Abstract
The corrosion behavior and electrochemical performance of Mg-La-Zr and Mg-La alloys were studied. Microstructural observation indicated that the trace alloying of Zr refined the grain size of Mg-La alloy, which improved the discharge activity of Mg-La alloys. At the same time, the addition of Zr led to a transformation of the second-phase distribution from intracrystalline to grain boundary central distribution. This change inhibited the self-corrosion of the alloy during discharge and improved the anode utilization efficiency. Therefore, an air battery based on a Mg-La-Zr alloy anode with a unique microstructure demonstrated a high discharge performance. In this paper, the relationship between the microstructure and anodic properties of Mg-La-Zr alloy are systematically elucidated.
1. Introduction
In recent years, Mg-air batteries have attracted the attention of scientists around the world because of their excellent theoretical performance, such as a theoretical discharge voltage of 3.1 V and a theoretical energy density of 6.8 W h g−1 [1,2]. In addition, metal Mg, as the anode of Mg-air batteries, is abundant in nature, has low acquisition costs, and is an environmentally friendly element [3,4]. However, the low utilization rate of the Mg anode has limited the development of Mg-air batteries [5,6]. This problem is mainly caused by the insufficient dissolution-induced anode detachment and self-corrosion in the electrolyte [7,8,9].
Numerous studies have focused on the aforementioned problems, seeking to narrow the gap between the actual performance and the theoretical values of Mg-air batteries [7,10,11]. Anode performance, encompassing discharge voltage and utilization rate, is mainly influenced by the microstructure of the anode material. Thus, enhancing the anode performance of Mg-air batteries is typically achieved by regulating the anode microstructure, including its composition and element distribution, grain size, second phase distribution and type, crystal defects, etc. [11,12,13,14]. Generally, the performance of Mg anodes results from the combined effect of these microstructural factors and is not determined by a single factor. Currently, the main methods for adjusting the alloy microstructure include alloying, processing, and heat treatment technologies. Our previous work demonstrated that extruded Mg-La binary alloy exhibits a non-basal distribution surface, finer grain size, and more uniform second-phase distribution compared to the cast alloy, thereby showing higher discharge voltage and anode utilization rate in Mg-air battery discharge applications [15]. Wang et al. [16] employed Mg-Al-Pb-RE strips of different thicknesses to observe the anodic dissolution. Their results indicated the 5 mm-thick alloy strip possessed outstanding performance because of its relatively weaker (0001) orientation, relatively coarser grain size, and relatively lower dislocation density. Furthermore, a microstructure of a Mg-air battery anode material with high discharge performance was obtained by adjusting the cooling rate during preparation [17]. It can thus be concluded that the discharge performance of anode materials is closely related to their microstructure.
According to the formula of battery energy density, increasing this value requires improving the discharge voltage and avoiding anode self-corrosion and detachment [18]. Our previous study revealed that La can increase the discharge voltage of Mg and help reduce self-corrosion [19]. However, due to the large grain size and the characteristics of the second phase, the active site of Mg-La alloy would be further enhanced. A large number of studies and practices have shown that the addition of Zr has a significant grain-refining effect on Mg alloys without Al [20,21]. Therefore, in this study, we propose to refine the Mg-La alloy using Zr, aiming to increase the anode active sites by increasing the grain boundaries and thereby improve its comprehensive discharge performance.
2. Experimental
The raw materials used for casting the experimental alloys were pure Mg (99.99% by weight, hereafter specified in weight percent, supplied by Chongqing Yuhua New Materials Ltd., Chongqing, China), Mg-20%La master alloy (99.95%, supplied by Ganzhou Feiteng Light Alloy Co., Ltd., Ganzhou, China), and Mg-20%Zr master alloy ingots (99.96%, supplied by Ganzhou Feiteng Light Alloy Co., Ltd., Ganzhou, China). The required amounts of raw materials were placed together in a stainless-steel crucible, then melted under a protective gas atmosphere, cast, and cooled to obtain the ingots. The cast alloys were also subjected to homogenization treatment at 520 °C for 24 h. The nominal compositions of the alloys were Mg-0.4La (denoted as Mg-La) and Mg-0.4La-0.1Zr (denoted as Mg-La-Zr). An inductively coupled plasma-atomic emission spectrometer (ICP-AES, Thermo Fisher Scientific, Waltham, MA, USA) was used to quantify the chemical compositions of the two alloys, which are reported in Table 1. The content deviation of each element did not exceed 0.1%, and the content of each impurity element (such as Fe, Cu, Si, etc.) did not exceed 0.01%.

Table 1.
Chemical compositions of Mg-La and Mg-La-Zr alloys (wt.%).
For optical microscopy (OM) analysis, the surface of the alloy was polished to a metallic luster using sandpapers of different grades and etched to reveal the grain boundaries using a prepared picric acid etchant for 10~20 s. After each step, the sample was rinsed with ethanol and dried with cold air. The metallographic structure of the alloy was observed using an OM (ZEISS Axiovert A1, Carl Zeiss AG, Oberkochen, Germany). The surface morphology was characterized using scanning electron microscopy (SEM, TESCAN-VEGA-3-LMH, Brno, CZ, Germany), which enabled the observation of second-phase distribution via backscattered electron (BSE) mode and element distribution via X-ray energy-dispersive spectroscopy (EDS). An X-ray diffractometer (XRD, D/Max-2500X, Rigaku, Akishima-shi, Japan) was used to analyze the phase composition of the alloy, with scanning performed in the 2θ range of 10°~80° with a speed of 4 °/min.
The immersion test, polarization test, and electrochemical impedance (EIS) and half-cell discharge techniques were used to evaluate the chemical stability and activity of the alloys in NaCl solution. All the test alloy blocks were embedded in epoxy resin, leaving only a 15 mm × 15 mm surface exposed. For the immersion test, the exposed surface was placed facing upward in a beaker, and an inverted burette-funnel device was used to measure the volume of hydrogen gas generated [22]. The immersion time was 72 h. Electrochemical tests were conducted using an electrochemistry workstation (CHI660E, Chenhua, Shanghai, China) with a three electrode system with a Pt counter electrode (Chenhua, Shanghai, China), a saturated calomel electrode (SCE, Chenhua, Shanghai, China) as the reference electrode, and the Mg alloys as the work electrode. The polarization test was performed at a scan rate of 1 mV·s−1, with the potential ranging from −2.1 V to −1.2 V vs. SCE. The EIS test was performed by a 5-mV sinusoidal perturbation over a frequency ranging from 105 to 10−2 Hz. The half-cell discharge was conducted at constant currents (e.g., 2.5 mA cm−2 for 20 h and 10 mA cm−2 for 10 h) to assess the anodic activity during the discharge. The utilization rates of the anodes were calculated after the discharge [23]. Scanning electron microscopy (SEM) was used to observe the surface and the cross section. Additionally, the anode surface free of products was observed using a laser scanning confocal microscope (Olympus OLS 4000, Olympus Corporation, Shibuya, Tokyo, Japan).
The Mg-air battery was assembled using a C/MnO2 catalyst as a cathode, NaCl aqueous solution (3.5%) as the electrolyte, and different types of Mg alloys as anodes. The active area ratio of cathode to anode was 3:1, i.e., 3 cm2 and 1 cm2, respectively. To compare the discharge stability of anode materials, a constant current discharge test was carried out for 10 h in NaCl aqueous solution. Voltage–current and energy density-current tests were carried out to evaluate the discharge outputs of full battery with increasing current. The voltage value was the average of that for 10 h. The energy density was calculated by the amount of transferred charge and weight loss of the Mg anode [23].
3. Results and Discussion
3.1. Microstructure
Figure 1 presents the XRD spectrum of two prepared Mg alloys, which can be used to qualitatively analyze the crystal structure composition of each alloy. Combining the previous research results with the PDF card (#35-0821) of Mg, it could be seen that Mg-La and Mg-La-Zr alloys were mainly composed of α-Mg phase and Mg12La phase [19]. Except for the α-Mg phase peaks, others were Mg12La phase. The result shows that the homogenization treatment did not change the phase composition of the alloy, but the addition of Zr to the alloy made the intensity of each peak stronger, indicating that the Zr in the alloy promoted the alloy more crystalline. In addition, the diffraction peaks of Zr-containing compounds were not detected, because the solid solubility of Zr in Mg is very low and it tends to react with impurity elements such as Fe and Ni during the melting process [24,25].

Figure 1.
XRD patterns of Mg-La and Mg-La-Zr alloys.
Microstructure and element distribution of the two alloys are shown in Figure 2. By selecting three metallographic images of the same magnification, statistical analysis showed that the average grain sizes of Mg-La (Figure 2a) and Mg-La-Zr (Figure 2b) alloys were 2.25 mm and 130 μm, respectively. The results show that the grain size of the alloy can be refined from millimeter level to micron level by adding only 0.1 wt.% Zr to the Mg-La alloy. It can be seen that the addition of Zr significantly affected the grain size of the alloy. Refinement of the alloy in turn also increased the grain boundaries. Figure 2c,d are SEM images indicating the second phase distribution features on the surfaces of the two alloys. For Mg-La alloys, the second phase was mainly distributed inside the grains, which were mostly rod-shaped and granular (Figure 2c). In the Mg-La-Zr alloy, the second phase was distributed continuously at the grain boundaries. Moreover, it can be seen from the element surface distribution diagram that the Zr element was evenly distributed inside the alloy. The EDS results also showed that the atomic ratio of Mg and La elements in the two alloys was close to 12:1 (not presenting the exact value here), so the second phase of the two alloys was Mg12La phase, which was consistent with the XRD results and previous research [19].
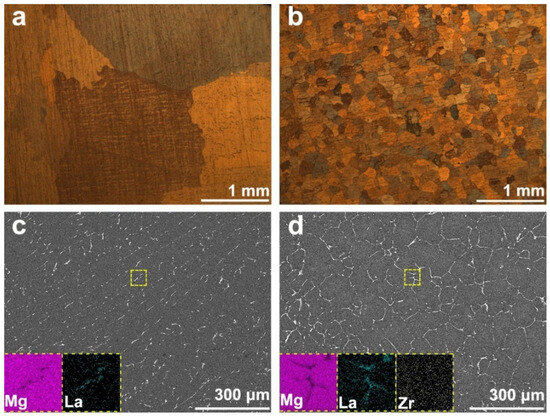
Figure 2.
OM (a,b) and SEM images of alloys (c,d) with the inset in c and d referring to the EDX mapping of each element in the area marked with the yellow rectangle: (a,c) for Mg-La, (b,d) for Mg-La-Zr alloy.
3.2. Chemical Stability and Activity in NaCl Aqueous Solution
The resistance to corrosion of Mg alloys in NaCl solution in an open circuit and the activity in high dissolution are critical air-battery properties. The correlation between alloy properties and their microstructures are discussed below. Figure 3 illustrates the hydrogen evolution profiles of Mg-La and Mg-La-Zr alloys under open-circuit conditions. Three parallel samples of each alloy were selected and tested under the same conditions. It can be seen from the total hydrogen evolution curves of the two alloys (Figure 3a) that the initial generation rate of hydrogen on the surface of the two alloys was smaller than that in the later stage. This was attributed to corrosion product film formation on the alloy surface during the initial immersion [19]. Afterwards, as the corrosion progressed, the film broke, resulting in an increase in the amount of hydrogen evolution. In addition, in the early stage of immersion, that is, within nearly 8 h, the hydrogen evolution rate of Mg-La-Zr alloy was higher than that of Mg-La alloy, indicating that the self-corrosion rate of the former was higher than that of the latter. This was because the Mg-La-Zr alloy had more grain boundaries than the Mg-La alloy. Grain boundaries are crystal defects with relatively high energy. Correspondingly, the grain boundaries were more active than the interior of the crystal. In the electrolyte, galvanic pairs formed at the grain boundaries and inside the grains, causing the galvanic corrosion [26]. However, the trend changed in the hydrogen evolution curves collected after the longer immersion time than 8 h. The amount of hydrogen evolution of the Mg-La-Zr alloy increased slowly. This was due to the second phase distributing at the grain boundary, which can slow the self-corrosion of the alloy to a certain extent [27]. At the same time, the hydrogen evolution amount of Mg-La alloy was higher than that of Mg-La-Zr alloy until the end of the reaction. A reasonable explanation for this is that the driving force for corrosion caused by the galvanic couple formed by the second phase (cathode phase) and the matrix (anode phase) in the Mg-La alloy was greater than the protective effect of the oxide film generated on the alloy surface. Therefore, in the later stage of immersion, the amount of hydrogen evolution of the Mg-La-Zr alloy was surpassed by that of the Mg-La alloy. Throughout the entire hydrogen evolution process that lasted for 72 h, the total hydrogen evolution volume of the Mg-La-Zr alloy was smaller than that of the Mg-La alloy, suggesting that the average corrosion rate of the former in 3.5 wt.% NaCl solution was lower than that of the latter. The self-corrosion rates of the two alloys could be directly calculated based on the volume of hydrogen released during the immersion process. That is, according to the following formula [28], the average corrosion rate (rc) of the Mg-La and Mg-La-Zr alloy was 4.02 mm y−1 and 3.18 mm y−1, respectively.
where ∆m is the mass loss (mg), S is the surface area (cm2), and t is the time of immersion (d).
rc = 2.1 ∆m/(S·t)
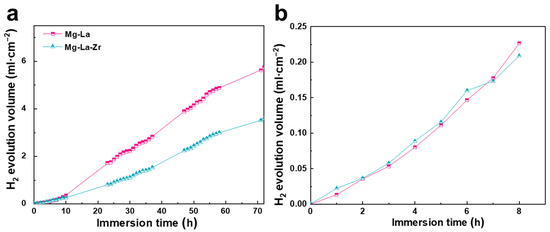
Figure 3.
Hydrogen evolution results of alloys in NaCl aqueous solution for 72 h (a) and the initial hydrogen evolution for 8 h (b).
The impedance curves of the alloy in NaCl solution were obtained using conventional EIS technology. Figure 4 presents the Nyquist curves of Mg-La and Mg-La-Zr alloys. The curve presents a typical, two semi-circular arc shape. It can be clearly seen that the impedance arc diameter of the total Nyquist curve of Mg-La-Zr alloy was smaller than that of Mg-La alloy, especially in the high-frequency area of the first loop. This area indicated that the alloy had film resistance in the solution [29]. The larger the alloy of the radius of the loop, the more corrosion-resistant it is, that is, the corrosion rate is smaller [4]; in contrast, the corrosion rate is larger. This test result also explains why the amount of hydrogen that evolved from Mg-La-Zr alloy was greater than that of Mg-La alloy in the early stage of hydrogen evolution. In addition, the small radius of the loop meant that the dissolution resistance of the alloy in the solution was small, which meant that the anode overvoltage was low [30]. The result shows that the addition of Zr reduced the overvoltage of the alloy dissolving in the solution, which contributed to improving the discharge activity of the alloy when used as an anode.
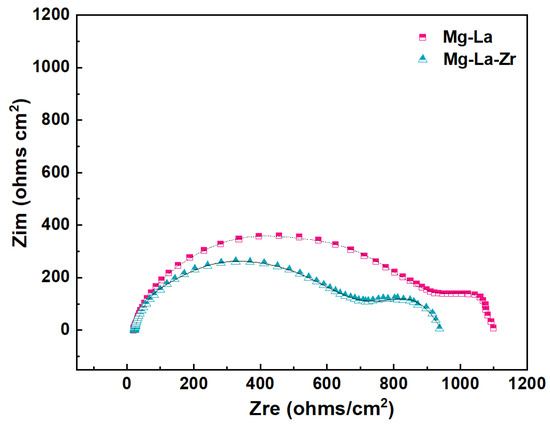
Figure 4.
Nyquist plots of Mg-La and Mg-La-Zr alloys at OCP.
Potentiodynamic polarization curves are an effective tool with which to study the dissolution behavior and corrosion resistance of Mg alloys within a given voltage range. Figure 5 shows the Tafel curves of the two alloys, and Table 2 lists the relevant parameters. The addition of Zr to the Mg-La alloy caused the corrosion potential to shift negatively, indicating that the addition of Zr improved the electrochemical activity of the Mg-La alloy [23]. The cathode process reflected the kinetics of the reduction reaction of water in the solution [31]. The corrosion current density of the alloy could be calculated by extrapolation [32]. That is, the linear Tafel region of the cathode polarization process of the polarization curve extended back to the corrosion potential value. The linear region selected in this study was between −120 and −250 mV relative to the corrosion potential value; the corrosion current density values are listed in Table 1. The Mg-La-Zr alloy had a smaller corrosion current density and could be stably present in the electrolyte when the battery was not working. This experimental result shows that the Mg-La-Zr alloy had better electrochemical activity and a lower corrosion rate than the Mg-La alloy in 3.5 wt.% NaCl electrolyte. However, the corrosion rate calculated by the polarization curve only represented the instantaneous corrosion rate of the alloy and could not directly predict the true corrosion behavior of the alloy. Therefore, the prediction of the corrosion behavior of the two alloys was still based on the hydrogen evolution test [33]. In addition, the different anodic polarization processes of the two alloys meant that they exhibited different anodic dissolution behaviors. This polarization process was mainly related to the oxidation reaction on the alloy surface [31]. Figure 5b shows the anodic polarization branch derived from the Tafel curve. It can be seen that the slope of the anodic polarization zone of the Mg-La alloy was small, i.e., 40.29 mA V−1 cm−2, indicating that the current density of the alloy increased slowly with the increase of voltage. While the larger slope value (50.28 mA V−1 cm−2) indicated that at the same potential, the current density of the Mg-La-Zr alloy was higher than that of the Mg-La alloy, and the corresponding oxidation reaction activity on the surface of the Mg-La-Zr alloy was stronger than that of the Mg-La alloy. Therefore, when the Mg-La-Zr alloy was used as the anode of the battery, it helped to increase the discharge voltage of the battery.
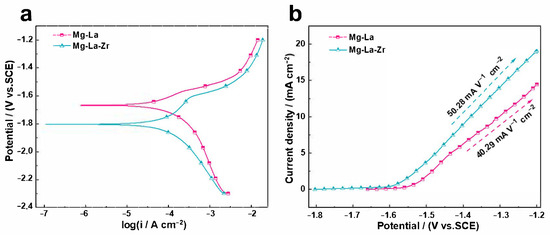
Figure 5.
Potentiodynamic polarization curves (a) and anodic polarization branches (b) of Mg-La and Mg-La-Zr alloys.

Table 2.
Electrochemical parameters of the polarization curves.
The half-cell discharge performance of Mg-La and Mg-La-Zr alloys at different current densities was evaluated by constant current discharge test, and the dissolution behavior of the alloy in the solution was further explored. Figure 6 presents the half-cell discharge results of each alloy. The average discharge voltage and anode utilization rate after the entire discharge test are listed in Table 3. It can be seen from Figure 6 that the Mg-La-Zr alloy showed a more negative discharge potential than the Mg-La alloy when discharging at different current densities, i.e., the average discharge potential of the Mg-La alloy was −1.56 V (vs. SCE), while that of the Mg-La-Zr alloy was −1.59 V (vs. SCE) at a current density of 10 mA cm−2. The result indicated that the Mg-La-Zr alloy helped to increase the discharge voltage of the battery when it was used as the anode of the air battery. The different discharge behaviors of the two alloy anodes in the half-cell reaction were mainly affected by the microstructure. The grain size of Mg-La-Zr alloy was relatively small, so it had more grain boundaries than the Mg-La alloy. As crystal defects, grain boundaries have higher energy than those inside the crystal, which helped to improve the overall activity of the alloy surface [34]. They can serve as channels for dissolution reactions during the discharge process, promoting rapid dissolution of the anode, and ultimately contributing to increasing the discharge potential. In addition, at the initial discharge, the anode surface is activated, and the potential shifts negatively. Then the potential shifts positively before maintaining stable discharge. This phenomenon is related to the oxidation products generated on the alloy surface during the reaction [35]. Figure 7a–d are product distribution morphologies of Mg-La alloy and Mg-La-Zr alloy after discharging at the current density of 10 mA cm−2 for 1 h. It can be seen from the low-magnification images (Figure 7a,c) that the products on the surfaces of the two alloys were loose, but the product distribution on the surface of the Mg-La-Zr alloy was more sparse when comparing the product morphologies with high magnifications (Figure 7b,d). Not only could the products fall off from the substrate surface by themselves, but more reaction channels promoted effective contact between the electrolyte and the substrate, which correspondingly reduced the dissolution resistance of the alloy surface and ultimately increased the discharge potential.
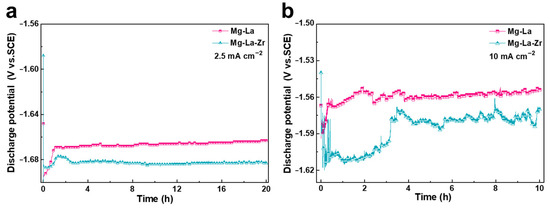
Figure 6.
Galvanostatic discharge curves of the alloys in 3.5 wt% NaCl by the imposition of current densities of (a) 2.5 mA cm−2 and (b) 10 mA cm−2.

Table 3.
Discharge performance parameters of the alloys.
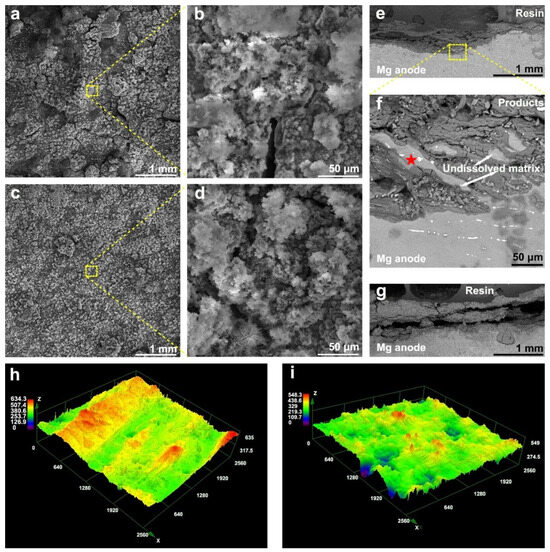
Figure 7.
Surface morphology after discharge at 10 mA cm−2 for 1 h corresponds to (a,b) Mg-La (c,d) Mg-La-Zr; Cross-sectional morphology of (e,f) Mg-La and (g) Mg-La-Zr after discharge at 10 mA cm−2 for 10 h; The undissolved matrix and the second phase are indicated by white arrows and red stars, respectively. Three-dimensional surface morphologies after discharge at 10 mA cm−2 for 10 h without the oxidation products: (h) Mg-La and (i) Mg-La-Zr.
Mg-air batteries are mainly used in low-power and long-term operation applications. If the battery needs to have a high capacity, the anode needs to have a high utilization rate. From the utilization values of each anode listed in Table 2, it can be seen that the alloying Zr in Mg-La alloy improved its anodic utilization. At 10 mA cm−2, the anode utilization rate was as high as 68.9%, which was higher than the utilization values of the alloys that have been studied [36,37,38]. Previous self-corrosion test results have shown that Mg-La-Zr alloy has a low self-corrosion rate, but the anode utilization rate of the alloy is also affected by the self-detachment of the matrix. This part of detachment will cause anode waste, thereby reducing the anode utilization rate and battery energy density [29]. The cross-sectional product morphology of the alloy after discharge given in Figure 7 could be used to predict whether the above phenomenon would occur during the reaction process. As can be seen from Figure 7e,f, there was some undissolved matrix in the product on the surface of the Mg-La alloy. EDS analysis was conducted on the positions of the red marked region, which were mainly composed of three elements, with mass percentages of Mg (98.92%), La (0.32%), and O (0.76%), which proved that most of this area was an undissolved matrix. This free matrix was the loss of anode utilization. In contrast, no matrix shedding phenomenon was found in the cross-sectional view of the product of the Mg-La-Zr alloy in Figure 7g. The results showed that the anode utilization rate of this alloy was less affected by metal shedding than that of the Mg-La alloy. In addition, the surface products of the two alloys after 10 h of reaction were removed, and the dissolution behavior of the alloys could be explained by observing the three-dimensional macroscopic morphology (Figure 7h,i). It can be seen that the surface roughness of the Mg-La-Zr alloy was smaller than that of the Mg-La alloy. This was because the former had a small grain size and a uniform distribution of the second phase, and thus underwent uniform dissolution during the reaction. The uneven distribution of the surface of the Mg-La alloy after removing the product was also the main reason for the metal shedding, resulting in a relatively low anode utilization rate. In short, the self-corrosion rate of the Mg-La-Zr alloy was lower than that of the Mg-La alloy, and the metal shedding phenomenon of the former was weaker than that of the latter. Therefore, the Mg-La-Zr alloy was expected to improve the energy density of the Mg-air battery.
3.3. Mg-Air Battery Anode Property Test
The application of Mg-La and Mg-La-Zr alloys as anode materials in Mg-air batteries was studied, and commercial pure Mg and AZ31 were selected for comparison. Figure 8a,b shows the discharge voltage curves of different anode materials at current densities of 2.5 and 10 mA cm−2. During the first few hours of discharge, the voltage dropped, which was due to the NDE effect, i.e., the accumulation of discharge products and corrosion products on the surface [39]. At 2.5 mA cm−2, other anodes except AZ31 kept the battery voltage stable within a reaction time of 20 h. However, the voltage values of batteries with pure Mg and AZ31 were significantly reduced in the later stage of the reaction at the current density of 10 mA cm−2. Both Mg-La alloy and Mg-La-Zr alloy demonstrated stable discharge. Figure 8c,d show the discharge performance parameters of Mg-air batteries using different anodes at different current densities, including the average discharge voltage and energy density during the entire discharge process. From Figure 8c, it can be seen that the Mg-air battery using the Mg-La-Zr alloy anode presented the highest discharge voltage at different current densities. At low current density, the difference in discharge voltage of each anode was small, but when the current density increased to 20 mA cm−2, the battery voltage using other anodes was obviously below that of the battery using the Mg-La-Zr alloy. For example, the average discharge voltage of the air-battery using Mg-La-Zr alloy anode was as high as 1.2 V at a current density of 20 mA cm−2, while that value of the battery using Mg-La alloy was 1 V, that with pure Mg was 0.94 V, and that with AZ31 was 0.91 V under the same conditions.
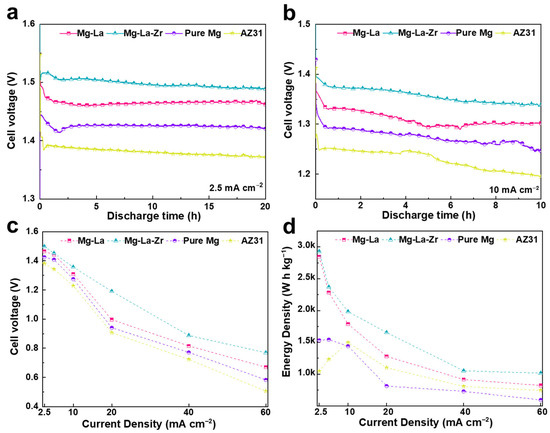
Figure 8.
Mg-air cell performance using different anodes during the battery discharge tests at different current densities, (a,b) for voltage–time curves, (c) for average cell voltage, and (d) for Energy density.
In addition, it can be seen from Figure 8d that when the Mg-air battery discharged using Mg-La-Zr alloy, the energy density provided in all current ranges was higher than that of Mg-La alloy, pure Mg, and AZ31. At 2.5 mA cm−2, the battery energy density was as high as 2929 W h kg−1 after 20 h of discharge with Mg-La-Zr as the anode. Within the current test range selected in this experiment, the energy density decreased with the increase of current density when the battery anode was Mg-La and Mg-La-Zr alloy. This was because the discharge voltage decreased with the increase of current density (Figure 8c). However, when the air battery used pure Mg and AZ31 as the anode, the energy density of the battery showed a different trend of change, unlike when Mg-La and Mg-La-Zr alloy were used. In particular, the energy density peak occurred at a current density of 10 mA cm−2, when the Mg-air battery had an AZ31 anode. From the calculation formula of the energy density, it can be seen that its value mainly depended on the anode utilization value under the condition of the same current density, the same cathode, the same reaction time, and the small discharge voltage difference at low current densities, while the anode utilization was mainly affected by self-corrosion and metal shedding. In addition, self-corrosion became more serious with an increase of current density, in line with NDE theory [7,40,41]. The self-corrosion rate of the alloy was relatively small when discharging at a small current density. Collectively, these results indicate that the low energy densities of batteries with AZ31 and pure Mg at a small current reaction were caused by serious matrix shedding. Furthermore, the energy density based on AZ31 was higher than that of pure Mg at a large current density (greater than 10 mA cm−2). This was because the Mg17Al12 phase in AZ31 could inhibit self-corrosion during the discharge process, which correspondingly improved the anode utilization and the the energy density. In summary, micro alloying with Zr could boost the properties of Mg-La alloy and thus, the discharge voltage and energy density were relatively high, so such an alloy could be used as an anode material for Mg-air batteries.
4. Conclusions
Continuing the work described in a previous study [19], a Mg-La alloy with La addition of 0.4% (wt.%) was micro-alloyed with Zr and evaluated in terms of the effect of Zr addition on the microstructure, chemical stability, activity in NaCl solution, and discharge performance in a Mg-air battery with a homogenized Mg-La alloy. The addition of a trace amount of Zr significantly inhibited the self-corrosion of Mg-La alloy and improved the electrochemical activity. Moreover, compared with commercial pure Mg and AZ31, the Mg-La-Zr alloy showed significantly superior Mg-air battery discharge performance.
The suppressed self-corrosion rate of the Mg-La alloy after Zr addition was based on the aggregation of the second phase at the grain boundaries. The activation of Mg-La anode by Zr was associated with the refinement of the grain via the increase of the grain boundary. In half-cell discharge, the discharge potential of Mg-La-Zr alloy was more negative than that of Mg-La alloy. In addition, due to the low self-corrosion rate and a small amount of metal shedding, the Mg-La-Zr alloy had a high anode utilization rate.
Compared with Mg-La alloy, pure Mg, and AZ31, the air battery using Mg-La-Zr anode showed high voltage and high energy density owing to the improved microstructure. For example, the average battery voltage reached 1.2 V at a current density of 20 mA cm−2 and achieved a remarkable energy density of 2929 W h kg−1 at 2.5 mA cm−2 after discharge for 20 h. Therefore, Mg-La-Zr alloy demonstrated superior potential as a next-generation anode material.
Author Contributions
Conceptualization, Y.G. and B.J.; Methodology, Y.S., G.F., J.Z., G.C. and A.Z.; Validation, Y.S. and G.F.; Investigation, Y.S., G.F., J.Z., G.C. and A.Z.; Resources, Y.S.; Writing—original draft, Y.S.; Writing—review & editing, Y.S., G.F., J.Z., G.C., P.W., A.Z., Y.G. and B.J.; Supervision, P.W., Y.G. and B.J.; Funding acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (U2037601, 52101126, and 52371094), the Postdoctoral Science Foundation of Chongqing (CSTB2023NSCQ-BHX0182), Chongqing Natural Science Fund (CSTB2024NSCQ-LZX0006).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Authors Yan Song, Gang Fang, Junping Zhang, Guanrun Chu were employed by the company China Automotive Engineering Research Institute Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Frei, M.; Martin, J.; Kindler, S.; Cristiano, G.; Zengerle, R.; Kerzenmacher, S. Power supply for electronic contact lenses: Abiotic glucose fuel cells vs. Mg/air batteries. J. Power Sources 2018, 401, 403–414. [Google Scholar] [CrossRef]
- Goel, P.; Dobhal, D.; Sharma, R.C. Aluminum-air batteries: A viability review. J. Energy Storage 2020, 28, 101287. [Google Scholar] [CrossRef]
- Li, D.; Yuan, Y.; Liu, J.; Fichtner, M.; Pan, F. A review on current anode materials for rechargeable Mg batteries. J. Magnes. Alloy 2020, 8, 963–979. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Deng, M.; Wang, L.; Höche, D.; Lamaka, S.V.; Snihirova, D.; Vaghefinazari, B.; Zheludkevich, M.L. Clarifying the decisive factors for utilization efficiency of Mg anodes for primary aqueous batteries. J. Power Sources 2019, 441, 227201. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, C.; Huang, J.; Zhao, Y.; Huang, G.; Wu, L.; Wang, G.; Zhang, H. Effect of Zn concentration on microstructure and corrosion resistance of Mg-Zn alloys microalloyed with Ca and Ce. Anti-Corros. Methods Mater. 2021, 68, 130–136. [Google Scholar] [CrossRef]
- Deng, M.; Wang, L.; Höche, D.; Lamaka, S.V.; Jiang, P.; Snihirova, D.; Scharnagl, N.; Zheludkevich, M.L. Ca/In micro alloying as a novel strategy to simultaneously enhance power and energy density of primary Mg-air batteries from anode aspect. J. Power Sources 2020, 472, 228528. [Google Scholar] [CrossRef]
- Chen, X.; Jia, Y.; Shi, Z.; Le, Q.; Li, J.; Zhang, M.; Liu, M.; Atrens, A. Understanding the discharge behavior of an ultrahigh-purity Mg anode for Mg–air primary batteries. J. Mater. Chem. A 2021, 9, 21387. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Y.; Wu, X.; Fang, H.; Chu, X.; Huang, J.; Zhang, J.; Song, J.; Yu, K. Effects of Al and Sn on microstructure, corrosion behavior and electrochemical performance of Mg-Al-based anodes for magnesium-air batteries. J. Alloys Compd. 2021, 859, 157755. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Wei, Q.; Wang, N.; Hou, B. Electrochemical behavior of Mg-Al-Zn-In alloy as anode materials in 3.5 wt.% NaCl solution. Electrochim. Acta 2017, 238, 156–167. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Q.; Le, Q.; Zou, Q.; Wang, H.; Atrens, A. The influence of samarium (Sm) on the discharge and electrochemical behaviors of the magnesium alloy AZ80 as an anode for the Mg-air battery. Electrochim. Acta 2020, 348, 136315. [Google Scholar] [CrossRef]
- Deng, M.; Höche, D.; Lamaka, S.V.; Wang, L.; Zheludkevich, M.L. Revealing the impact of second phase morphology on discharge properties of binary Mg-Ca anodes for primary Mg-air batteries. Corros. Sci. 2019, 153, 225–235. [Google Scholar] [CrossRef]
- Deng, M.; Wang, L.; Höche, D.; Lamaka, S.V.; Snihirova, D.; Jiang, P.; Zheludkevich, M.L. Corrosion and discharge properties of Ca/Ge micro-alloyed Mg anodes for primary aqueous Mg batteries. Corros. Sci. 2020, 177, 108958. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, Y.; Wang, Y.; Zhang, H.; Pan, F. Performance of Mg-air battery based on AZ31 alloy sheet with twins. Mater. Lett. 2013, 113, 46–49. [Google Scholar] [CrossRef]
- Song, Y.; Yao, K.; Zou, Q.; Yang, Q.; Jiang, B.; Wang, L.; Yuan, M.; Wang, Q.; Huang, G.; Pan, F. Processing Micro-Alloyed Mg-La Binary Alloy into a High-Performance Mg-Air Battery Anode via Extrusion. J. Electrochem. Soc. 2022, 169, 020575. [Google Scholar] [CrossRef]
- Wang, N.G.; Li, W.P.; Huang, Y.X.; Wu, G.; Hu, M.C.; Li, G.Z.; Shi, Z.C. Wrought Mg-Al-Pb-RE alloy strips as the anodes for Mg-air batteries. J. Power Sources 2019, 436, 226855. [Google Scholar] [CrossRef]
- Le, T.; Mao, P.; Hu, W.; Le, Q. The electrochemical corrosion behaviors and Mg-air battery anode performance of Mg–1Sn–1Ca-0.3Mn alloy with different grain size and Mg2Ca morphology. J. Power Sources 2024, 608, 234297. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; Yuan, S.; Bai, J.; Wang, J.; Cao, Y.; Hong, Q. Performance of Mg-9Al-1In alloy as anodes for Mg-air batteries in 3.5 wt% NaCl solutions. J. Electrochem. Soc. 2017, 164, A3131–A3137. [Google Scholar] [CrossRef]
- Song, Y.; Yang, H.; Chai, Y.; Wang, Q.; Jiang, B.; Wu, L.; Zou, Q.; Huang, G.; Pan, F.; Atrens, A. Corrosion and discharge behavior of Mg-xLa alloys (x = 0.0−0.8) as anode materials. Trans. Nonferrous Met. Soc. China 2021, 31, 1979–1992. [Google Scholar] [CrossRef]
- Stjohn, D.H.; Qian, M.; Easton, M.A.; Cao, P.; Hildebrand, Z. Grain refinement of magnesium alloys. Metall. Mater. Trans. A 2005, 36, 1669–1679. [Google Scholar] [CrossRef]
- Peng, Z.K.; Zhang, X.M.; Chen, J.M.; Xiao, Y.; Jiang, H. Grain refining mechanism in Mg-9Gd-4Y alloys by zirconium, Mater. Sci. Technol. 2013, 21, 722–726. [Google Scholar]
- Song, G.; Atrens, A.; St John, D. An hydrogen evolution method for the estimation of the corrosion rate of magnesium alloys. In Essential Readings in Magnesium Technology; Springer: Cham, Switzerland, 2011; pp. 255–262. [Google Scholar]
- Ma, J.; Zhang, Y.; Ma, M.; Qin, C.; Ren, F.; Wang, G. Corrosion and discharge performance of a magnesium aluminum eutectic alloy as anode for magnesium-air batteries. Corros. Sci. 2020, 170, 108695. [Google Scholar] [CrossRef]
- StJohn, D.H.; Cao, P.; Qian, M.; Easton, M.A. A new analytical approach to reveal the mechanisms of grain refinement. Adv. Eng. Mater. 2007, 9, 739–746. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Luo, Y.; Deng, Y.; Guan, L.; Ye, L.; Guo, X.; Luo, A. Effect of grain size and crystal orientation on the corrosion behavior of as-extruded Mg-6Gd-2Y-0.2Zr alloy. Corros. Sci. 2020, 164, 108338. [Google Scholar] [CrossRef]
- Hamu, G.B.; Eliezer, D.; Shin, K.S.; Cohen, S. The relation between microstructure and corrosion behavior of Mg-Y-RE-Zr alloys. J. Alloys Compd. 2007, 431, 269–276. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Song, G.; Liu, M.; Atrens, A. Corrosion behaviour in salt spray and in 3.5% NaCl solution saturated with Mg(OH)2 of as-cast and solution heat-treated binary Mg-X alloys: X=Mn, Sn, Ca, Zn, Al, Zr, Si, Sr. Corros. Sci. 2013, 76, 60–97. [Google Scholar] [CrossRef]
- Deng, M.; Höche, D.; Lamaka, S.V.; Snihirova, D.; Zheludkevich, M.L. Mg-Ca binary alloys as anodes for primary Mg-air batteries. J. Power Sources 2018, 396, 109–118. [Google Scholar] [CrossRef]
- Gomes, M.P.; Costa, I.; Pébère, N.; Rossi, J.L.; Tribollet, B.; Vivier, V. On the corrosion mechanism of Mg investigated by electrochemical impedance spectroscopy. Electrochim. Acta 2019, 306, 61–70. [Google Scholar] [CrossRef]
- Atrens, A.D.; Gentle, I.; Atrens, A. Possible dissolution pathways participating in the Mg corrosion reaction. Corros. Sci. 2015, 92, 173–181. [Google Scholar] [CrossRef]
- Zhao, M.; Schmutz, P.; Brunner, S.; Liu, M.; Song, G.; Atrens, A. An exploratory study of the corrosion of Mg alloys during interrupted salt spray testing. Corros. Sci. 2009, 51, 1277–1292. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H. The effect of grain size on aluminum anodes for Al-air batteries in alkaline electrolytes. J. Power Sources 2015, 284, 409–415. [Google Scholar] [CrossRef]
- Pino, M.; Chacón, J.; Fatás, E.; Ocón, P. Performance of commercial aluminium alloys as anodes in gelled electrolyte aluminium-air batteries. J. Power Sources 2015, 299, 195–201. [Google Scholar] [CrossRef]
- Cheng, S.; Cheng, W.; Gu, X.; Yu, H.; Wang, Z.; Wang, H.; Wang, L. Discharge properties of low-alloyed Mg-Bi-Ca alloys as anode materials for Mg-air batteries: Influence of Ca alloying. J. Alloys Compd. 2020, 823, 153779. [Google Scholar] [CrossRef]
- Wang, N.G.; Wang, R.C.; Peng, C.Q.; Peng, B.; Feng, Y.; Hu, C.W. Discharge behaviour of Mg-Al-Pb and Mg-Al-Pb-In alloys as anodes for Mg-air battery, Electrochim. Acta 2014, 149, 193–205. [Google Scholar]
- Wang, N.G.; Wang, R.C.; Feng, Y.; Xiong, W.H.; Zhang, J.C.; Deng, M. Discharge and corrosion behaviour of Mg-Li-Al-Ce-Y-Zn alloy as the anode for Mg-air battery. Corros. Sci. 2016, 112, 13–24. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Frankel, G.S.; Samaniego, A.; Birbilis, N. Evolution of hydrogen at dissolving magnesium surfaces. Corros. Sci. 2013, 70, 104–111. [Google Scholar] [CrossRef]
- Song, G.L.; Unocic, K.A. The anodic surface film and hydrogen evolution on Mg. Corros. Sci. 2015, 98, 758–765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).