Reduction Disintegration Behavior and Mechanism of Vanadium–Titanium Magnetite Pellets During Hydrogen-Based Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Vanadium–Titanium Magnetite Concentrate
2.1.2. Binder
2.2. Experimental Methods
2.2.1. Reduction Disintegration Experiment
2.2.2. Analytical Methods
- (1)
- X-ray Diffraction Analyzer (XRD)
- (2)
- Scanning Electron Microscope (SEM)
3. Results and Discussion
3.1. Reduction Disintegration Behavior
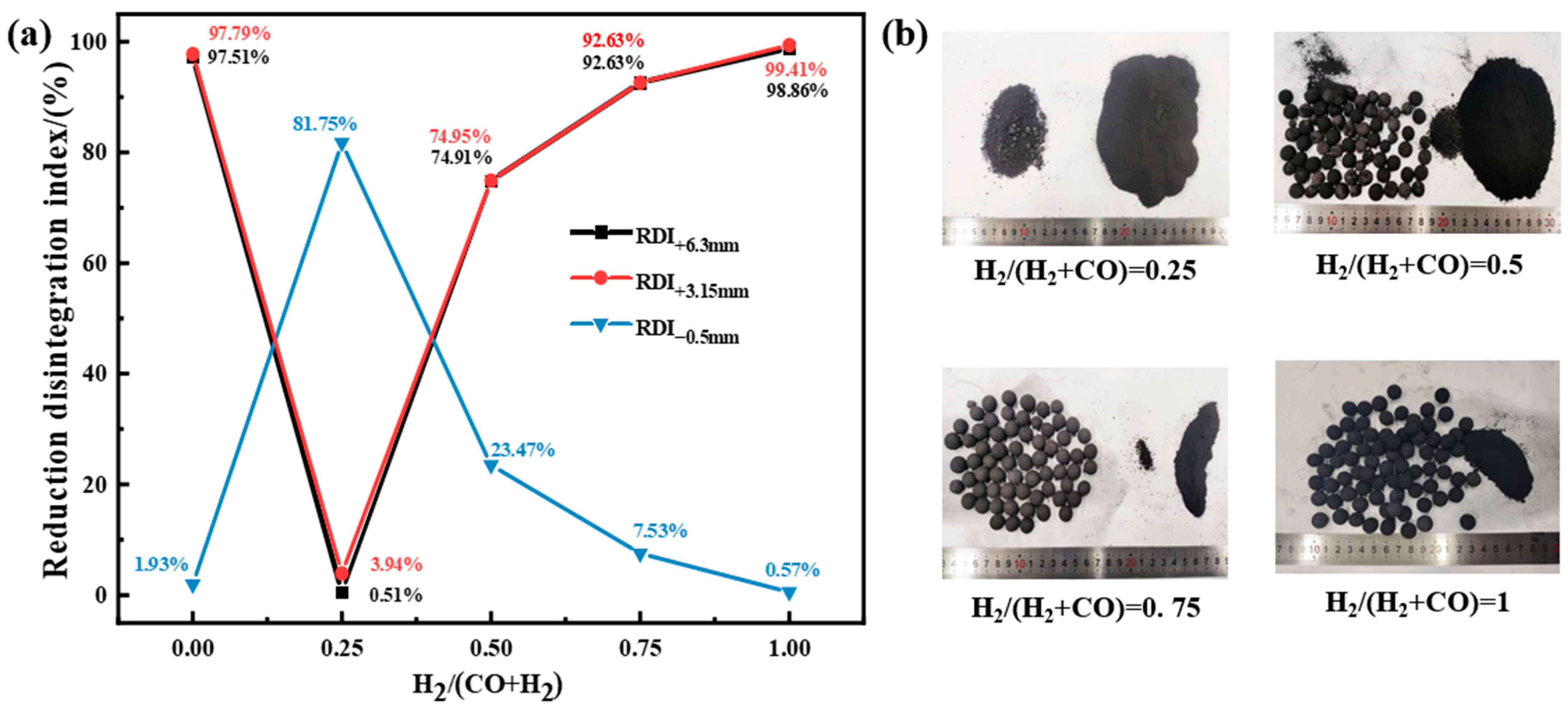
3.1.1. Effect of Reduction Atmosphere
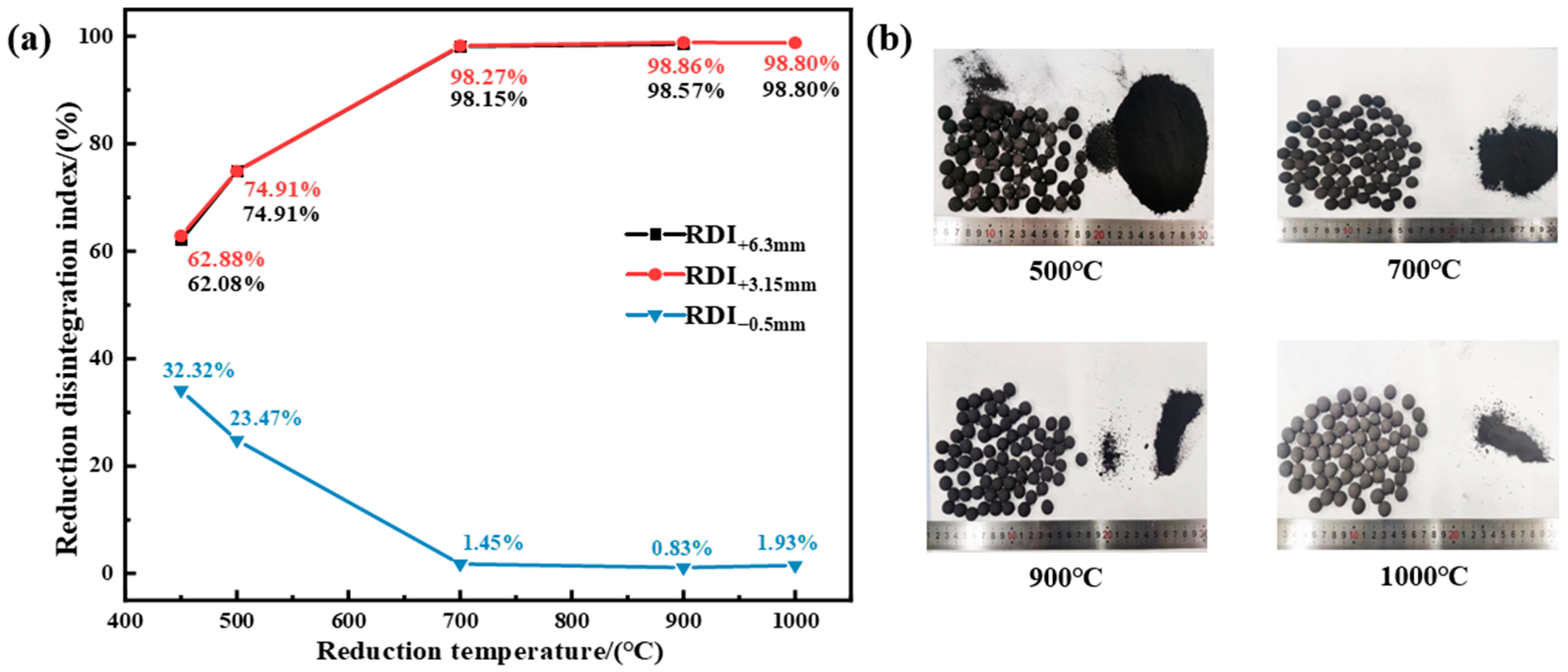
3.1.2. Effect of Reduction Temperature
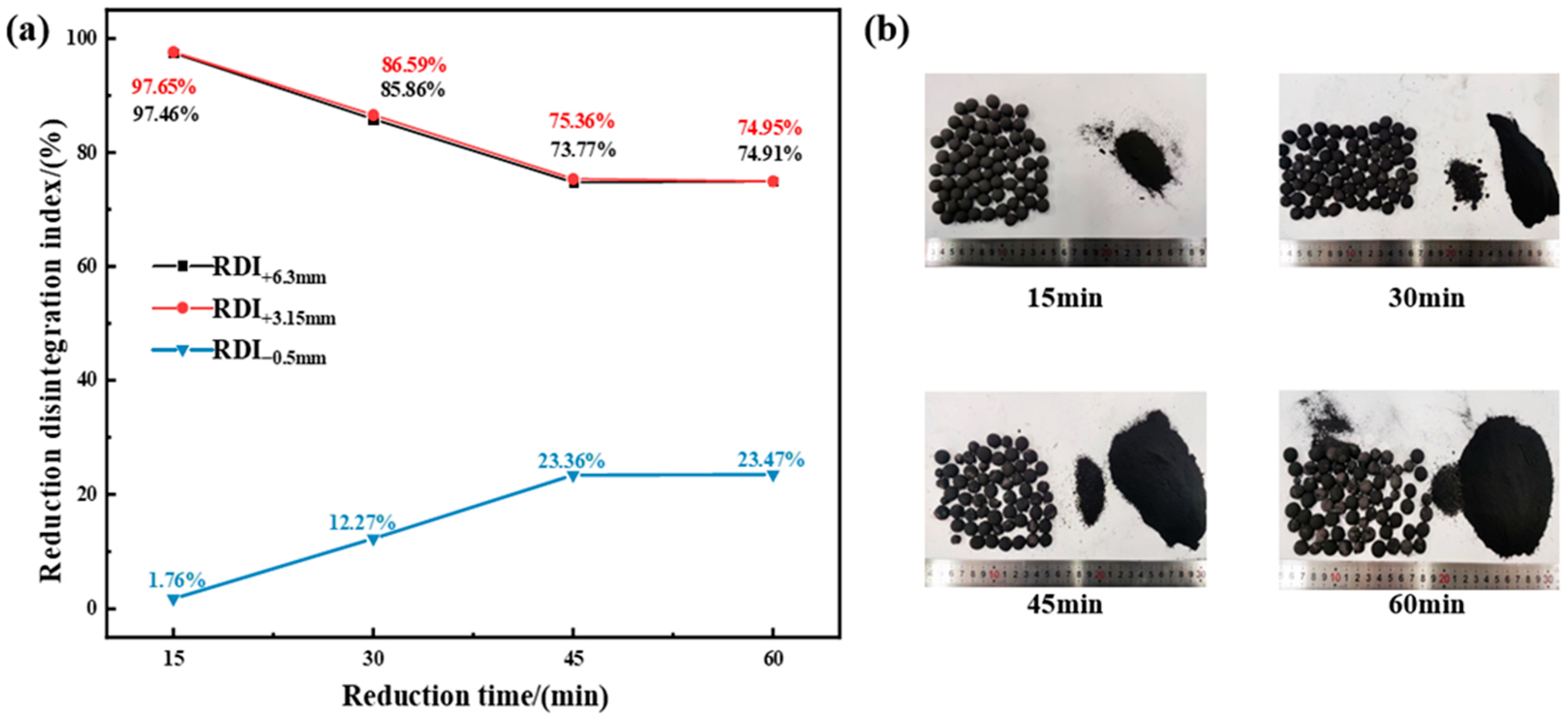
3.1.3. Effect of Reduction Time
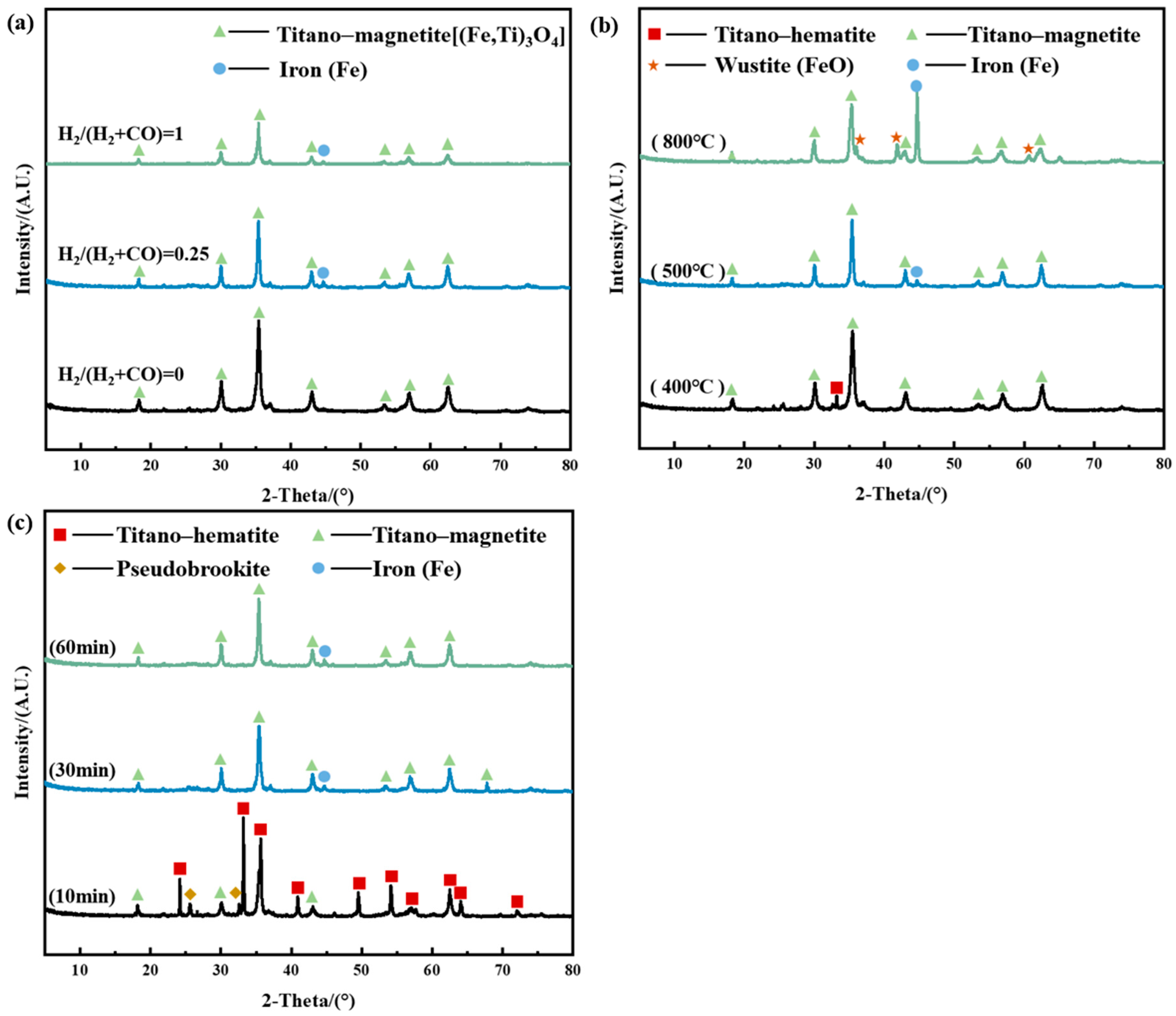
3.2. Phase Transformation
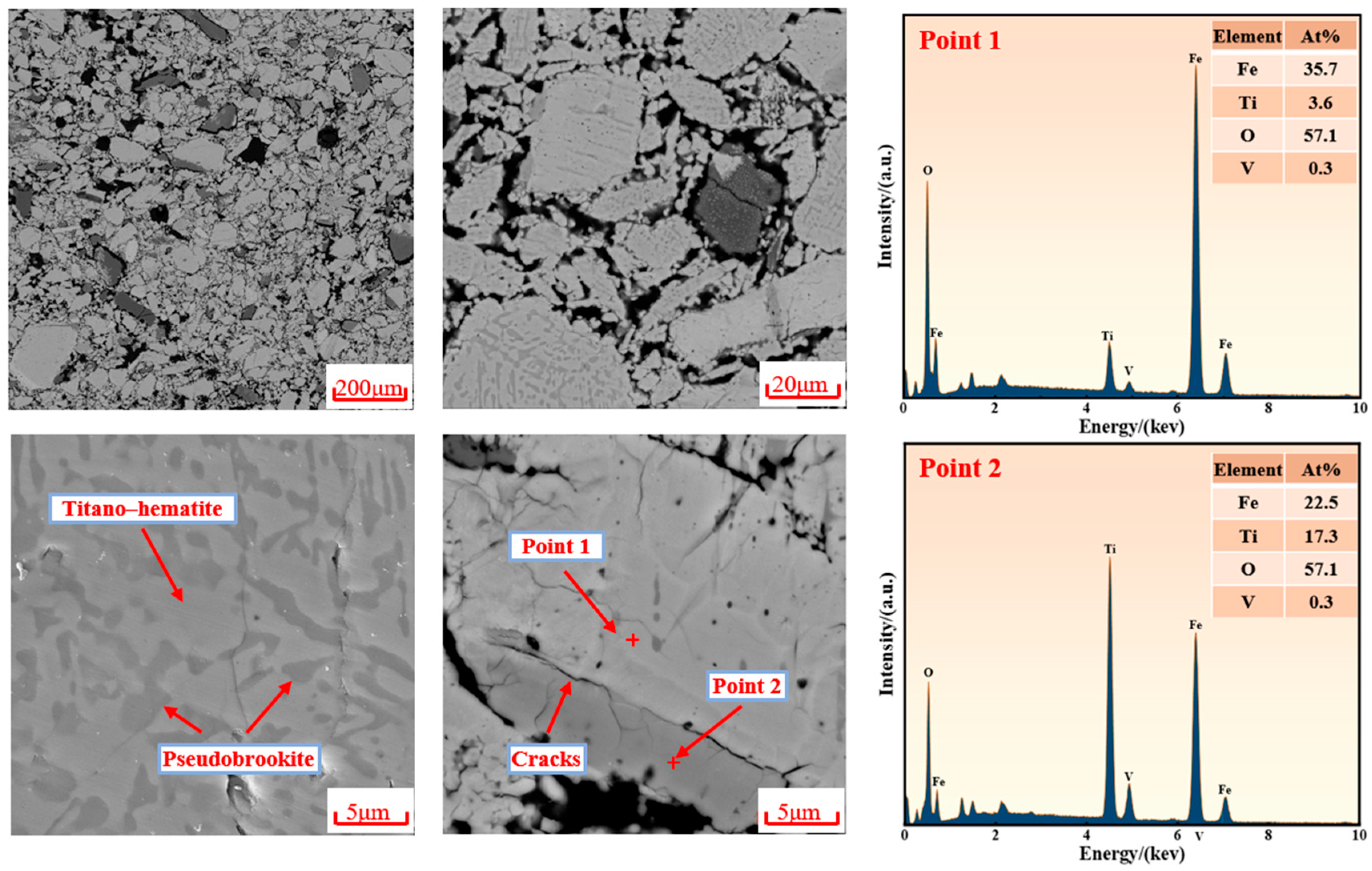
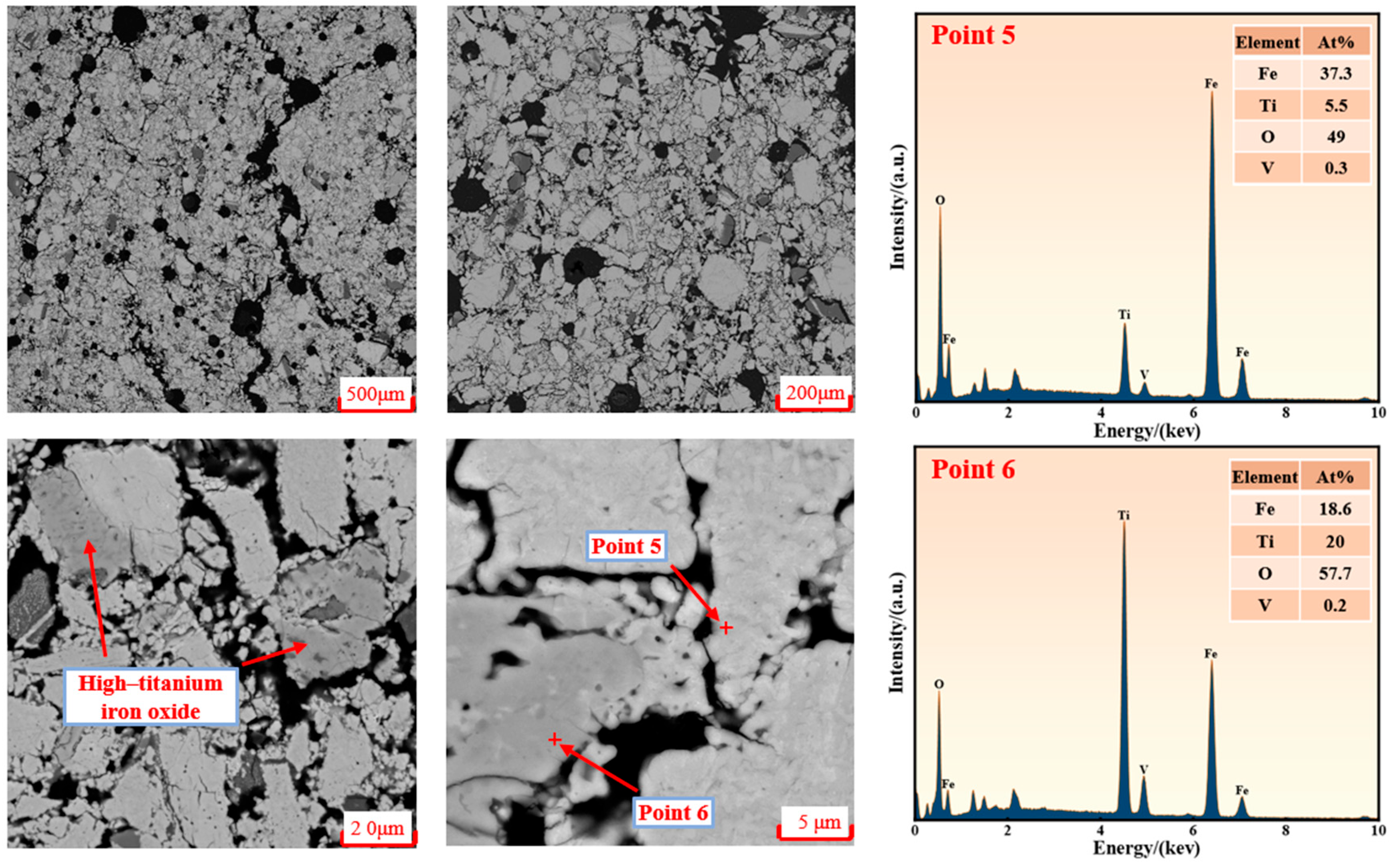
3.3. Microstructural Evolution
- Volume expansion, which was caused by crystallographic phase transformations during the reduction of iron–titanium oxides, which generated internal stress.
- Stress amplification, which was due to the differential reduction rates between low-Ti iron oxides and high-Ti iron oxides.
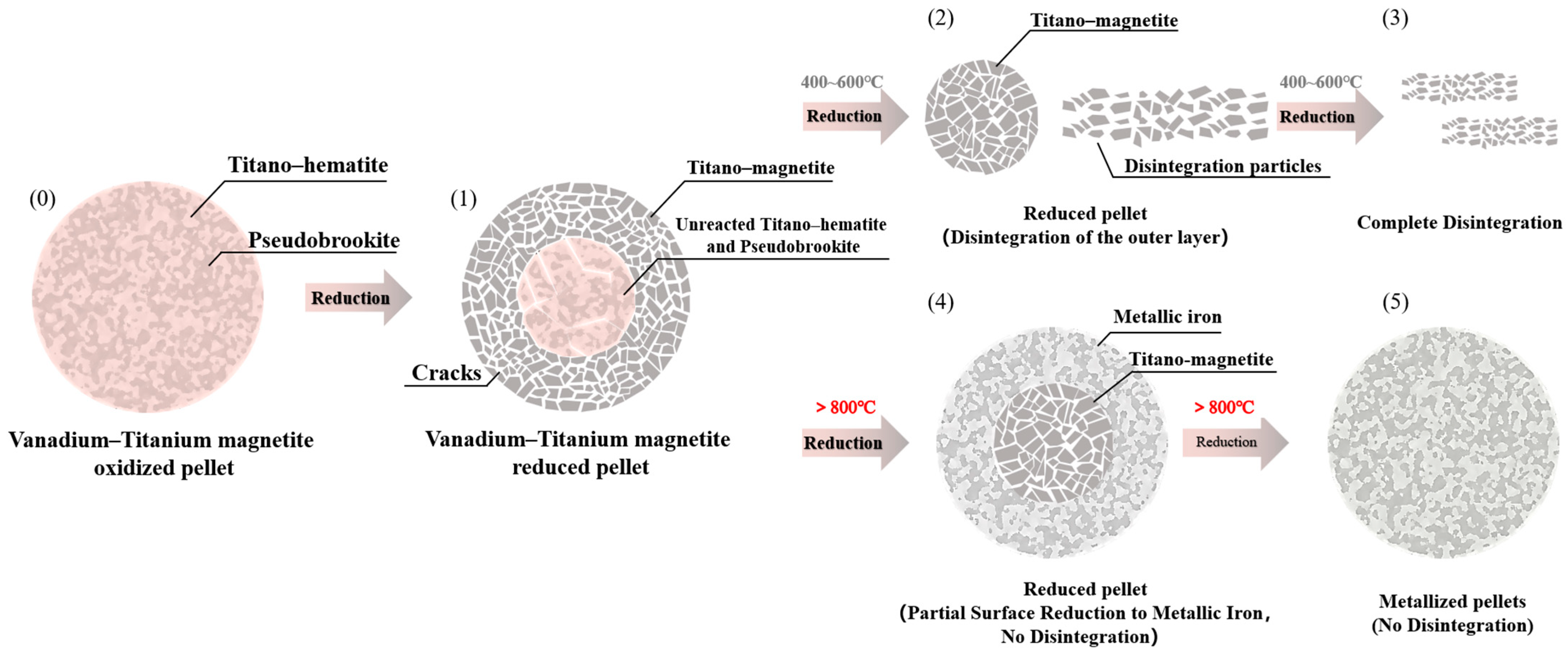
3.4. Reduction Disintegration Mechanism
- The reduction process was initially observed to occur at the pellet surface, where expansion stresses from phase transformation caused structural failure. Cracks were generated, and mineral particles were exfoliated from the surface. Meanwhile, the pellet core remained unreacted, with titano–hematite preserved as the main component, maintaining structural integrity.
- At low reduction temperatures (400–600 °C), crack formation on the pellet surface was found to accelerate reducing gas diffusion. The core region was subsequently reduced, resulting in complete surface disintegration and exposure of the original core material, which developed extensive cracking.
- With continued low-temperature reduction, complete pellet disintegration was ultimately achieved.
- When the reduction temperature exceeded 800 °C, surface titano–magnetite was initially reduced to metallic iron. The resulting fine metallic iron particles formed interparticle connections that effectively accommodated phase transformation stresses. As a result, interparticle cracking was minimized, and the pellet structure became re-densified, thereby significantly suppressing reduction disintegration. However, unreduced titano–magnetite still remained in the pellet core at this stage.
- Prolonged high-temperature reduction ultimately produced structurally dense metalized pellets that maintained complete integrity without disintegration.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, F.; Olayiwola, A.U.; Liu, B.; Wang, S.; Du, H.; Li, J.; Wang, W.; Chen, D.; Zhang, Y. Review of vanadium production part I: Primary resources. Miner. Process. Extr. Metall. Rev. 2022, 43, 466–488. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, Y.; Wu, C.; Han, X.; Zhao, N.; Zhang, Z.; Dai, L.; Wang, L.; He, Z. A review on vanadium extraction techniques from major vanadium-containing resources. Rare Met. 2024, 43, 4115–4131. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Qi, T.; Chen, D.; Zhao, H.; Liu, Y. A novel method to extract iron, titanium, vanadium, and chromium from high-chromium vanadium-bearing titanomagnetite concentrates. Hydrometallurgy 2014, 149, 106–109. [Google Scholar] [CrossRef]
- Fu, W.; Xie, H. Progress in Technology of Vanadium-Bearing Titanomagnetite Smelting in Pangang. J. Iron Steel Res. Int. 2009, 16, 554–559. [Google Scholar] [CrossRef]
- Qiu, G.; Guo, Y. Current situation and development trend of titanium metal industry in China. Int. J. Miner. Metall. Mater. 2022, 29, 599–610. [Google Scholar] [CrossRef]
- Xie, H.; Yu, W.; You, Z.; Lv, X.; Bai, C. The effect of titanium carbonitride on the viscosity of high-titanium-type blast furnace slag. Metals 2019, 9, 395. [Google Scholar] [CrossRef]
- Cai, Y.; Song, N.; Yang, Y.; Sun, L.; Hu, P.; Wang, J. Recent progress of efficient utilization of titanium-bearing blast furnace slag. Int. J. Miner. Metall. Mater. 2022, 29, 22–31. [Google Scholar] [CrossRef]
- Jiang, T.; Chu, M.; Li, F.; Tang, Y.; Liu, Z.; Xue, X. Reduction mechanism of high-chromium vanadium-titanium magnetite pellets by H2-CO-CO2 gas mixtures. Int. J. Miner. Metall. Mater. 2015, 22, 562–572. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.; Chu, M.; Zhu, M. An effective and cleaner process to recovery iron, titanium, vanadium, and chromium from Hongge vanadium titanomagnetite with hydrogen-rich gases. Ironmak. Steelmak. 2021, 48, 33–39. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.; Jiang, T.; Chen, F.; Zheng, F.; Yang, L. Behavior of silicon during reduction and smelting of vanadium titanomagnetite metallized pellets in an electric furnace. Jom 2019, 71, 329–335. [Google Scholar] [CrossRef]
- Sharma, T.; Gupta, R.C.; Prakash, B. Effect of reduction rate on the swelling behaviour of iron ore pellets. ISIJ Int. 1992, 32, 812–818. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, J.; Zuo, H.; Jiao, K.; Hu, Z.; Xing, X. Mechanisms of swelling of iron ore oxidized pellets in high reduction potential atmosphere. J. Iron Steel Res. Int. 2015, 22, 1–8. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, S.; Wang, X.; Chen, B.; Yang, H.; Wang, S.; Luo, P. Recovery of Iron from Chromium Vanadium-Bearing Titanomagnetite Concentrate by Direct Reduction. JOM 2016, 68, 2698–2703. [Google Scholar] [CrossRef]
- Yu, W.; Wen, X.; Chen, J.; Kuang, J.; Tang, Q.; Tian, Y.; Fu, J.; Huang, W.; Qiu, T. Preparation of direct reduced iron and titanium nitride from panzhihua titanomagnetite concentrate through carbothermic reduction-magnetic separation. Minerals 2017, 7, 220. [Google Scholar] [CrossRef]
- Chen, D.; Song, B.; Wang, L.; Qi, T.; Wang, Y.; Wang, W. Solid state reduction of Panzhihua titanomagnetite concentrates with pulverized coal. Miner. Eng. 2011, 24, 864–869. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, X.; Cao, M.; Jiao, K.; Wang, C.; Ren, S. Reduction kinetics of vanadium titano-magnetite carbon composite pellets adding catalysts under high temperature. J. Iron Steel Res. Int. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Han, Y.; She, X.; Xue, Q. Reduction mechanism of titanomagnetite concentrate by hydrogen. Int. J. Miner. Process. 2013, 125, 122–128. [Google Scholar] [CrossRef]
- Sui, Y.; Guo, Y.; Jiang, T.; Qiu, G. Reduction kinetics of oxidized vanadium titano-magnetite pellets using carbon monoxide and hydrogen. J. Alloys Compd. 2017, 706, 546–553. [Google Scholar] [CrossRef]
- Gan, M.; Sun, Y.; Fan, X.; Ji, Z.; Lv, W.; Chen, X. Preparing high-quality vanadium titano-magnetite pellets for large-scale blast furnaces as ironmaking burden. Ironmak. Steelmak. 2020, 47, 130–137. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Liang, Z.; Peng, X.; Deng, X.; Li, J. Direct reduction of iron ore pellets by H2-CO mixture: An in-situ investigation of the evolution and dynamics of swelling. Mater. Today Commun. 2023, 36, 106940. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.; Chu, M.; Zhu, M. Effect of porosity of Hongge vanadium titanomagnetite-oxidized pellet on its reduction swelling behavior and mechanism with hydrogen-rich gases. Powder Technol. 2019, 343, 194–203. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Jiang, T.; Zhong, R.; Liang, Z. Iron ore pellet disintegration mechanism in simulated shaft furnace conditions. Powder Technol. 2017, 317, 89–94. [Google Scholar] [CrossRef]
- Hu, Q.; Xin, R.; Gao, X.; Wang, Y.; You, Y.; You, Z.; Lv, X. Reduction disintegration behavior of vanadium titanomagnetite pellets in H2-CO-CO2-N2 mixtures. Powder Technol. 2023, 413, 118073. [Google Scholar] [CrossRef]
- Chen, F.; Li, H.; Guo, Y.; Liu, K.; Wang, S.; Yang, L.; Wen, Y.; Zhang, M. Reduction disintegration behavior and crystallographic transformation of vanadium–titanium magnetite pellets during hydrogen-based reduction. J. Mater. Res. Technol.-JMRT 2024, 34, 152–163. [Google Scholar] [CrossRef]
- Wang, H.; Sohn, H.Y. Effects of Reducing Gas on Swelling and Iron Whisker Formation during the Reduction of Iron Oxide Compact. Steel Res. Int. 2012, 83, 903–909. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, M.; Liu, Z.; Chen, S.; Xue, X. Effects of Temperature and Atmosphere on Pellets Reduction Swelling Index. J. Iron Steel Res. Int. 2012, 19, 7–19. [Google Scholar] [CrossRef]
- Sesen, M.K. The influence of CaO on the precipitation behaviour of iron in the reduction of iron oxide. Scand. J. Metall. 2001, 30, 1–7. [Google Scholar] [CrossRef]
- Li, G.; Tang, Z.; Zhang, Y.; Cui, Z.; Jiang, T. Reduction swelling behaviour of haematite/magnetite agglomerates with addition of MgO and CaO. Ironmak. Steelmak. 2010, 37, 393–397. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, G.; Liu, Z.; Chu, M.; Xue, X. Reduction process of pellet containing high chromic vanadium-titanium magnetite in cohesive zone. Steel Res. Int. 2015, 86, 808–816. [Google Scholar] [CrossRef]
- Rang, J.; Chu, M.; Feng, J.; Zhao, Z.; Tian, H. Preparation of Oxidized Pellets of Vanadium Titanium Magnetite and Direct Reduction Behavior in Hydrogen-Based Shaft Furnace. Steel Res. Int. 2024, 96, 2400480. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Wang, S.; Li, L. Direct reduction of vanadium titanium pellets using ammonia as a reductant: Thermodynamics, characteristics, and kinetics analysis. Fuel Process. Technol. 2025, 270, 108202. [Google Scholar] [CrossRef]
- ISO 11257-2022; Iron Ores for Shaft Direct-Reduction Feedstocks—Determination of the Low-Temperature Reduction Disintegration Index and Degree of Metallization. International Organization for Standardization: Geneva, Switzerland, 2022.




| Chemical Composition | TFe | FeO | TiO2 | V2O5 | CaO | MgO | Al2O3 | SiO2 | S |
|---|---|---|---|---|---|---|---|---|---|
| Content % | 57.03 | 34.61 | 10.12 | 3.78 | 2.34 | 1.93 | 3.36 | 2.76 | 0.35 |
| Chemical Composition | SiO2 | Al2O3 | Na2O | CaO | Fe2O3 | MgO | K2O |
|---|---|---|---|---|---|---|---|
| Content/% | 62.23 | 12.91 | 1.66 | 4.68 | 2.93 | 3.09 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Li, H.; Wang, S.; Chen, M.; Tang, W.; Guo, Y.; Wen, Y.; Yang, L. Reduction Disintegration Behavior and Mechanism of Vanadium–Titanium Magnetite Pellets During Hydrogen-Based Reduction. Metals 2025, 15, 700. https://doi.org/10.3390/met15070700
Chen F, Li H, Wang S, Chen M, Tang W, Guo Y, Wen Y, Yang L. Reduction Disintegration Behavior and Mechanism of Vanadium–Titanium Magnetite Pellets During Hydrogen-Based Reduction. Metals. 2025; 15(7):700. https://doi.org/10.3390/met15070700
Chicago/Turabian StyleChen, Feng, Hao Li, Shuai Wang, Mao Chen, Wenbo Tang, Yufeng Guo, Yuekai Wen, and Lingzhi Yang. 2025. "Reduction Disintegration Behavior and Mechanism of Vanadium–Titanium Magnetite Pellets During Hydrogen-Based Reduction" Metals 15, no. 7: 700. https://doi.org/10.3390/met15070700
APA StyleChen, F., Li, H., Wang, S., Chen, M., Tang, W., Guo, Y., Wen, Y., & Yang, L. (2025). Reduction Disintegration Behavior and Mechanism of Vanadium–Titanium Magnetite Pellets During Hydrogen-Based Reduction. Metals, 15(7), 700. https://doi.org/10.3390/met15070700











