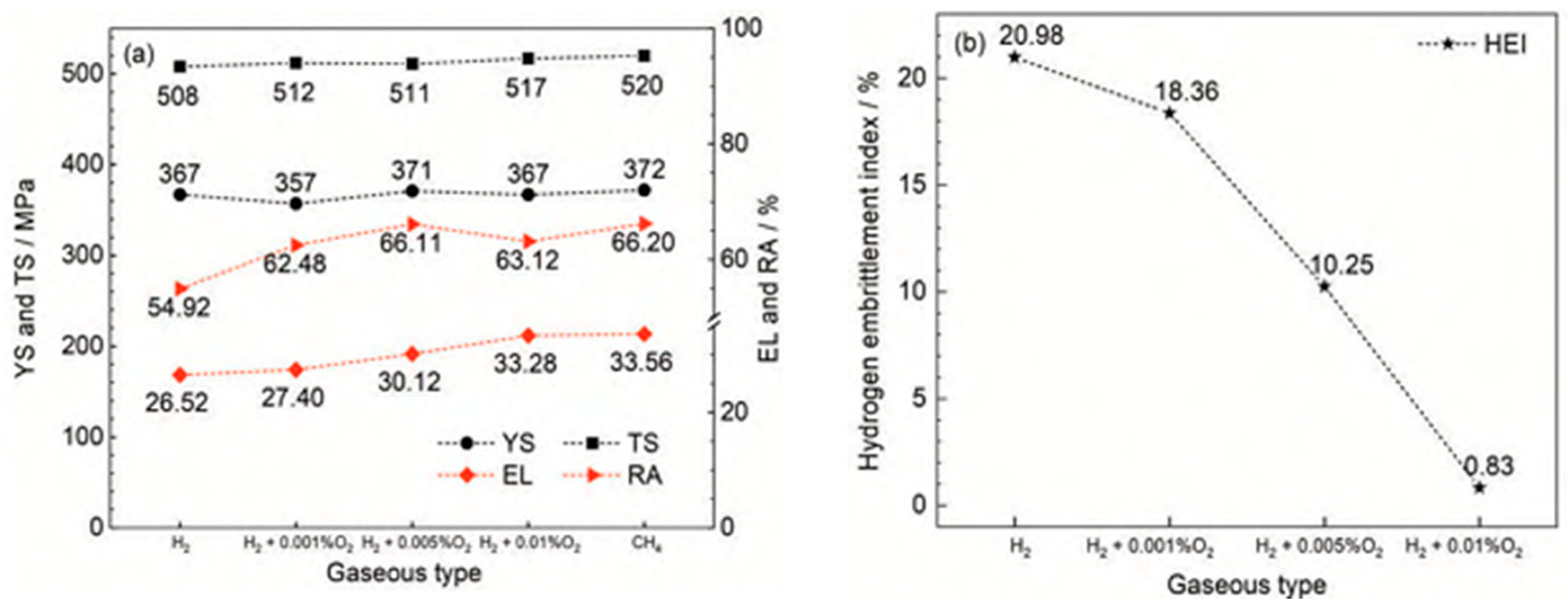
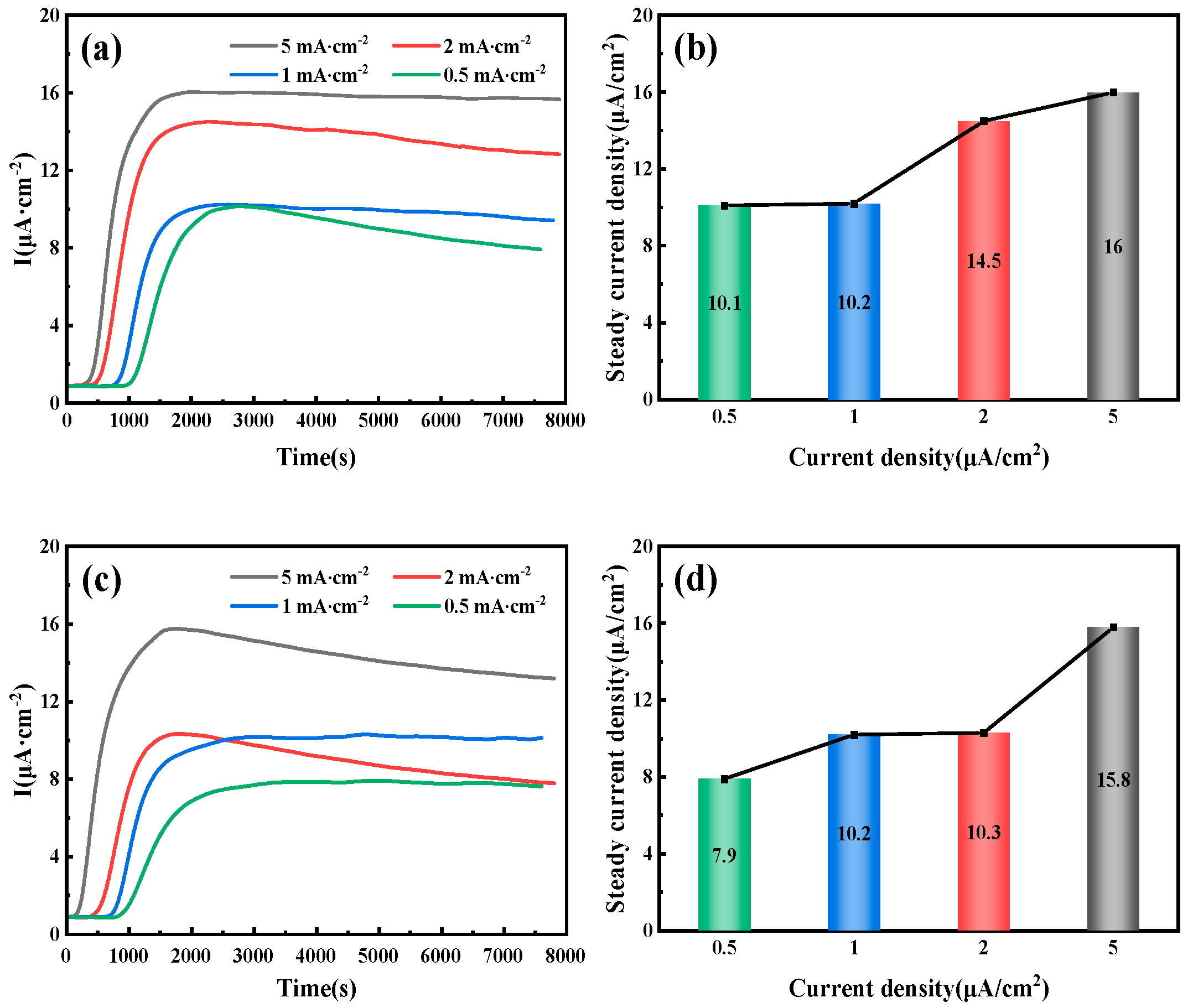
3.2. Hydrogen Permeation Behavior of Pipeline Steel Under Different Current Densities
An electrochemical workstation is used to apply a constant current to the hydrogen charging chamber, initiating the electrolysis of water to produce H+. Initially, the current density remains below 1 µA/cm2 and shows a decreasing trend. This is because the hydrogen generated during the electrochemical reaction at the beginning of the hydrogen filling side of the adsorption process on the surface of the specimen preferentially fills the hydrogen traps that exist inside the specimen and does not pass through the specimen into the hydrogen diffusion chamber. As a result, the hydrogen diffusion chamber side of the basic current signal can not be detected. As time progresses, hydrogen atoms continuously penetrate the specimen and enter the hydrogen traps, an oxidation reaction occurs in the hydrogen diffusion chamber, and a reduction reaction occurs in the hydrogen charging chamber, which generates a current. The current density gradually increases over time. When the current density on the anode side of the specimen stabilizes, it indicates that the hydrogen atoms have reached saturation.
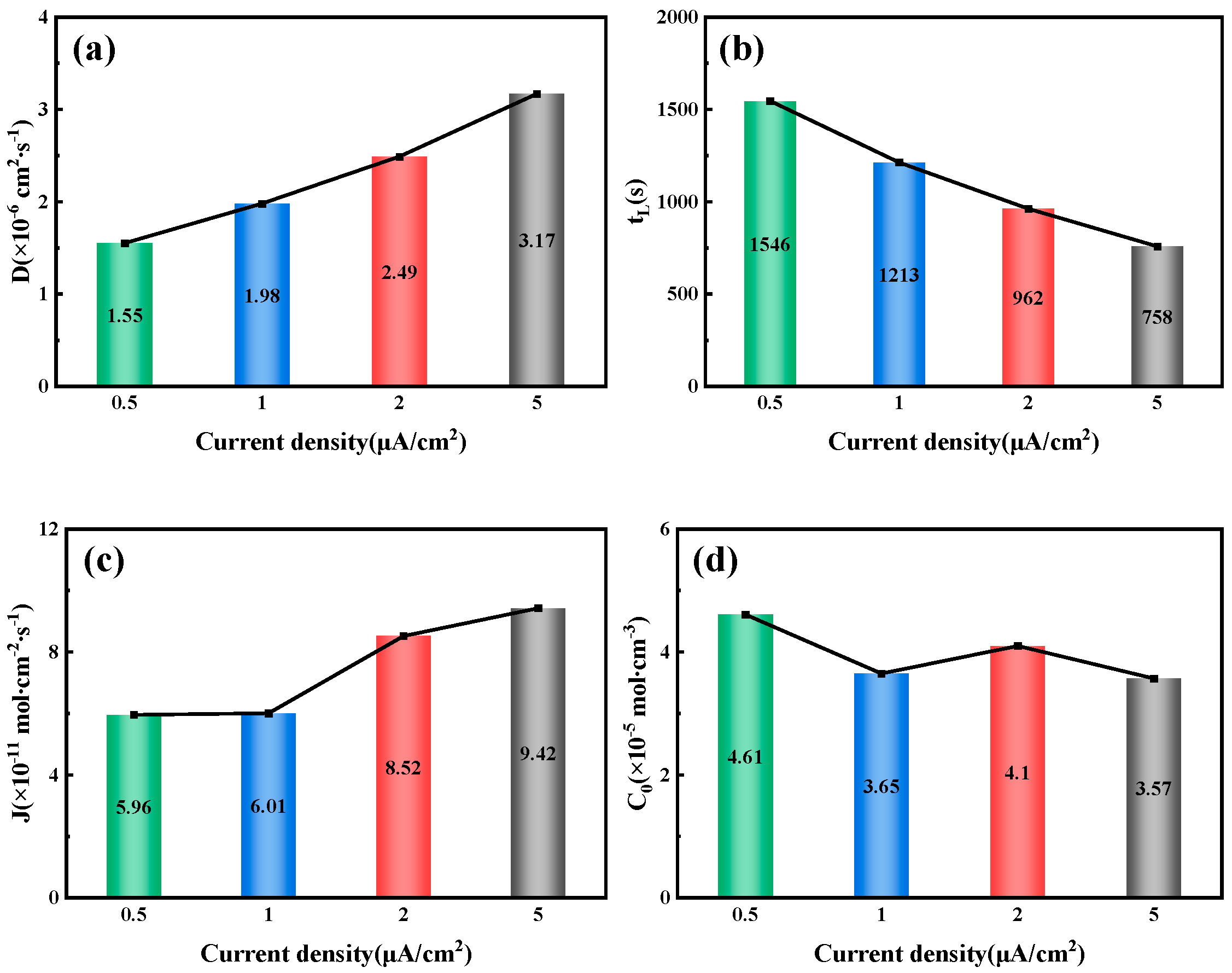
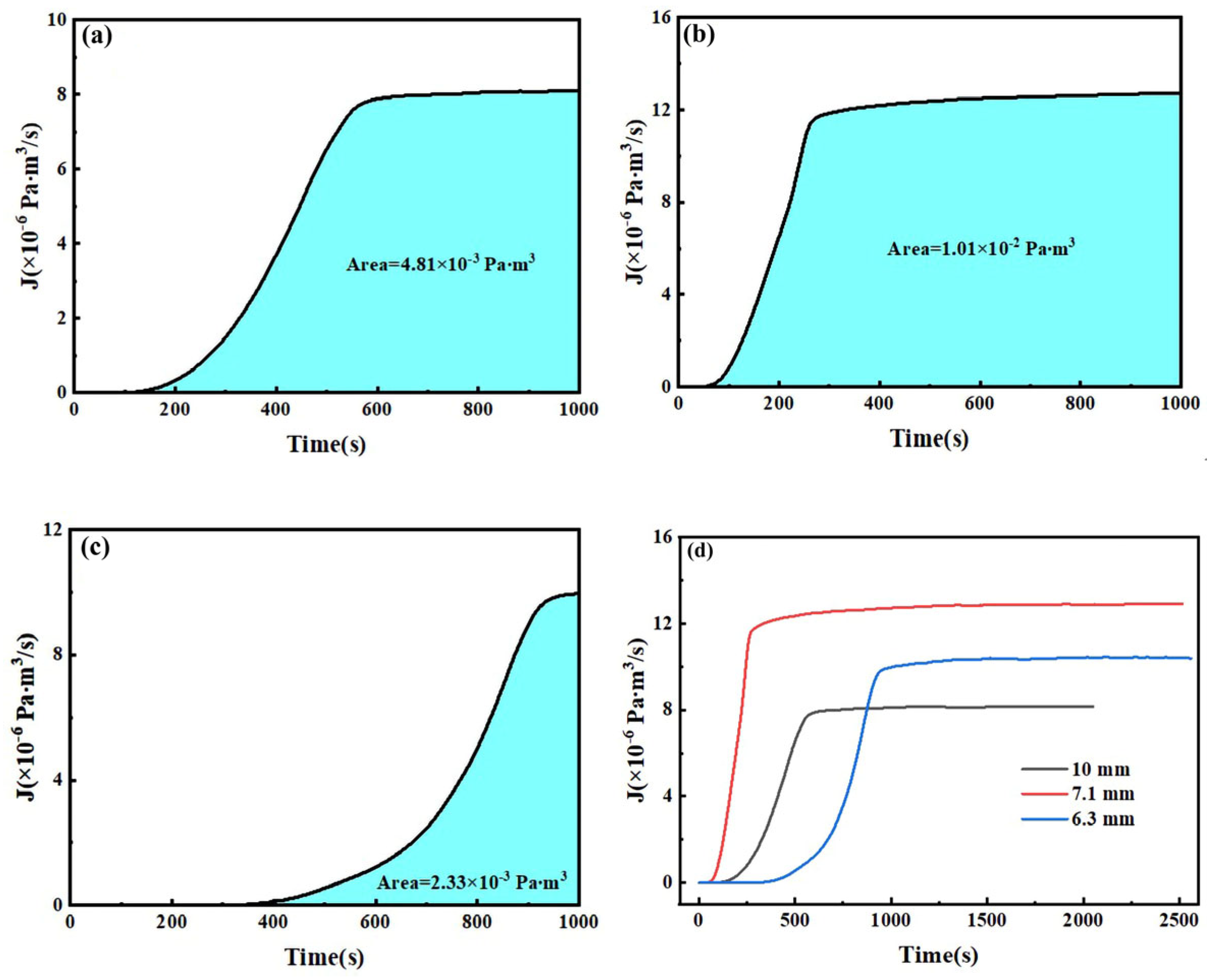
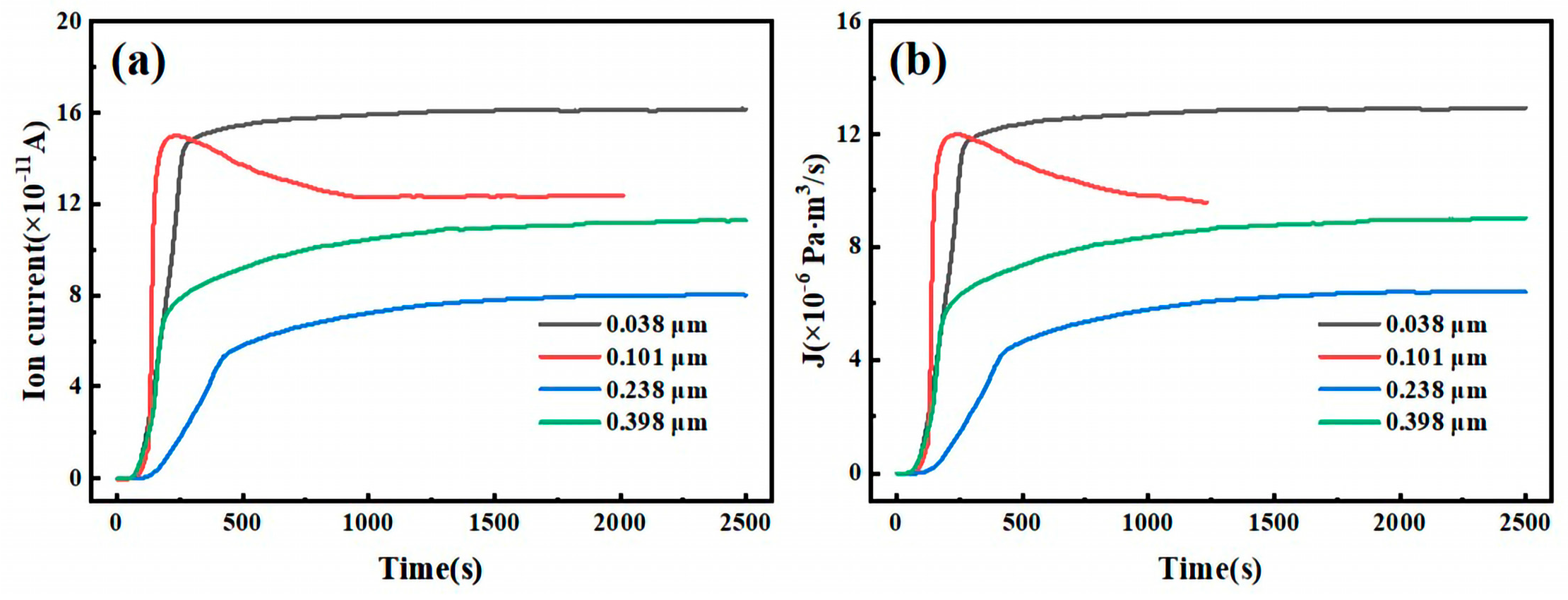
Figure 6 illustrates the hydrogen permeation curves and steady-state current relationships of three different thicknesses of test steel at room temperature. As shown in
Figure 6a,c,e, during the initial phase of the hydrogen permeation experiment, the anode current density is below 1 µA/cm
2. After a certain period, the anode current begins to increase with the hydrogen charging time, and the rate of change of the anode current gradually decreases, eventually stabilizing at a value of
I∞. The time required for hydrogen atoms to penetrate and reach a steady-state current decreases as the hydrogen charging current density increases. The penetration time and time to reach steady-state current for three thicknesses of pipeline steel under different hydrogen charging current densities are detailed in
Table 4. Among these, the corresponding penetration times for 10 mm test steel at hydrogen charging current densities of 0.5, 1, 2, and 5 mA/cm
2 are 1002, 786, 443, and 296 s, respectively, and the times to reach a steady-state current are 2785, 2590, 2280, and 2010 s, respectively. The corresponding penetration times for 7.1 mm test steel are 856, 702, 421, and 143 s, respectively, and the times to reach a steady-state current are 3975, 3035, 1805, and 1755 s, respectively. The corresponding penetration times for the 6.3 mm test steel are 1046, 983, 676, and 157 s, respectively, and the times to reach a steady-state current are 4640, 2890, 4125, and 3385 s, respectively.
Figure 6b,d,f show the steady-state hydrogen permeation current density. For the same test steel, as the current density increases, the anode current reaches a stable value of
I∞. The increase in hydrogen charging current density leads to a higher concentration of hydrogen atoms on the hydrogen side. Due to the increased chemical gradient, according to Fick’s first law, the diffusion flux for the same test steel also increases. Consequently, more hydrogen atoms reach the hydrogen diffusion side within a unit of time, leading to an increase in the
I∞ value. It is evident that under different current density conditions, the penetration time for the 7.1 mm test steel is the shortest, followed by the 10 mm specimen steel, and the penetration time is the longest for the 6.3 mm test steel.
Hydrogen diffusion refers to the movement of hydrogen atoms along the gaps in an ideal crystal lattice. However, in actual steel, hydrogen traps (such as dislocations and inclusions) can capture hydrogen atoms through physical or chemical adsorption, significantly altering their diffusion characteristics. When the current density is low, the number of hydrogen atoms released is limited, leading to insufficient penetration of hydrogen atoms into the steel. As a result, hydrogen traps cannot quickly reach saturation, resulting in a significant increase in the delay time of hydrogen diffusion. Additionally, because diffused hydrogen atoms are preferentially captured by irreversible traps, the hydrogen concentration gradient in the steel is reduced, further exacerbating the lag effect of the diffusion process. This also results in a lower anode saturation current value, which appears as a decrease in the apparent hydrogen diffusion coefficient. When the current density reaches a certain threshold, the number of hydrogen atoms diffusing through the lattice gaps far exceeds the trapping capacity of the traps, stabilizing the hydrogen diffusion process. At this point, the apparent hydrogen diffusion coefficient no longer changes significantly with the increase in current density.
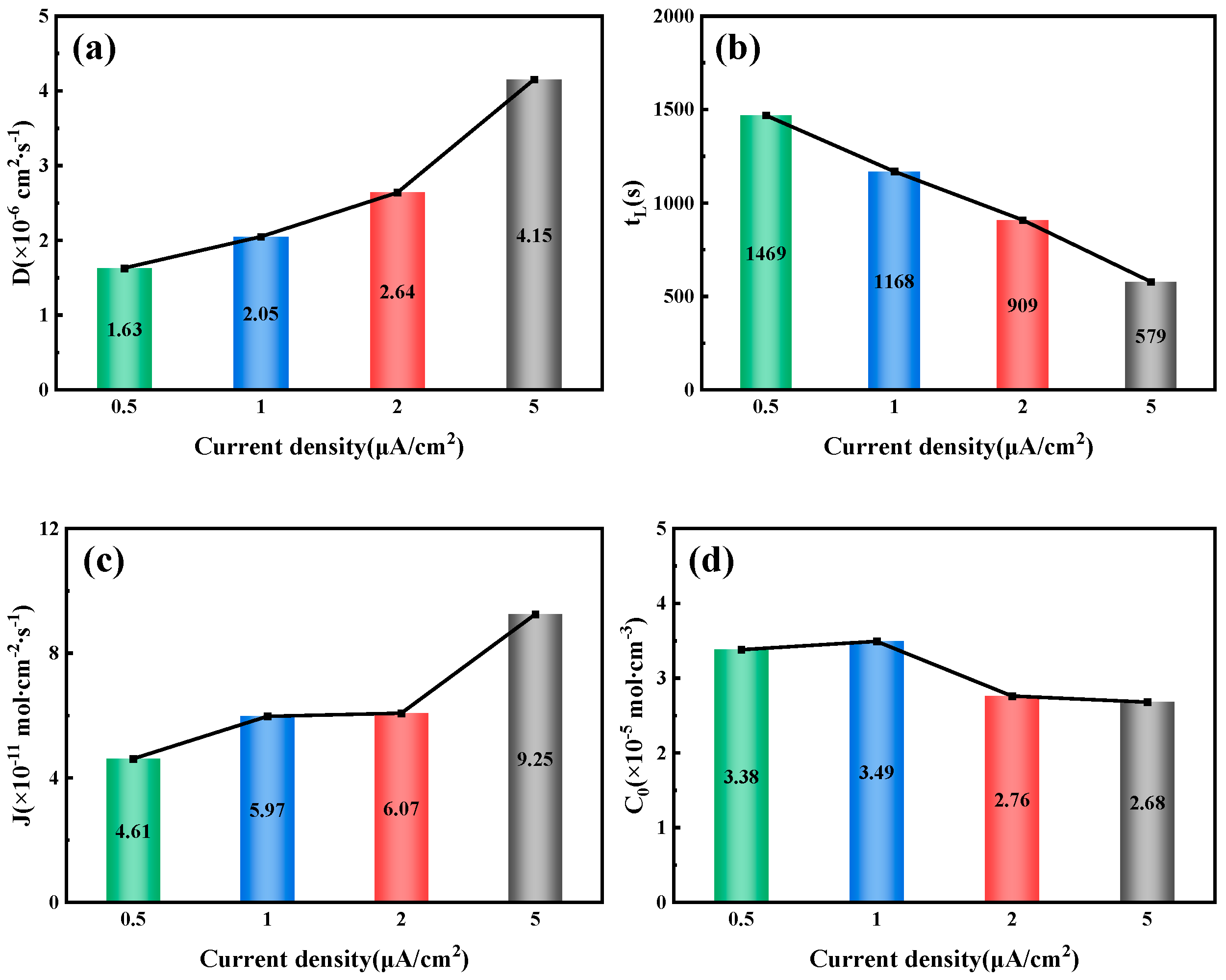
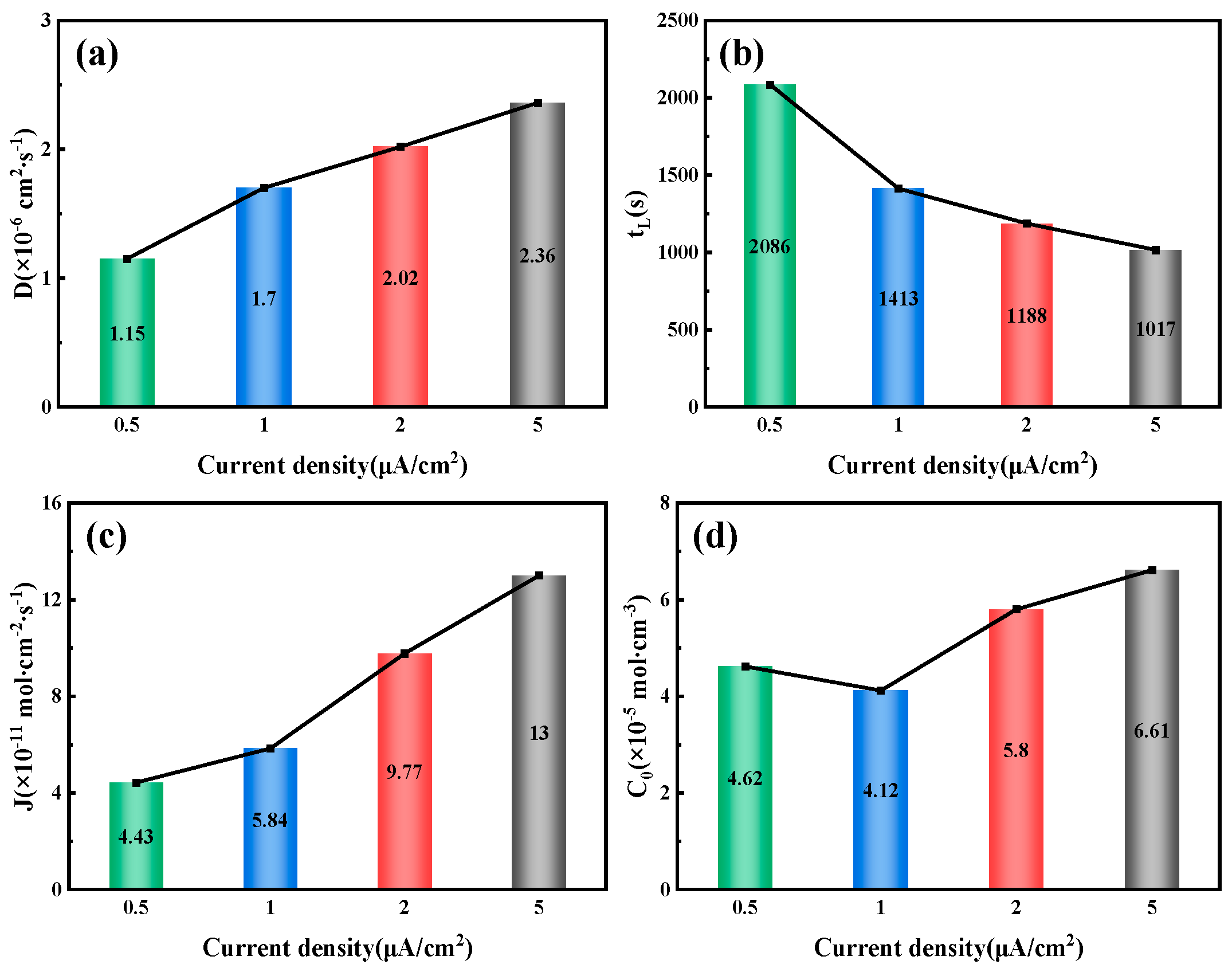
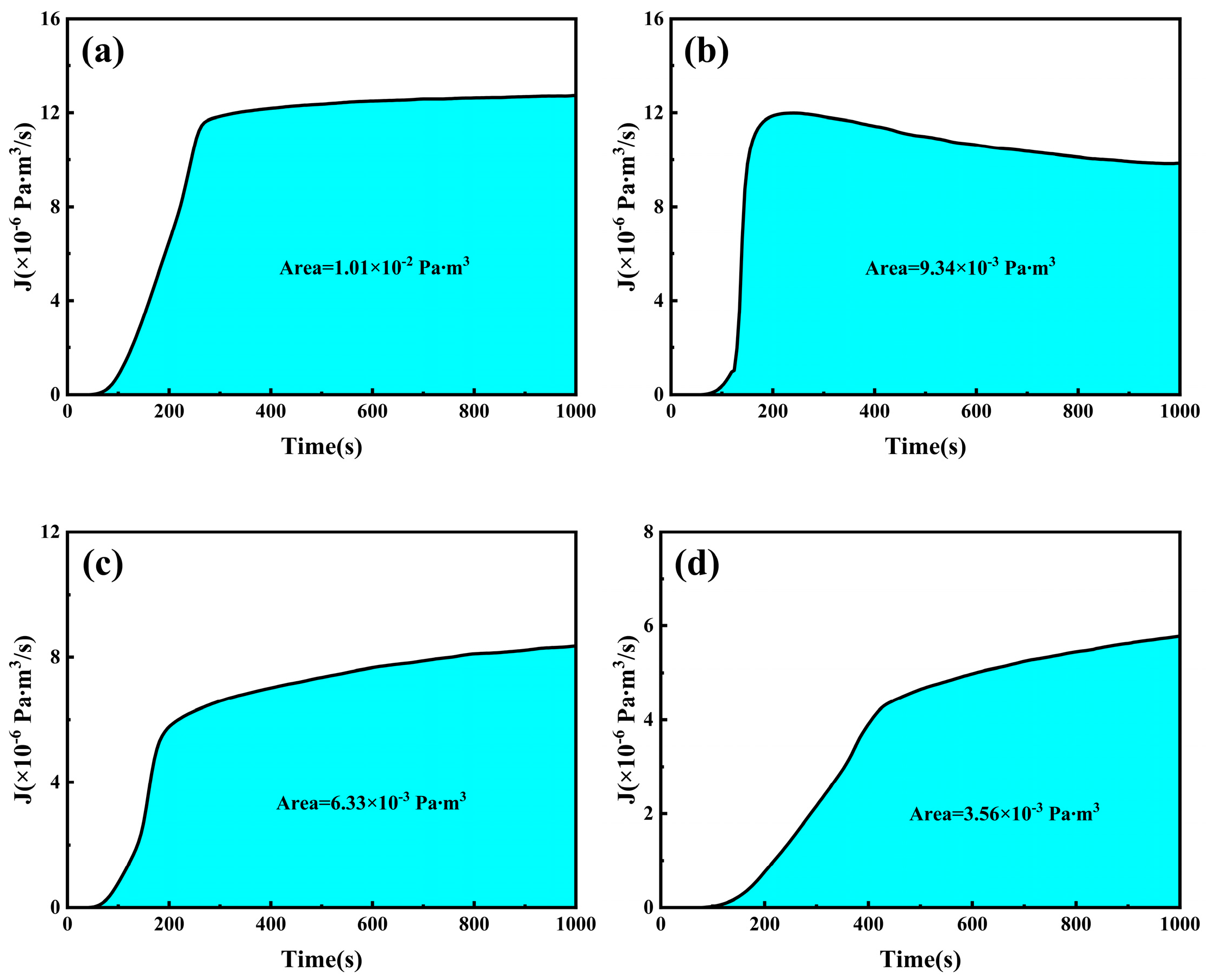
By fitting and calculating the hydrogen permeation curves at different current densities on the hydrogen charging side, we can obtain the hydrogen permeation parameters for three types of test steels, as shown in
Figure 7,
Figure 8 and
Figure 9. These figures illustrate the relationship between the hydrogen charging current density and the relevant parameters for the three thicknesses of the test steels. It is evident that the trends are consistent across all three types of steel. Specifically, the hydrogen diffusion coefficient and flux increase gradually with the rise in hydrogen charging current density, while the hydrogen permeation lag time decreases progressively. When the hydrogen charging current density is 0.5 mA/cm
2, the hydrogen diffusion coefficients of the 10 mm, 7.1 mm, and 6.3 mm test steels stabilize at 1.55 × 10
−6, 1.63 × 10
−6, and 1.15 × 10
−6 cm
2/s, respectively. The hydrogen diffusion lag times are 1546, 1469, and 2086 s, respectively, and the hydrogen diffusion fluxes are 5.96 × 10
−11, 4.61 × 10
−11, and 4.43 × 10
−11 mol·cm
−2/s, respectively. The hydrogen concentrations on the cathode side are 4.61 × 10
−5, 3.38 × 10
−5, and 4.62 × 10
−5 mol·cm
−3, respectively. When the hydrogen charging current density increases to 5 mA/cm
2, the hydrogen diffusion coefficients of the test steels increase to 3.17 × 10
−6, 4.15 × 10
−6, and 2.36 × 10
−6 cm
2/s, respectively. The lag times decrease to 758, 579, and 1017 s, respectively, and the hydrogen diffusion fluxes increase to 9.42 × 10
−11, 9.25 × 10
−11, and 13 × 10
−11 mol·cm
−2/s, respectively. The hydrogen concentration on the cathode side changes to 3.57 × 10
−5, 2.68 × 10
−5, and 6.61 × 10
−5 mol cm
−3, respectively. Overall, among the three types of test steels, the 7.1 mm steel exhibits the highest hydrogen diffusion coefficient and the shortest hydrogen absorption lag time at various current densities; in contrast, the 6.3 mm steel shows the opposite characteristics. As the hydrogen charging current density increases, the concentration of hydrogen atoms in the hydrogen charging chamber rises, leading to a greater concentration difference between the two electrolytic cells. Consequently, more hydrogen atoms pass through the specimen per unit time, increasing the hydrogen diffusion flux. The rate of this increase corresponds to the rate at which the stable current value, I∞, at the anode rises. The trend in hydrogen concentration on the cathode side is influenced by the product of the steady-state current value’s (
I∞) rise rate and the rate at which the lag time shortens. When this product is greater than 1, the hydrogen concentration on the cathode side increases; otherwise, it decreases. The results indicate that the 6.3 mm steel exhibits better resistance to hydrogen, while the 7.1 mm steel performs worse.
3.3. Hydrogen Permeation Behavior of Pipeline Steel Under Different Surface Roughness Conditions
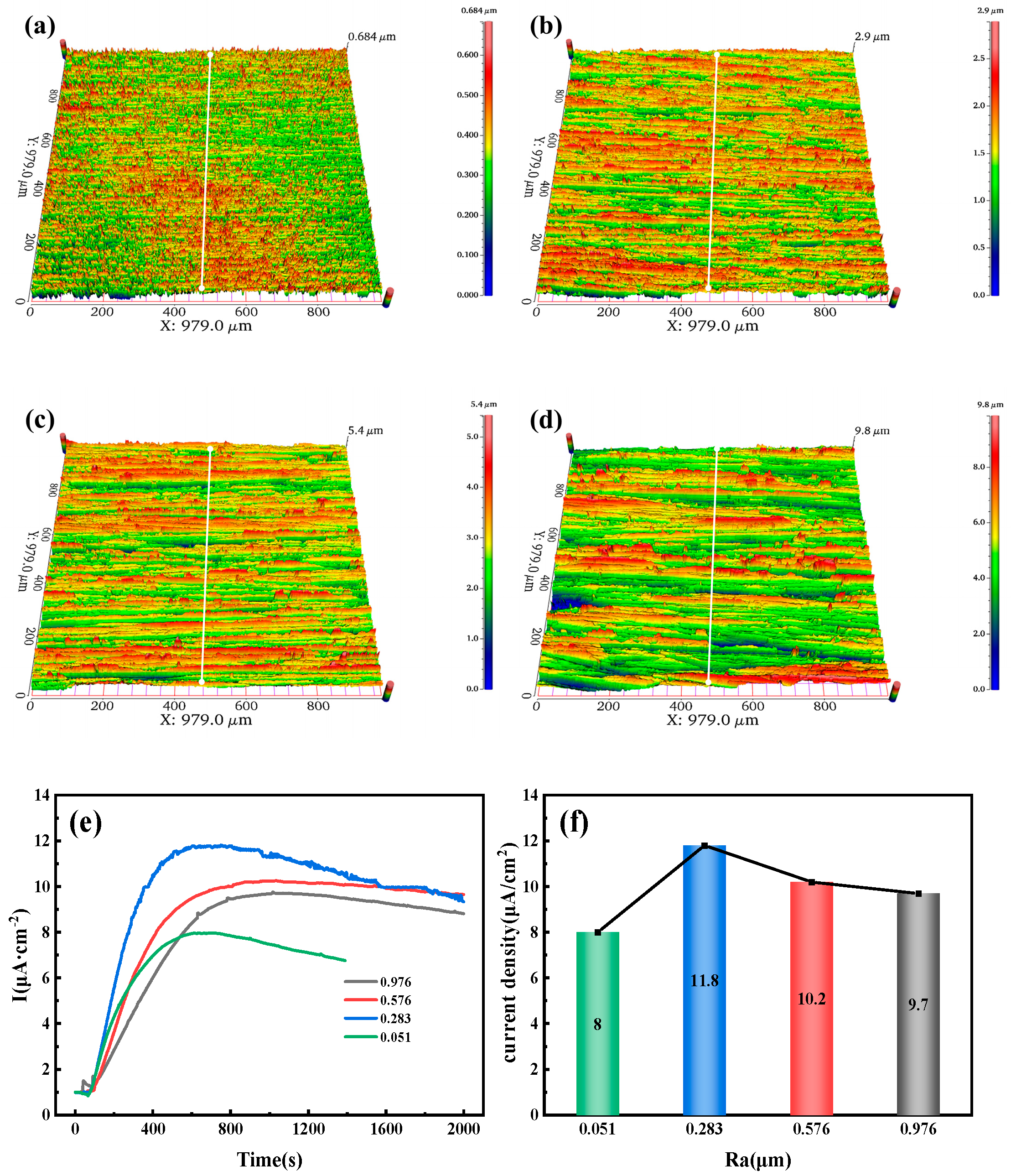
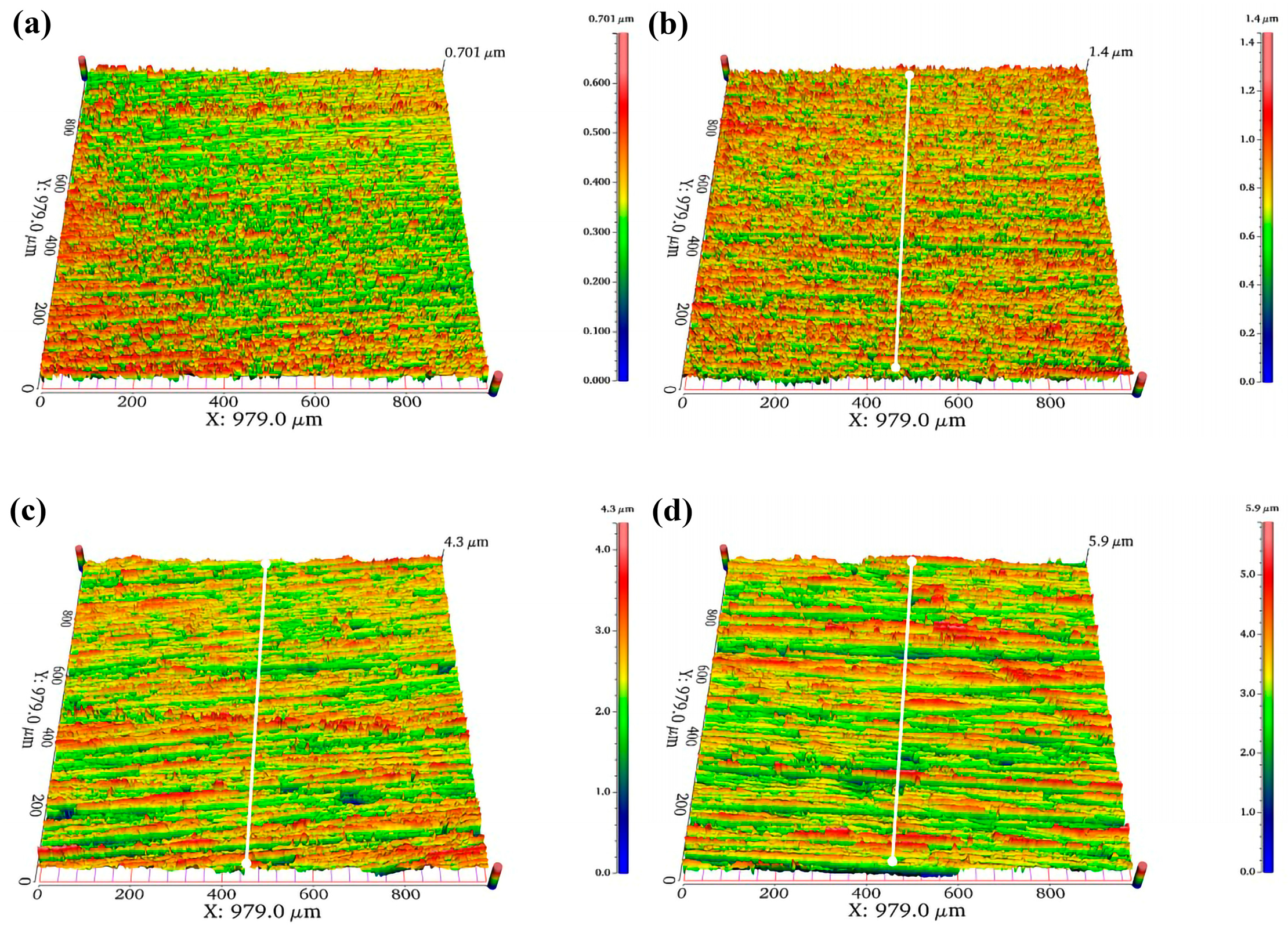
Figure 10a–d show the three-dimensional surface morphology of the 10 mm test steel with different roughness. This morphology is measured using the Ra (arithmetic mean deviation of the profile). Ra refers to the arithmetic mean of the absolute values of the distances from each point on the measured surface profile curve to a reference midline (profile midline), where the profile curve is compared against this midline. The Ra is determined using a white light interferometer, with values of 0.026, 0.245, 0.566, and 0.76 μm. As the roughness increases, more red areas appear on the specimen surface, forming a ridge-like pattern.
Figure 10e,f present the hydrogen permeation curves and steady-state current density under different surface Ra conditions.
Figure 10e shows the relationship between current density and time after a hydrogen charging current of 20 mA/cm
2 is applied to the cathode of the test steel. At this point, hydrogen evolution begins in the hydrogen charging chamber, and the hydrogen permeation curves within the first 30 s are in the initial stage of hydrogen charging, during which the anode barely detects any change in current. As time progresses, the anode current gradually increases and eventually stabilizes. It is observed that the hydrogen penetration time decreases as the surface Ra increases. When the Ra is 0.026 μm, the penetration time is 65 s, whereas when the Ra is 0.76 μm, it is only 34 s. The hydrogen permeation curve shows an increase in the time required to reach the steady-state current from the start of hydrogen charging. For specimens with roughness values of 0.026, 0.245, 0.566, and 0.76 μm, the times to reach the steady-state current are 492, 1193, 982, and 1435 s, respectively. As the hydrogen permeation current increases, the slope of the curve gradually decreases, indicating that the hydrogen permeation rate decreases as the specimen roughness increases.
Figure 10f shows that in this set of experimental data, the steady-state current density of hydrogen permeation first decreases and then increases with the increase in the specimen surface roughness. Under steady-state diffusion conditions, the minimum steady-state current density of hydrogen permeation for a specimen with a roughness of 0.245 μm is 7.2 µA/cm
2. It can be concluded that a higher Ra value shortens the hydrogen penetration time but reduces the hydrogen permeation rate.
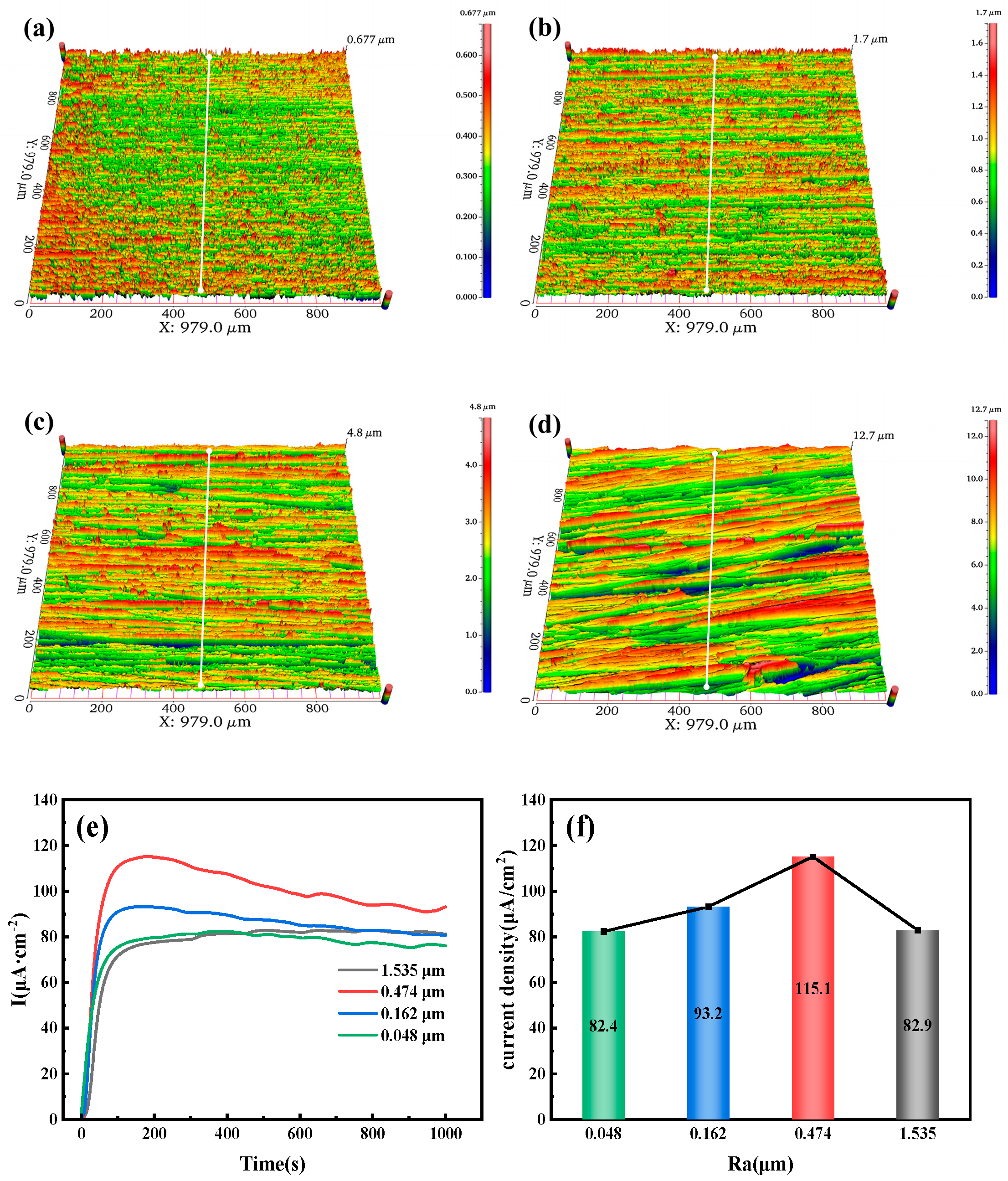
Figure 11a–d show the three-dimensional morphology of the 7.1 mm test steel, with four specimens having roughness values of 0.048, 0.162, 0.474, and 1.535 μm, respectively. As the roughness increases, the red areas on the surface also expand. When the roughness reaches 1.535 μm, a layered ridge-like appearance with a certain angle in the horizontal direction is observed.
Figure 11e,f show the hydrogen permeation curves and steady-state current density of the test steel.
Figure 11e indicates that during the initial hydrogen charging phase, the anode quickly detects the oxidation current for the 7.1 mm test steel, taking only a few seconds to do so. The time from the start of hydrogen charging to reaching a steady-state current first decreases and then increases. For roughness values of 0.048, 0.162, 0.474, and 1.535 μm, the times to reach a steady-state current are 202, 164, 179, and 506 s, respectively, indicating that the time to reach steady-state current increases as the Ra increases.
Figure 11f shows that as the surface roughness of the specimen increases, the steady-state current density of hydrogen permeation first increases and then decreases. Under steady-state diffusion conditions, the steady-state current density of hydrogen permeation is highest at a roughness value of 0.474 μm, corresponding to a current density of 115.1 µA/cm
2, and is lowest at a roughness value of 0.048 μm, corresponding to a current density of 82.4 µA/cm
2.
Figure 12a–d show the three-dimensional morphology of the 6.3 mm test steel, with Ra values of 0.051, 0.238, 0.576, and 0.976 μm. As the surface roughness increases, the red areas on the surface gradually expand. However, when the roughness is 0.576 or 0.238 μm, the red areas partially decrease.
Figure 12e,f present the hydrogen permeation curves and steady-state current density of the test steel.
Figure 12e indicates that the hydrogen atom penetration time decreases as the surface roughness increases. At a roughness of 0.051 μm, the penetration time is 78 s, while at a roughness of 0.976 μm, the penetration time is only 18 s. Additionally, the time required for hydrogen permeation to reach a steady state from the start of hydrogen charging increases with the increase in roughness. For roughness values of 0.051, 0.238, 0.576, and 0.976 μm, the times to reach a steady-state current are 606, 642, 897, and 996 s, respectively.
Figure 12f shows that the steady-state current density of hydrogen permeation first increases and then decreases as the surface roughness of the specimen increases. Under steady-state diffusion conditions, the maximum steady-state current density of hydrogen permeation is 11.8 µA/cm
2 at a roughness of 0.283 μm, and the minimum steady-state current density is 8 µA/cm
2 at a roughness of 0.051 μm.
To further investigate the impact of different roughness levels on hydrogen permeation in the specimens, the hydrogen permeation curves are fitted and calculated to determine the relationship between surface roughness and hydrogen permeation parameters, as shown in
Figure 13,
Figure 14 and
Figure 15.
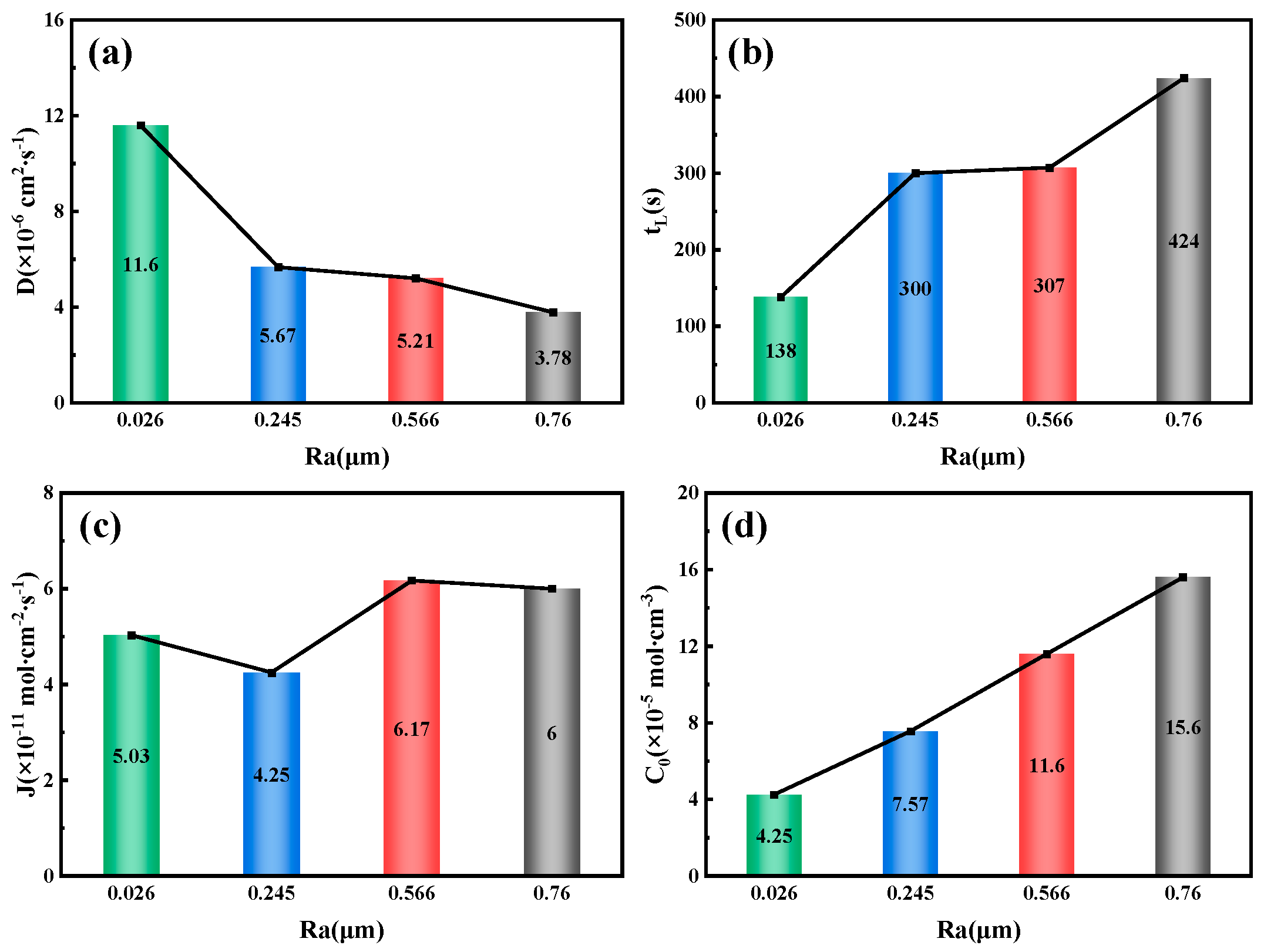
Figure 13 shows that for a 10 mm steel specimen, as the surface roughness increases, the hydrogen diffusion coefficient decreases from 11.6 × 10
−6 cm
2/s
−1 at an Ra value of 0.026 μm to 3.78 × 10
−6 cm
2/s
−1 at a roughness value of 0.76 μm, which is halved. Surface roughness can inhibit the hydrogen diffusion process to some extent, primarily due to the influence of surface microstructure characteristics, internal defect density, grain size, and the density of hydrogen capture sites [
6]. Conversely, as the surface roughness of the specimen increases, both the lag time of hydrogen permeation and the hydrogen concentration at the cathode side increase. At an Ra value of 0.026 μm, the lag time is only 138 s, while at an Ra value of 0.76 μm, the lag time increases to 424 s. The hydrogen concentration at the cathode side rises from 4.25 × 10
−5 mol/cm
−3 at an Ra value of 0.026 μm to 15.6 × 10
−5 mol/cm
−3 at an Ra value of 0.76 μm, representing a 2.67-fold increase. It is worth noting that the variation law of the hydrogen diffusion flux and the steady-state current density of hydrogen permeation correspond to each other. When the Ra value is 0.245 μm, the hydrogen diffusion flux is the smallest at 4.25 × 10
−11 mol·cm
−2 s
−1; when the Ra value is 0.566 μm, the hydrogen diffusion flux reaches a maximum at 6.17 × 10
−11 mol·cm
−2 s
−1.
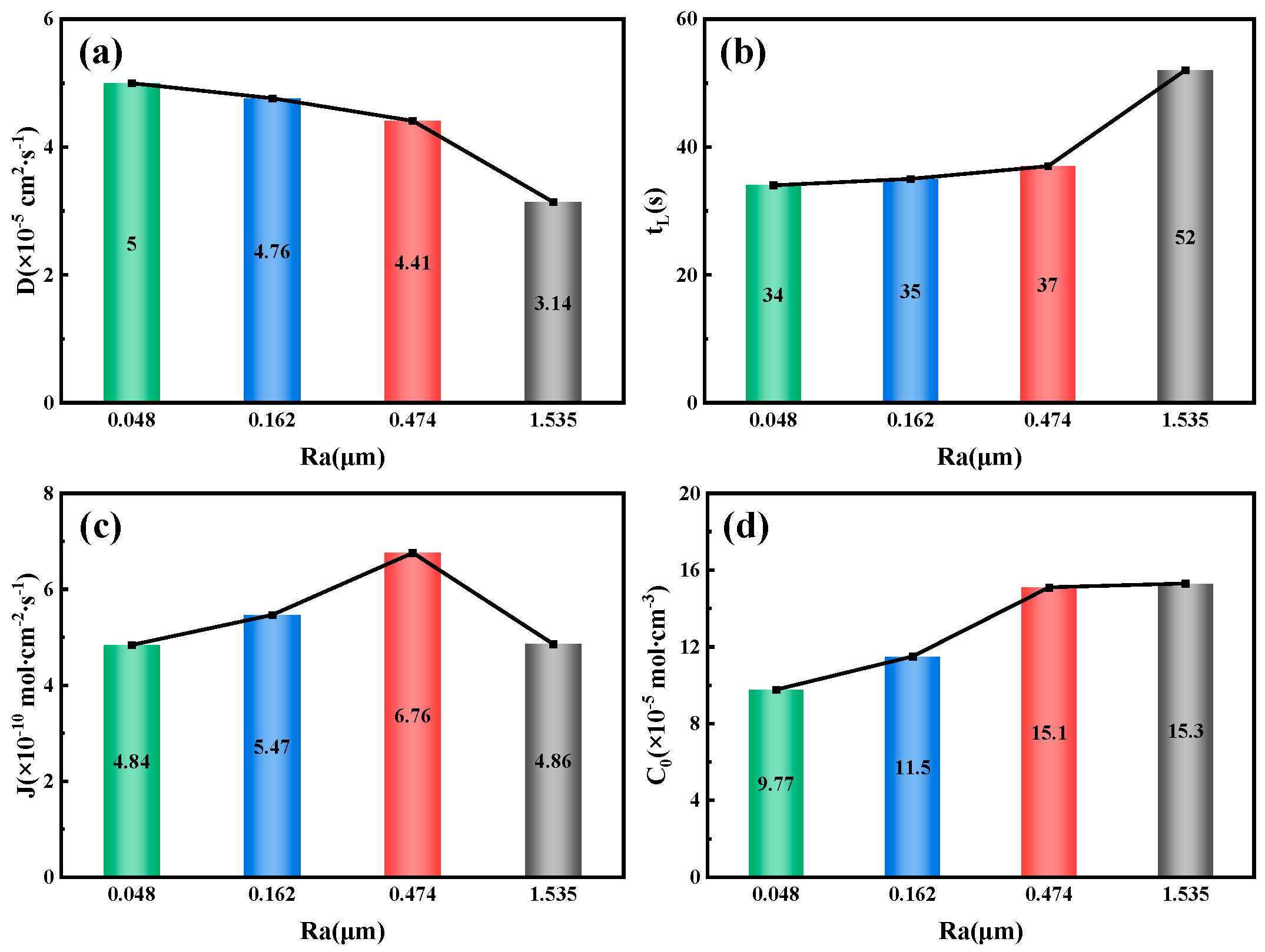
Figure 14 illustrates the relationship between the surface roughness of the 7.1 mm test steel and the parameters related to hydrogen permeation. As the surface roughness increases, the hydrogen diffusion coefficient decreases gradually, from 5 × 10
−5 cm
2/s
−1 at an Ra value of 0.048 μm to 3.14 × 10
−5 cm
2/s
−1 at an Ra value of 1.535 μm. Conversely, the lag time for hydrogen permeation and the hydrogen concentration on the cathode side both increase with the surface roughness. The lag time increases from 34 s at an Ra value of 0.048 μm to 52 s at an Ra value of 1.535 μm; the hydrogen concentration on the cathode side rises from 9.77 × 10
−5 mol/cm
−3 at an Ra value of 0.048 μm to 15.3 × 10
−5 mol/cm
−3 at an Ra value of 1.535 μm. Similarly, the changes in the hydrogen diffusion flux and the hydrogen permeation current density follow a similar pattern, peaking at 6.67 × 10
−10 mol/cm
−2/s
−1 at an Ra value of 0.474 μm.
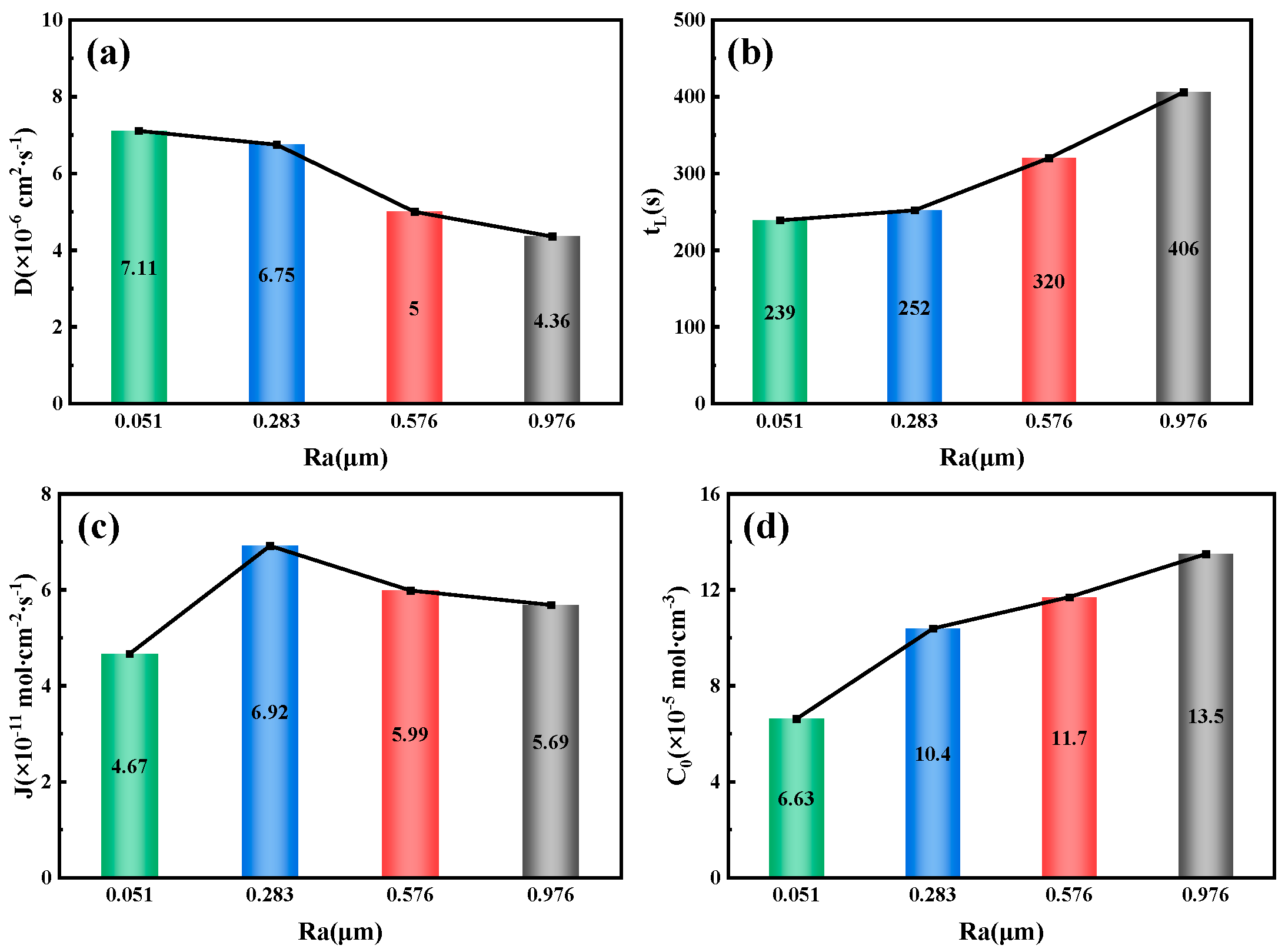
Figure 15 illustrates the relationship between the surface roughness of the 6.3 mm test steel and the parameters related to hydrogen permeation. The results show that the hydrogen diffusion coefficient decreases as the surface roughness increases, from 7.11 × 10
−6 cm
2/s
−1 at an Ra value of 0.051 μm to 4.36 × 10
−6 cm
2/s
−1 at an Ra value of 0.976 μm. Conversely, as the specimen’s surface roughness increases, both the hydrogen permeation lag time and the hydrogen concentration on the cathode side increase. At an Ra value of 0.051 μm, the lag time is 239 s, while at an Ra value of 0.976 μm, it increases to 406 s. The hydrogen concentration on the cathode side also doubles, from 6.63 × 10
−5 mol/cm
−3 at an Ra value of 0.051 μm to 13.5 × 10
−5 mol/cm
−3 at an Ra value of 0.976 μm. The variation in the hydrogen diffusion flux follows the same pattern as the change in the hydrogen permeation current density, reaching a maximum of 6.92 × 10
−11 mol/cm
−2/s
−1 at an Ra value of 0.283 μm.
The results indicate that as the surface roughness of the specimens increases, the hydrogen diffusion coefficients of the three test steels gradually decrease. Meanwhile, the penetration time, lag time, and hydrogen concentration on the cathode side all increase. This suggests that surface roughness has a certain inhibitory effect on the hydrogen diffusion process, with a stronger inhibitory effect as the surface roughness increases. When the surface roughness is high, the specimen surface exhibits more unevenness and microscopic defects, which can impede the penetration of hydrogen atoms. More obstacles and torturous paths increase the difficulty of hydrogen permeation. After hydrogen atoms enter these traps, they are temporarily captured or adsorbed, making it difficult for them to continue to penetrate into the specimen’s depth. The “mountains and valleys” regions of rough surfaces cause hydrogen atoms to accumulate, which hinders further diffusion into the material’s interior, thus inhibiting hydrogen permeation to a certain extent.
In a gaseous environment, for pipeline steel, the rough surface provides more active sites for hydrogen adsorption due to the increase in microscopic peaks and valleys, significantly promoting the dissociative adsorption of hydrogen molecules (H
2→2H), increasing the concentration of hydrogen atoms on the surface, and accelerating the penetration of hydrogen into the matrix [
33]. In a liquid phase environment, however, the rough surface promotes the recombination of hydrogen atoms (2H→H
2), which instead reduces the hydrogen permeation flux. Within a certain range, an increase in surface roughness causes more unevenness and microscopic defects on the sample surface, which makes hydrogen atoms encounter more obstacles and torturous paths during the penetration process, thereby increasing the difficulty of hydrogen penetration. Once hydrogen atoms enter these traps, they are temporarily captured or adsorbed, making it difficult for them to continue penetrating deeper into the sample. The “valley” areas on rough surfaces cause hydrogen atoms to accumulate and make it difficult for them to continue diffusing into the material, thereby inhibiting the penetration of hydrogen to some extent.
Compared to 10 mm and 6.3 mm steel, the hydrogen permeation steady-state current density, hydrogen diffusion coefficient, and hydrogen diffusion flux of the 7.1 mm steel are all one order of magnitude higher. Additionally, the hydrogen atom penetration time and lag time of 7.1 mm steel are significantly shorter compared to those of 10 mm and 6.3 mm steel, indicating that 7.1 mm steel has the poorest hydrogen resistance. For the other two steel thicknesses, a comparison between 10 mm steel with an Ra value of 0.566 μm and 6.3 mm steel with an Ra value of 0.576 μm shows that the latter has a lower hydrogen diffusion coefficient and hydrogen diffusion flux, as well as longer hydrogen atom penetration time and lag time, even when the Ra value is below 0.1 μm. It can be preliminarily concluded that the 6.3 mm-thick steel has the best hydrogen resistance among the three thicknesses, followed by the 10 mm-thick steel, and the 7.1 mm-thick steel has the worst hydrogen resistance.
To intuitively analyze hydrogen penetration behavior, the surface morphology is characterized microscopically.
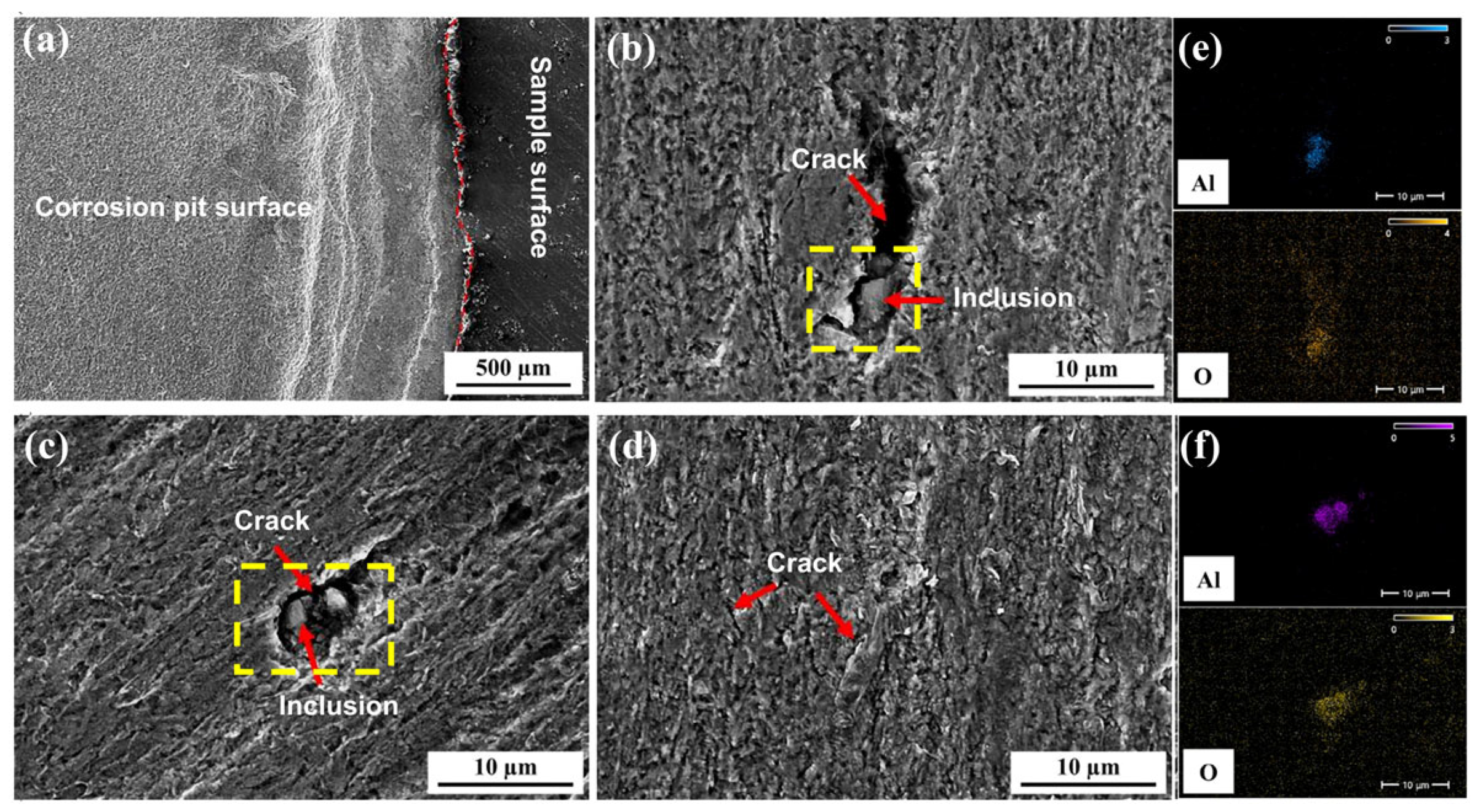
Figure 16 shows the surface morphology and EDS analysis of inclusions after hydrogen penetration tests on 10 mm test steel with varying roughness. The figure indicates that when the Ra value is 0.026 μm, the entire effective contact area of the specimen develops corrosion pits after hydrogen penetration, forming a certain step height relative to the specimen surface. These pits contain numerous hydrogen-induced cracks, mostly at grain boundaries. The reasons for this are as follows: (1) At grain boundaries, the atomic arrangement is irregular, with many defects, such as vacancies and dislocations, which make it easier for hydrogen atoms to accumulate, forming localized high-hydrogen concentration areas. (2) Grain boundaries have higher energy, making the adsorption energy of hydrogen atoms lower, thus facilitating their adsorption and gradual accumulation at these boundaries. (3) When hydrogen atoms accumulate to a certain concentration at grain boundaries, they create hydrogen pressure, leading to changes in the local stress field and a decrease in the bonding force at grain boundaries [
7,
34,
35]. (4) Uneven deformation during processing can lead to residual stresses within the material, which interact with hydrogen, thereby increasing the sensitivity of crack formation at grain boundaries. When the Ra value is 0.245 μm, numerous small corrosion pits and hydrogen-induced cracks form on the specimen surface, with inclusions serving as crack sources, leading to the development of longer and deeper hydrogen-induced cracks. When the Ra value is 0.566 μm, many corrosion pits also appear on the specimen surface, with hydrogen-induced cracks forming around inclusions.
Figure 16e,f show the EDS analysis of the inclusions in the yellow boxes of
Figure 16b,c, respectively. This figure reveals that all inclusions are Al
2O
3, which are regions where hydrogen atoms tend to accumulate. In the hydrogen permeation test, hydrogen ions generated by the hydrogen charging chamber accumulate at the tips of Al
2O
3 inclusions, forming hydrogen molecules [
3,
4,
5,
6]. Due to their large size, these hydrogen molecules cannot diffuse between grain boundaries. As hydrogen atoms continue to diffuse, they create significant hydrogen pressure. When this pressure exceeds the tensile strength of the steel, cracks begin to form at this location and extend along the defect [
7,
34,
35]. Therefore, the presence of large Al
2O
3 inclusions in the steel is a key factor in the formation of cracks. When the Ra value increases to 0.76 μm, numerous small corrosion pits and hydrogen-induced cracks form on the specimen surface, with no large-scale hydrogen-induced cracks observed. Overall, these hydrogen damage patterns are predominantly characterized by cracks, with rare hydrogen bubble formation. This is due to the high concentration of atomic hydrogen within the material. When the hydrogen atom concentration exceeds the critical threshold, it rapidly recombines into hydrogen molecules and escapes from the material surface, forming localized high pressure that directly damages the internal lattice structure and surface integrity of the material, resulting in these corrosion patterns [
3,
4,
5,
6].
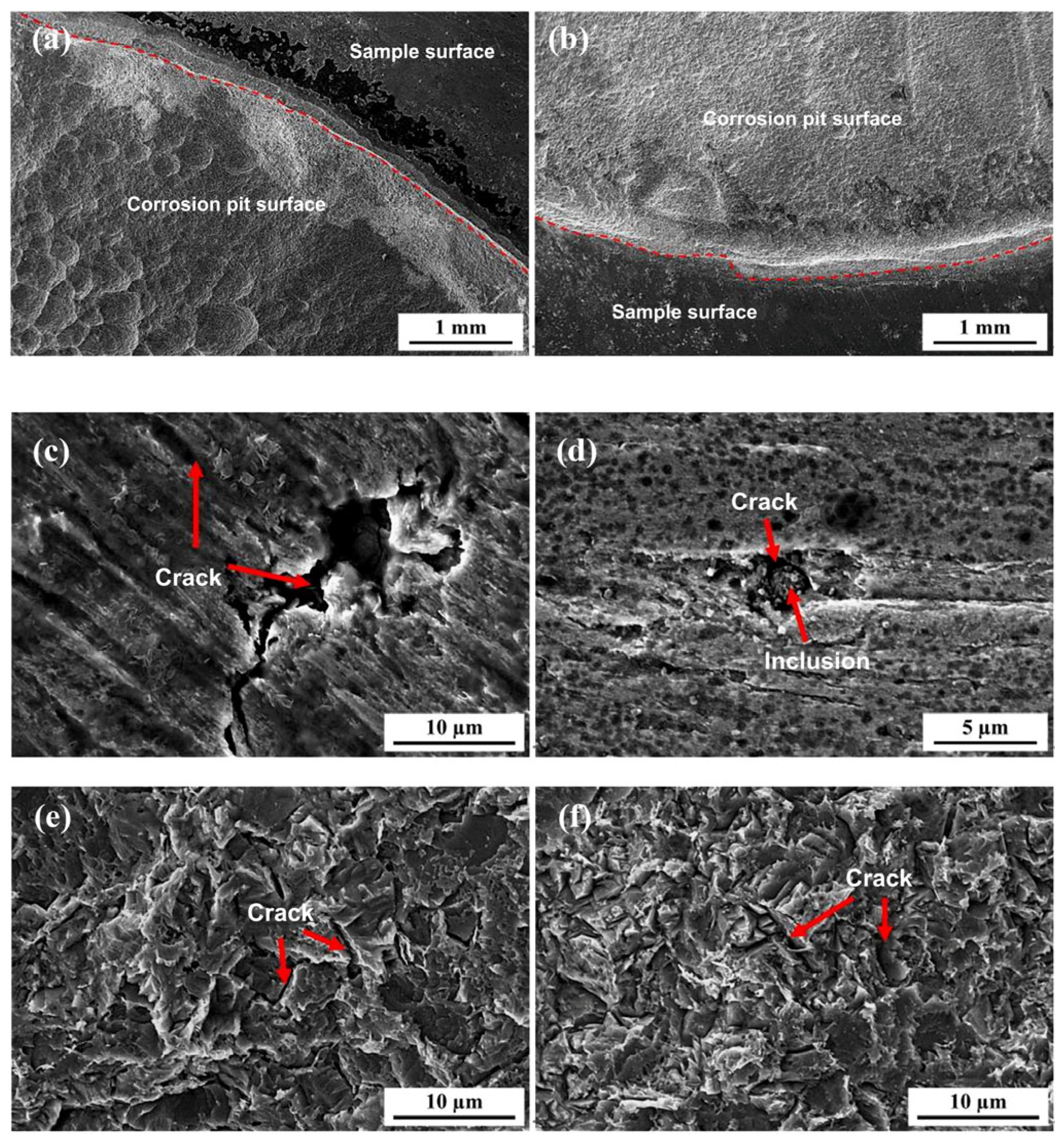
Figure 17 shows the surface morphology of the 7.1 mm test steel after hydrogen penetration at different Ra values. It is evident that when the Ra value of the specimen is 0.048 μm or 0.162 μm, corrosion pits have formed across the entire effective area of the specimen after hydrogen penetration, creating a step height on the specimen surface. The step height is higher at an Ra value of 0.048 μm, and numerous hydrogen-induced cracks are present in the corrosion pits. When the Ra value of the specimen is 0.474 μm, longer and deeper hydrogen-induced cracks appear on the specimen surface. As the surface roughness increases to 1.535 μm, dense and smaller corrosion pits appear on the specimen surface, with hydrogen-induced cracks forming around inclusions.
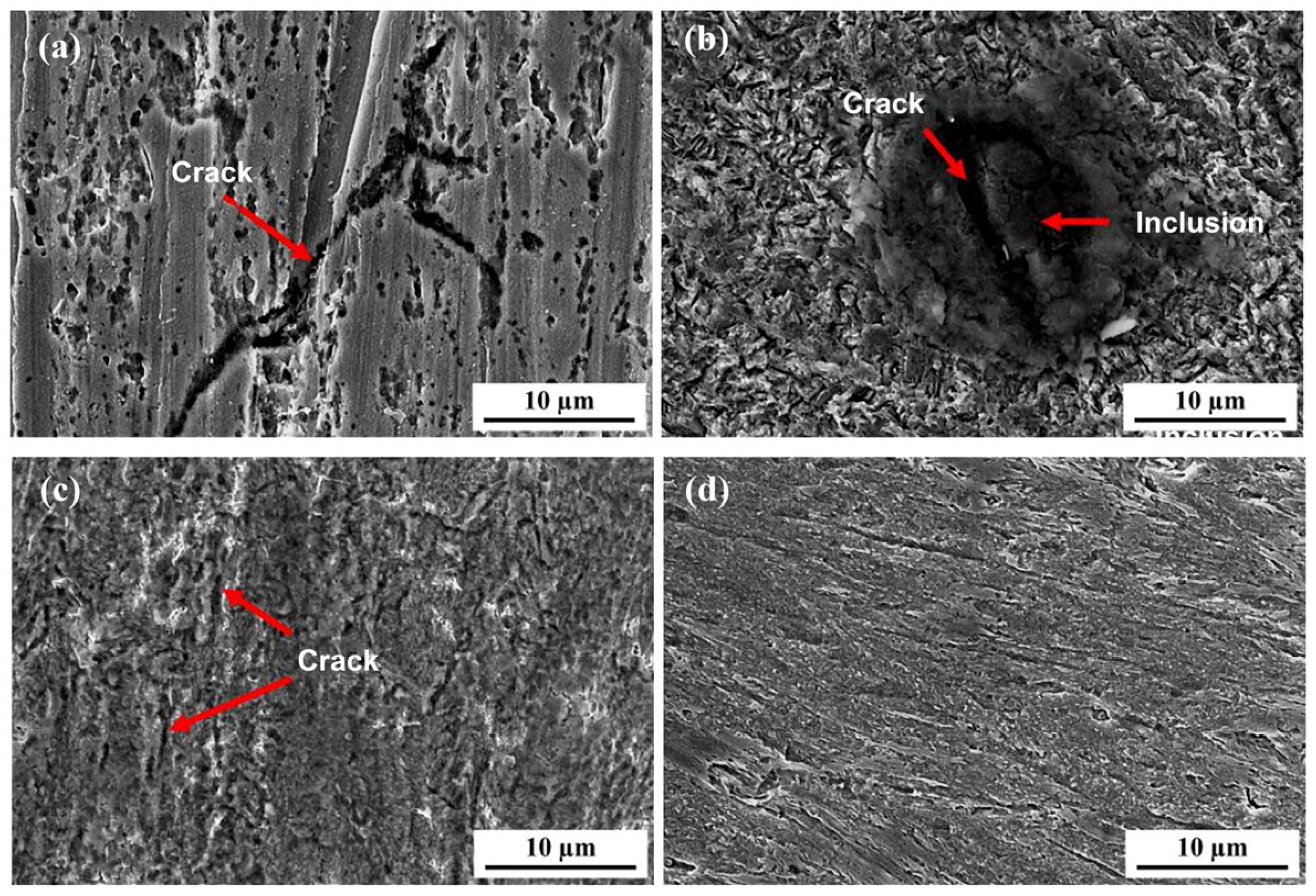
Figure 18 shows the surface morphology of the 6.3 mm test steel after hydrogen penetration at different Ra values. When the Ra value of the specimen surface is 0.051 μm, numerous small corrosion pits and larger pits formed by merging these smaller ones appear on the surface, along with longer and deeper hydrogen-induced cracks. At an Ra value of 0.238 μm, many hydrogen-induced cracks, resulting from the expansion and merging of small secondary cracks, appear on the surface, and hydrogen-induced cracks also form around inclusions. At an Ra value of 0.576 μm, numerous shallow corrosion pits and cracks are observed on the surface. When the surface roughness increases to 0.976 μm, only point corrosion occurs on the surface, with almost no significant changes in the macroscopic morphology, indicating an enhanced resistance to hydrogen.
The results described above indicate that for the three types of test steels, as the Ra value of the specimen’s surface increases, the degree of corrosion on the surface after hydrogen penetration decreases. This is because a higher Ra value leads to more hydrogen adsorption at surface defects, reducing the amount of hydrogen that penetrates the specimen and lowering the hydrogen diffusion coefficient. Therefore, the surface roughness of the specimen has a certain inhibitory effect on hydrogen diffusion, with a stronger inhibitory effect as the surface roughness increases. The morphology of the hydrogen-filled side shows that the 7.1 mm test steel exhibits the most severe hydrogen corrosion. When the Ra value is 0.162 μm, large corrosion pits form across the entire area, creating a step height relative to the specimen surface. When the Ra value increases to 0.474 μm, significant hydrogen-induced cracks appear. In contrast, the 6.3 mm test steel shows the least hydrogen corrosion. At an Ra value of 0.051 μm, only partial hydrogen-induced cracks are observed. As the Ra value increases, the number of cracks gradually decreases, and at an Ra value of 0.976 μm, only pitting corrosion is observed. This indicates that the 6.3 mm test steel has the best resistance to hydrogen, while the 7.1 mm test steel has the poorest resistance.