Massive Fluctuations in the Derivatives of Pair Distribution Function Minima and Maxima During the Glass Transition
Abstract
1. Introduction
2. Theoretical Considerations
- (i)
- Increasing its amplitude;
- (ii)
- Shifting its position to larger values.The shift of the position of PDFmin reflects [14]
- (iii)
- The formation of configurons (broken chemical bonds);
- (iv)
- Enlarging the size of the first coordination shell (FCS).
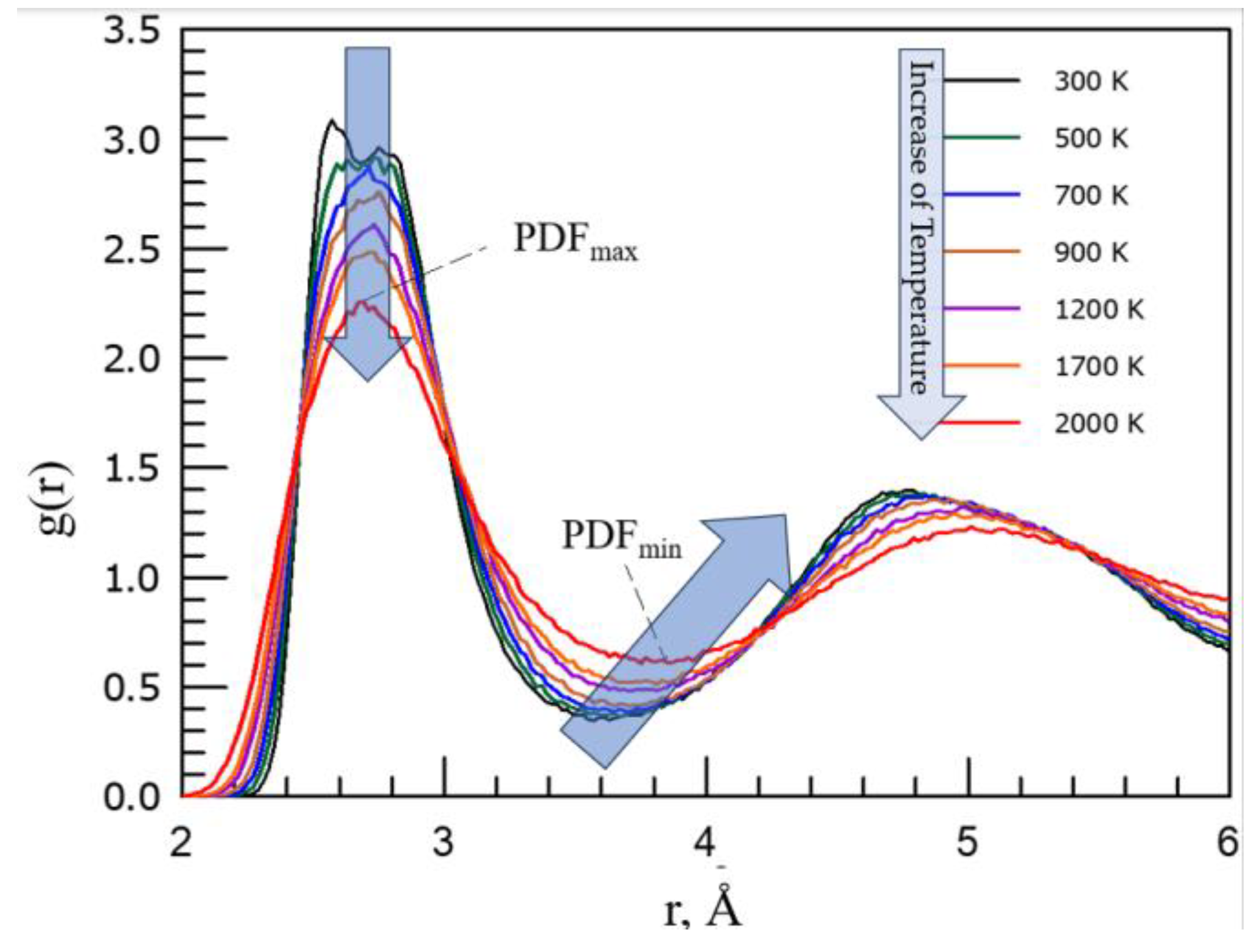
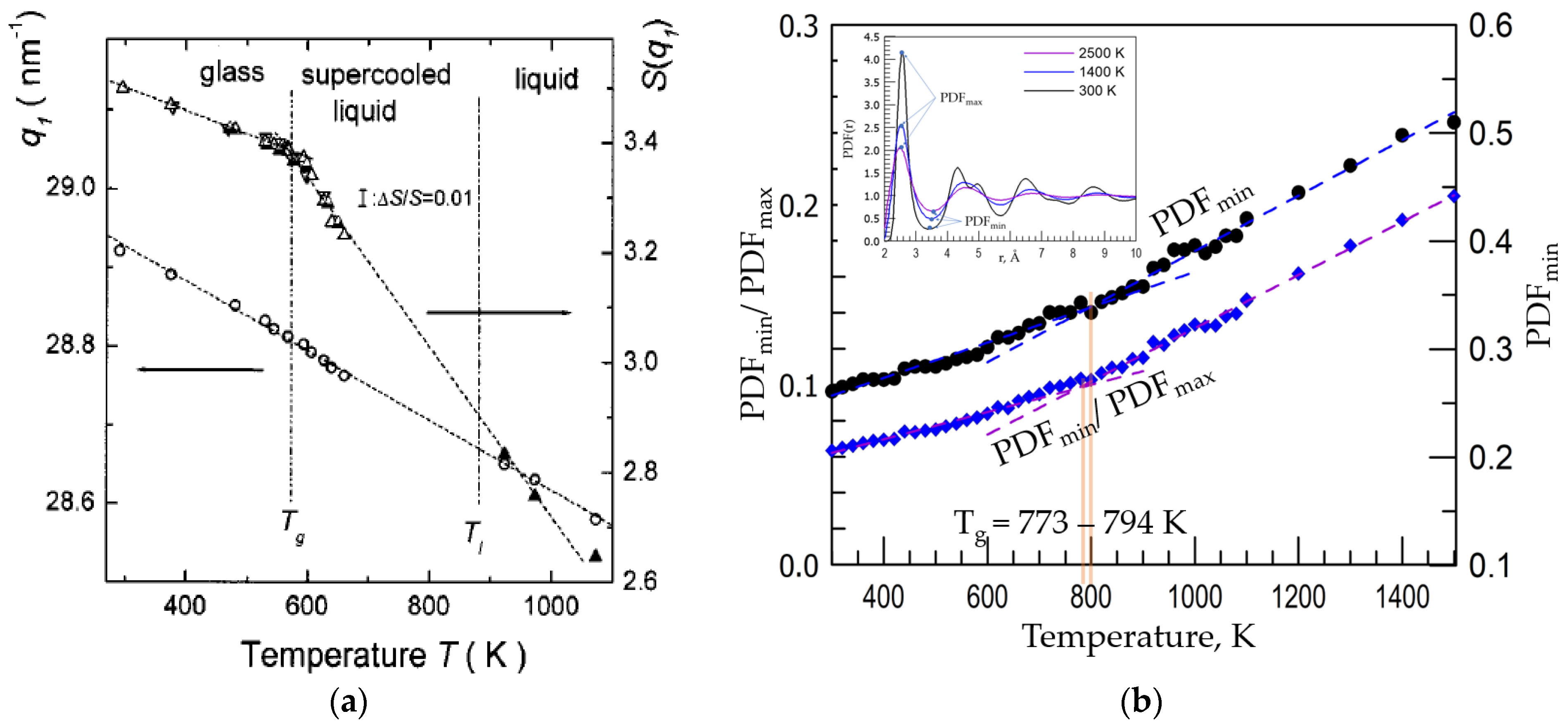
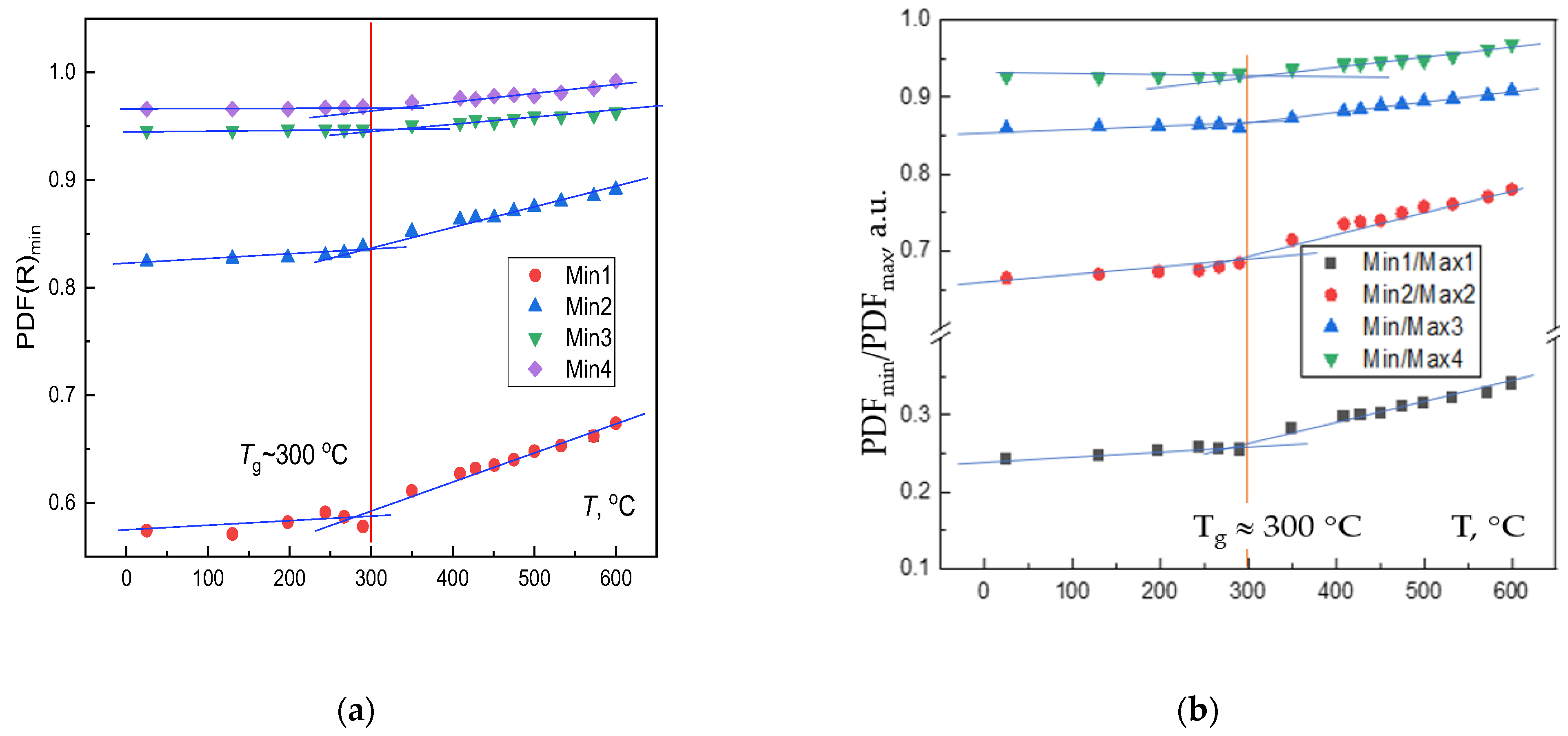
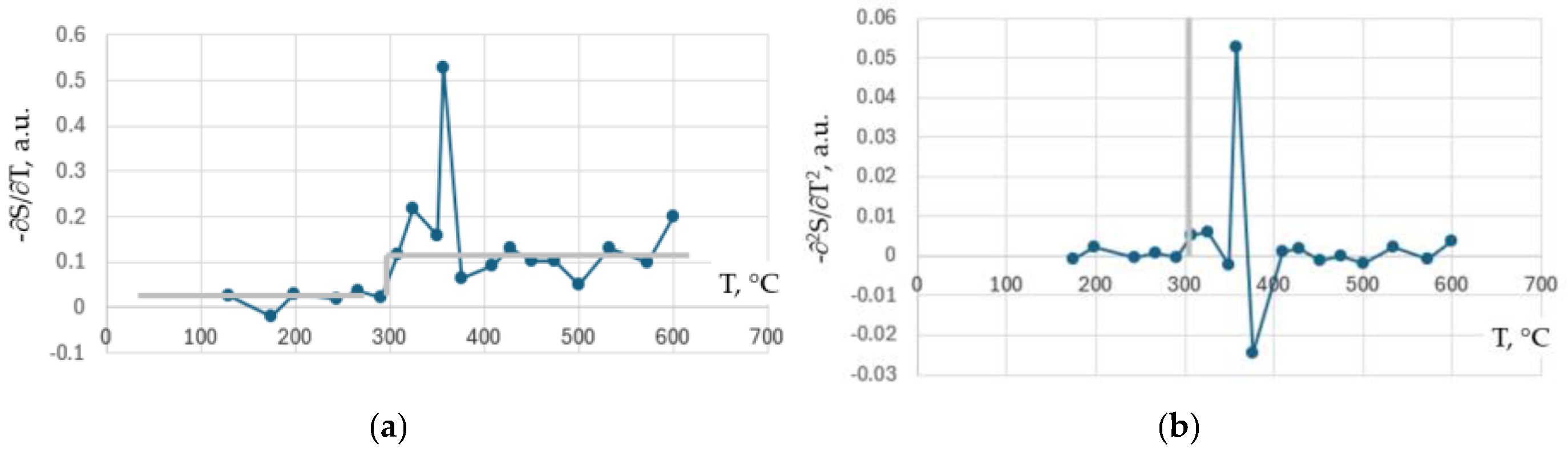
3. Experimental Results
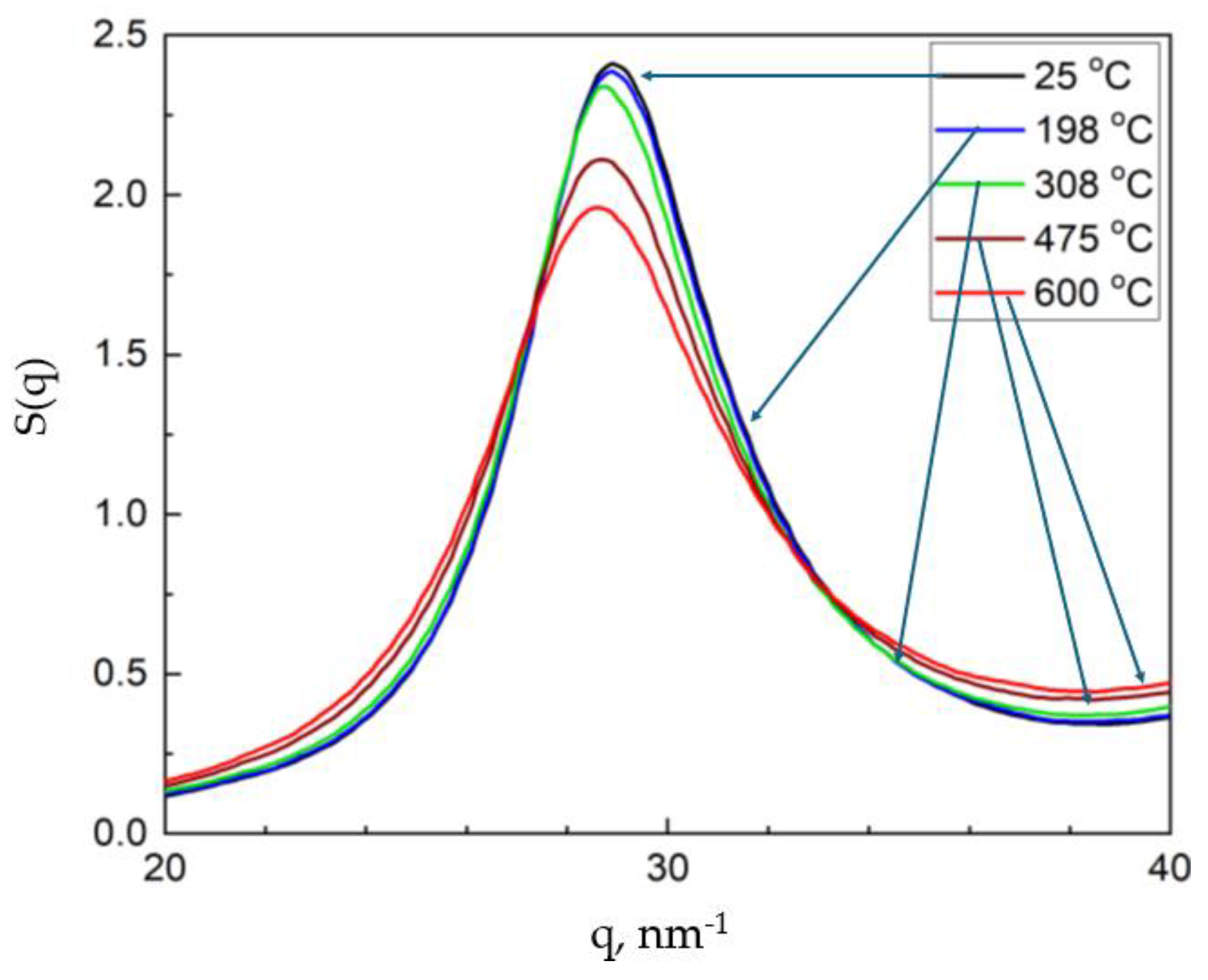
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPT | Configuron percolation theory |
| FCS | First coordination shell |
| FSDM | First sharp diffraction minimum |
| DMA | Dynamic mechanical analysis, |
| DTA | Differential thermal analysis |
| Pair distribution function | |
| RDF | Radial distribution function |
| TMA | Thermal mechanical analysis |
References
- Kauzmann, W. The nature of the glassy state and the behavior of liquids at low temperatures. Chem. Rev. 1948, 43, 219–256. [Google Scholar] [CrossRef]
- Martinez, L.-M.; Angell, C.A. A thermodynamic connection to the fragility of glass-forming liquids. Nature 2001, 410, 663–667. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Montazerian, M.; Gulbiten, O.; Mauro, J.C.; Zanotto, E.D.; Yue, Y. Understanding glass through differential scanning calorimetry. Chem. Rev. 2019, 119, 7848–7939. [Google Scholar] [CrossRef]
- ASTM E1356-08; Standard Test Method for Assignment of the Glass Transition Temperatures by Differential Scanning Calorimetry. American Society of Testing and Materials: Philadelphia, PA, USA, 2014. Available online: https://www.astm.org/e1356-08r14.html (accessed on 29 July 2025).
- ASTM E794-06; Standard Test Method for Melting and Crystallization Temperatures by Thermal Analysis. American Society of Testing and Materials: Philadelphia, PA, USA, 2018. Available online: https://www.astm.org/e0794-06r18.html (accessed on 29 July 2025).
- ASTM E1640-13; Standard Test Method for Assignment of the Glass Transition Temperature by Dynamic Mechanical Analysis. American Society of Testing and Materials: Philadelphia, PA, USA, 2018.
- Kato, H.; Yavari, A.R.; Le Moulec, A.; Inoue, A.; Nishiyama, N.; Lupu, N.; Matsubara, E.; Botta, W.J.; Vaughan, G.; Di Michiel, M.; et al. Excess free volume in metallic glasses measured by X-ray diffraction. Acta Mater. 2005, 53, 1611. [Google Scholar] [CrossRef]
- Chen, H.-S.; Inoue, A. Relationship between thermal expansion coefficient and glass transition temperature in metallic glasses. Scr. Mater. 2008, 58, 1106–1109. [Google Scholar] [CrossRef]
- Glass Transition Temperature. Omnexus. Available online: https://www.specialchem.com/plastics/guide/glass-transition-temperature (accessed on 16 July 2025).
- Suryanarayana, C.; Inoue, A. Bulk metallic Glasses, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 520. [Google Scholar]
- Louzguine-Luzgin, D.V.; Belosludov, R.V.; Ojovan, M.I. Room-temperature pressure-induced phase separation in glassy alloys. Mater. Today Commun. 2024, 40, 109453. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V. Crystallization of metallic glasses and supercooled liquids. Materials 2024, 17, 3573. [Google Scholar] [CrossRef] [PubMed]
- Wendt, H.H.; Abraham, F.F. Empirical criterion for the glass transition region based on monte carlo simulations. Phys. Rev. Lett. 1978, 41, 1244–1246. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Louzguine-Luzgin, D.V. Revealing structural changes at glass transition via radial distribution functions. J. Phys. Chem. B 2020, 124, 3186–3194. [Google Scholar] [CrossRef]
- Kantor, Y.; Webman, I. Elastic properties of random percolating systems. Phys. Rev. Lett. 1984, 52, 1891–1894. [Google Scholar] [CrossRef]
- Ojovan, M.I. Glass formation in amorphous SiO2 as a percolation phase transition in a system of network defects. J. Exp. Theor. Phys. Lett. 2004, 79, 632–634. [Google Scholar] [CrossRef]
- Scher, H.; Zallen, R. Critical Density in Percolation Processes. J. Chem. Phys. 1970, 53, 3759–3761. [Google Scholar] [CrossRef]
- Isichenko, M.B. Percolation, Statistical Topography, and Transport in Random Media. Rev. Mod. Phys. 1992, 64, 961–1043. [Google Scholar] [CrossRef]
- Mattern, N.; Hermann, H.; Roth, S.; Sakowski, J.; Macht, M.-P.; Jovari, P.; Jiang, J. Structural behavior of Pd40Cu30Ni10P20 bulk metallic glass below and above the glass transition. Appl. Phys. Lett. 2003, 82, 2589–2591. [Google Scholar] [CrossRef]
- Hansen, J.-P.; McDonald, I.R. Theory of Simple Liquids, 3rd ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 78–108. [Google Scholar] [CrossRef]
- Kitamura, T. Liquid Glass Transition. A Unified Theory from the Two Band Model; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Fischer, H.E.; Barnes, A.C.; Salmon, P.S. Neutron and X-ray Diffraction Studies of Liquids and Glasses. Rep. Prog. Phys. 2006, 69, 233–299. [Google Scholar] [CrossRef]
- Ojovan, M.I. The Modified Random Network (MRN) Model within the Configuron Percolation Theory (CPT) of Glass Transition. Ceramics 2021, 4, 121–134. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Tournier, R.F. On structural rearrangements near the glass transition temperature in amorphous silica. Materials 2021, 14, 19. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Louzguine-Luzgin, D.V. On Structural Rearrangements during the Vitrification of Molten Copper. Materials 2022, 15, 1313. [Google Scholar] [CrossRef]
- Soper, A.K. The Radial Distribution Functions of Water as Derived from Radiation Total Scattering Experiments: Is There Anything We Can Say for Sure? ISRN Phys. Chem. 2013, 2013, 279463. [Google Scholar] [CrossRef]
- Stoch, P.; Krakowiak, I. Thermal properties of Sr-containing iron-phosphate glasses experimental and theoretical approach. J. Therm. Anal. Calorim. 2025. [Google Scholar] [CrossRef]
- Georgarakis, K.; Louzguine-Luzgin, D.V.; Antonowicz, J.; Vaughan, G.; Yavari, A.R.; Egami, T.; Inoue, A. Variations in atomic structural features of a supercooled Pd–Ni–Cu–P glass forming liquid during in situ vitrification. Acta Mater. 2011, 59, 708–716. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Belosludov, R.A.; Yavari, R.; Georgarakis, K.; Vaughan, G.; Kawazoe, Y.; Egami, T.; Inoue, A. Structural basis for supercooled liquid fragility established by synchrotron-radiation method and computer simulation. J. Appl. Phys. 2011, 110, 043519. [Google Scholar] [CrossRef]
- Sanditov, D.S.; Ojovan, M.I. Relaxation aspects of the liquid—Glass transition. Phys. Uspekhi 2019, 62, 111–130. [Google Scholar] [CrossRef]
- Gallino, I.; Busch, R. Thermodynamic and kinetic Aspects of the Glass Transition. In Physical Metallurgy of Bulk Metallic Glass-Forming Liquids; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2024; Volume 341. [Google Scholar]
- Yue, Y.-Z. Characteristic temperatures of enthalpy relaxation in glass. J. Non-Cryst. Solids 2008, 354, 1112–1118. [Google Scholar] [CrossRef]
- Varshneya, A.K. Fundamentals of Inorganic Glasses; Society of Glass Technology: Sheffield, UK, 2006. [Google Scholar]
- Zarzycki, J. Glasses and the Vitreous State; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Ehrenfest, P. Phasenumwandlungen im Ueblichen und Erweiterten Sinn, Classifiziert nach den Entsprechenden SINGULARITAETEN des Thermodynamischen Potentiales; N. V. Noord-Hollandsche Uitgevers Maatschappij: Amsterdam, The Netherlands, 1933. [Google Scholar]
- Landau, L.D.; Lifshitz, E.M. Course of Theoretical Physics Volume 5: Statistical Physics Part 1; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Stanley, H.E. Introduction to Phase Transitions and Critical Phenomena; International Series of Monographs on Physics; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Albert, S.; Bauer, T.H.; Michl, M.; Biroli, G.; Bouchaud, J.-P.; Loidl, A.; Lunkenheimer, P.; Tourbot, R.; Wiertel-Gasquet, C.; Ladieu, F. Fifth-order susceptibility unveils growth of thermodynamic amorphous order in glass-formers. Science 2016, 352, 1308−1311. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Compendium of Chemical Terminology; Royal Society of Chemistry: Cambridge, UK, 1997; Volume 66, p. 583. [Google Scholar]
- Ojovan, M. Glass formation., Chapter 3.1. In Encyclopedia of Glass Science, Technology, History, and Culture; Richet, P., Conradt, R., Takada, A., Dyon, J., Eds.; Wiley: Hoboken, NJ, USA, 2021; Volume 1568, pp. 249–259. [Google Scholar]
- Egami, T.; Ryu, C.W. Origin of medium-range atomic correlation in simple liquids: Density wave theory. AIP Adv. 2023, 13, 085308. [Google Scholar] [CrossRef]
- Patashinsky, A.Z.; Pokrovsky, V.L. Fluctuation Theory of Phase Transition; Nauka: Moscow, Russia, 1982; p. 382. [Google Scholar]
- Kuhner, C.L. Phase Transitions and Critical Phenomena. Statistical Physics Seminar SS2023. Seminar Talk Summary for the Seminar of Statistical Physics Held by Prof. Wolschin; Department of Physics, Heidelberg University: Heidelberg, Germany, 2023; Volume 25. [Google Scholar]
- Bokov, N.A. Non-equilibrium fluctuations as a plausible reason of the light scattering intensity peak in the glass transition region. J. Non-Cryst. Solids 2008, 354, 1119–1122. [Google Scholar] [CrossRef]
- Goldenfeld, N. Lectures on Phase Transitions and the Renormalization Group; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Haken, H. Synergetics: An Introduction: Nonequilibrium Phase Transitions and Self-Organization in Physics; Chemistry and Biology Springer: Berlin, Germany, 2004. [Google Scholar]
- Henkel, M.; Pleimling, M. Non-Equilibrium Phase Transitions: Volume 2: Ageing and Dynamical Scaling Far from Equilibrium; Springer Science & Business Media: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Vadakkayil, N.; Esposito, M.; Meibohm, J. Critical fluctuations at a finite-time dynamical phase transition. Phys. Re. E 2024, 110, 064156. [Google Scholar] [CrossRef]
- Bray, A.J. Theory of phase-ordering kinetics. Adv. Phys. 2002, 51, 481. [Google Scholar] [CrossRef]
- Lu, Z.; Raz, O. Nonequilibrium thermodynamics of the Markovian Mpemba effect and its inverse. Proc. Natl. Acad. Sci. USA 2017, 114, 5083. [Google Scholar] [CrossRef]
- Vadakkayil, N.; Das, S.K. Should a hotter paramagnet transform quicker to a ferromagnet? Monte Carlo simulation results for Ising model. Phys. Chem. Chem. Phys. 2021, 23, 11186. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Building and Breaking Bonds by Homogenous Nucleation in Glass-Forming Melts Leading to Transitions in Three Liquid States. Materials 2021, 14, 2287. [Google Scholar] [CrossRef]
- Holtzman, R.; Raz, O. Landau theory for the Mpemba effect through phase transitions. Commun. Phys. 2022, 5, 280. [Google Scholar] [CrossRef]
- Kovacs, A.J.; Aklonis, J.J.; Hutchinson, J.M.; Ramos, A.R. Isobaric volume and enthalpy recovery of glasses. II. A transparent multiparameter theory. J. Polym. Sci. Polym. Phys. 1979, 17, 1097. [Google Scholar] [CrossRef]
- Bertin, E.; Bouchaud, J.; Drouffe, J.; Godreche, C. The Kovacs effect in model glasses. J. Phys. A 2003, 36, 10701. [Google Scholar] [CrossRef]
- Meibohm, J.; Forastiere, D.; Adeleke-Larodo, T.; Proesmans, K. Relaxation-speed crossover in anharmonic potentials. Phys. Rev. E 2021, 104, L032105. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.; Dieball, C.; Lasanta, A.; Godec, A.; Rica, R.A. Heating and cooling are fundamentally asymmetric and evolve along distinct pathways. Nat. Phys. 2024, 20, 135. [Google Scholar] [CrossRef]
- Fan, G.J.; Löffler, J.F.; Wunderlich, R.K.; Fecht, H.J. Thermodynamics, enthalpy relaxation and fragility of the bulk metallic glass-forming liquid Pd43Ni10Cu27P20. Acta Mater. 2004, 52, 667. [Google Scholar] [CrossRef]
- Mauro, N.A.; Blodgett, M.; Johnson, M.L.; Vogt, A.J.; Kelton, K.F. A structural signature of liquid fragility. Nat. Commun. 2014, 5, 4616. [Google Scholar] [CrossRef]
- Scopigno, T.; Ruocco, G.; Sette, F.; Monaco, G. Is the fragility of a liquid embedded in the properties of its glass? Science 2003, 302, 849–852. [Google Scholar] [CrossRef]
- Tanaka, H.; Kawasaki, T.; Shintani, H.; Watanabe, K. Critical-like behaviour of glass-forming liquids. Nat. Mater. 2010, 9, 324–331. [Google Scholar] [CrossRef]
- Vasin, M.G. Glass transition as a topological phase transition. Phys. Rev. E 2022, 106, 044124. [Google Scholar] [CrossRef]
- Ojovan, M.I. The Flow of Glasses and Glass–Liquid Transition under Electron Irradiation. Int. J. Mol. Sci. 2023, 24, 12120. [Google Scholar] [CrossRef]
- Rusinowicz, M.; Sao-Joao, S.; Bourguignon, M.; Rosales-Sosa, G.; Kato, Y.; Volpi, F.; Barthel, E.; Kermouche, G. Electric charges as an apparent governing parameter for electron induced stress relaxation in amorphous silica micropillars. Scr. Mater. 2025, 261, 116628. [Google Scholar] [CrossRef]
- Bruns, S.; Kang, S.-G.; Choi, I.S.; Durst, K. The role of electron-beam irradiation on small-scale deformation: Challenges and benefits in in situ SEM indentation tests. MRS Bull. 2025, 50, 735–748. [Google Scholar] [CrossRef]
- Ulitin, N.V.; Shadrina, G.R.; Anisimova, V.I.; Rodionov, I.S.; Baldinov, A.A.; Lyulinskaya, Y.L.; Tereshchenko, K.A.; Shiyan, D.A. Interpretation of the Structure–Glass Transition Temperature Relationship for Organic Homopolymers with the Use of Increment, Random Forest, and Density Functional Theory Methods. J. Struct. Chem. 2025, 66, 1095–1109. [Google Scholar] [CrossRef]
- Qiao, J.C.; Wang, Q.; Pelletier, J.M.; Kato, H.; Casalini, R.; Crespo, D.; Pineda, E.; Yao, Y.; Yang, Y. Structural heterogeneities and mechanical behavior of amorphous alloys. Prog. Mater. Sci. 2019, 104, 250–329. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Seki, I.; Yamamoto, T.; Kawaji, H.; Suryanarayana, C.; Inoue, A. Double-stage glass transition in a metallic glass. Phys. Rev. B 2010, 81, 144202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojovan, M.I.; Lu, A.K.A.; Louzguine-Luzgin, D.V. Massive Fluctuations in the Derivatives of Pair Distribution Function Minima and Maxima During the Glass Transition. Metals 2025, 15, 869. https://doi.org/10.3390/met15080869
Ojovan MI, Lu AKA, Louzguine-Luzgin DV. Massive Fluctuations in the Derivatives of Pair Distribution Function Minima and Maxima During the Glass Transition. Metals. 2025; 15(8):869. https://doi.org/10.3390/met15080869
Chicago/Turabian StyleOjovan, Michael I., Anh Khoa Augustin Lu, and Dmitri V. Louzguine-Luzgin. 2025. "Massive Fluctuations in the Derivatives of Pair Distribution Function Minima and Maxima During the Glass Transition" Metals 15, no. 8: 869. https://doi.org/10.3390/met15080869
APA StyleOjovan, M. I., Lu, A. K. A., & Louzguine-Luzgin, D. V. (2025). Massive Fluctuations in the Derivatives of Pair Distribution Function Minima and Maxima During the Glass Transition. Metals, 15(8), 869. https://doi.org/10.3390/met15080869







