Ultrasonic Enhancement of Tin Dissolution in NaOH/H2O2 System: Electrochemical and Passivation Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Characterization Equipment
3. Results and Discussion
3.1. Dissolution Behavior of Tin in NaOH/H2O2 Solutions Under Ultrasonic Treatment
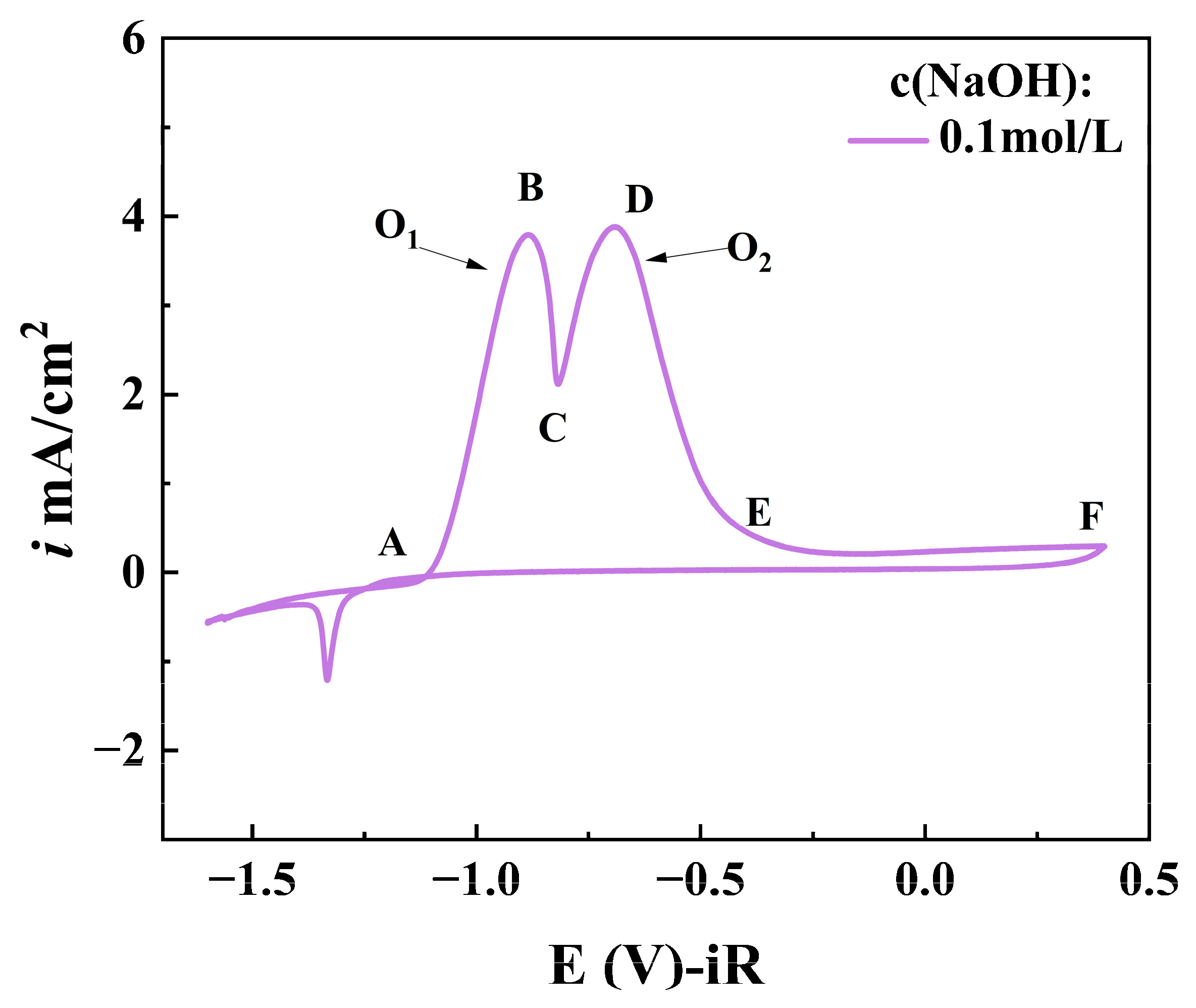
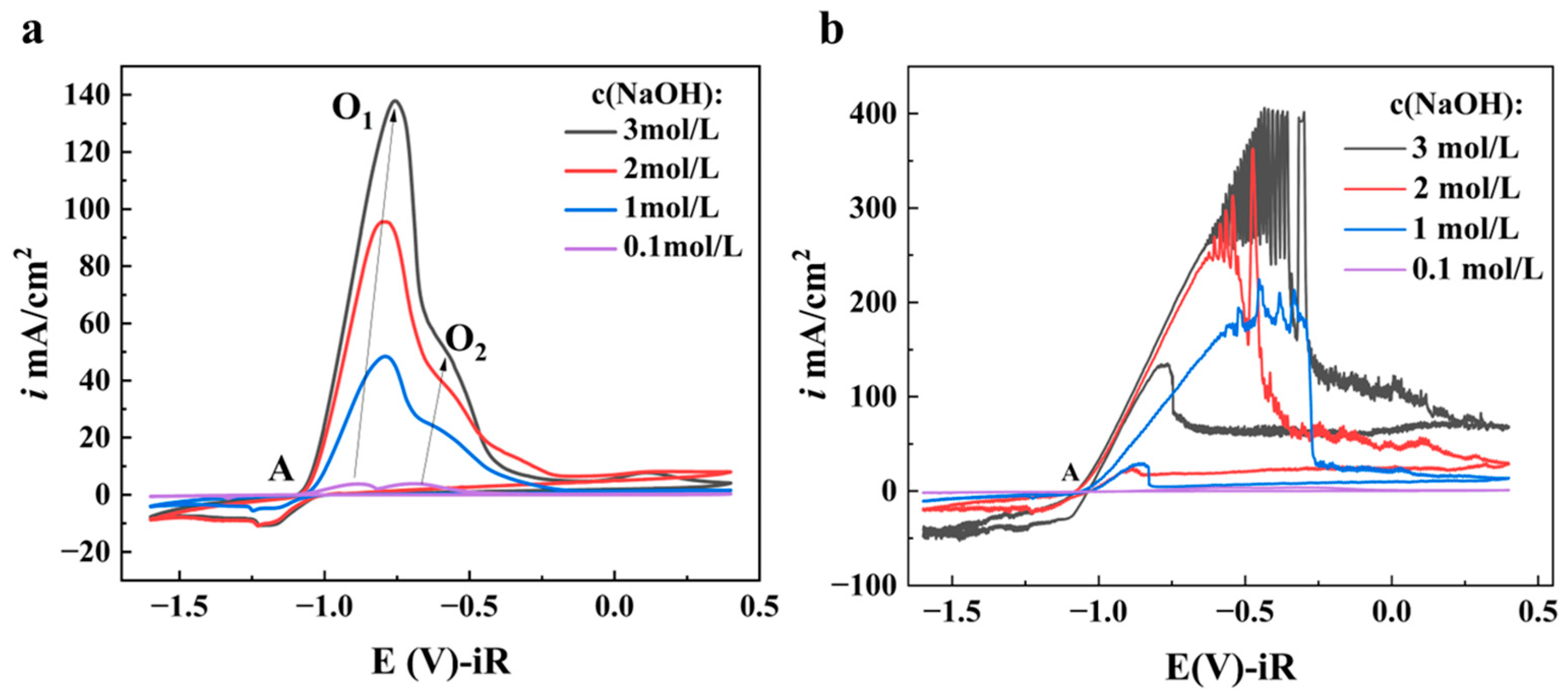
3.1.1. Effect of NaOH Concentration
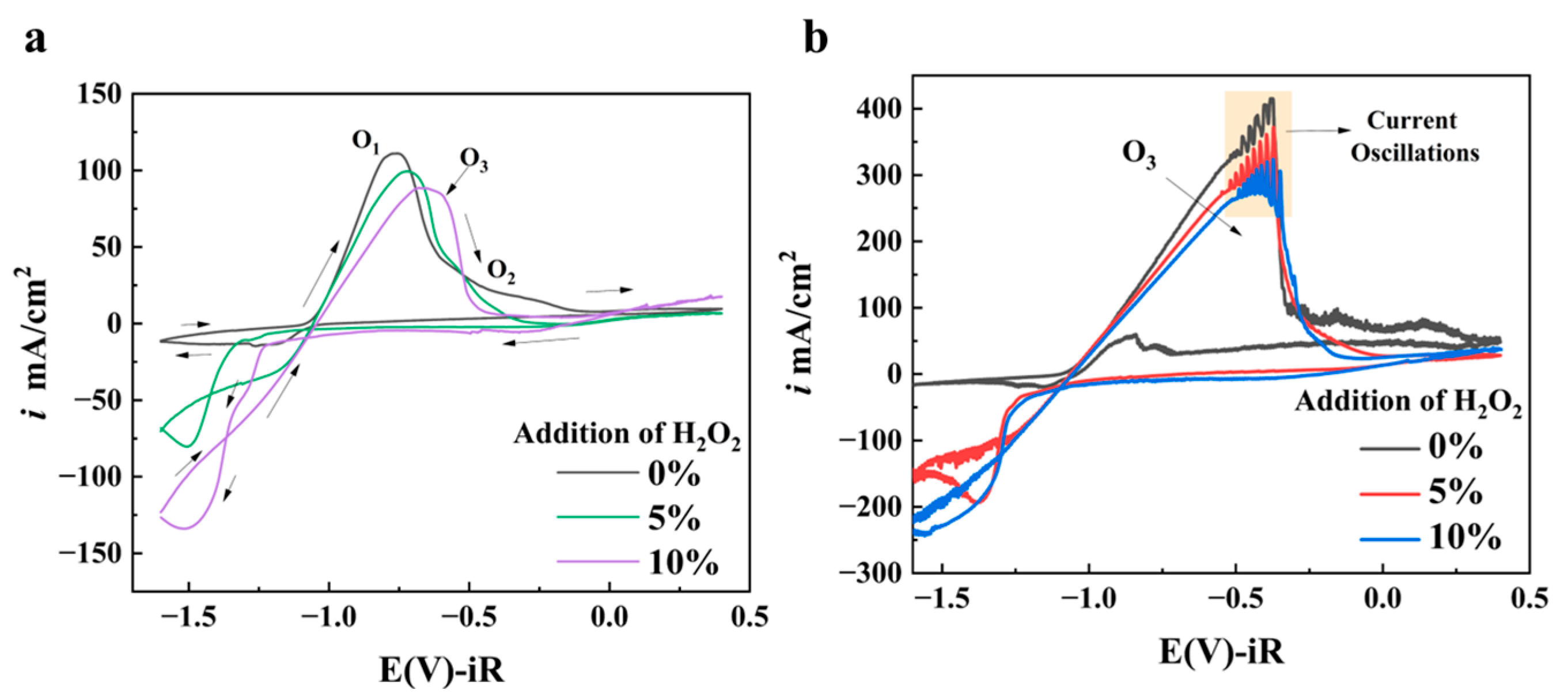
3.1.2. Effect of H2O2 Additive Amount
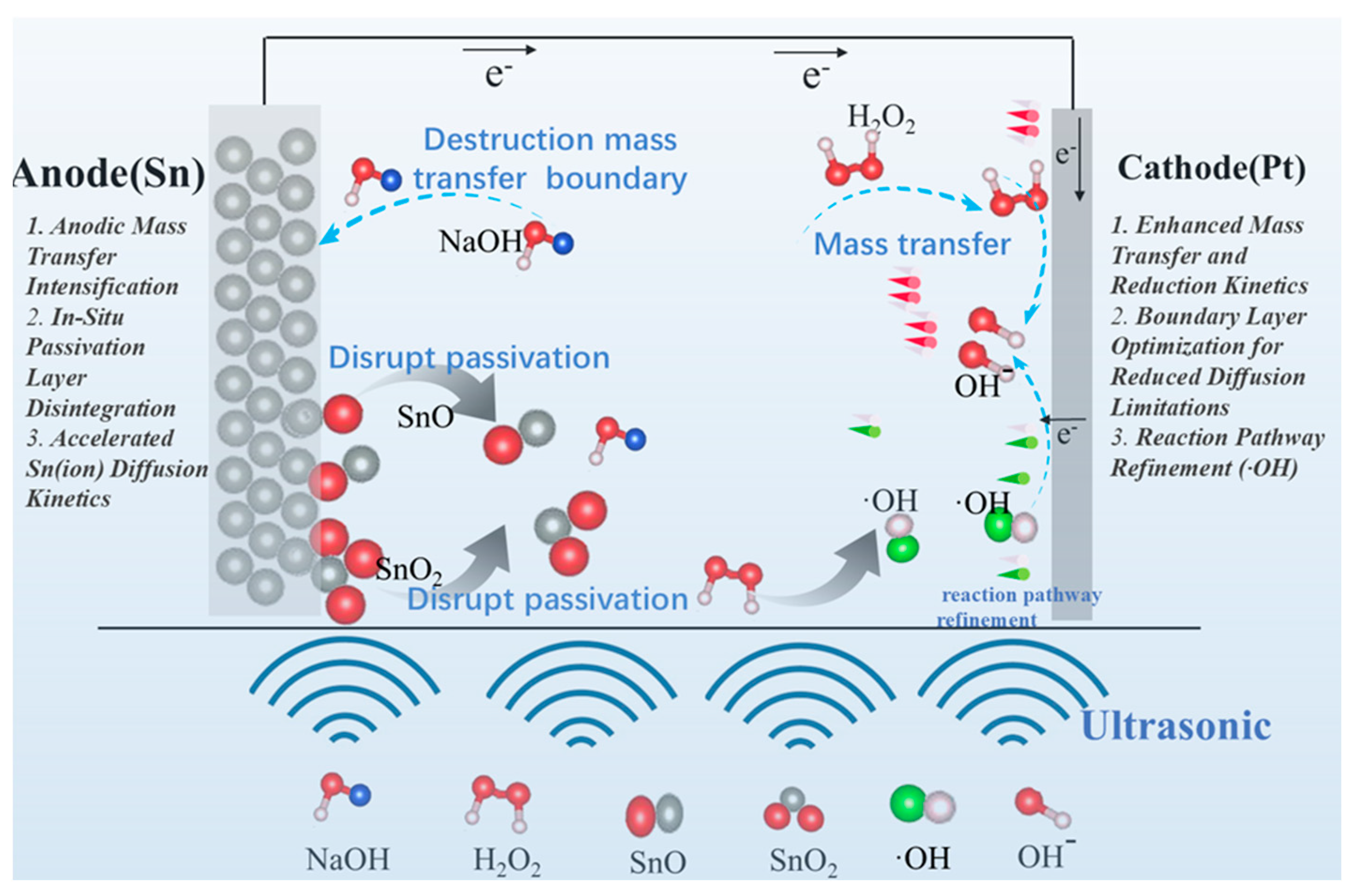
3.2. Mechanism of Ultrasonic Enhancement on Tin Dissolution in NaOH/H2O2
3.2.1. Mass Transfer Intensification
3.2.2. Reaction Path Optimization and Kinetic Enhancement
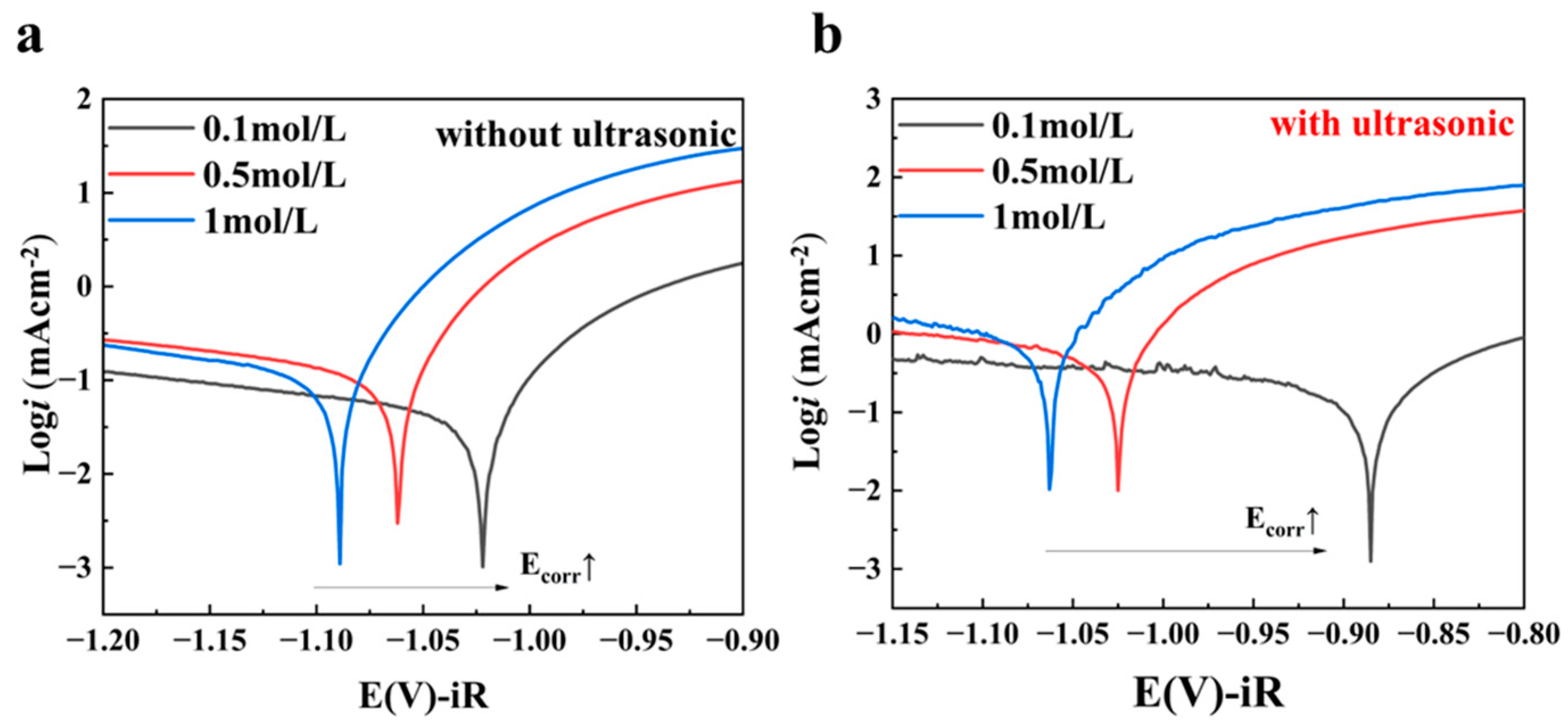
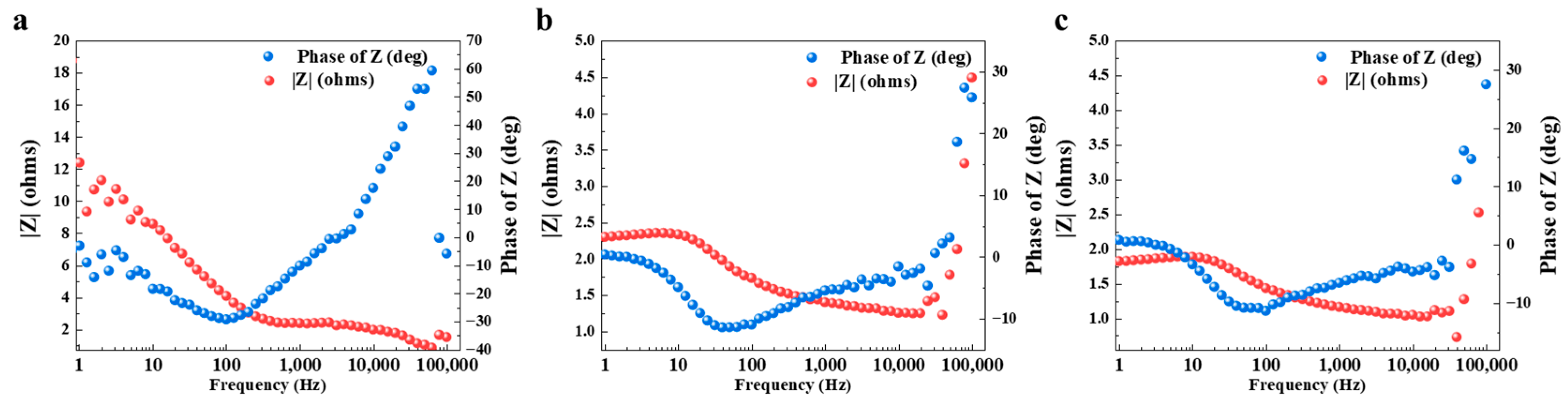
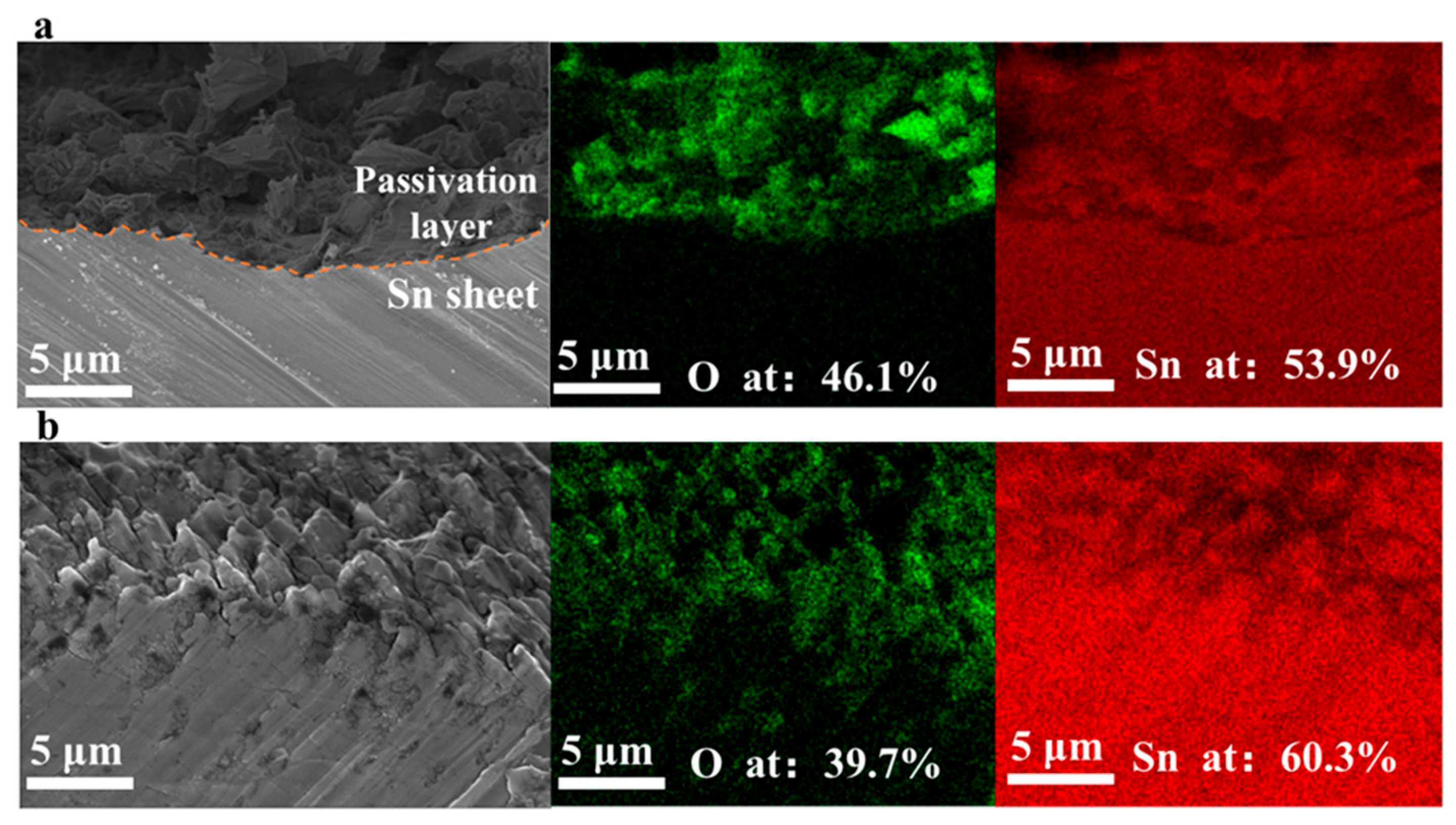
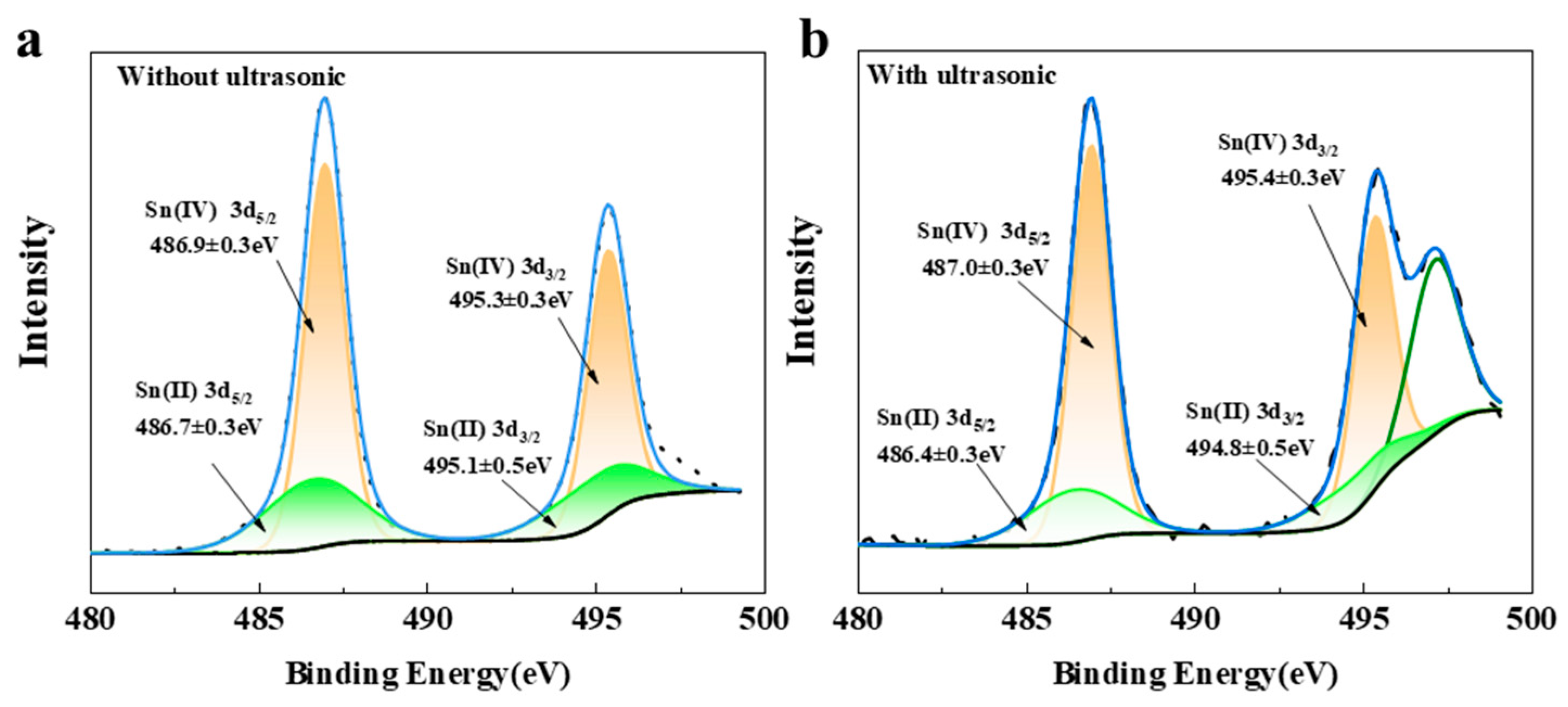
3.2.3. Passivation Layer
3.2.4. The Impact of Tin Dissolution Rate
4. Conclusions
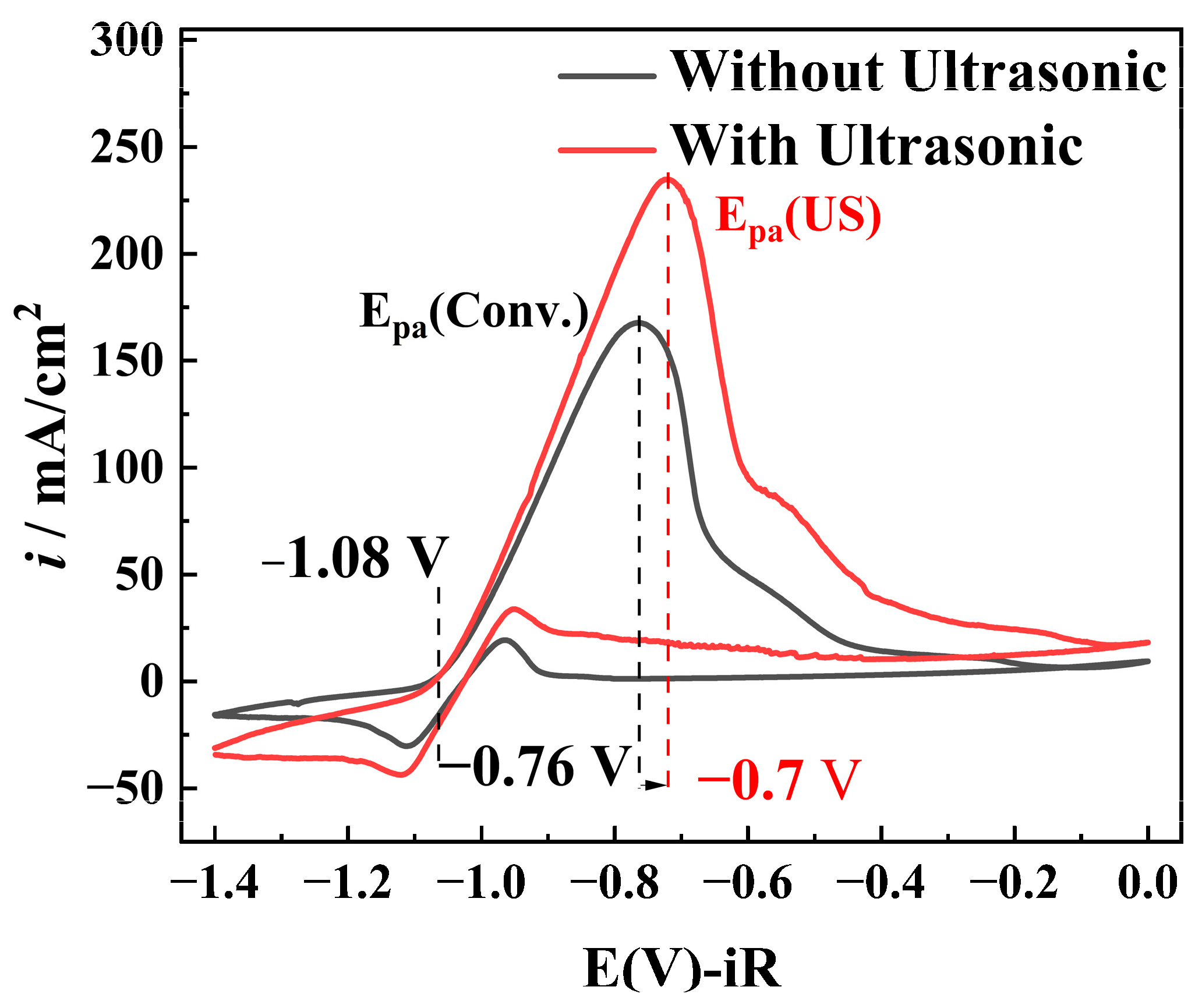
- Mass transfer intensification: The cavitation microjet generated by ultrasound has been shown to enhance the diffusion process. This acceleration of the electron transfer has been demonstrated to increase the peak current from 165.9 mA/cm2, to 234 mA/cm2. Furthermore, the microjet has been observed to destroy the mass-transfer boundary layer and to promote the dissolution of passivation products, with the oxidation peak potential increasing from −0.76 V to −0.70 V, which resulted in a weakening of the passivation.
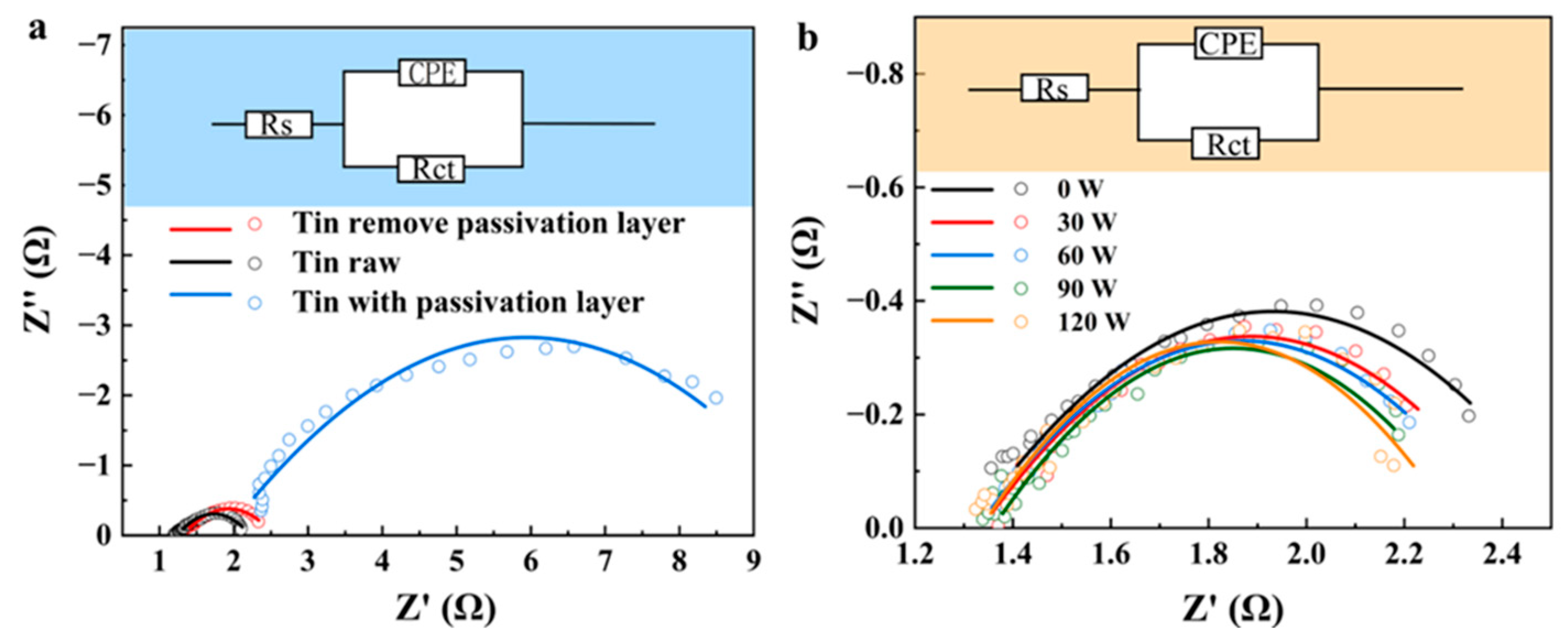
- Passivation suppression: Ultrasound effectively removes the passivation layer on the surface of the tin sheet through the action of micro-jets generated by cavitation. Consequently, the charge transfer resistance (Rct) decreases from 8.09 ± 0.01 Ω to 1.15 ± 0.01 Ω. The removal of the passivation layer by ultrasound reduces the activation barrier.
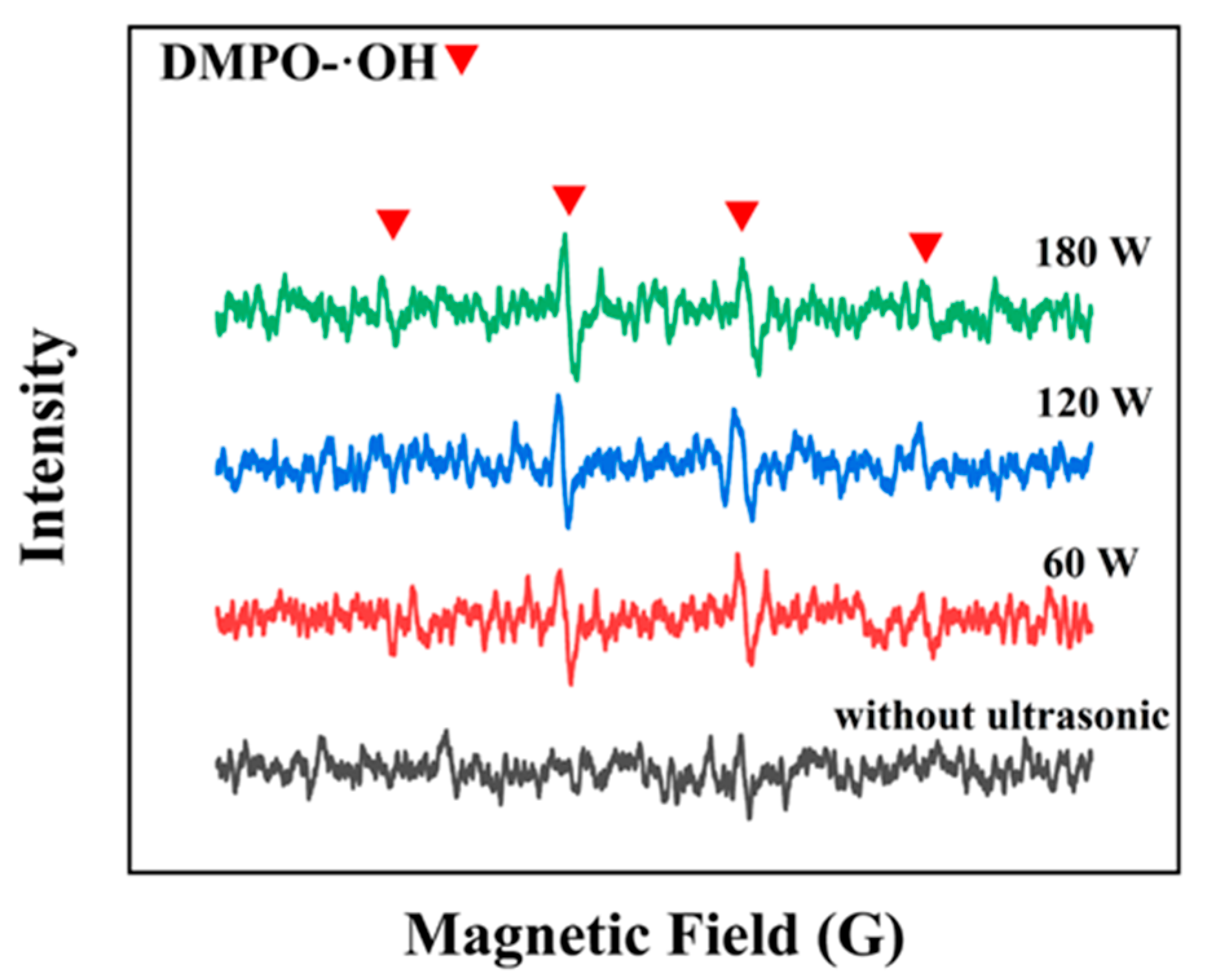
- Reaction path optimization and kinetic enhancement: The generation of hydroxyl radicals under ultrasound optimizes the reaction path, accelerates the diffusion of oxidant to the cathode surface, increases the self-corrosion current from 0.286 mA/cm2 to 15.8 mA/cm2, and improves the cathodic reaction kinetics.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pal, A.; Samanta, A.K.; Kar, T.R. Eco-friendly fire-retardant finishing of cotton fabric with mixture of ammonium sulfamate and sodium Stannate with and without zinc acetate as external reagent. Cellulose 2023, 30, 11813–11828. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.; Ma, Z.; Lu, D.; Hou, B. Na2SnO3 functions as outstanding magnesium alloy passivator by synergistic effect with trace carboxymethyl chitosan for Mg–air batteries for standby protection. New J. Chem. 2021, 46, 2105–2127. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, Y.; Yin, S.; Au, C.-T. Solid sodium stannate as a high-efficiency superbase catalyst for anti-Markovnikov hydroamination and hydroalkoxylation of electron-deficient olefins under mild conditions. Catal. Commun. 2011, 12, 712–716. [Google Scholar] [CrossRef]
- Taninouchi, Y.-K.; Uda, T. Rapid Oxidative Dissolution of Metallic Tin in Alkaline Solution Containing Iodate Ions. J. Sustain. Met. 2021, 7, 1762–1771. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Wang, S.; Xia, H.; Miao, W.; Le, T.; Zhang, L. Ultrasonic-Catalyzed Oxidation and Dissolution of Tin Using Hydrogen Peroxide. Molecules 2025, 30, 1591. [Google Scholar] [CrossRef]
- Kékesi, T.; Török, T.I.; Kabelik, G. Extraction of tin from scrap by chemical and electrochemical methods in alkaline media. Hydrometallurgy 2000, 55, 213–222. [Google Scholar] [CrossRef]
- Liu, B.; Chu, Q.; Huang, Y.; Han, G.; Sun, H.; Zhang, L. Ultrasound-assisted extraction of Sn from tinplate scraps by alkaline leaching: Novel acoustoelectric synergy effect underlying intensifying mechanism. Ultrason. Sonochem. 2023, 100, 106631. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, D.; Buonincontri, M.; Mapelli, C.; Gruttadauria, A.; Barella, S.; Fusari, F.; Rinaldini, D. Effect of tinplated scraps surface-to-volume ratio on the efficiency of the electrolytic detinning process. J. Mater. Res. Technol. 2022, 19, 1217–1230. [Google Scholar] [CrossRef]
- Palacios-Padrós, A.; Caballero-Briones, F.; Díez-Pérez, I.; Sanz, F. Tin passivation in alkaline media: Formation of SnO microcrystals as hydroxyl etching product. Electrochim. Acta 2013, 111, 837–845. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Li, C.; Fu, L.; Huang, W.; Li, Z. Fabrication of pompon-like and flower-like SnO microspheres comprised of layered nanoflakes by anodic electrocrystallization. Electrochim. Acta 2012, 72, 94–100. [Google Scholar] [CrossRef]
- El Din, A.; El Wahab, F. On the anodic passivity of tin in alkaline solutions. Electrochim. Acta 1964, 9, 883–896. [Google Scholar] [CrossRef]
- Jiang, L.; Volovitch, P.; Sundermeier, U.; Wolpers, M.; Ogle, K. Dissolution and passive film formation of Sn and Sn coated steel using atomic emission spectroelectrochemistry. Electrochim. Acta 2011, 58, 322–329. [Google Scholar] [CrossRef]
- Begum, S.N.; Basha, A.; Muralidharan, V.; Lee, C.W. Electrochemical behaviour of tin in alkali solutions containing halides. Mater. Chem. Phys. 2012, 132, 1048–1052. [Google Scholar] [CrossRef]
- Cao, J.; Gao, Z.; Wang, C.; Muzammal, H.M.; Wang, W.; Gu, Q.; Dong, C.; Ma, H.; Wang, Y. Morphology evolution of the anodized tin oxide film during early formation stages at relatively high constant potential. Surf. Coat. Technol. 2020, 388, 125592. [Google Scholar] [CrossRef]
- Devos, C.; Bampouli, A.; Brozzi, E.; Stefanidis, G.D.; Dusselier, M.; Van Gerven, T.; Kuhn, S. Ultrasound mechanisms and their effect on solid synthesis and processing: A review. Chem. Soc. Rev. 2025, 54, 85–115. [Google Scholar] [CrossRef]
- Chen, J.; Geng, J.; Li, Y.; Xia, P.; Li, X.; Wang, F.; Chen, D.; Wang, M.; Wang, H. Investigation of incipient cavitation in various liquids based on PIV quantification and numerical simulations. Sci. Rep. 2025, 15, 9390. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Xia, H.; Zhang, Q.; Zhang, L.; Zhang, W. Recovery of Zn and Ge from zinc oxide dust by ultrasonic-H2O2 enhanced oxidation leaching. RSC Adv. 2021, 11, 33788–33797. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Liu, C.; Zhang, L.; Kong, D. Mechanism and kinetics of synergistic decopperization from copper anode slime by ultrasound and ozone. J. Clean. Prod. 2021, 322, 129058. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Zhu, R.; Fu, L.; Liu, J.; Zhang, G.; Lin, G. Electrochemical behavior and the mechanism of nickel ion removal during zinc sulfate electrolysis via ultrasonic purification. J. Water Process. Eng. 2025, 71, 107214. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, R.; Xiang, D.; Zhu, M.; Wang, S.; Fu, L.; Zhang, G. Ultrasonically enhanced zinc powder replacement method for cobalt removal: Electrochemical behavior, numerical simulation. J. Clean. Prod. 2024, 467, 142847. [Google Scholar] [CrossRef]
- Liu, B.; Shi, C.; Huang, Y.; Han, G.; Sun, H.; Zhang, L. Intensifying separation of Pb and Sn from waste Pb-Sn alloy by ultrasound-assisted acid leaching: Selective dissolution and sonochemistry mechanism. Ultrason. Sonochem. 2024, 102, 106758. [Google Scholar] [CrossRef] [PubMed]
- GB/T 3260.11-2023; Methods for Chemical Analysis of tin-Part 11: Determination of Copper, Iron, Bismuth, Lead, Antimony, Arsenic, Aluminum, Zinc, Cadmium, Silver, Nickel and Cobalt Contents. Inductively Coupled Plasma Atomic Emission Spectrometry. Standardization Administration of China: Beijing, China, 2023.
- GB/T 3260.9-2023; Methods for Chemical analysis of tin-Part 9. Determination of Sulphur Content-Infra-Red Absorption Method after High Frequency Induction Furnace Combustion. Standardization Administration of China: Beijing, China, 2023.
- Du, Y.; Kou, M.; Tu, J.; Wang, M.; Jiao, S. An investigation into the anodic behavior of TiB2 in a CaCl2-based molten salt. Corros. Sci. 2021, 178, 109089. [Google Scholar] [CrossRef]
- Li, L.; King, A.; Davis, K.; Yu, B. Electrochemical Kinetics Study of Ultrasound-Assisted Chalcopyrite Oxidation. J. Sustain. Met. 2023, 9, 678–687. [Google Scholar] [CrossRef]
- Berezhnaya, A.G.; Kaz’mina, M.A.; Volochaev, V.A. Evaluation of the Effect of the Concentration of Sodium Hydroxide with and without Potassium Oleate on the Behavior of Lead by Cyclic Voltammetry. Prot. Met. Phys. Chem. Surfaces 2017, 53, 1193–1198. [Google Scholar] [CrossRef]
- Fuentes-García, J.; Santoyo-Salzar, J.; Rangel-Cortes, E.; Goya, G.; Cardozo-Mata, V.; Pescador-Rojas, J. Effect of ultrasonic irradiation power on sonochemical synthesis of gold nanoparticles. Ultrason. Sonochem. 2021, 70, 105274. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, P.; Dai, Y.; Luo, X.; Manickam, S.; Li, D.; Han, Y.; Show, P.L. Bridge between mass transfer behavior and properties of bubbles under two-stage ultrasound-assisted physisorption of polyphenols using macroporous resin. Chem. Eng. J. 2022, 436, 135158. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Fan, J.; Gao, C.; Yuan, X.; Wang, G.; Zhang, Q. Dual effects of ultrasound on fabrication of anodic aluminum oxide. Ultrason. Sonochem. 2023, 96, 106431. [Google Scholar] [CrossRef]
- Wen, J.-Y.; Huang, H.-H. Synergetic effects of H2O2 concentration and pH value on corrosion behavior of biomolecular coatings on 3D-printed porous low elastic modulus titanium alloy scaffolds under equilibrium conditions. J. Mater. Res. Technol. 2025, 34, 2000–2014. [Google Scholar] [CrossRef]
- Zhu, H.; Ke, B.; Lei, L.; Wan, J.; Feng, H.; Yao, L. Insight into the effect of ultrasonic treatment on surface properties and flotation behavior of chalcopyrite after galvanic corrosion of grinding media and chalcopyrite. Mater. Today Commun. 2024, 41, 110745. [Google Scholar] [CrossRef]
- Starchuk, Y.; Budzulyak, I.; Popovych, O.; Rachiy, B.; Yablon, L. Electrochemical behavior of NiWO4 modified by ultrasonic and laser irradiation. Full-Nanotub. Carbon Nanostruct. 2023, 31, 459–463. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, T.; Gong, X.; Wang, Z.; Wang, M.; Zhang, S. Roles of ultrasound on hydroxyl radical generation and bauxite desulfurization from water electrolysis. J. Electrochem. Soc. 2018, 165, E177–E183. [Google Scholar] [CrossRef]
- Zhang, H.; Han, Z.; Fang, X. Enhancement of wire electrochemical micromachining of 40CrNiMoA with electrolyte ultrasonic assistance. Int. J. Adv. Manuf. Technol. 2024, 133, 3715–3725. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Yang, C. Measurement of hydroxyl radical production in ultrasonic aqueous solutions by a novel chemiluminescence method. Ultrason. Sonochem. 2008, 15, 665–672. [Google Scholar] [CrossRef]
- Ma, F.; Tang, X.; Yang, D.; Xu, Z.; Cheng, W.; Liu, X.; Wang, G. Utilization of ultrasonic electrodeposition for fabricating isotopic nickel targets. J. Radioanal. Nucl. Chem. 2025, 334, 3071–3082. [Google Scholar] [CrossRef]
- Kulakarni, Y.A.; Jagadeesh, M.R.; Almalki, A.S.A.; Prasanna, B.M.; Kumar, D.G.P.; Alhadhrami, A.; Alsubaie, A.; Vasanthkumar, M.S.; Shivakumara, S. AC conductivity and dielectric investigations of amorphous manganese oxide and amorphous manganese oxide/conducting polymer nanocomposites. J. Mater. Sci. Mater. Electron. 2023, 34, 176. [Google Scholar] [CrossRef]
- Tageldeen, M.; Pagkalos, I.; Ghoreishizadeh, S.; Drakakis, E. Analogue circuit realisation of surface-confined redox reaction kinetics. Biosens. Bioelectron. 2023, 228, 115190. [Google Scholar] [CrossRef]
- Elgar, C.E.; Ravenhill, S.; Hunt, P.; Jacobson, B.; Feeney, A.; Prentice, P.; Ryder, K.S.; Abbott, A.P. Using ultrasound to increase copper and nickel dissolution and prevent passivation using concentrated ionic fluid. Electrochim. Acta 2024, 476, 143707. [Google Scholar] [CrossRef]
- Che, J.; Shi, G.; Zhou, T. Dynamical model and numerical study of cavitation bubble in ultrasonic assisted electrochemical polishing solution of selective laser melting NiTi alloy. Phys. Scr. 2023, 98, 115980. [Google Scholar] [CrossRef]
- Attias, R.; Dlugatch, B.; Chae, M.S.; Goffer, Y.; Aurbach, D. Changes in the interfacial charge-transfer resistance of Mg metal electrodes, measured by dynamic electrochemical impedance spectroscopy. Electrochem. Commun. 2021, 124, 106952. [Google Scholar] [CrossRef]


| Without Ultrasound | With Ultrasound | |||||
|---|---|---|---|---|---|---|
| CNaOH (mol/L) | 0.1 mol/L | 0.5 mol/L | 1 mol/L | 0.1 mol/L | 0.5 mol/L | 1 mol/L |
| Ecorr (V) | −1.022 ± 0.001 | −1.062 ± 0.001 | −1.089 ± 0.001 | −0.088 ± 0.001 | −1.025 ± 0.001 | −1.063 ± 0.001 |
| Log icorr (mA/cm2) | 0.06 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.06 ± 0.01 | 0.76 ± 0.01 | 1.11 ± 0.01 |
| Without Ultrasound | Ultrasound-Assisted | |||||
|---|---|---|---|---|---|---|
| H2O2 addition | 0% | 5% | 10% | 0% | 5% | 10% |
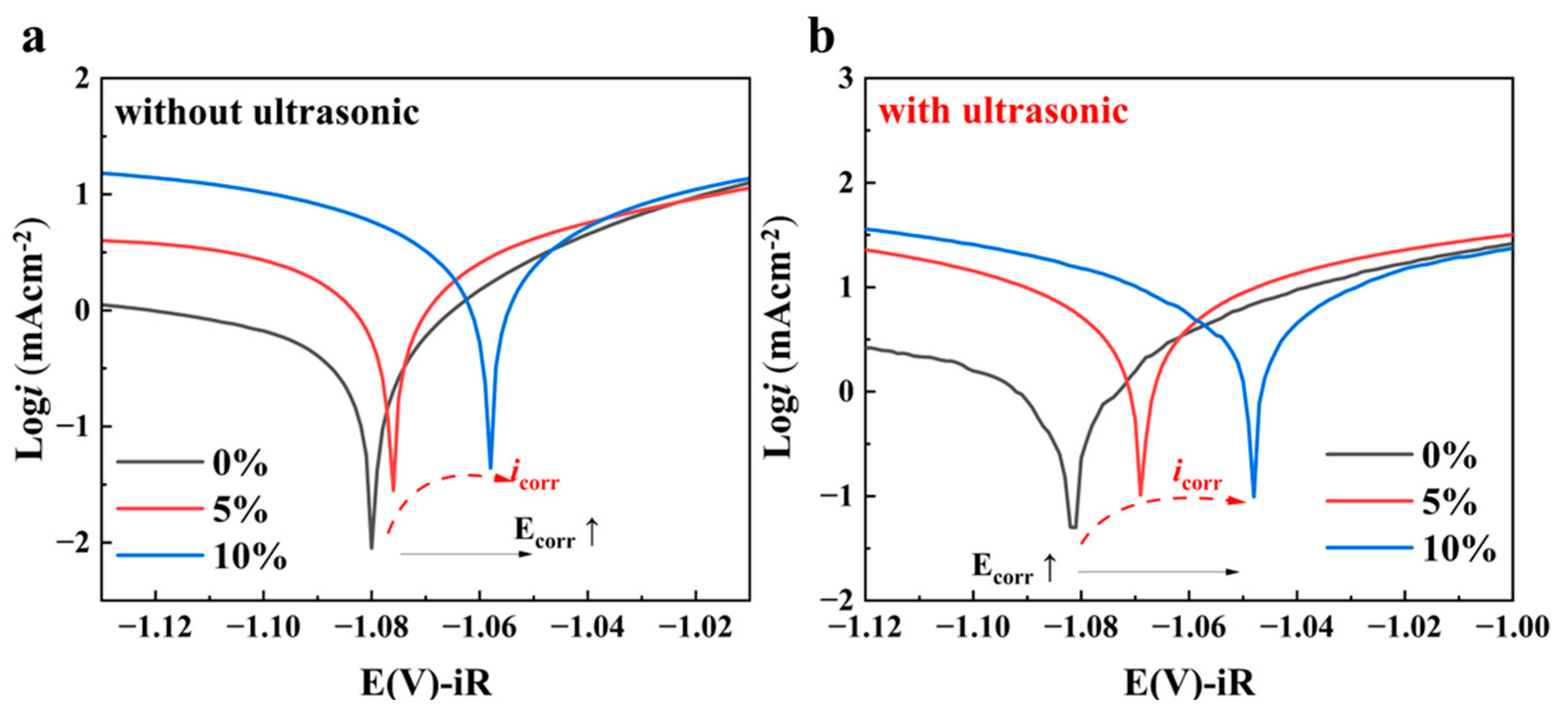
| Ecorr (V) | −1.081 ± 0.001 | −1.076 ± 0.001 | −1.067 ± 0.001 | −1.081 ± 0.001 | −1.036 ± 0.001 | −1.048 ± 0.001 |
| Log icorr (mA/cm2) | 1.02 ± 0.01 | 8.08 ± 0.01 | 6.20 ± 0.01 | 2.93 ± 0.01 | 32.84 ± 0.01 | 24.32 ± 0.01 |
| 0 W | 30 W | 60 W | 90 W | 120 W | 150 W | |
|---|---|---|---|---|---|---|
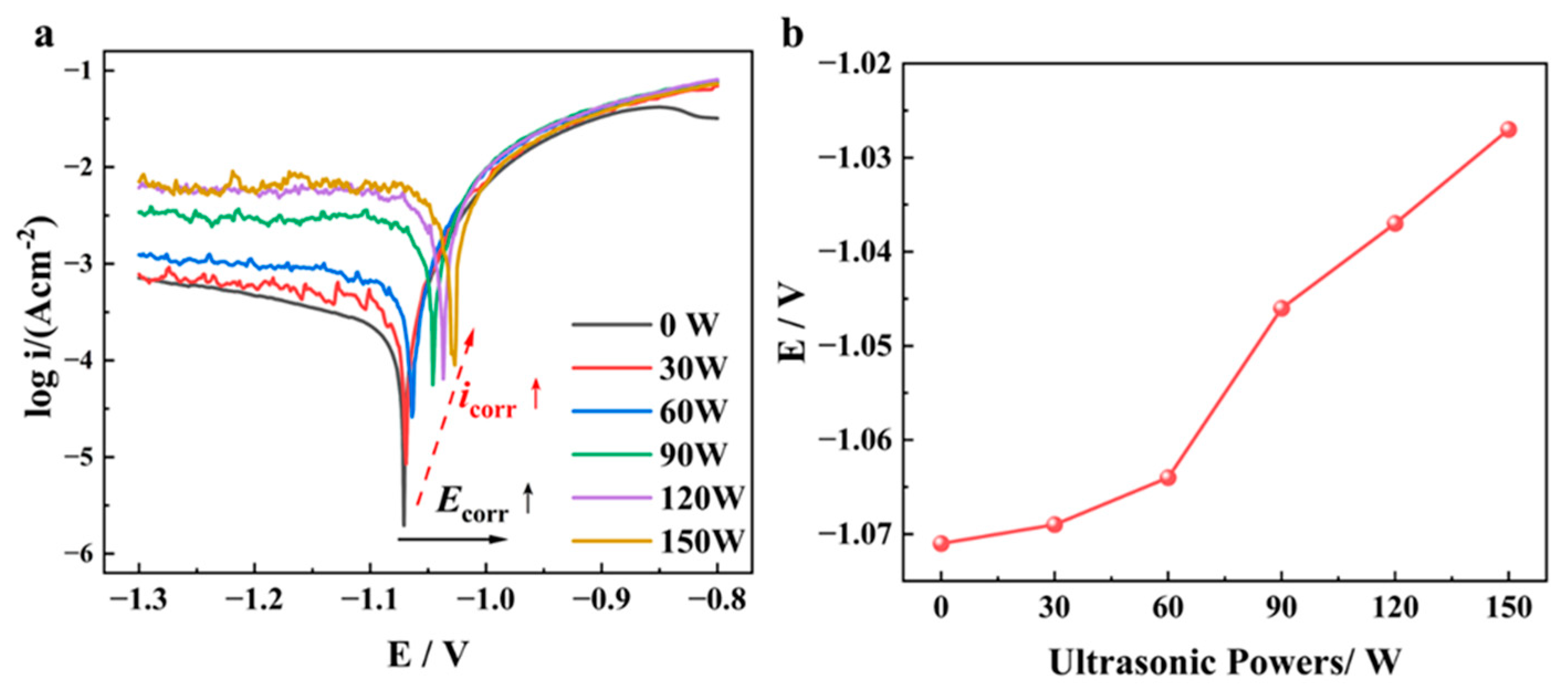
| Ecorr (V) | −1.071 ± 0.001 | −1.069 ± 0.001 | −1.063 ± 0.001 | −1.045 ± 0.001 | −1.030 ± 0.001 | −1.027 ± 0.001 |
| icorr (mA/cm2) | 0.29 ± 0.01 | 0.48 ± 0.01 | 0.90 ± 0.01 | 5.79 ± 0.01 | 13.67 ± 0.01 | 15.82 ± 0.01 |
| Tin Remove Passivation Layer | Tin Raw | Tin with Passivation Layer | |
|---|---|---|---|
| Rs (Ω) | 1.355 ± 0.02 | 1.34 ± 0.01 | 2.10 ± 0.01 |
| Rct (Ω) | 1.145 ± 0.01 | 1.07 ± 0.01 | 8.09 ± 0.02 |
| CPE1-T | 0.014 ± 0.01 | 0.018 ± 0.01 | 0.015 ± 0.01 |
| CPE1-P | 0.737 ± 0.01 | 0.7115 ± 0.01 | 0.706 ± 0.01 |
| χ2 | 0.00148 | 0.0015 | 0.0015 |
| 0 W | 30 W | 60 W | 90 W | 120 W | |
|---|---|---|---|---|---|
| Rs (Ω) | 1.36 ± 0.02 | 1.35 ± 0.01 | 1.35 ± 0.02 | 1.37 ± 0.01 | 1.35 ± 0.01 |
| Rct (Ω) | 1.15 ± 0.01 | 1.07 ± 0.01 | 1.04 ± 0.02 | 0.94 ± 0.01 | 0.93 ± 0.02 |
| CPE1-T | 0.014 ± 0.01 | 0.020 ± 0.01 | 0.019 ± 0.01 | 0.017 ± 0.01 | 0.014 ± 0.01 |
| CPE1-P | 0.737 ± 0.01 | 0.700 ± 0.01 | 0.702 ± 0.01 | 0.728 ± 0.01 | 0.756 ± 0.01 |
| χ2 | 0.00148 | 0.00120 | 0.00162 | 0.001836 | 0.00159 |
| Time | Tin Dissolution Rate (Ultrasonic) | Tin Dissolution Rate (Without Ultrasound) |
|---|---|---|
| 10 min | 27.15% | 13.49% |
| 20 min | 56.74% | 30.95% |
| 30 min | 78.61% | 45.66% |
| 40 min | 91.38% | 54.43% |
| 50 min | 95.22% | 61.48% |
| 60 min | 98.16% | 68.27% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Fu, M.; Wang, T.; Miao, W.; Xiang, L.; Le, T.; Zhang, L. Ultrasonic Enhancement of Tin Dissolution in NaOH/H2O2 System: Electrochemical and Passivation Modulation. Metals 2025, 15, 1016. https://doi.org/10.3390/met15091016
Wang D, Fu M, Wang T, Miao W, Xiang L, Le T, Zhang L. Ultrasonic Enhancement of Tin Dissolution in NaOH/H2O2 System: Electrochemical and Passivation Modulation. Metals. 2025; 15(9):1016. https://doi.org/10.3390/met15091016
Chicago/Turabian StyleWang, Dongbin, Mingge Fu, Tian Wang, Wenlong Miao, Liuxin Xiang, Thiquynhxuan Le, and Libo Zhang. 2025. "Ultrasonic Enhancement of Tin Dissolution in NaOH/H2O2 System: Electrochemical and Passivation Modulation" Metals 15, no. 9: 1016. https://doi.org/10.3390/met15091016
APA StyleWang, D., Fu, M., Wang, T., Miao, W., Xiang, L., Le, T., & Zhang, L. (2025). Ultrasonic Enhancement of Tin Dissolution in NaOH/H2O2 System: Electrochemical and Passivation Modulation. Metals, 15(9), 1016. https://doi.org/10.3390/met15091016








