Influence of Pre-Corrosion in NaCl Solution on Cavitation Resistance of Steel Samples (42CrMo4)
Abstract
1. Introduction
- -
- Vibratory Cavitation Apparatus (ASTM G32-16(2021)e1 [11]);
- -
- Cavitating Liquid Jets (ASTM G134-17 [12] and Variants);
- -
- High-speed Cavitation Tunnels.
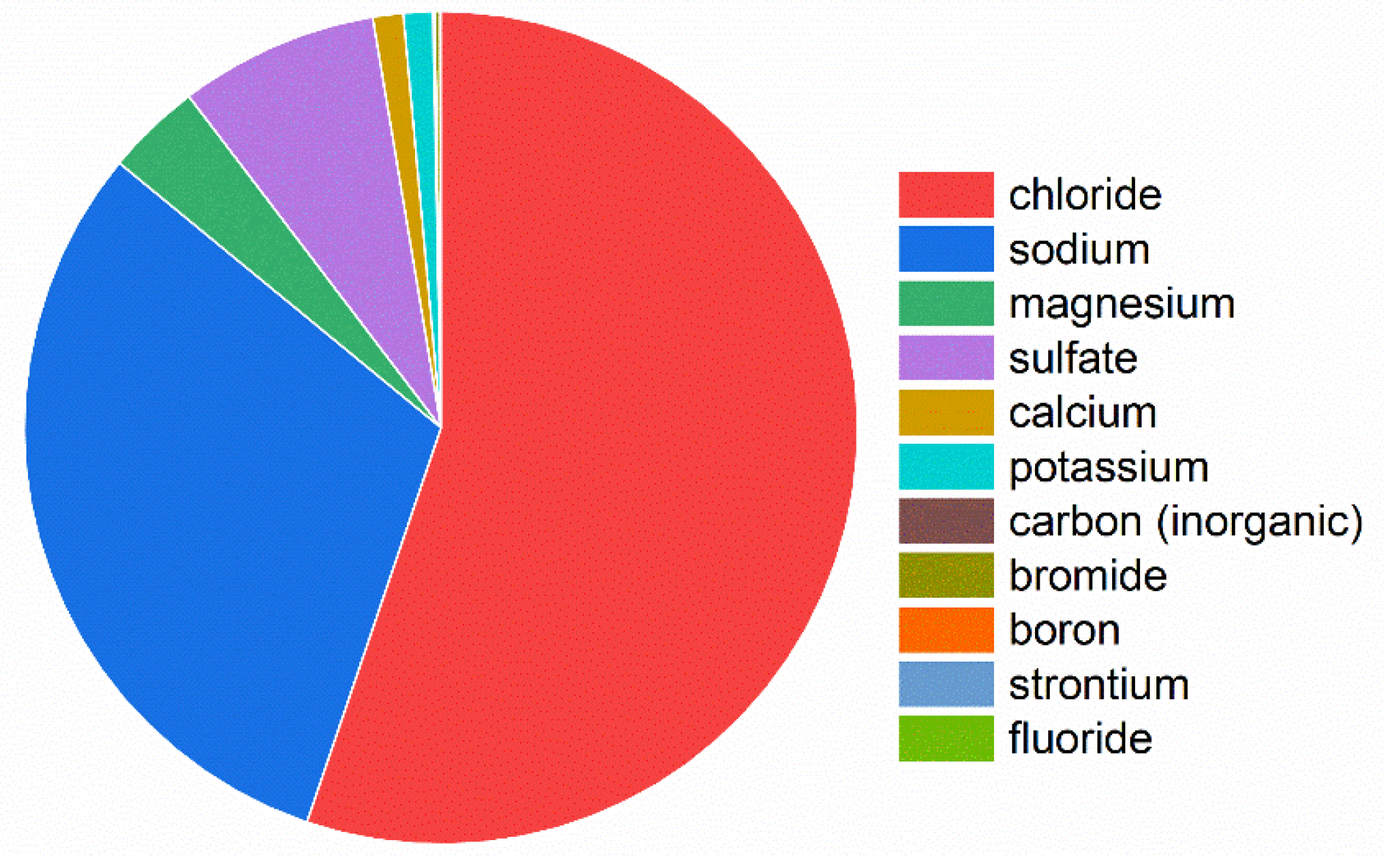
2. Materials and Methods
3. Results and Discussion
3.1. Cavitation Before Pre-Corrosion
3.1.1. Mass Loss and Mass Loss Rate
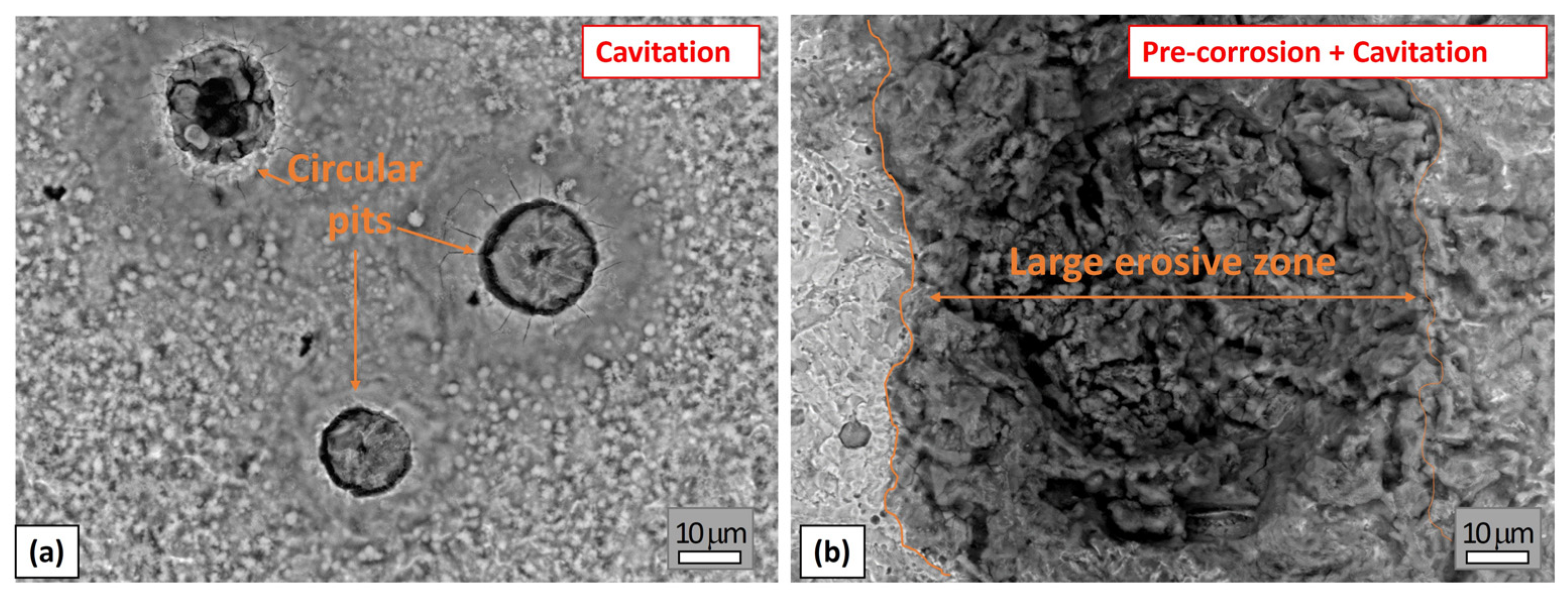
3.1.2. Image and Morphological Characterization of the Samples
3.2. Cavitation After Immersion in NaCl Solution
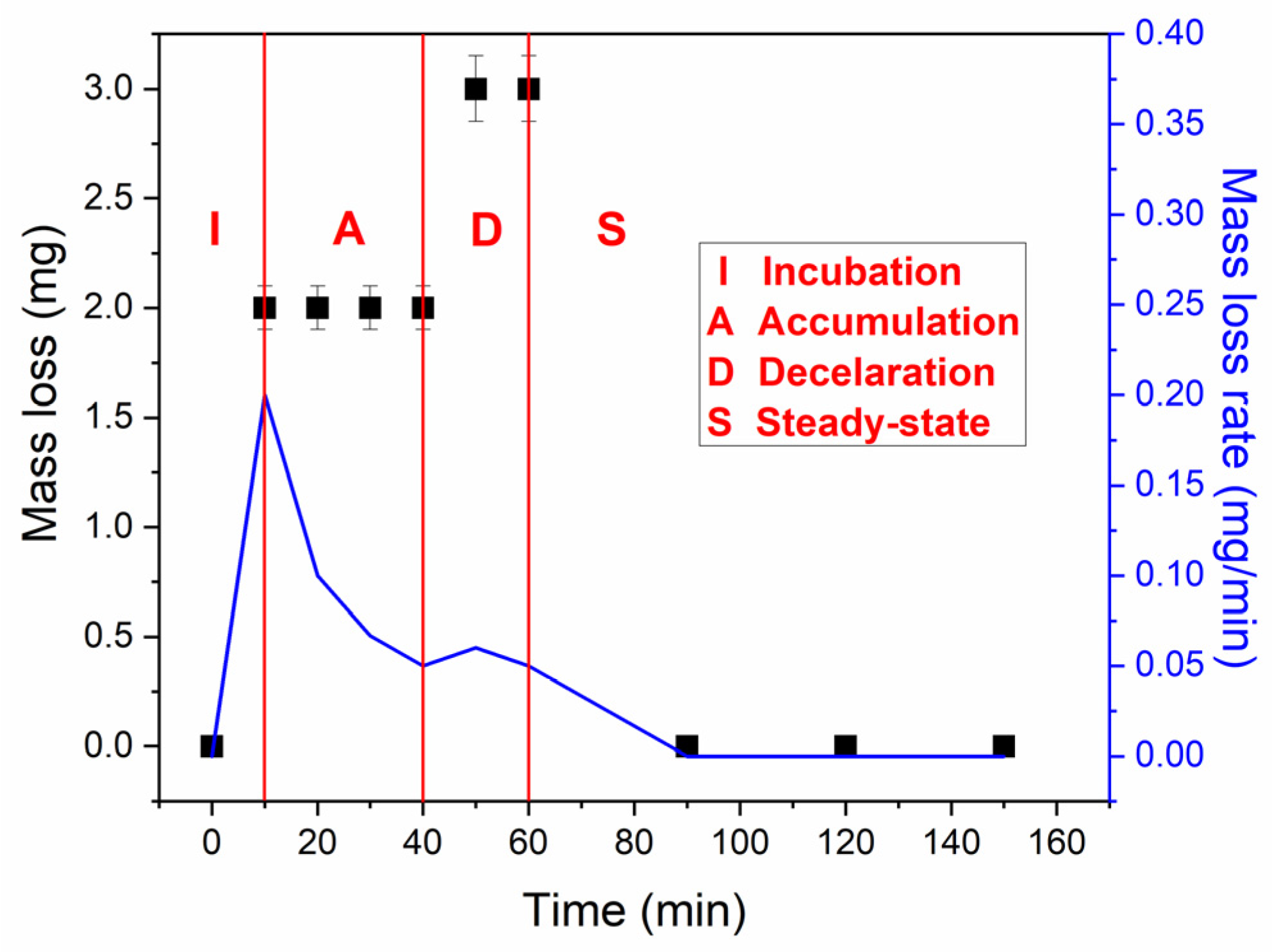
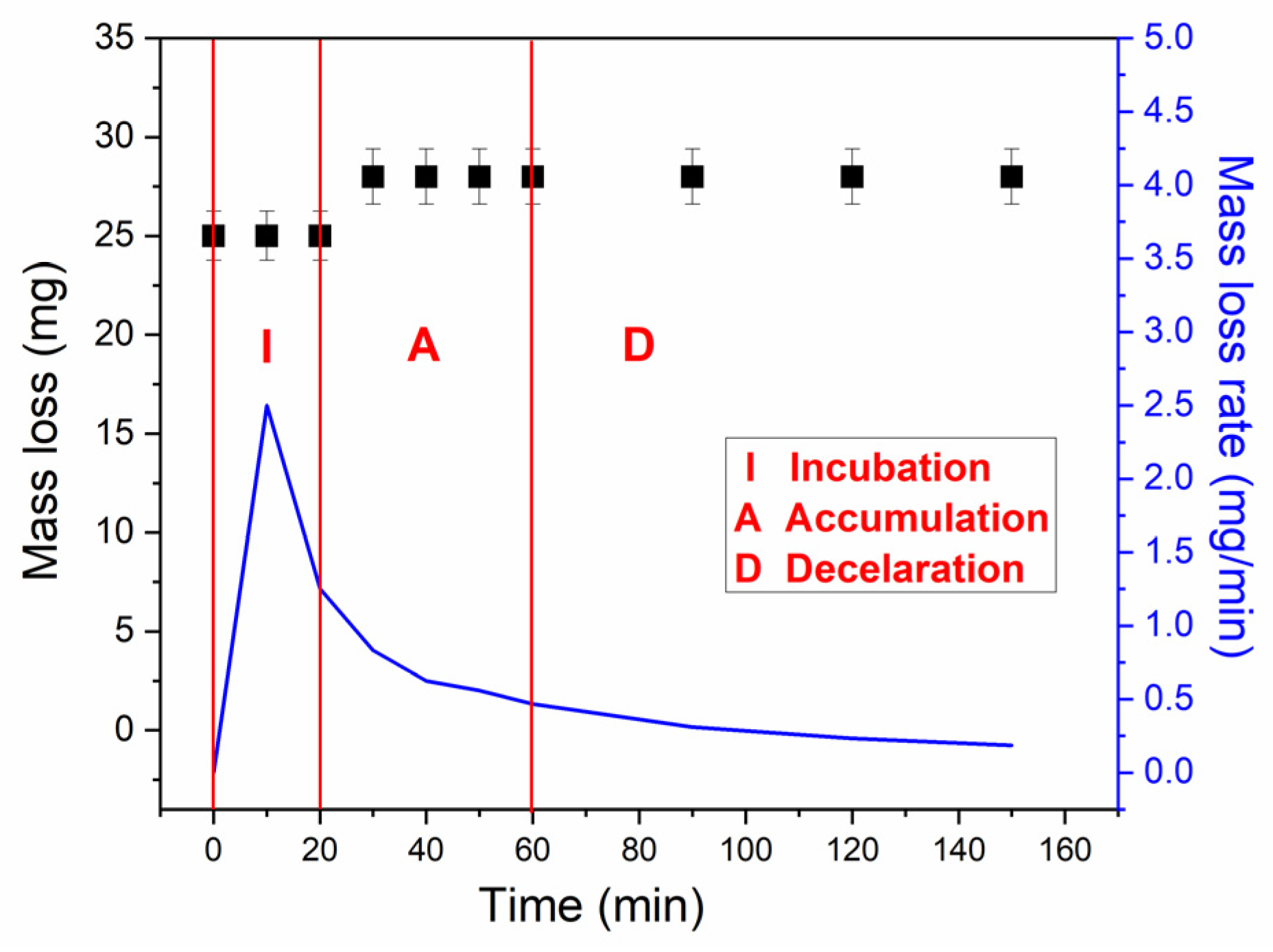
3.2.1. Mass Loss and Mass Loss Rate
- -
- Initialization (I), which was observed till 40 min of testing,
- -
- Acceleration (A) observed from 40 to 60 min of testing, and
- -
- Deceleration (D) observed from 60 min of testing.
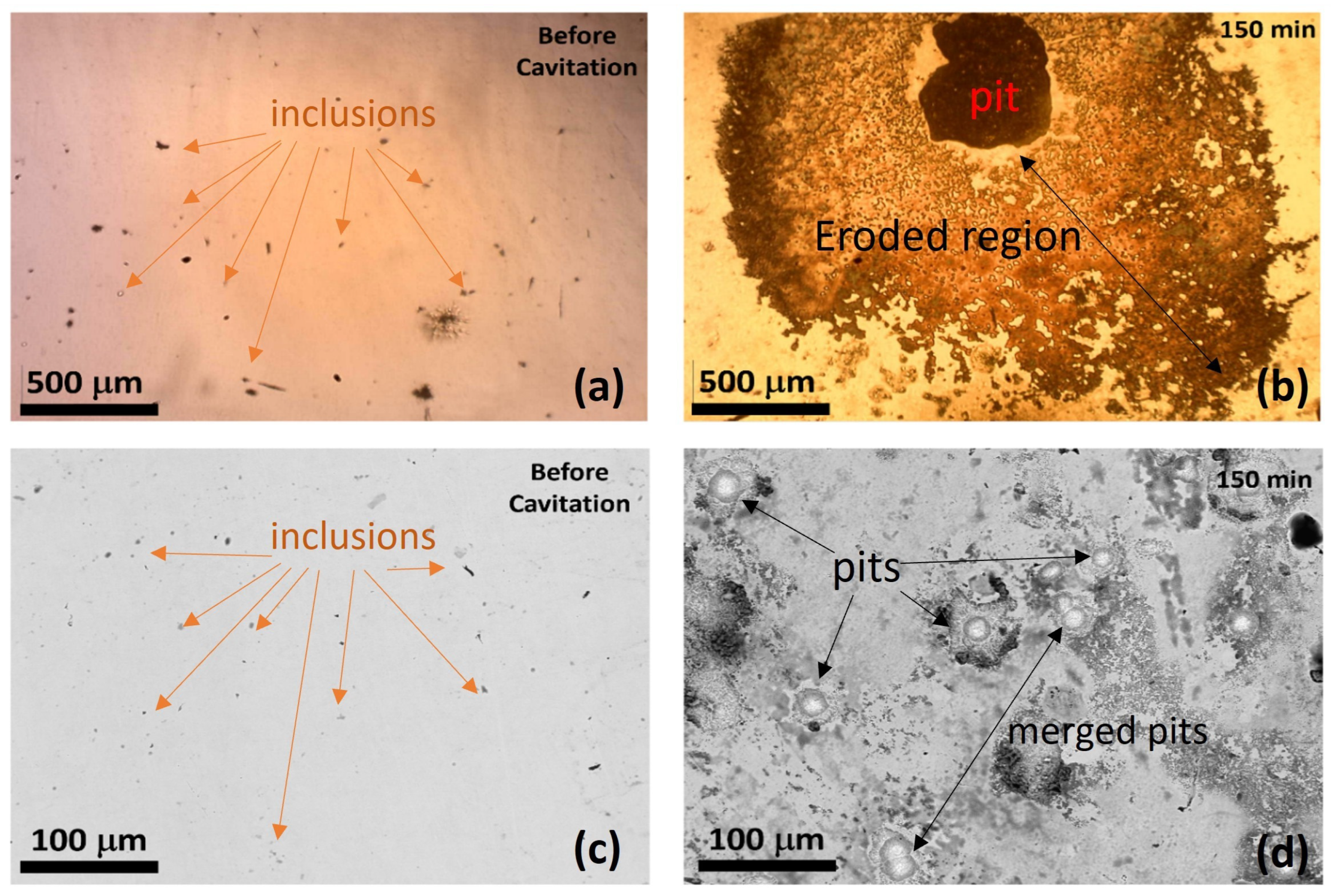
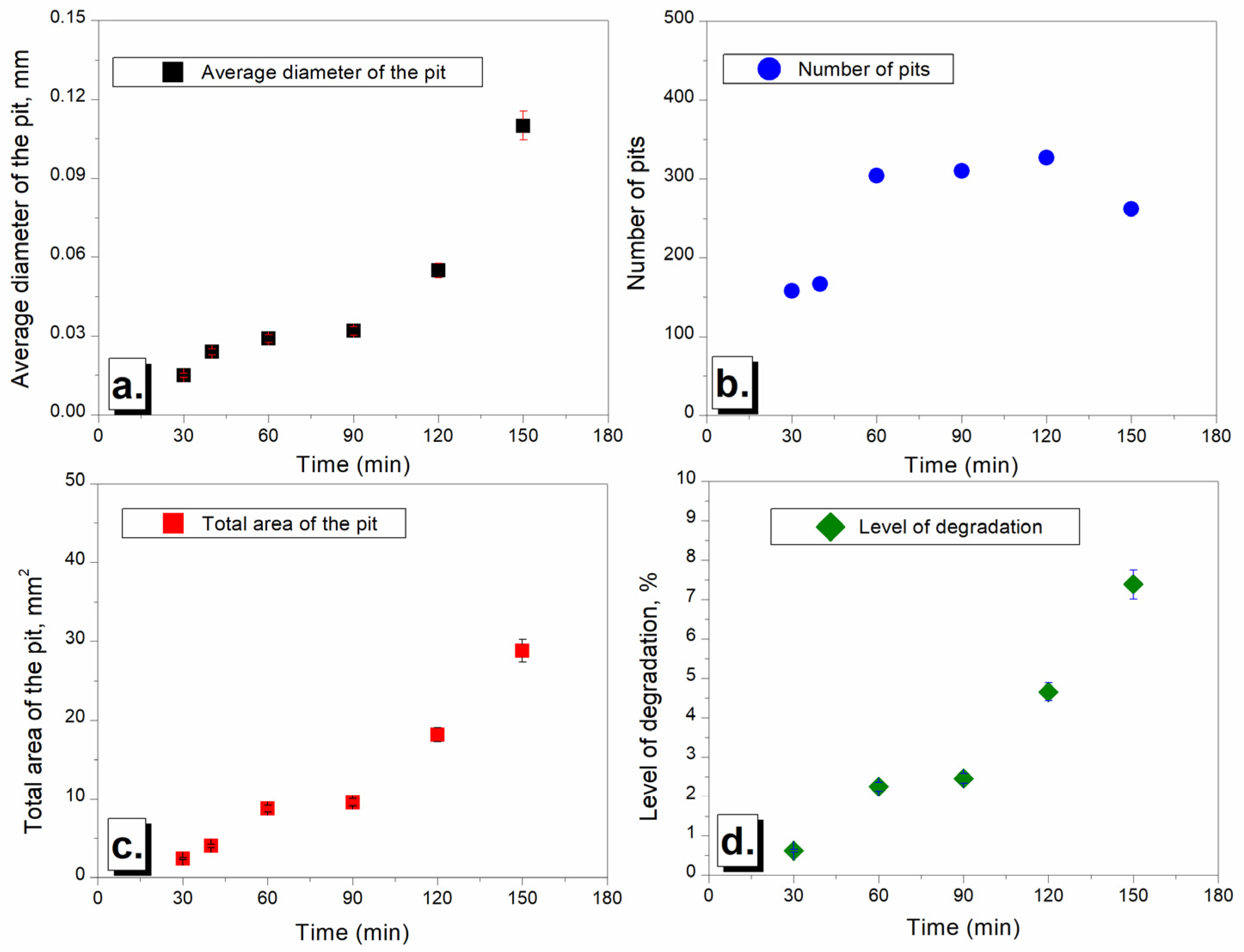
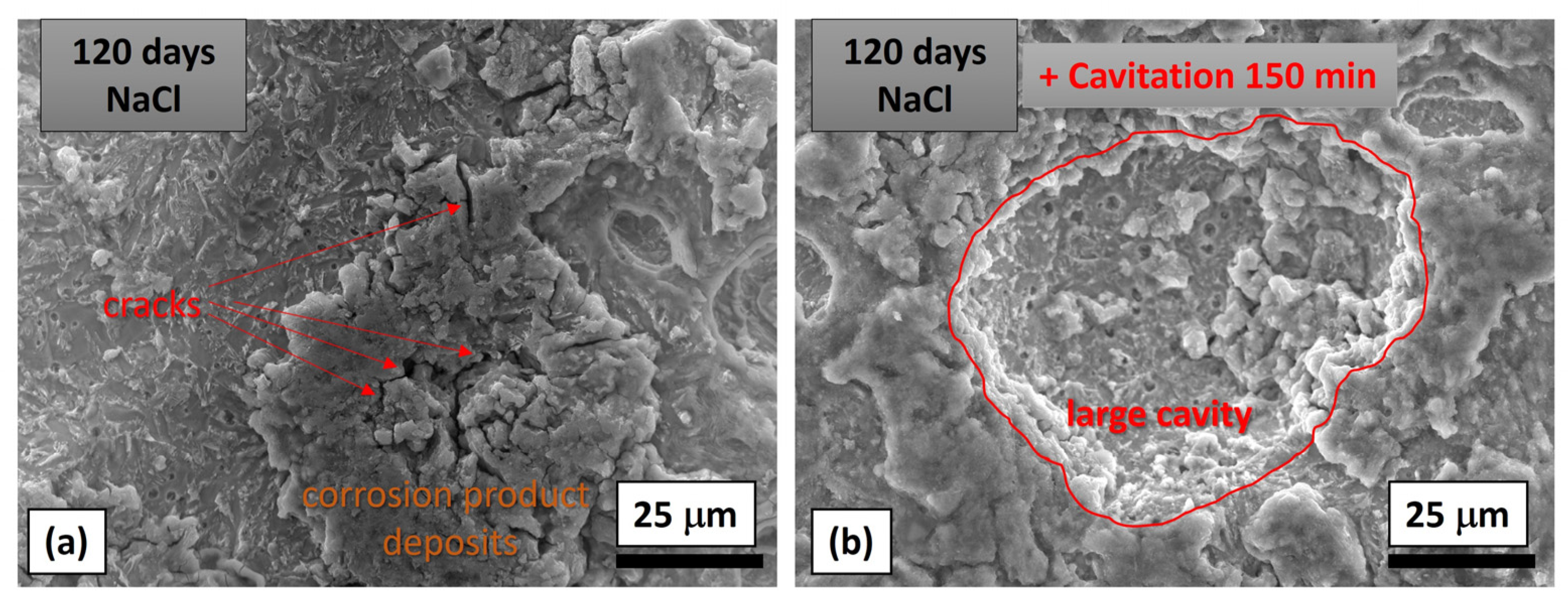
3.2.2. Image and Morphological Characterization of the Samples
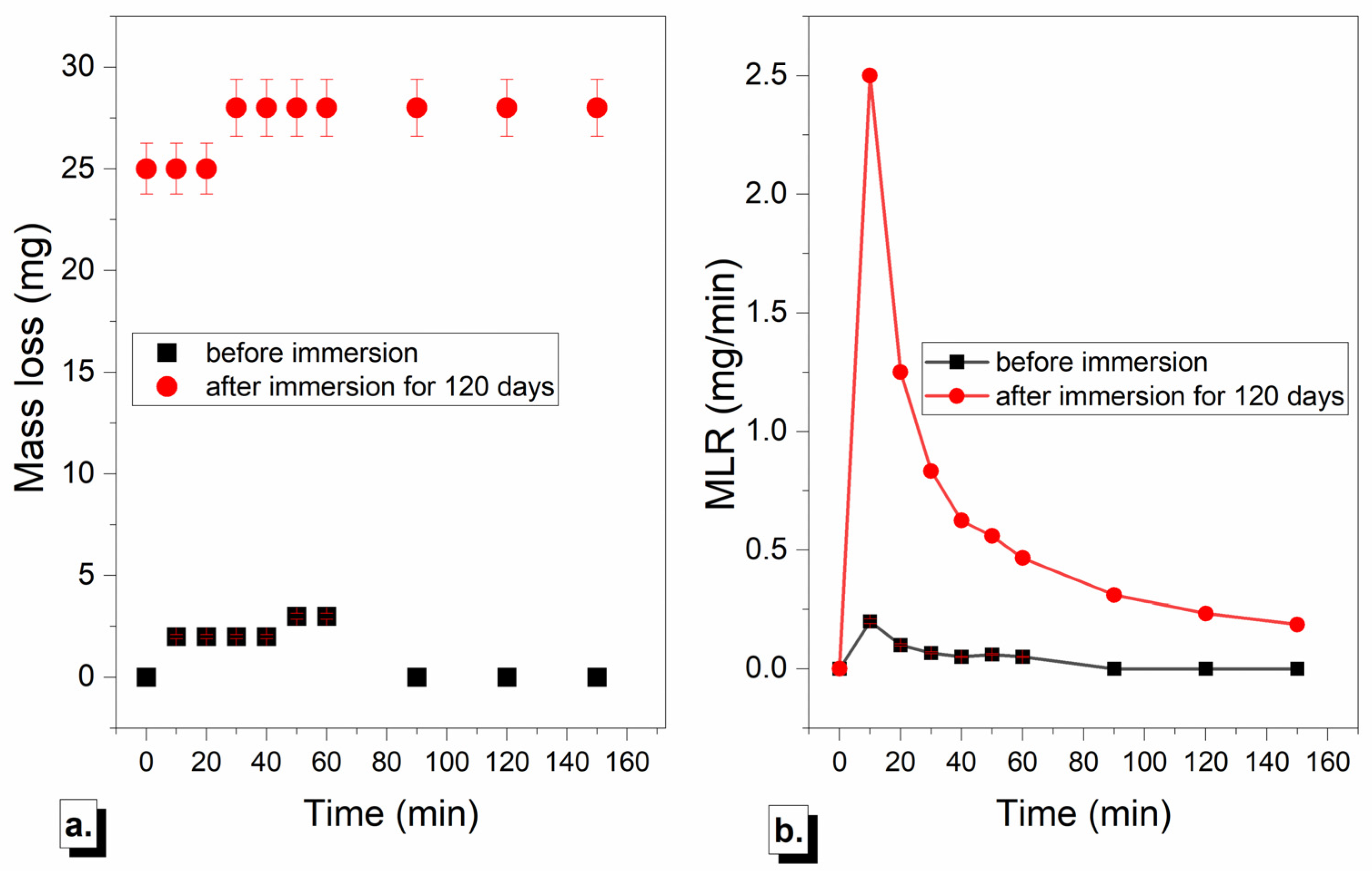
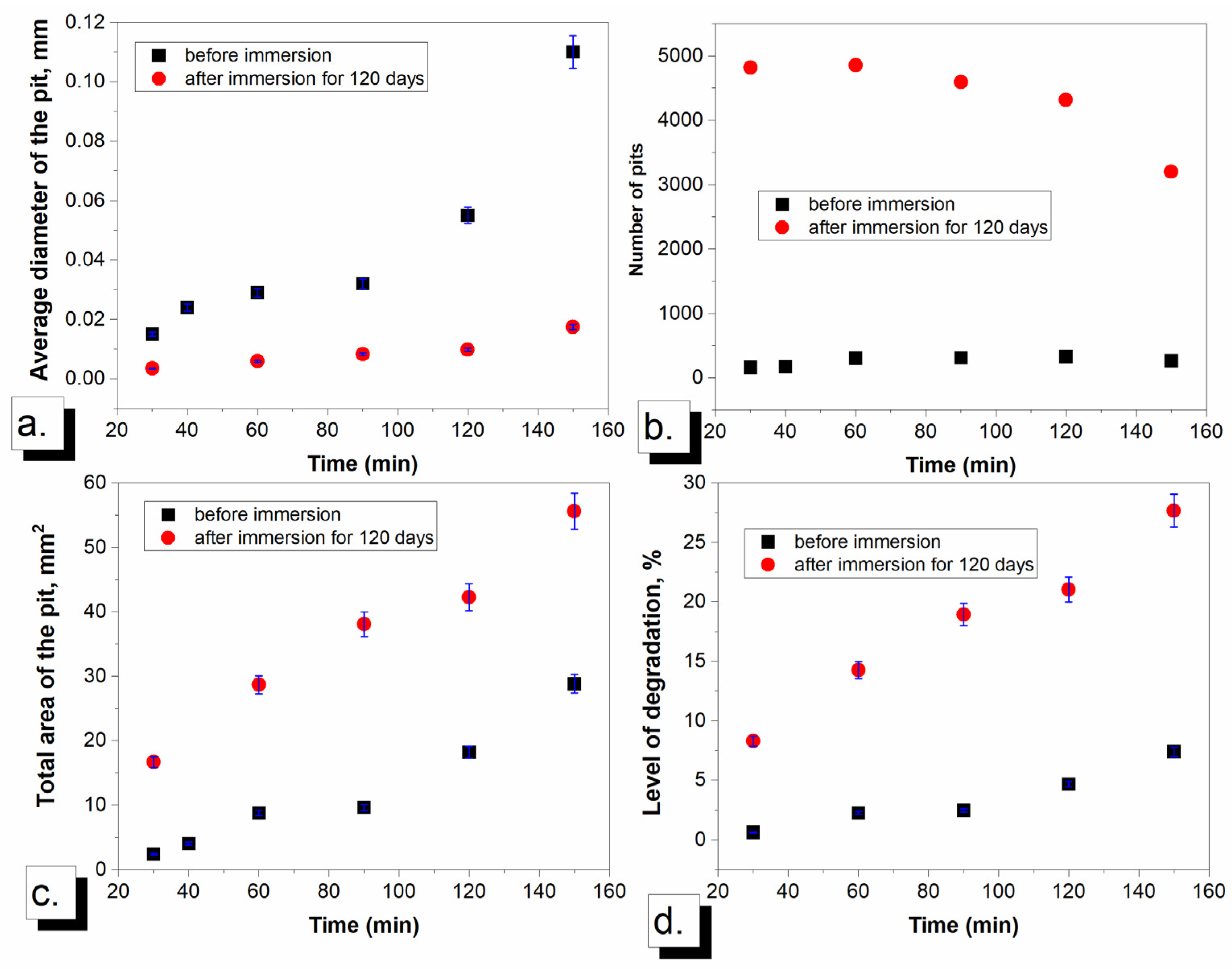
3.3. Comparison of Cavitation Behavior Before and After Immersion in NaCl Solution
3.3.1. Mass Loss and Mass Loss Rate
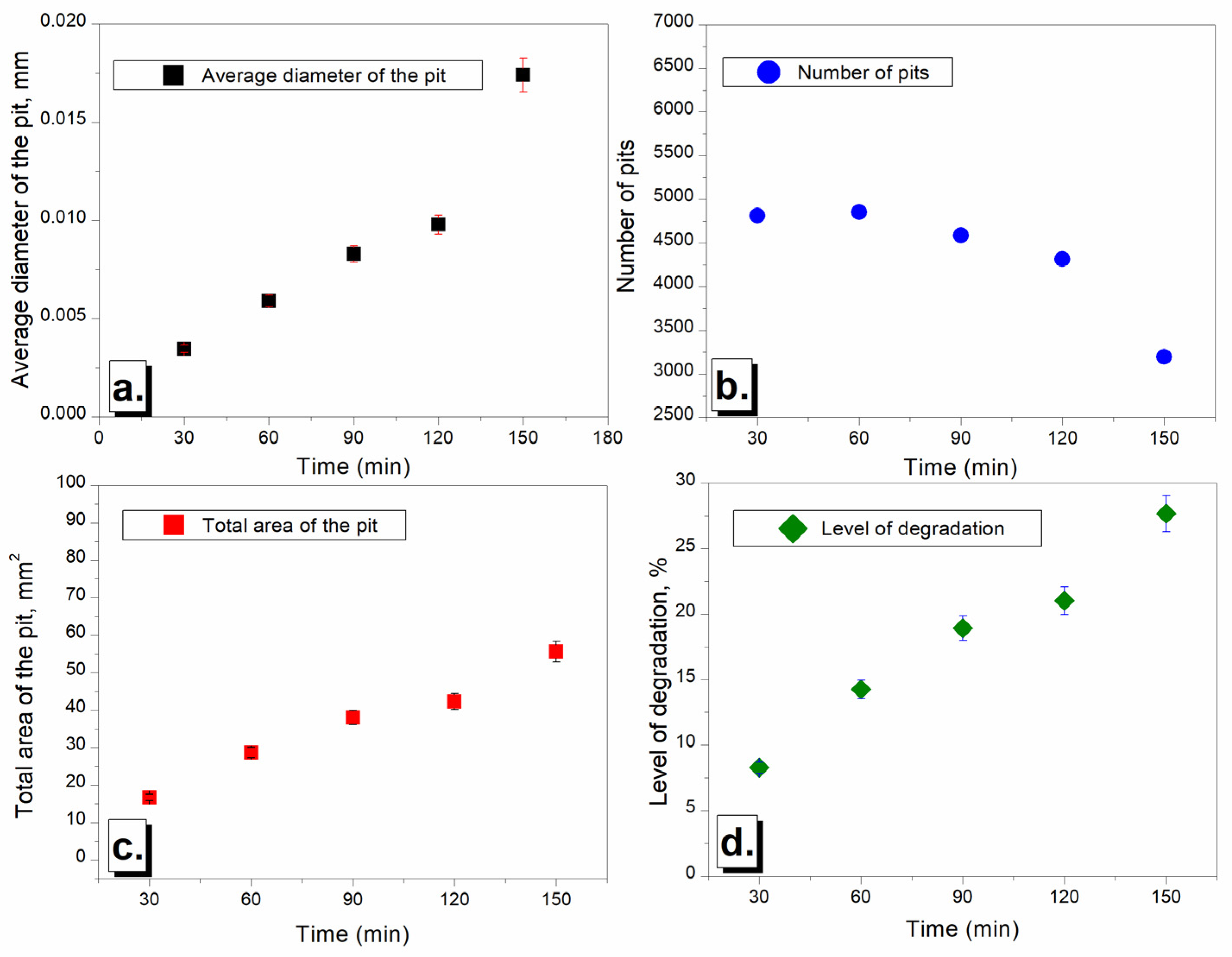
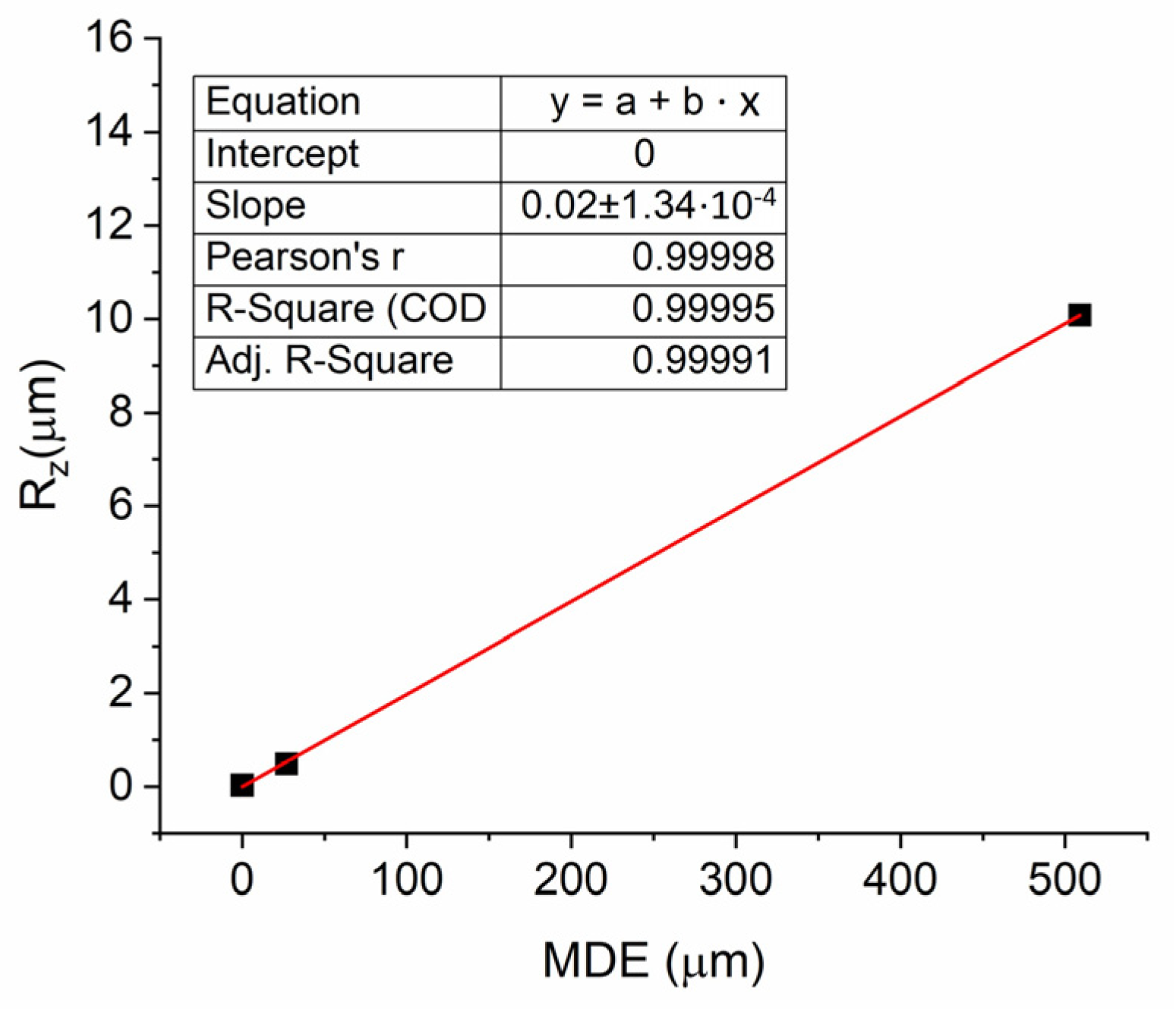
3.3.2. Mean Depth of Erosion and Surface Roughness
3.3.3. Morphological Characterization of the Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CE | Cavitation Erosion |
| ML | Mass Loss |
| MLR | Mass Loss Rate |
| MDE | Mean Depth of Erosion |
| LoD | Level of Degradation |
References
- Hammitt, F.G. Cavitation and Multiphase Flow Phenomena; McGraw-Hill Companies: New York, NY, USA, 1980. [Google Scholar]
- Brennen, C.E. Cavitation and Bubble Dynamics; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Volkov-Husović, T.; Ivanić, I.; Kožuh, S.; Stevanović, S.; Vlahović, M.; Martinović, S.; Stopic, S.; Gojić, M. Microstructural and Cavitation Erosion Behavior of the CuAlNi Shape Memory Alloy. Metals 2021, 11, 997. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, Z. Cavitation Erosion Mechanism of 2Cr13 Stainless Steel. Wear 2021, 488–489, 204137. [Google Scholar] [CrossRef]
- Zakrzewska, D.E.; Krella, A.K. Cavitation Erosion Resistance Influence of Material Properties. Adv. Mater. Sci. 2019, 19, 18–34. [Google Scholar] [CrossRef]
- Mohammadizadeh, S.; Dalfré Filho, J.G.; Sampaio Descovi, C.; Genovez, A.I.B.; Teixeira Buttignol, T.E. Experimental Analysis of Cavitation Erosion: Parameter Sensitivity and Testing Protocols. Coatings 2024, 14, 1288. [Google Scholar] [CrossRef]
- Basumatary, J.; Nie, M.; Wood, R.J.K. The Synergistic Effects of Cavitation Erosion–Corrosion in Ship Propeller Materials. J. Bio- Tribo-Corros. 2015, 1, 12. [Google Scholar] [CrossRef]
- Raami, L.; Varis, T.; Valtonen, K.; Wendler, M.; Volkova, O.; Peura, P. Enhancing the Cavitation Erosion Resistance of AISI 420-Type Stainless Steel with Quenching and Partitioning. Wear 2023, 526–527, 204897. [Google Scholar] [CrossRef]
- Chahine, G.L.; Franc, J.-P.; Karimi, A. Laboratory Testing Methods of Cavitation Erosion. In Advanced Experimental and Numerical Techniques for Cavitation Erosion Prediction; Springer: Cham, Switzerland, 2014; pp. 21–35. [Google Scholar] [CrossRef]
- Volkov-Husović, T.; Martinović, S.; Alil, A.; Vlahović, M.; Dimitrijević, B.; Ivanić, I.; Pavkov, V. Comparative investigation of ultrasonic cavitation erosion for two engineering materials. J. Min. Metall. Sect. B-Metall. 2024, 60, 295–304. [Google Scholar] [CrossRef]
- ASTM G32-16(2021)e1; Standard Test Method for Cavitation Erosion Using Vibratory Apparatus. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM G134-17; Standard Test Method for Erosion of Solid Materials by Cavitating Liquid Jet. ASTM International: West Conshohocken, PA, USA, 2017.
- Yang, R.; Chen, X.; Tian, Y.; Chen, H.; Boshkov, N.; Li, H. An Attempt to Improve Cavitation Erosion Resistance of UHMWPE Coatings through Enhancing Thermal Conductivity via the Incorporation of Copper Frames. Surf. Coat. Technol. 2021, 425, 127705. [Google Scholar] [CrossRef]
- Alil, A.; Martinović, S.; Živojinović, D.; Volkov Husović, T. Analysis of the Behavior of the Mullite Samples Surface Exposed to the Cavitation Using a Statistical Analysis Approach. Eng. Fail. Anal. 2025, 171, 109402. [Google Scholar] [CrossRef]
- Russ, J.C.; Neal, F.B. The Image Processing Handbook; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Vlahović, M.; Alil, A.; Devečerski, A.; Živojinović, D.; Volkov-Husović, T. Non-Destructive Examination for Cavitation Resistance of Talc-Based Refractories with Different Zeolite Types Intended for Protective Coatings. Materials 2023, 16, 5577. [Google Scholar] [CrossRef]
- Martinović, S.; Alil, A.; Milićević, S.; Živojinović, D.; Volkov Husović, T. Morphological Assessment of Cavitation Caused Damage of Cordierite and Zircon Based Materials Using Principal Component Analysis. Eng. Fail. Anal. 2023, 148, 107224. [Google Scholar] [CrossRef]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Xu, Q.; Yang, X.; Liu, J.; Jiang, D.; Qiu, Z. Improved Corrosion Resistance of 42CrMo4 Steel by Reconstructing Surface Integrity Using Ultrasonic Surface Rolling Process. Mater. Today Commun. 2023, 35, 105932. [Google Scholar] [CrossRef]
- Bartošák, M.; Horváth, J.; Španiel, M. Isothermal Low-Cycle Fatigue and Fatigue-Creep of a 42CrMo4 Steel. Int. J. Fatigue 2020, 135, 105538. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, Y.; Xie, Q.; Li, D.; Gao, N. Mechanical Properties and Dynamic Constitutive Model of 42CrMo Steel. Mater. Des. 2017, 119, 171–179. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Lu, H.; Liu, J.; Cai, G. Surface Integrity and Corrosion Resistance of 42CrMo4 High-Strength Steel Strengthened by Hard Turning. Materials 2021, 14, 6995. [Google Scholar] [CrossRef]
- Maskavizan, A.J.; Quintana, J.P.; Dalibón, E.L.; Márquez, A.B.; Brühl, S.P.; Farina, S.B. Evaluation of wear and corrosion resistance in acidic and chloride solutions of Cathodic Arc PVD chromium nitride coatings on untreated and plasma nitrided AISI 4140 steel. Surf. Coatings Technol. 2024, 494, 131476. [Google Scholar] [CrossRef]
- Roberge, P. Handbook of Corrosion Engineering; McGraw-Hill: Columbus, OH, USA, 1999. [Google Scholar]
- Li, N.; Chen, Y.-J.; Kong, D.-D. Wear Mechanism Analysis and Its Effects on the Cutting Performance of PCBN Inserts during Turning of Hardened 42CrMo. Int. J. Precis. Eng. Manuf. 2018, 19, 1355–1368. [Google Scholar] [CrossRef]
- Abbas, M.; Rizvi, S.H.M.; Sarfraz, S.; Raza, A.; Khan, A.; Loya, A.; Najib, A. Evaluation of the influence of dissolved nitrates on corrosion behaviour of ship structural steel exposed to seawater environment. Ocean Eng. 2024, 298, 117268. [Google Scholar] [CrossRef]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on Corrosion Inhibitors: A Short View. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Fernandes, J.S.; Montemor, F. Corrosion. In Materials for Construction and Civil Engineering: Science, Processing, and Design; Gonçalves, M.C., Margarido, F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 679–716. [Google Scholar] [CrossRef]
- Nodoushan, E.J.; Lee, Y.J.; Na, H.-J.; You, B.-H.; Lee, M.-Y.; Kim, N. Effects of NaCl and temperature on rheological characteristics and structures of CTAB/NaSal wormlike micellar solutions. J. Ind. Eng. Chem. 2021, 98, 458–464. [Google Scholar] [CrossRef]
- Que, Z.; Saario, T.; Toivonen, A.; Ehrnstén, U. Stress corrosion cracking initiation susceptibility of Alloy 182 with different surface treatments. Corros. Sci. 2022, 196, 110037. [Google Scholar] [CrossRef]
- Balangao, J.K. Corrosion of Metals: Factors, Types and Prevention Strategies. J. Chem. Health Risks 2024, 14, 79–87. [Google Scholar]
- Maurya, P.M. Effect of Corrosion on Metals and Its Prevention. Res. Anal. J. 2022, 5, 5–9. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J. Materials Corrosion and Protection; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018; pp. 5–7, 59, 78–81. [Google Scholar] [CrossRef]
- Causes of Corrosion. Available online: https://www.avdec.com/about/articles/causes_of_corrosion (accessed on 13 May 2025).
- Gudić, S.; Nagode, A.; Šimić, K.; Vrsalović, L.; Jozić, S. Corrosion Behavior of Different Types of Stainless Steel in PBS Solution. Sustainability 2022, 14, 8935. [Google Scholar] [CrossRef]
- Refait, P.; Chaves, I. Corrosion and Protection of Steels in Marine Environments: State-of-The-Art and Emerging Research Trends; MDPI: Basel, Switzerland, 2022. [Google Scholar]
- ASTM G1-03(2017)e1; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2003.
- ISO 11130 (E):2017; Corrosion of Metals and Alloys—Alternate Immersion Test in Salt Solution. The International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Seawater Composition. Available online: https://www.eps.mcgill.ca/~courses/c542/CHOC_Week%202a(Seawater&Salinity)_2020.pdf (accessed on 28 April 2025).
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The Composition of Standard Seawater and the Definition of the Reference-Composition Salinity Scale. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Physical, Chemical, and Biological Aspects of Water—Volume I; EOLSS Publications: Oxford, UK, 2010.
- Duxbury, A.C.; Mackenzie, F.T.; Byrne, R.H. Seawater|Composition, Salinity, Distribution, & Facts. In Encyclopedia Britannica; Encyclopedia Britannica, Inc.: Chicago, IL, USA, 2018. [Google Scholar]
- Earth Guide: Educational Resources in Earth, Marine, Environmental, and Planetary Sciences. Available online: https://earthguide.ucsd.edu/ (accessed on 26 April 2025).
- Gotbooks.miracosta.edu. Available online: https://gotbooks.miracosta.edu/oceans/chapter7.html (accessed on 27 April 2025).
- Available online: https://www.usna.edu/NAOE/_files/documents/Courses/EN380/Course_Notes/Marine%20Environment%20and%20Seawater%20Properties.pdf (accessed on 24 April 2025).
- Rajput, A.; Ramkumar, J.; Mondal, K. Cavitation Behavior of Various Microstructures Made from a C–Mn Eutectoid Steel. Wear 2021, 486–487, 204056. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Huang, C.; Guo, H.; Man, J.; Yu, P. Experimental Study of Surface Damage of Stainless Steel Subjected to Cavitation Collapse in Aqueous Environment. Coatings 2023, 13, 1356. [Google Scholar] [CrossRef]
- Ding, H.; Tang, Q.; Zhu, Y.; Zhang, C.; Yang, H. Cavitation Erosion Resistance of 316L Stainless Steel Fabricated Using Selective Laser Melting. Friction 2021, 9, 1580–1598. [Google Scholar] [CrossRef]

| Morphological Parameter | Definition | Image |
|---|---|---|
| Area | Area of object. Does not include the hole area |  |
| Diameter (mean) | Average length of diameters measured at 2-degree intervals and passing through the object’s centroid |  |
| Radius (max) | Maximal distance between the object’s centroid and outline |  |
| Radius (min) | Minimal distance between the object’s centroid and outline |  |
| Radius ratio | The ratio between the maximal and minimal radius |  |
| Roundness | The measure of how closely the shape of an object approaches that of a mathematically perfect circle |  |
| Number of pits/defects | Number of selected objects |  |
| 42CrMo4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element (wt.%) | C | Cr | Mo | Mn | Si | Ni | Cu | Al | S | P |
| 0.40 | 0.93 | 0.20 | 0.65 | 0.29 | 0.03 | 0.04 | 0.044 | 0.003 | 0.009 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedović, S.; Alil, A.; Martinović, S.; Dikić, S.; Volkov-Husović, T. Influence of Pre-Corrosion in NaCl Solution on Cavitation Resistance of Steel Samples (42CrMo4). Metals 2025, 15, 1041. https://doi.org/10.3390/met15091041
Nedović S, Alil A, Martinović S, Dikić S, Volkov-Husović T. Influence of Pre-Corrosion in NaCl Solution on Cavitation Resistance of Steel Samples (42CrMo4). Metals. 2025; 15(9):1041. https://doi.org/10.3390/met15091041
Chicago/Turabian StyleNedović, Stanica, Ana Alil, Sanja Martinović, Stefan Dikić, and Tatjana Volkov-Husović. 2025. "Influence of Pre-Corrosion in NaCl Solution on Cavitation Resistance of Steel Samples (42CrMo4)" Metals 15, no. 9: 1041. https://doi.org/10.3390/met15091041
APA StyleNedović, S., Alil, A., Martinović, S., Dikić, S., & Volkov-Husović, T. (2025). Influence of Pre-Corrosion in NaCl Solution on Cavitation Resistance of Steel Samples (42CrMo4). Metals, 15(9), 1041. https://doi.org/10.3390/met15091041