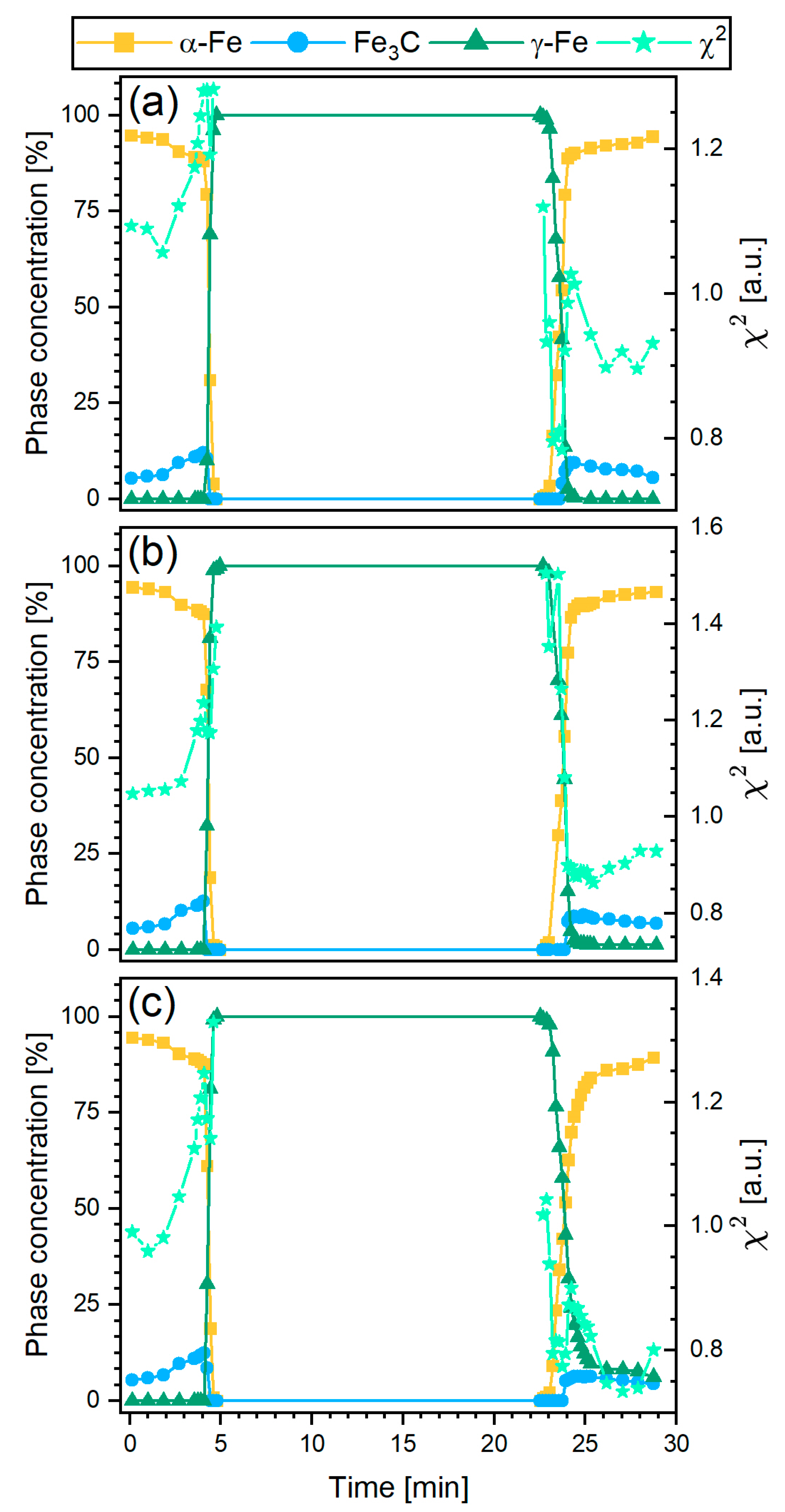
3.3.3. Quantitative Phase Analysis Using Rietveld Refinement
3.4. Hardness Testing
4. Discussion
4.1. Phase Transformation Behavior
4.2. Phase Composition Evolution
4.3. Hardness
4.4. Impact of This Study on Future Alloy Designs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| α-Fe | Fe3C | γ-Fe | ||||
|---|---|---|---|---|---|---|
| Parameter | Min | Max | Min | Max | Min | Max |
| Lattice Parameter (a) [nm] | 0.28 | 0.32 | 0.5 | 0.52 | 0.35 | 0.39 |
| Lattice Parameter (b) [nm] | - | - | 0.67 | 0.69 | - | - |
| Lattice Parameter (c) [nm] | - | - | 0.44 | 0.46 | - | - |
| Scale Factor [a.u.] | 0 | 3 | 0 | 1 | 0 | 3 |
| Crystalline Size Distribution [a.u.] | 0 | 1 | 0 | 1 | 0 | 1 |
| Micro Strain [nm−2 × 10−3] | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 |
| Gewicht Parameter | SPHAR8 | SPHAR8 | SPHAR8 | |||

References
- World Steel Association. Available online: https://worldsteel.org/data/world-steel-in-figures-2023/ (accessed on 18 December 2024).
- Maier, R.; Gerres, T.; Tuerk, A.; Mey, F. Finding Tipping Points in the Global Steel Sector: A Comparison of Companies in Australia, Austria, South Korea and the USA. Glob. Environ. Change 2024, 86, 102846. [Google Scholar] [CrossRef]
- Sun, G.; Li, B.; Guo, H.; Yang, W.; Li, S.; Guo, J. Thermodynamic Study of Energy Consumption and Carbon Dioxide Emission in Ironmaking Process of the Reduction of Iron Oxides by Carbon. Energies 2021, 14, 1999. [Google Scholar] [CrossRef]
- Boretti, A. The Perspective of Hydrogen Direct Reduction of Iron. J. Clean. Prod. 2023, 429, 139585. [Google Scholar] [CrossRef]
- Chevrier, V. Ultra-Low CO2 Ironmaking. Steel Times Int. 2020, 44, 29–34. [Google Scholar]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Raabe, D.; Jovičević-Klug, M.; Ponge, D.; Gramlich, A.; Kwiatkowski Da Silva, A.; Grundy, A.N.; Springer, H.; Souza Filho, I.; Ma, Y. Circular Steel for Fast Decarbonization: Thermodynamics, Kinetics, and Microstructure Behind Upcycling Scrap into High-Performance Sheet Steel. Annu. Rev. Mater. Res. 2024, 54, 247–297. [Google Scholar] [CrossRef]
- Dworak, S.; Rechberger, H.; Fellner, J. How Will Tramp Elements Affect Future Steel Recycling in Europe?—A Dynamic Material Flow Model for Steel in the EU-28 for the Period 1910 to 2050. Resour. Conserv. Recycl. 2022, 179, 106072. [Google Scholar] [CrossRef]
- Li, G. Chapter 1.4.3—Steel Scraps. In Treatise on Process Metallurgy; Seetharaman, S., McLean, A., Guthrie, R., Seetharaman, S., Sohn, H.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 151–165. ISBN 978-0-443-33732-1. [Google Scholar]
- Daigo, I.; Tajima, K.; Hayashi, H.; Panasiuk, D.; Takeyama, K.; Ono, H.; Kobayashi, Y.; Nakajima, K.; Hoshino, T. Potential Influences of Impurities on Properties of Recycled Carbon Steel. ISIJ Int. 2021, 61, 498–505. [Google Scholar] [CrossRef]
- Bell, S.; Davis, B.; Javaid, A.; Essadiqi, E. Final Report on Effect of Impurities in Steel; Natural Resources Canada: Ottawa, ON, Canada, 2006. [Google Scholar] [CrossRef]
- Gao, Z.; Sridhar, S.; Spiller, D.E.; Taylor, P.R. Review of Impurity Removal Methods in Steel Scrap Recycling. J. Solid Waste Technol. Manag. 2021, 47, 732–745. [Google Scholar] [CrossRef]
- Aktas, S.; Ma, E.A. Recovery of Zinc from Galvanized Scraps. Turk. J. Eng. Env. Sci. 2002, 26, 395–402. [Google Scholar]
- Chin, D.-T. Electrochemical Extraction of Copper from Scrap Steel. AIChE J. 1977, 23, 434–440. [Google Scholar] [CrossRef]
- Cho, W.D.; Fan, P. Methods and Systems for Removing Copper from Ferrous Scrap. US7789936B2, 7 September 2010. [Google Scholar]
- Sano, N.; Katayama, H.; Sasabe, M.; Matsuoka, S. Research Activities on Removal of Residual Elements from Steel Scrap in Japan. Scand. J. Metall. 1998, 27, 24–30. [Google Scholar]
- Savov, L.; Janke, D. Evaporation of Cu and Sn from Induction-Stirred Iron-Based Melts Treated at Reduced Pressure. ISIJ Int. 2000, 40, 95–104. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kang, Y.-B. Simultaneous Evaporation of Cu and Sn from Liquid Steel. Metall. Mater. Trans. B 2016, 47, 2564–2570. [Google Scholar] [CrossRef]
- Davis, J.R. (Ed.) Alloying: Understanding the Basics; ASM International: Almere, The Netherlands, 2001; ISBN 978-1-62708-297-6. [Google Scholar]
- Houpert, C.; Lanteri, V.; Jolivet, J.M.; Guttmann, M.; Birat, J.P.; Jallon, M.; Confente, M. Influence of Tramp Elements in the Production of High Quality Steels Using the Scrap/Electric Arc Furnace Route. Rev. Met. Paris 1997, 94, 1369–1384. [Google Scholar] [CrossRef]
- Kim, S.-J.; Gil Lee, C.; Lee, T.-H.; Oh, C.-S. Effect of Cu, Cr and Ni on Mechanical Properties of 0.15 Wt.% C TRIP-Aided Cold Rolled Steels. Scr. Mater. 2003, 48, 539–544. [Google Scholar] [CrossRef]
- Bargel, H.-J.; Schulze, G. (Eds.) Werkstoffkunde; Springer-Lehrbuch; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-17716-3. [Google Scholar]
- Sekunowo, O.I.; Durowaye, S.I.; Gbenebor, O.P. Effect of Copper on Microstructure and Mechanical Properties of Construction Steel. Int. J. Chem. Nucl. Metall. Mater. Eng. 2014, 8, 785–789. [Google Scholar]
- Inujima, K.; Ichikawa, K. Influence of Sn on Practical Performances of Structural Steels. IJMMME 2020, 6, 27–34. [Google Scholar] [CrossRef]
- Madias, J. Electric Furnace Steelmaking. In Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 243–265. ISBN 978-0-323-85373-6. [Google Scholar]
- Nowell, M.; Wright, S.; Carpenter, J. Differentiating Ferrite and Martensite in Steel Microstructures Using Electron Backscatter Diffraction. In Proceedings of the Materials Science and Technology Conference and Exhibition 2009, MS and T’09, Pittsburgh, PA, USA, 25–29 October 2009; Volume 2. [Google Scholar]
- Calcagnotto, M.; Ponge, D.; Raabe, D. Microstructure Control during Fabrication of Ultrafine Grained Dual-Phase Steel: Characterization and Effect of Intercritical Annealing Parameters. ISIJ Int. 2012, 52, 874–883. [Google Scholar] [CrossRef]
- Lin, S.; Borggren, U.; Stark, A.; Borgenstam, A.; Mu, W.; Hedström, P. In-Situ High-Energy X-Ray Diffraction Study of Austenite Decomposition During Rapid Cooling and Isothermal Holding in Two HSLA Steels. Metall. Mater. Trans. A 2021, 52, 1812–1825. [Google Scholar] [CrossRef]
- Kieffer, J.; Karkoulis, D. PyFAI, a Versatile Library for Azimuthal Regrouping. J. Phys. Conf. Ser. 2013, 425, 202012. [Google Scholar] [CrossRef]
- Ashiotis, G.; Deschildre, A.; Nawaz, Z.; Wright, J.P.; Karkoulis, D.; Picca, F.E.; Kieffer, J. The Fast Azimuthal Integration Python Library: pyFAI. J. Appl. Crystallogr. 2015, 48, 510–519. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld Refinement Guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Balzar, D.; Popa, N. Analyzing Microstructure by Rietveld Refinement. Rigaku J. 2005, 22, 16–25. [Google Scholar]
- Toby, B.H. R Factors in Rietveld Analysis: How Good Is Good Enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Stinton, G.W.; Evans, J.S.O. Parametric Rietveld Refinement. J. Appl. Crystallogr. 2007, 40, 87–95. [Google Scholar] [CrossRef]
- Jiao, H.; Xie, X.; Hu, X.; Zhao, L.; Misra, R.D.K.; Liu, D.; Tang, Y.; Hu, Y. Role of Hot Rolling in Microstructure and Texture Development of Strip Cast Non-Oriented Electrical Steel. Metals 2022, 12, 354. [Google Scholar] [CrossRef]
- Liu, Z.; Kobayashi, Y.; Yin, F.; Kuwabara, M.; Nagai, K. Nucleation of Acicular Ferrite on Sulfide Inclusion during Rapid Solidification of Low Carbon Steel. ISIJ Int. 2007, 47, 1781–1788. [Google Scholar] [CrossRef]
- Hatzenbichler, L.; Vincely, C.; Haslberger, P.; Galler, M.; Glushko, O.; Holec, D.; Clemens, H.; Schnitzer, R. Effect of tramp elements on the microstructural evolution of a ferritic-pearlitic steel. Pract. Metallogr. 2025, 62, 148–175. [Google Scholar] [CrossRef]
- Collins, J.; Taylor, M.; Scarlett, A.L.; Palmiere, E.J.; Pickering, E.J. Prior Austenite Grain Measurement: A Direct Comparison of EBSD Reconstruction, Thermal Etching and Chemical Etching. Mater. Charact. 2024, 208, 113656. [Google Scholar] [CrossRef]
- Mehta, B.; Yang, X.; Höglund, L.; Mu, W.; Hedström, P. Toward Scrap-Tolerant Steels: Investigating the Role of Cu and Sn Micro-Segregations on Solidification Microstructure and Cracking. J. Sustain. Metall. 2025, 11, 1908–1921. [Google Scholar] [CrossRef]
- Bloder, B.; Scheiber, D.; Raninger, P.; Ecker, W.; Antretter, T. Modeling Solute Drag during Austenite–Ferrite Transformation with Ab Initio Binding Energies. Materialia 2024, 36, 102128. [Google Scholar] [CrossRef]
- Park, M.; Shibata, A.; Tsuji, N. Challenging Ultra Grain Refinement of Ferrite in Low-C Steel Only by Heat Treatment. Front. Mater. 2020, 7, 604792. [Google Scholar] [CrossRef]
- García De Andrés, C. Application of Dilatometric Analysis to the Study of Solid–Solid Phase Transformations in Steels. Mater. Charact. 2002, 48, 101–111. [Google Scholar] [CrossRef]
- Trzaska, J. Empirical Formulas for Calculating Continuous Cooling Transformation Diagrams. Available online: https://journalamme.org/article/01.3001.0013.7946/en (accessed on 2 July 2025).
- Zhou, L.; Fang, F.; Kumagai, M.; Pickering, E.; Yu, T.; Zhang, X. Structure Restoration and Coarsening of Nanocrystalline Cementite in Cold Drawn Pearlitic Wire Induced by Low Temperature Annealing. Scr. Mater. 2022, 215, 114696. [Google Scholar] [CrossRef]
- Nielsen, J.; McMorrow, D. Kinematical Scattering II: Crystalline Order. In Elements of Modern X-Ray Physics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 147–205. ISBN 978-1-119-99836-5. [Google Scholar]
- Stephenson, E.T. Effect of Recycling on Residuals, Processing, and Properties of Carbon and Low-Alloy Steels. Metall. Trans. A 1983, 14, 343–353. [Google Scholar] [CrossRef]
- Krauss, G. (Ed.) Steels: Processing, Structure, and Performance; ASM International: Materials Park, OH, USA, 2010; ISBN 978-0-87170-817-5. [Google Scholar]
- Bhadeshia, H.; Honeycombe, R. Chapter 3—Iron-Carbon Equilibrium and Plain Carbon Steels. In Steels: Microstructure and Properties, 4th ed.; Bhadeshia, H., Honeycombe, R., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 59–100. ISBN 978-0-08-100270-4. [Google Scholar]
- Zhang, M.; Xu, H.; Cao, W.; Dong, H. Effect of Hot-Rolling Parameters on the Microstructure and Property of Fe–Mn–Al–C Micro–Laminated Dual Phase Steels. ISIJ Int. 2016, 56, 861–867. [Google Scholar] [CrossRef]
- Malyshevskii, V.A.; Semicheva, T.G.; Khlusova, E.I. Effect of Alloying Elements and Structure on the Properties of Low-Carbon Heat-Treatable Steel. Met. Sci. Heat. Treat. 2001, 43, 331–335. [Google Scholar] [CrossRef]
- Devra, V.K.; Maity, J. Solute Drag Effect on Austenite Grain Growth in Hypoeutectoid Steel. Philos. Mag. Lett. 2020, 100, 245–259. [Google Scholar] [CrossRef]
- Kern, M.; Bernhard, M.; Kang, Y.-B.; Bernhard, C. In Situ Study and Assessment of the Phosphorus-Induced Solute Drag Effect on the Grain Boundary Mobility of Austenite. Acta Mater. 2024, 269, 119826. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, J.; Zhao, H.; Guan, D.; Zhang, Y.; Huang, Y.; Li, S.; Yang, W.; Wang, K.; Wang, S.; et al. Effects of Residual Elements on the Microstructure and Mechanical Properties of a Q&P Steel. J. Mater. Sci. Technol. 2025, 221, 143–154. [Google Scholar] [CrossRef]
- Neetu; Sangal, S.; Mondal, K. Effect of Various Phase Fractions of Bainite, Retained Austenite, Intercritical Ferrite and Pearlite on the Wear Behaviour of Multiphase Steels. Wear 2022, 500–501, 204355. [Google Scholar] [CrossRef]
- Llopis, A.M. Effect of Alloying Elements in Steels on the Kinetics of the Austenite to Bainite Transformation; Lawrence Berkeley Laboratory: Berkeley, CA, USA, 1975; Report No. LBL-4555. [Google Scholar]
- Kapoor, I.; Davis, C.; Li, Z. Effects of Residual Elements during the Casting Process of Steel Production: A Critical Review. Ironmak. Steelmak. 2021, 48, 712–727. [Google Scholar] [CrossRef]
- Shibata, K.; Seo, S.-J.; Kaga, M.; Uchino, H.; Sasanuma, A.; Asakura, K.; Nagasaki, C. Suppression of Surface Hot Shortness Due to Cu in Recycled Steels. Mater. Trans. 2002, 43, 292–300. [Google Scholar] [CrossRef]


| Trial Alloy | P | S | Ni | Cu | Mo | Sn | Co |
|---|---|---|---|---|---|---|---|
| Medium-Scrap | −0.0035 | −0.0017 | +0.03 | +0.06 | +0.15 | +0.002 | −0.004 |
| High-Scrap | +0.0035 | −0.0047 | +0.1 | +0.12 | +0.037 | +0.019 | +0.006 |
| Heating | Cooling | Ms | ||||
|---|---|---|---|---|---|---|
| Sample | Ac1 [°C] | Ac3 [°C] | AR1 [°C] | AR3 [°C] | Msemp [°C] | Msexp [°C] |
| Reference | 742 ± 5 | 810 ± 5 | 580 ± 5 | 691 ± 5 | 319 | — |
| Medium | 745 ± 5 | 813 ± 5 | 578 ± 5 | 690 ± 5 | 323 | — |
| High | 738 ± 5 | 807 ± 5 | 447 ± 5 | 662 ± 5 | 321 | 292 |
| Before Annealing | After Annealing | |||||
|---|---|---|---|---|---|---|
| Intensity [a.u.] | FWHM [deg. × 10−3 ] | Intensity [a.u.] | FWHM [deg. × 10−3 ] | |||
| α-Fe (200) | α-Fe (200) | α-Fe (200) | γ-Fe (200) | α-Fe (200) | γ-Fe (200) | |
| Reference | 847 ± 3 | 27.58 ± 0.13 | 786 ± 2.0 | — | 30.77 ± 0.11 | — |
| Medium | 827 ± 3 | 27.86 ± 0.15 | 730 ± 2.0 | — | 32.28 ± 0.12 | — |
| High | 837 ± 3 | 28.89 ± 0.12 | 759 ± 2.6 | 82 ± 1 | 33.60 ± 0.16 | 45.23 ± 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gocnik, M.; Hatzenbichler, L.; Meindlhumer, M.; Haslberger, P.; Galler, M.; Stark, A.; Olsson, C.-O.A.; Keckes, J.; Schnitzer, R. Influence of Tramp Elements on Phase Transformations, Microstructure and Hardness of a 0.3 wt.%C Low-Alloyed Steel. Metals 2025, 15, 1053. https://doi.org/10.3390/met15091053
Gocnik M, Hatzenbichler L, Meindlhumer M, Haslberger P, Galler M, Stark A, Olsson C-OA, Keckes J, Schnitzer R. Influence of Tramp Elements on Phase Transformations, Microstructure and Hardness of a 0.3 wt.%C Low-Alloyed Steel. Metals. 2025; 15(9):1053. https://doi.org/10.3390/met15091053
Chicago/Turabian StyleGocnik, Marek, Lukas Hatzenbichler, Michael Meindlhumer, Phillip Haslberger, Matthew Galler, Andreas Stark, Claes-Olof A. Olsson, Jozef Keckes, and Ronald Schnitzer. 2025. "Influence of Tramp Elements on Phase Transformations, Microstructure and Hardness of a 0.3 wt.%C Low-Alloyed Steel" Metals 15, no. 9: 1053. https://doi.org/10.3390/met15091053
APA StyleGocnik, M., Hatzenbichler, L., Meindlhumer, M., Haslberger, P., Galler, M., Stark, A., Olsson, C.-O. A., Keckes, J., & Schnitzer, R. (2025). Influence of Tramp Elements on Phase Transformations, Microstructure and Hardness of a 0.3 wt.%C Low-Alloyed Steel. Metals, 15(9), 1053. https://doi.org/10.3390/met15091053







