A Recycling-Oriented Approach to Rare Earth Element Recovery Using Low-Cost Agricultural Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biosorbent Preparation
2.3. Biosorbent Characterization
2.4. Design of the Sorption Experiments
2.5. Quantification of REEs
2.6. Data Analysis
2.7. Response Surface Methodology
2.8. Statistical Analysis
3. Results and Discussion
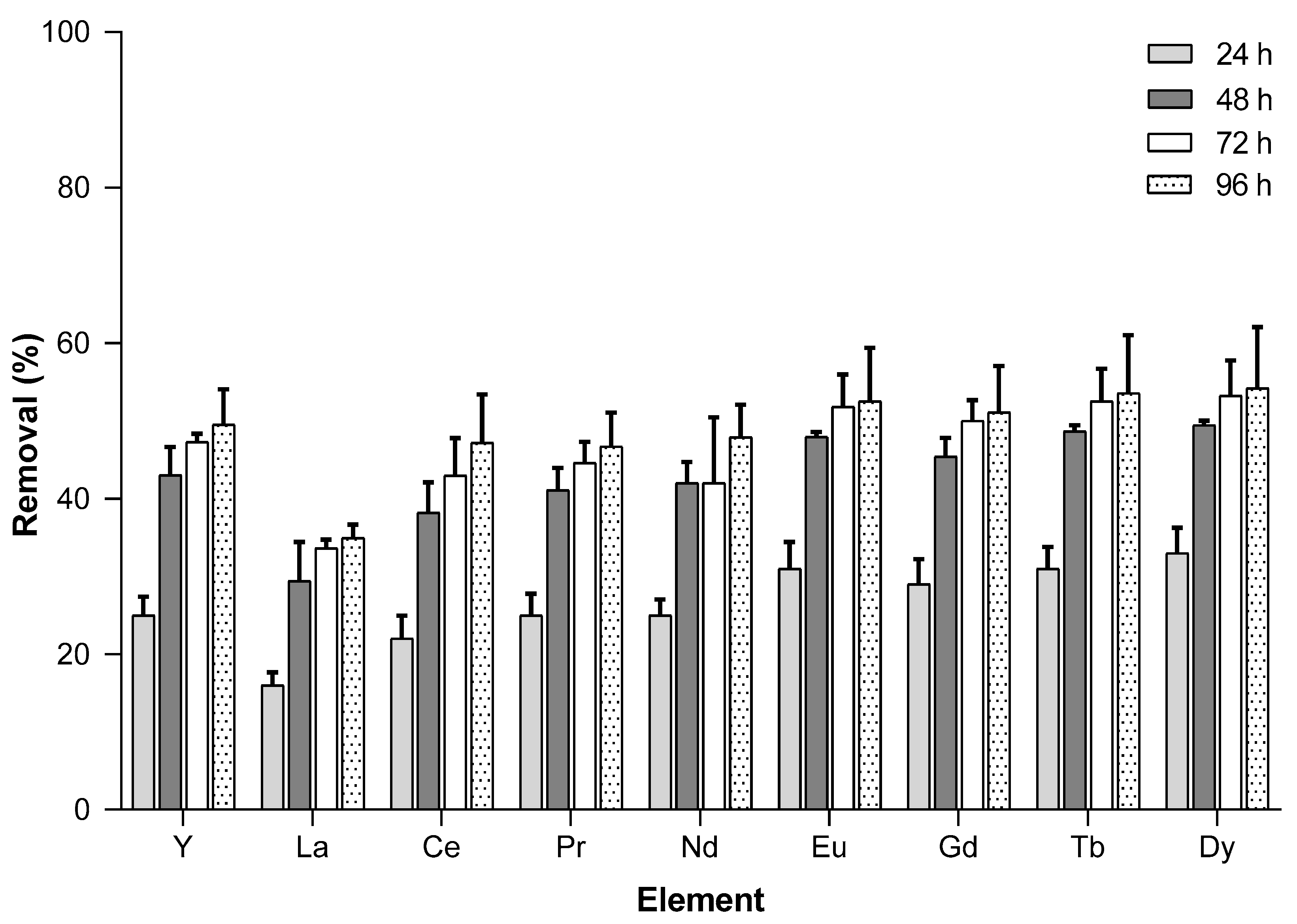
3.1. Removal Percentage of Rare Earth Elements by Hazelnut Shells
3.2. Time Exposure Impact
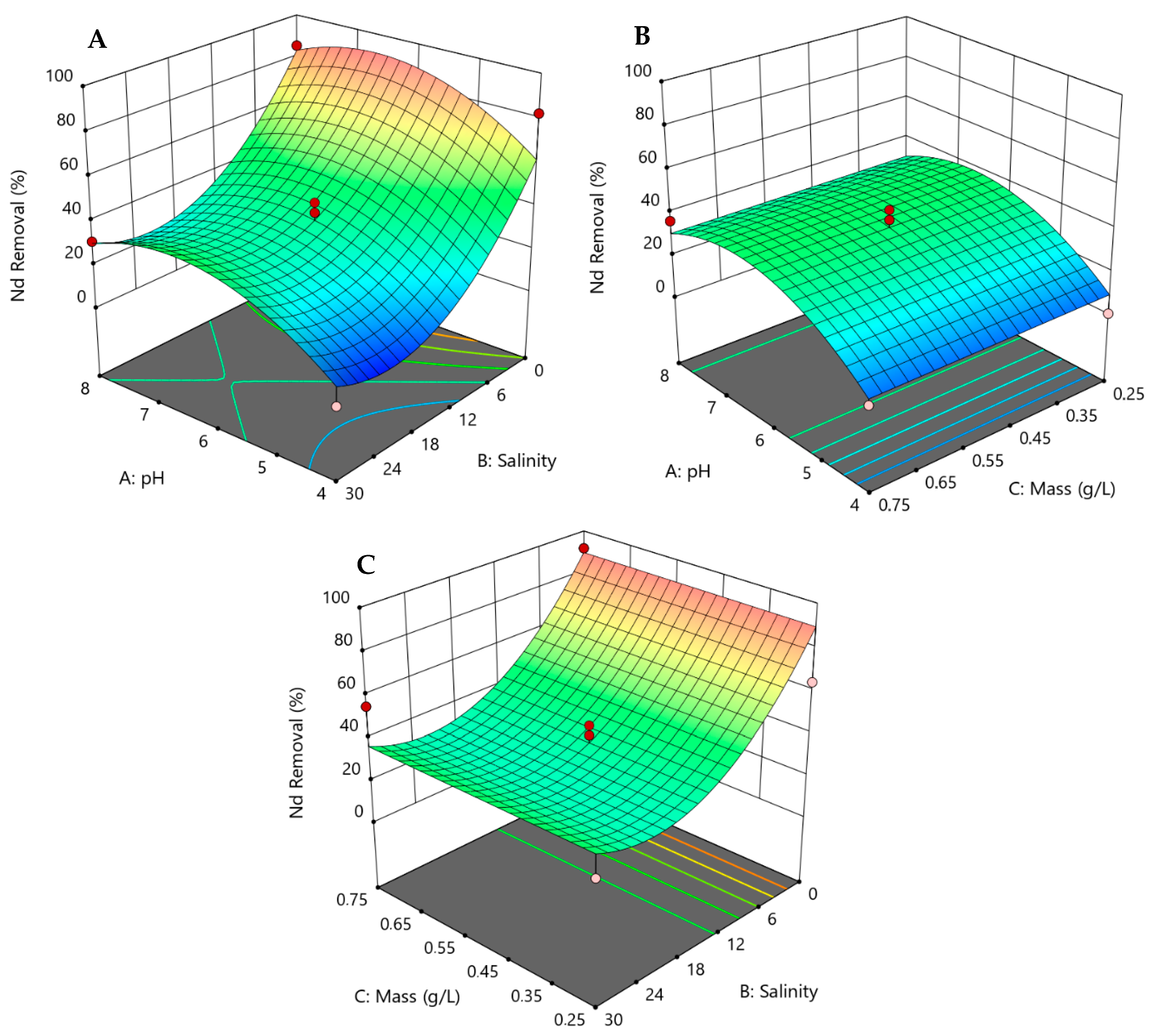
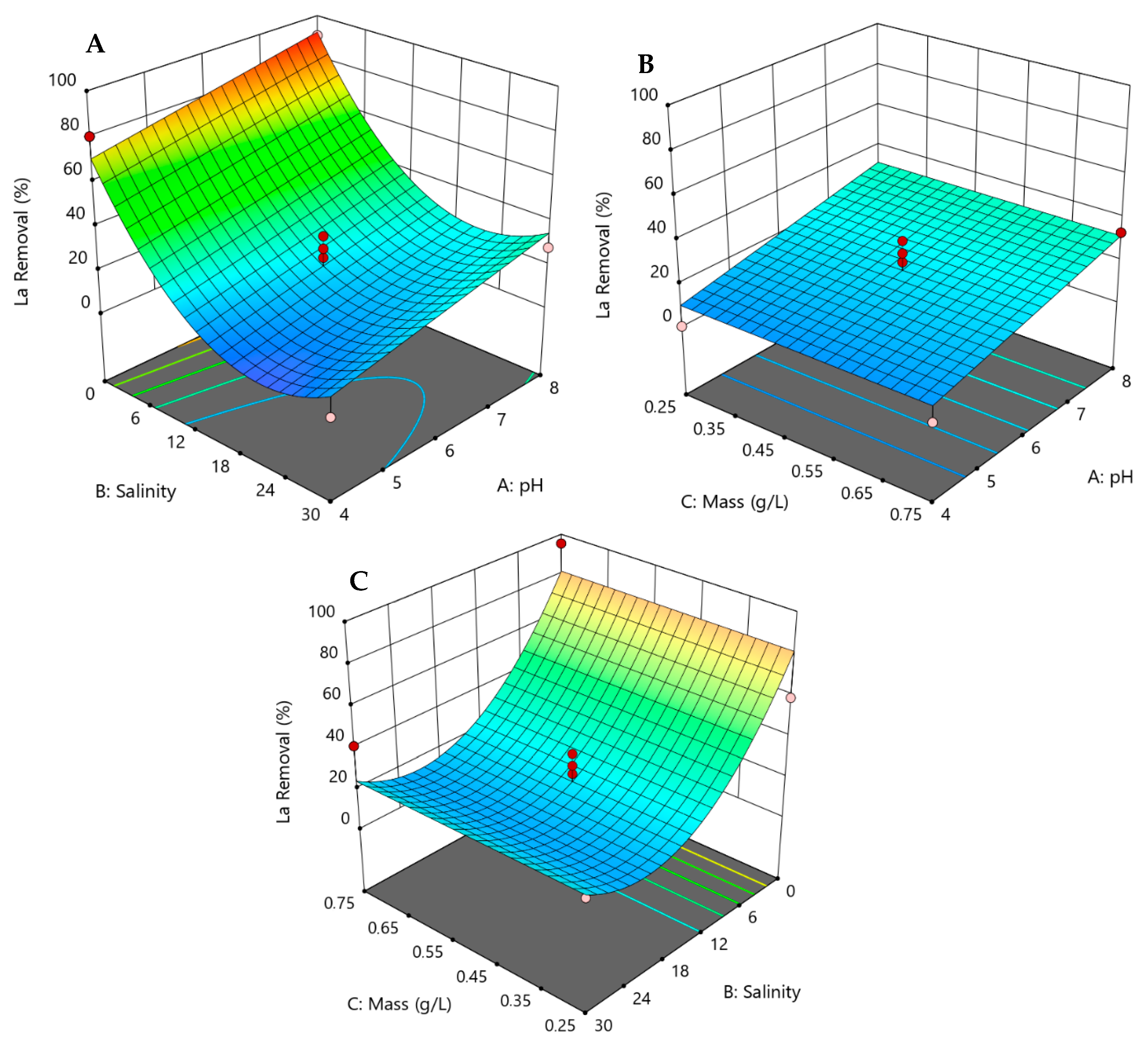
3.3. Response Surface Models and Statistical Analysis
3.4. 3D Responses for the Removal of REEs by Hazelnut Shells
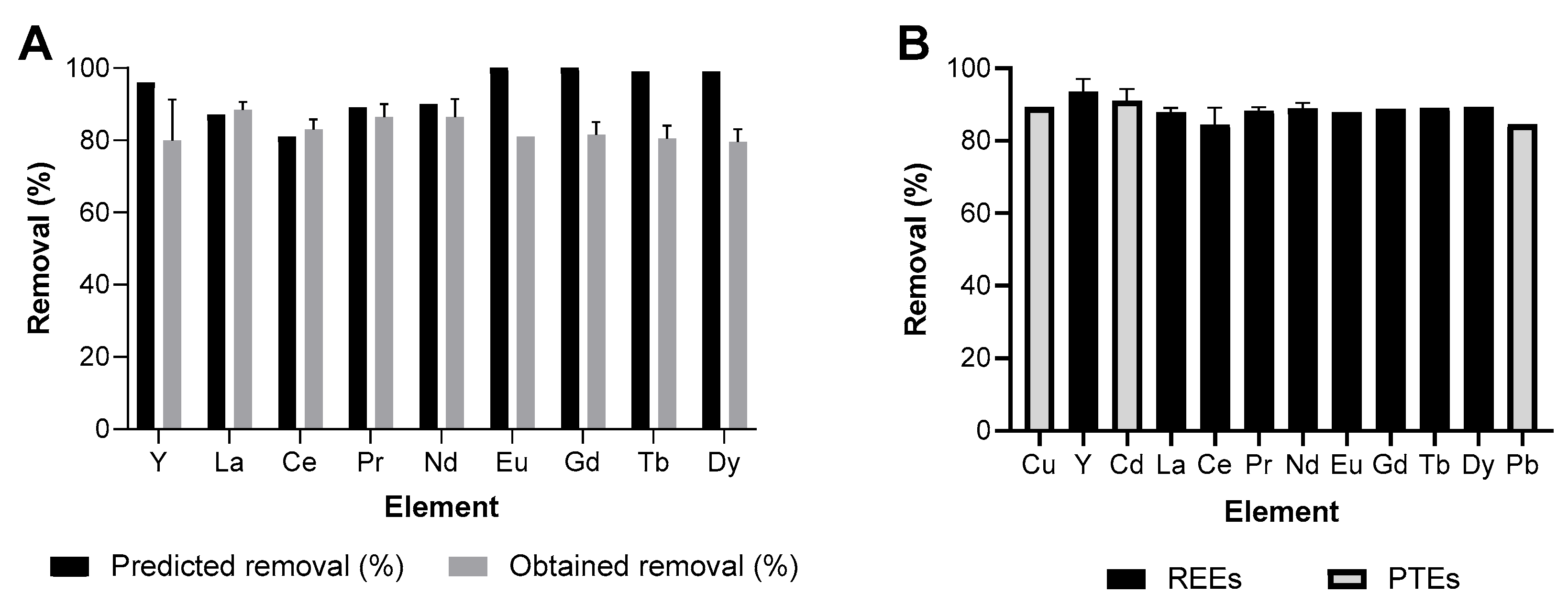
3.5. Optimization of Operational Parameters Affecting REEs Removal by Response Surface Methodology
3.6. Hazelnut Shell Removal of Rare Earth Elements Under Optimal Operational Conditions in Presence and Absence of Potentially Toxic Elements
3.7. Hazelnut Shell Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-Doped Electrospun Nanofibrous Membrane for Photocatalytic Water Treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef]
- Tsani, S.; Koundouri, P.; Akinsete, E. Resource management and sustainable development: A review of the European water policies in accordance with the United Nations’ Sustainable Development Goals. Environ. Sci. Policy 2020, 114, 570–579. [Google Scholar] [CrossRef]
- UNESCO. The United Nations World Water Development Report 2024—Water for Prosperity and Development; UNESCO: Paris, France, 2024. [Google Scholar] [CrossRef]
- UNESCO. The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; UNESCO: Paris, France, 2023. [Google Scholar]
- Tiwari, J.; Ankit; Sweta; Kumar, S.; Korstad, J.; Bauddh, K. Ecorestoration of Polluted Aquatic Ecosystems Through Rhizofiltration. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–201. [Google Scholar] [CrossRef]
- Sarkar, S.; Gill, S.S.; Das Gupta, G.; Kumar Verma, S. Water toxicants: A comprehension on their health concerns, detection, and remediation. Environ. Sci. Pollut. Res. 2022, 29, 53934–53953. [Google Scholar] [CrossRef]
- Baldé, A.C.P.; Kuehr, R.; Yamamoto, T.; Mcdonald, R.; Angelo, E.D.; Althaf, S.; Bel, G.; Deubzer, O.; Fernandez-cubillo, E.; Forti, V.; et al. Global E-Waste Monitor 2024; Geneva/Bonn: Brisbane, QLD, Australia, 2024; Available online: https://ewastemonitor.info/wp-content/uploads/2024/12/GEM_2024_EN_11_NOV-web.pdf (accessed on 23 July 2025).
- Xavier, L.H.; Giese, E.C.; Ribeiro-Duthie, A.C.; Lins, F.A.F. Sustainability and the circular economy: A theoretical approach focused on e-waste urban mining. Resour. Policy 2021, 74, 101467. [Google Scholar] [CrossRef]
- The E-Waste Coalition. A New Circular Vision for Electronics Time for a Global Reboot; The E-Waste Coalition: East Falmouth, MA, USA, 2019; Available online: www.weforum.org (accessed on 1 February 2025).
- Chakraborty, S.C.; Qamruzzaman, M.; Zaman, M.W.U.; Alam, M.M.; Hossain, M.D.; Pramanik, B.K.; Nguyen, L.N.; Nghiem, L.D.; Ahmed, M.F.; Zhou, J.L.; et al. Metals in e-waste: Occurrence, fate, impacts and remediation technologies. Process Saf. Environ. Prot. 2022, 162, 230–252. [Google Scholar] [CrossRef]
- European Commission. Study on the Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2023. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials for the EU, Report of the Ad-Hoc Working Group on Defining Critical Raw Materials; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Wall, F. Rare Earth Elements. In Encyclopedia of Geology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 680–693. [Google Scholar] [CrossRef]
- Dagwar, P.P.; Iqbal, S.S.; Dutta, D. Sustainable recovery of rare Earth elements from industrial waste: A path to circular economy and environmental health. Waste Manag. Bull. 2025, 3, 373–390. [Google Scholar] [CrossRef]
- Mudali, U.K.; Patil, M.; Saravanabhavan, R.; Saraswat, V.K. Review on E-waste Recycling: Part II—Technologies for Recovery of Rare Earth Metals. Trans. Indian Natl. Acad. Eng. 2021, 6, 613–631. [Google Scholar] [CrossRef]
- Rautela, R.; Arya, S.; Vishwakarma, S.; Lee, J.; Kim, K.-H.; Kumar, S. E-waste management and its effects on the environment and human health. Sci. Total Environ. 2021, 773, 145623. [Google Scholar] [CrossRef] [PubMed]
- El Ouardi, Y.; Virolainen, S.; Massima Mouele, E.S.; Laatikainen, M.; Repo, E.; Laatikainen, K. The recent progress of ion exchange for the separation of rare earths from secondary resources—A review. Hydrometallurgy 2023, 218, 106047. [Google Scholar] [CrossRef]
- Patil, A.B.; Struis, R.P.W.J.; Ludwig, C. Opportunities in Critical Rare Earth Metal Recycling Value Chains for Economic Growth with Sustainable Technological Innovations. Circ. Econ. Sustain. 2022, 3, 1127–1140. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Anupan, A.; Imyim, A.; Fuangswasdi, S. Selective separation of neodymium(III) using dual function DEHPA-menthol deep eutectic solvent. J. Mol. Liq. 2025, 432, 127760. [Google Scholar] [CrossRef]
- Yurramendi, L.; Gijsemans, L.; Forte, F.; Aldana, J.L.; del Río, C.; Binnemans, K. Enhancing rare-earth recovery from lamp phosphor waste. Hydrometallurgy 2019, 187, 38–44. [Google Scholar] [CrossRef]
- Silva, R.G.; Morais, C.A.; Oliveira, É.D. Selective precipitation of rare earth from non-purified and purified sulfate liquors using sodium sulfate and disodium hydrogen phosphate. Miner. Eng. 2019, 134, 402–416. [Google Scholar] [CrossRef]
- Thakkar, J.; Wissler, B.; Dudenas, N.; Yin, X.; Vailhe, M.; Bricker, J.; Zhang, X. Recovery of Critical Rare-Earth Elements Using ETS-10 Titanosilicate. Ind. Eng. Chem. Res. 2019, 58, 11121–11126. [Google Scholar] [CrossRef]
- Giese, E.C. Biosorption as green technology for the recovery and separation of rare earth elements. World J. Microbiol. Biotechnol. 2020, 36, 52. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Devi, A.P.; Mishra, P.M. Biosorption of dysprosium (III) using raw and surface-modified bark powder of Mangifera indica: Isotherm, kinetic and thermodynamic studies. Environ. Sci. Pollut. Res. 2019, 26, 6545–6556. [Google Scholar] [CrossRef] [PubMed]
- Swain, K.K.; Mishra, P.M.; Devi, A.P. Biosorption of praseodymium (III) using Terminalia arjuna bark powder in batch systems: Isotherm and kinetic studies. Water Sci. Technol. 2018, 77, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rao, Y.; Tian, C.; Huang, T.; Lu, J.; Zhang, M.; Han, M. Adsorption Performance of La(III) and Y(III) on Orange Peel: Impact of Experimental Variables, Isotherms, and Kinetics. Adsorpt. Sci. Technol. 2021, 2021. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Saravanan, A.; Vo, D.-V.N. Advances in biosorbents for removal of environmental pollutants: A review on pretreatment, removal mechanism and future outlook. J. Hazard. Mater. 2021, 420, 126596. [Google Scholar] [CrossRef] [PubMed]
- Shahbandeh, M. Nuts: Global Production 2023/24, Statista. 2025. Available online: https://www.statista.com/statistics/1030933/tree-nut-global-production/ (accessed on 23 May 2025).
- Khir, R.; Pan, Z. (Eds.) Walnuts. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 391–411. [Google Scholar] [CrossRef]
- Dias, M.; Pinto, J.; Henriques, B.; Figueira, P.; Fabre, E.; Tavares, D.; Vale, C.; Pereira, E. Nutshells as Efficient Biosorbents to Remove Cadmium, Lead, and Mercury from Contaminated Solutions. Int. J. Environ. Res. Public Health 2021, 18, 1580. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Chojnacka, K.; Podstawczyk, D.; Dawiec, A.; Pokomeda, K. Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour. Technol. 2014, 160, 150–160. [Google Scholar] [CrossRef]
- Sahu, J.N.; Acharya, J.; Meikap, B.C. Response surface modeling and optimization of chromium(VI) removal from aqueous solution using Tamarind wood activated carbon in batch process. J. Hazard. Mater. 2009, 172, 818–825. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Turan, A.Z.; Turan, M. Removal of Heavy Metals and Dyes from Wastewaters by Raw and Activated Carbon Hazelnut Shells. In Topics in Applied Physics; Ünlü, H., Horing, N.J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 907–933. [Google Scholar] [CrossRef]
- Fatahi, A.; Ziarati, P.; Jafarpour, A.; Cruz-Rodriguez, L. Heavy Metal Removal from Edible Leafy Vegetable by Low Cost Novel Adsorbents: Hazelnut Shell. Cit. J. Sci. Discov. 2020, 4, 20039. [Google Scholar] [CrossRef]
- Figueira, P.; Henriques, B.; Teixeira, F.; Afonso, N.; Pinto, J.; Tavares, D.; Vale, C.; Pereira, E. Potentialities of Agro-Based Wastes to Remove Cd, Hg, Pb, and As from Contaminated Waters. Water Air Soil Pollut. 2022, 233, 78. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D.; Bertrand, G.; Soulié, J. 1.11 Bioactive Calcium Phosphate Compounds: Physical Chemistry. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 244–290. [Google Scholar] [CrossRef]
- Demirbaş, Ö.; Karadağ, A.; Alkan, M.; Doğan, M. Removal of copper ions from aqueous solutions by hazelnut shell. J. Hazard. Mater. 2008, 153, 677–684. [Google Scholar] [CrossRef]
- Al-Ajji, M.A.; Al-Ghouti, M.A. Novel insights into the nanoadsorption mechanisms of crystal violet using nano-hazelnut shell from aqueous solution. J. Water Process Eng. 2021, 44, 102354. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Moschopoulou, E.; Klepetsanis, P.G.; Tsakiroglou, C.D. Valorization of fruit wastes (pistachio shells) as adsorbent for the removal of Zn from aqueous solutions under adverse acidic conditions. Desalin. Water Treat. 2017, 74, 174–183. [Google Scholar] [CrossRef]
- Ferreira, N.; Fabre, E.; Henriques, B.; Viana, T.; Costa, M.; Pinto, J.; Tavares, D.; Carvalho, L.; Pinheiro-Torres, J.; Pereira, E. Response surface approach to optimize the removal of the critical raw material dysprosium from water through living seaweeds. J. Environ. Manag. 2021, 300, 113697. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Varshini C, J.S.; Das, D.; Das, N. Optimization of parameters for praseodymium(III) biosorption onto biowaste materials using response surface methodology: Equilibrium, kinetic and regeneration studies. Ecol. Eng. 2015, 81, 321–327. [Google Scholar] [CrossRef]
- Keshtkar, A.R.; Moosavian, M.A.; Sohbatzadeh, H.; Mofras, M. La(III) and Ce(III) biosorption on sulfur functionalized marine brown algae Cystoseira indica by xanthation method: Response surface methodology, isotherm and kinetic study. Groundw. Sustain. Dev. 2019, 8, 144–155. [Google Scholar] [CrossRef]
- Fabre, E.; Henriques, B.; Viana, T.; Pinto, J.; Costa, M.; Ferreira, N.; Tavares, D.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. Optimization of Nd(III) removal from water by Ulva sp. and Gracilaria sp. through Response Surface Methodology. J. Environ. Chem. Eng. 2021, 9, 105946. [Google Scholar] [CrossRef]
- Raji, M.; Abolghasemi, H.; Safdari, J.; Kargari, A. Response Surface Optimization of Dysprosium Extraction Using an Emulsion Liquid Membrane Integrated with Multi-Walled Carbon Nanotubes. Chem. Eng. Technol. 2018, 41, 1857–1870. [Google Scholar] [CrossRef]
- He, C.; Salih, K.A.M.; Wei, Y.; Mira, H.; Abdel-Rahman, A.A.H.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Efficient Recovery of Rare Earth Elements (Pr(III) and Tm(III)) From Mining Residues Using a New Phosphorylated Hydrogel (Algal Biomass/PEI). Metals 2021, 11, 294. [Google Scholar] [CrossRef]
- Xiqiang, L.; Hui, Z.; Yong, T.; Yunlong, L. REE Geochemical Characteristic of Apatite: Implications for Ore Genesis of the Zhijin Phosphorite. Minerals 2020, 10, 1012. [Google Scholar] [CrossRef]
- Figueira, P.; Vale, C.; Pereira, E. Factors influencing sorption of trace elements in contaminated waters onto ground nut shells. J. Environ. Manag. 2022, 308, 114618. [Google Scholar] [CrossRef]
- Maina, I.W.; Obuseng, V.; Nareetsile, F. Use of Moringa oleifera (Moringa) Seed Pods and Sclerocarya birrea (Morula) Nut Shells for Removal of Heavy Metals from Wastewater and Borehole Water. J. Chem. 2016, 2016, 9312952. [Google Scholar] [CrossRef]
- McNeill, D.C.; Pal, A.K.; Nath, D.; Rodriguez-Uribe, A.; Mohanty, A.K.; Pilla, S.; Gregori, S.; Dick, P.; Misra, M. Upcycling of ligno-cellulosic nutshells waste biomass in biodegradable plastic-based biocomposites uses—A comprehensive review. Compos. Part C Open Access 2024, 14, 100478. [Google Scholar] [CrossRef]
- Şencan, A.; Karaboyacı, M.; Kılıç, M. Determination of lead(II) sorption capacity of hazelnut shell and activated carbon obtained from hazelnut shell activated with ZnCl2. Environ. Sci. Pollut. Res. 2015, 22, 3238–3248. [Google Scholar] [CrossRef] [PubMed]
- Gozaydin, G.; Yuksel, A. Valorization of hazelnut shell waste in hot compressed water. Fuel Process. Technol. 2017, 166, 96–106. [Google Scholar] [CrossRef]
- da Silva Bruckmann, F.; Piazzi Fuhr, A.C.F.; Silva Oliveira, M.L.; Oliveira Silva, L.F.; Knani, S.; Graba, B.; Dotto, G.L. Recovery and concentration of Eu and Dy from real leachate using wastes as adsorbents: Standard adsorption study and fixed bed operation. Sep. Purif. Technol. 2025, 373, 133596. [Google Scholar] [CrossRef]
- Gallardo, K.; Castillo, R.; Mancilla, N.; Remonsellez, F. Biosorption of Rare-Earth Elements from Aqueous Solutions Using Walnut Shell. Front. Chem. Eng. 2020, 2, 4. [Google Scholar] [CrossRef]
- Jamali, H.A.; Mahvi, A.H.; Nazmara, S. Removal of cadmium from aqueous solutions by Hazel nut shell. World Appl. Sci. J. 2009, 2, 16–20. [Google Scholar]
- Pehlivan, E.; Altun, T.; Cetin, S.; Iqbal Bhanger, M. Lead sorption by waste biomass of hazelnut and almond shell. J. Hazard. Mater. 2009, 167, 1203–1208. [Google Scholar] [CrossRef]


| Variable | Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| pH | 4 | 6 | 8 |
| Salinity (g/L) | 0 | 15 | 30 |
| Hazelnut shell dose (g/L) | 0.25 | 0.50 | 0.75 |
| Experiment | pH | Salinity (g/L) | Hazelnut Shell Dose (g/L) |
|---|---|---|---|
| 1 | 4 | 15 | 0.25 |
| 2 | 4 | 15 | 0.75 |
| 3 | 6 | 30 | 0.75 |
| 4 | 4 | 30 | 0.50 |
| 5 | 8 | 30 | 0.50 |
| 6 | 8 | 15 | 0.25 |
| 7 | 6 | 15 | 0.50 |
| 8 | 4 | 0 | 0.50 |
| 9 | 6 | 15 | 0.50 |
| 10 | 6 | 0 | 0.25 |
| 11 | 8 | 0 | 0.50 |
| 12 | 6 | 15 | 0.50 |
| 13 | 6 | 30 | 0.25 |
| 14 | 8 | 15 | 0.75 |
| 15 | 6 | 0 | 0.75 |
| Experiment | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |||
| Removal (%) | Y | 24 h | 0 | 4 | 39 | 3 | 31 | 14 | 22 | 62 | 27 | 46 | 78 | 25 | 13 | 39 | 87 |
| 48 h | 0 | 5 | 53 | 6 | 40 | 30 | 46 | 70 | 44 | 54 | 87 | 39 | 21 | 53 | 91 | ||
| 72 h | 0 | 5 | 64 | 4 | 49 | 36 | 46 | 75 | 48 | 61 | 91 | 48 | 29 | 64 | 95 | ||
| La | 24 h | 0 | 1 | 30 | 3 | 16 | 8 | 14 | 69 | 17 | 48 | 84 | 17 | 21 | 17 | 82 | |
| 48 h | 0 | 3 | 41 | 4 | 28 | 22 | 35 | 80 | 29 | 59 | 91 | 25 | 23 | 33 | 95 | ||
| 72 h | 0 | 3 | 51 | 3 | 36 | 33 | 35 | 85 | 34 | 66 | 91 | 33 | 33 | 50 | 96 | ||
| Ce | 24 h | 0 | 4 | 41 | 4 | 17 | 6 | 18 | 71 | 23 | 48 | 78 | 24 | 19 | 16 | 89 | |
| 48 h | 0 | 4 | 53 | 3 | 28 | 21 | 42 | 82 | 38 | 62 | 86 | 34 | 26 | 34 | 93 | ||
| 72 h | 0 | 4 | 64 | 5 | 36 | 33 | 42 | 87 | 38 | 68 | 87 | 48 | 36 | 52 | 94 | ||
| Pr | 24 h | 0 | 4 | 45 | 5 | 17 | 5 | 22 | 72 | 26 | 48 | 77 | 27 | 21 | 17 | 88 | |
| 48 h | 0 | 6 | 55 | 5 | 30 | 20 | 44 | 82 | 40 | 62 | 85 | 39 | 27 | 36 | 92 | ||
| 72 h | 0 | 5 | 64 | 5 | 38 | 32 | 44 | 87 | 42 | 68 | 85 | 47 | 37 | 54 | 94 | ||
| Nd | 24 h | 0 | 5 | 45 | 6 | 18 | 5 | 23 | 72 | 26 | 51 | 76 | 27 | 23 | 18 | 86 | |
| 48 h | 0 | 6 | 55 | 0 | 31 | 22 | 45 | 82 | 41 | 64 | 85 | 41 | 26 | 36 | 92 | ||
| 72 h | 0 | 6 | 66 | 4 | 38 | 34 | 45 | 87 | 32 | 68 | 83 | 48 | 36 | 55 | 93 | ||
| Eu | 24 h | 0 | 5 | 52 | 6 | 21 | 7 | 27 | 71 | 34 | 51 | 73 | 33 | 26 | 21 | 86 | |
| 48 h | 2 | 8 | 61 | 7 | 34 | 22 | 49 | 81 | 47 | 64 | 82 | 48 | 34 | 41 | 99 | ||
| 72 h | 0 | 8 | 71 | 6 | 42 | 34 | 50 | 86 | 49 | 71 | 86 | 57 | 44 | 58 | 93 | ||
| Gd | 24 h | 0 | 3 | 47 | 3 | 21 | 9 | 25 | 70 | 31 | 48 | 76 | 30 | 20 | 23 | 87 | |
| 48 h | 0 | 7 | 60 | 7 | 35 | 23 | 48 | 80 | 45 | 61 | 85 | 43 | 28 | 42 | 92 | ||
| 72 h | 0 | 6 | 68 | 7 | 43 | 36 | 48 | 84 | 48 | 68 | 87 | 53 | 38 | 58 | 94 | ||
| Tb | 24 h | 0 | 4 | 51 | 4 | 25 | 10 | 28 | 66 | 33 | 48 | 73 | 33 | 22 | 27 | 86 | |
| 48 h | 0 | 6 | 61 | 6 | 38 | 25 | 50 | 77 | 48 | 61 | 84 | 48 | 32 | 46 | 91 | ||
| 72 h | 0 | 6 | 70 | 6 | 45 | 36 | 50 | 81 | 51 | 66 | 88 | 57 | 43 | 61 | 93 | ||
| Dy | 24 h | 0 | 6 | 49 | 3 | 27 | 12 | 29 | 63 | 35 | 46 | 73 | 34 | 22 | 32 | 85 | |
| 48 h | 0 | 7 | 61 | 5 | 39 | 28 | 49 | 74 | 50 | 58 | 84 | 49 | 31 | 49 | 90 | ||
| 72 h | 0 | 8 | 70 | 5 | 47 | 37 | 49 | 79 | 52 | 64 | 88 | 58 | 41 | 63 | 93 | ||
| Friedman Test with Dunn’s Multiple Comparisons Test (p-Value ≤ 0.05) | ||||||
|---|---|---|---|---|---|---|
| Element | 24 h vs. 48 h | 24 h vs. 72 h | 24 h vs. 96 h | 48 h vs. 72 h | 48 h vs. 96 h | 72 h vs. 96 h |
| Y | 0.0433 | 0.0002 | <0.0001 | 0.9438 | 0.0973 | >0.9999 |
| La | 0.0281 | 0.0003 | <0.0001 | >0.9999 | 0.3374 | >0.9999 |
| Ce | 0.3960 | 0.0006 | <0.0001 | 0.2418 | 0.0533 | >0.9999 |
| Pr | 0.0433 | 0.0008 | <0.0001 | >0.9999 | 0.0973 | >0.9999 |
| Nd | 0.2034 | 0.0019 | <0.0001 | 0.8254 | 0.1177 | >0.9999 |
| Eu | 0.0225 | 0.0003 | <0.0001 | >0.9999 | 0.4626 | >0.9999 |
| Gd | 0.0433 | <0.0001 | <0.0001 | 0.5381 | 0.2034 | >0.9999 |
| Tb | 0.0225 | <0.0001 | <0.0001 | 0.7188 | 0.3374 | >0.9999 |
| Dy | 0.0433 | 0.0001 | <0.0001 | 0.7188 | 0.0655 | >0.9999 |
| p-Value for Quadratic Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | Salinity | HN Mass | pH2 | Salinity2 | HN Mass2 | pH-Salinity | pH-HN Mass | Salinity-HN Mass | ||
| 48 h | Nd | 0.0496 | 0.0014 | 0.0659 | 0.0328 | 0.0090 | 0.2348 | 0.2881 | 0.7254 | 0.9332 |
| Gd | 0.0375 | 0.0021 | 0.0411 | 0.0294 | 0.0096 | 0.1692 | 0.3744 | 0.6139 | 0.9762 | |
| Eu | 0.0503 | 0.0018 | 0.0440 | 0.0140 | 0.0085 | 0.2326 | 0.3025 | 0.5624 | 0.7491 | |
| La | 0.0369 | 0.0007 | 0.0751 | 0.1568 | 0.0028 | 0.3679 | 0.5553 | 0.7031 | 0.4612 | |
| Dy | 0.0162 | 0.0031 | 0.0399 | 0.0222 | 0.0188 | 0.1708 | 0.3210 | 0.5267 | 0.9328 | |
| Tb | 0.0245 | 0.0029 | 0.0477 | 0.0221 | 0.0147 | 0.1726 | 0.3193 | 0.5243 | 0.9792 | |
| Pr | 0.0697 | 0.0017 | 0.0635 | 0.0408 | 0.0080 | 0.2310 | 0.3942 | 0.6762 | 0.9440 | |
| Y | 0.0066 | 0.0015 | 0.0221 | 0.0671 | 0.0131 | 0.1770 | 0.4627 | 0.4152 | 0.8326 | |
| Ce | 0.0679 | 0.0017 | 0.0801 | 0.0536 | 0.0076 | 0.3051 | 0.4312 | 0.7217 | 0.8979 | |
| Reduced Equation | Nº of Equation | R2 | Adjusted R2 |
|---|---|---|---|
| Equation (6) | 0.8615 | 0.8061 | |
| Equation (7) | 0.8673 | 0.8311 | |
| Equation (8) | 0.7150 | 0.6675 | |
| Equation (9) | 0.8387 | 0.7742 | |
| Equation (10) | 0.8440 | 0.7817 | |
| Equation (11) | 0.9063 | 0.8542 | |
| Equation (12) | 0.8978 | 0.8410 | |
| Equation (13) | 0.8870 | 0.8242 | |
| Equation (14) | 0.8879 | 0.8256 |
| Optimal Condition for Removal (%) | ||||||
| pH | Salinity | Sorbent Dose (g/L) | Removal (%) | Uptake (mg/g) | Total Uptake (mg/g) | |
| Y | 7.0 | 0 | 0.75 | 96 | 0.103 | 1.52 |
| La | 87 | 0.187 | ||||
| Ce | 81 | 0.103 | ||||
| Pr | 89 | 0.192 | ||||
| Nd | 90 | 0.141 | ||||
| Eu | 100 | 0.205 | ||||
| Gd | 100 | 0.194 | ||||
| Tb | 99 | 0.180 | ||||
| Dy | 99 | 0.220 | ||||
| Optimal Condition for Uptake (mg/g) | ||||||
| pH | Salinity | Sorbent dose (g/L) | Removal (%) | Uptake (mg/g) | Total Uptake (mg/g) | |
| Y | 7.0 | 0 | 0.25 | 72 | 0.170 | |
| La | 87 | 0.304 | ||||
| Ce | 81 | 0.170 | ||||
| Pr | 89 | 0.324 | ||||
| Nd | 90 | 0.312 | 2.69 | |||
| Eu | 81 | 0.360 | ||||
| Gd | 79 | 0.383 | ||||
| Tb | 78 | 0.302 | ||||
| Dy | 77 | 0.363 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, N.; Tavares, D.S.; Baptista, I.; Viana, T.; Jacinto, J.; Silva, T.S.C.; Pereira, E.; Henriques, B. A Recycling-Oriented Approach to Rare Earth Element Recovery Using Low-Cost Agricultural Waste. Metals 2025, 15, 842. https://doi.org/10.3390/met15080842
Ferreira N, Tavares DS, Baptista I, Viana T, Jacinto J, Silva TSC, Pereira E, Henriques B. A Recycling-Oriented Approach to Rare Earth Element Recovery Using Low-Cost Agricultural Waste. Metals. 2025; 15(8):842. https://doi.org/10.3390/met15080842
Chicago/Turabian StyleFerreira, Nicole, Daniela S. Tavares, Inês Baptista, Thainara Viana, Jéssica Jacinto, Thiago S. C. Silva, Eduarda Pereira, and Bruno Henriques. 2025. "A Recycling-Oriented Approach to Rare Earth Element Recovery Using Low-Cost Agricultural Waste" Metals 15, no. 8: 842. https://doi.org/10.3390/met15080842
APA StyleFerreira, N., Tavares, D. S., Baptista, I., Viana, T., Jacinto, J., Silva, T. S. C., Pereira, E., & Henriques, B. (2025). A Recycling-Oriented Approach to Rare Earth Element Recovery Using Low-Cost Agricultural Waste. Metals, 15(8), 842. https://doi.org/10.3390/met15080842








