Effect of Heat Treatment on Corrosion of an AlCoCrFeNi2.1 Eutectic High-Entropy Alloy in 3.5 wt% NaCl Solution
Abstract
1. Introduction
2. Experimental
2.1. Materials and Surface Preparation
2.2. Microstructure Characterization
2.3. Electrochemical Measurements
2.4. Surface Analyses
3. Results and Discussion
3.1. Microstructure Characterization
3.2. Corrosion Behavior
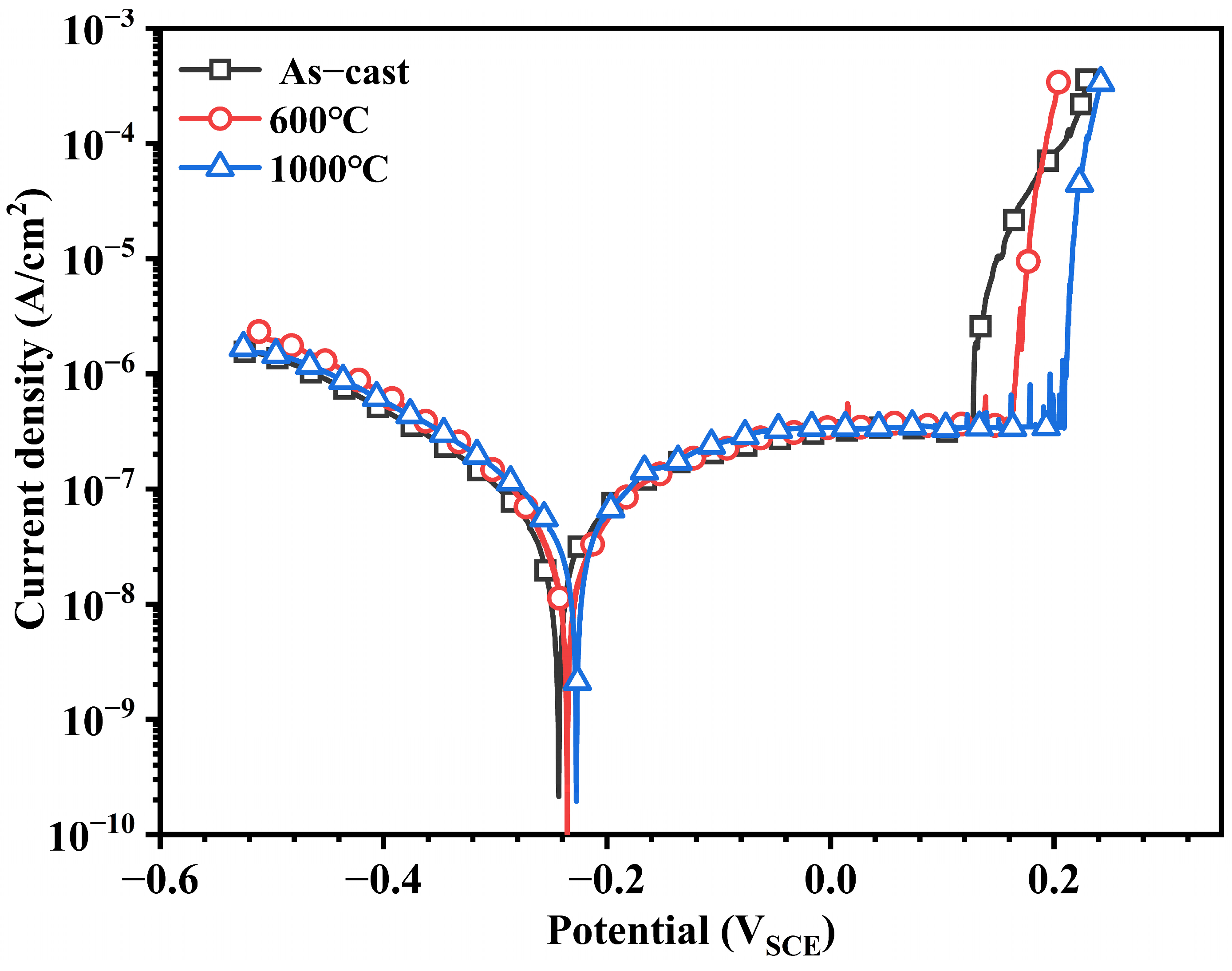
3.2.1. Potentiodynamic Polarization Tests
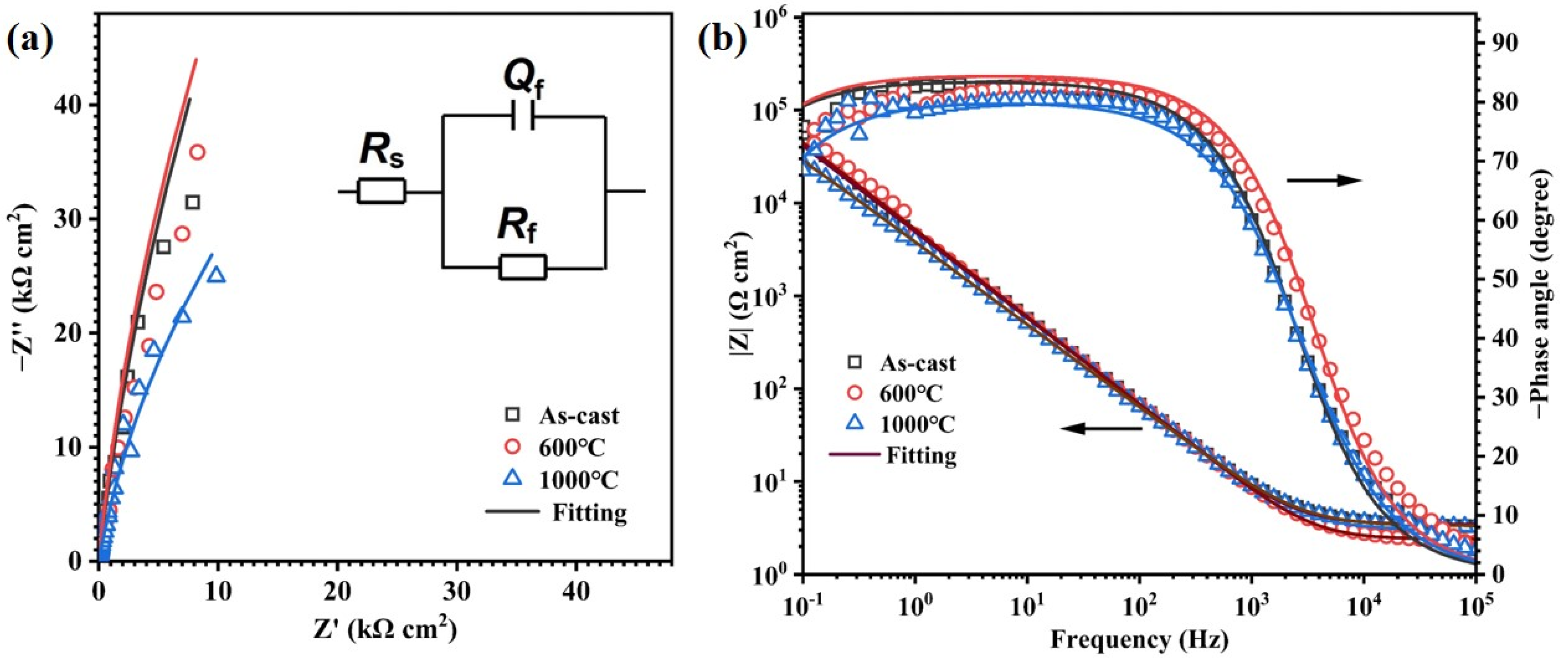
3.2.2. EIS Tests
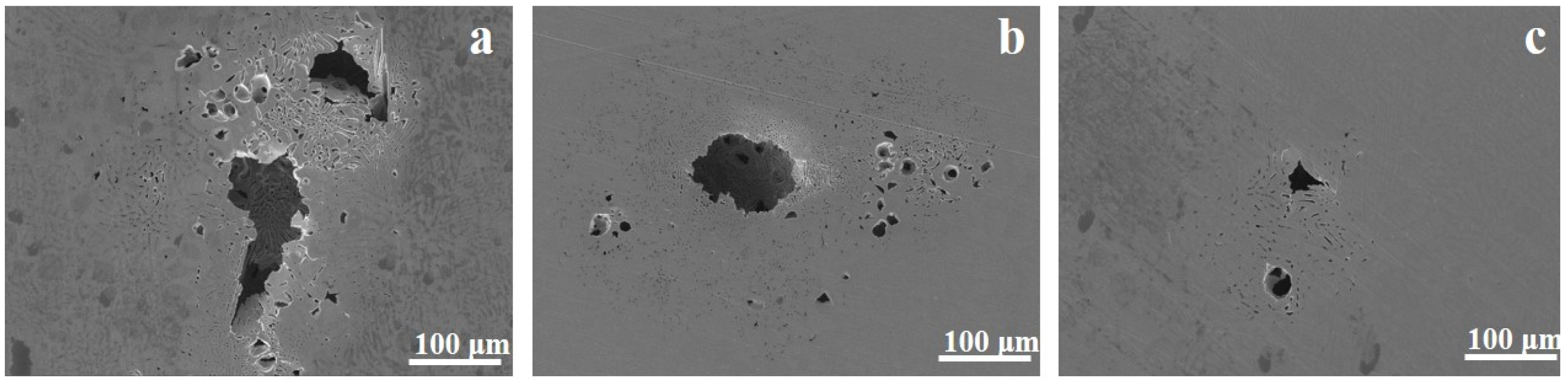
3.3. Corrosion Morphology Observation
3.4. XPS Analysis
4. Conclusions
- The microstructure of the as-cast AlCoCrFeNi2.1 EHEA changes after the heat treatment at 600 °C and 1000 °C. After the heat treatment, the proportion of the FCC phase enriched with Cr and Fe elements increases, while that of the BCC phase rich in Ni and Al elements decreases.
- Both the as-cast and heat-treated AlCoCrFeNi2.1 EHEAs exhibit spontaneous passive behavior in a 3.5 wt% NaCl solution. The pitting potential of the AlCoCrFeNi2.1 EHEA rises with the heat treatment temperature, while the passive current density and passive film resistance are almost independent of the heat treatment. These results demonstrate that corrosion resistance of the as-cast AlCoCrFeNi2.1 in a 3.5 wt% NaCl solution is improved by the heat treatment.
- The enhanced resistance to pitting corrosion results from the decrease in the content of the Al-rich B2 phase, which can act as the pit initiation site by preferential corrosion (evidenced by corrosion morphology observation) due to the higher electrochemical activity than the Cr-rich FCC phase, and is also detrimental for the protectiveness of the passive film (evidenced by XPS analysis).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Luo, H.; Sohn, S.S.; Lu, W.J.; Li, L.L.; Li, X.G.; Soundararajan, C.K.; Krieger, W.; Li, Z.M.; Raabe, D. A strong and ductile medium-entropy alloy resists hydrogen embrittlement and corrosion. Nat. Commun. 2020, 11, 3081. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Choi, W.S.; Jun, H.; Do, H.S.; Lee, B.J.; Choi, P.P.; Han, H.N.; Ko, W.S.; Sohn, S.S. Doubled strength and ductility via maraging effect and dynamic precipitate transformation in ultrastrong medium-entropy alloy. Nat. Commun. 2023, 14, 145. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.W.; Li, X.G. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Shi, P.J.; Ren, W.L.; Zheng, T.X.; Ren, Z.M.; Hou, X.L.; Peng, J.C.; Hu, P.F.; Gao, Y.F.; Zhong, Y.B.; Liaw, P.K. Enhanced strength-ductility synergy in ultrafine-grained eutectic high-entropy alloys by inheriting microstructural lamellae. Nat. Commun. 2019, 10, 489. [Google Scholar] [CrossRef]
- Nie, S.J.; Yi, X.N.; Zhou, H.L.; Zhu, H.J.; Yang, L.L.; Fu, F.L.; Li, J.Y.; Yang, H.K.; Xu, G.X.; Lu, S.; et al. Corrosion behavior of as-cast Al0.75CoFeCr1.25Ni high entropy alloy in 0.5 mol/L NaOH solution. J. Iron Steel Res. Int. 2024, 31, 2852–2863. [Google Scholar] [CrossRef]
- Nie, S.; Zheng, Z.; Qiao, Y.; Duan, Y.; Cui, J.; Mekkey, S.D.; Amin, M.A.; Melhi, S.; Yang, H.; Zhou, H.; et al. Corrosion behavior of as-cast Al0.75CoCr1.25FeNi high entropy alloy in 0.5 mol/L sulfuric acid. Adv. Compos. Hybrid Mater. 2024, 7, 138. [Google Scholar] [CrossRef]
- Zhu, H.J.; Han, J.Y.; Fang, K.W.; Zheng, Z.B.; Zhou, H.L.; Yang, L.L.; Qiao, Y.X.; Chen, J.; Cui, J.; Wang, Q. Corrosion behavior of CoCrCu0.1FeMoNi high entropy alloy in 0.5 mol/L NaOH solution. J. Iron Steel Res. Int. 2025, 32, 1163–1175. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A promising new class of high-temperature alloys: Eutectic high-entropy alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, X.; Jiang, L.; Chen, Z.; Wang, T.; Jie, J.; Kang, H.; Zhang, Y.; Guo, S.; Ruan, H.; et al. Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 2017, 124, 143–150. [Google Scholar] [CrossRef]
- Lu, Y.P.; Dong, Y.; Jiang, H.; Wang, Z.J.; Cao, Z.Q.; Guo, S.; Wang, T.M.; Li, T.J.; Liaw, P.K. Promising properties and future trend of eutectic high entropy alloys. Scr. Mater. 2020, 187, 202–209. [Google Scholar] [CrossRef]
- Song, L.F.; Hu, W.B.; Liao, B.K.; Wan, S.; Kang, L.; Guo, X.P. Corrosion behavior of AlCoCrFeNi2.1 eutectic high-entropy alloy in Cl--containing solution. J. Alloys Compd. 2023, 938, 168609. [Google Scholar]
- Hasannaeimi, V.; Mukherjee, S. Galvanic corrosion in a eutectic high entropy alloy. J. Electroanal. Chem. 2019, 848, 113331. [Google Scholar] [CrossRef]
- Shi, Z.J.; Wang, Z.B.; Wang, X.D.; Zhang, S.; Zheng, Y.G. Effect of thermally induced B2 phase on the corrosion behavior of an Al0.3CoCrFeNi high entropy alloy. J. Alloys Compd. 2022, 903, 163886. [Google Scholar] [CrossRef]
- Mao, T.; Chen, S.; Tang, L.; Mo, L.; Wang, Y.; Yan, J. Corrosion behavior of AlCoCrFeNi2.1 high entropy alloy in a simulated oilfield produced fluid environment compared to 2205 duplex stainless steel. Int. J. Electrochem. Sci. 2024, 19, 100405. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, H.; Chen, X.; Prabhakar, J.M.; Wang, X.; Cheng, H.; Du, C.; Hu, S.; Li, X. The corrosion behavior and passive film properties of the cast and annealed AlCoCrFeNi2.1 eutectic high-entropy alloy in sulfuric acid solution. Corros. Sci. 2024, 240, 112456. [Google Scholar] [CrossRef]
- Wang, Z.B.; Yang, J.; Xiao, Z.X.; Liu, Z.Y.; Xiao, B.L.; Ma, Z.Y.; Cheng, H.-M.; Zheng, Y.G. Nature-inspired incorporation of precipitants into high-strength bulk aluminum alloys enables life-long extraordinary corrosion resistance in diverse aqueous environments. Adv. Mater. 2024, 36, e2406506. [Google Scholar] [CrossRef]
- Duan, X.; Han, T.; Guan, X.; Wang, Y.; Su, H.; Ming, K.; Wang, J.; Zheng, S. Cooperative effect of Cr and Al elements on passivation enhancement of eutectic high-entropy alloy AlCoCrFeNi2.1 with precipitates. J. Mater. Sci. Technol. 2023, 136, 97–108. [Google Scholar] [CrossRef]
- Song, L.; Hu, W.; Huang, S.; Guo, X. Electrochemical behavior and passive film properties of Ce-added AlCoCrFeNi2.1 eutectic high-entropy alloys in sulfuric acid solution. J. Electroanal. Chem. 2023, 950, 117883. [Google Scholar] [CrossRef]
- Xiong, T.; Zheng, S.J.; Pang, J.Y.; Ma, X.L. High-strength and high-ductility AlCoCrFeNi2.1 eutectic high-entropy alloy achieved via precipitation strengthening in a heterogeneous structure. Scr. Mater. 2020, 186, 336–340. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Tailoring nanostructures and mechanical properties of AlCoCrFeNi2.1 eutectic high entropy alloy using thermo-mechanical processing. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2016, 675, 99–109. [Google Scholar] [CrossRef]
- Kumar, T.S.; Chauhan, L.; Chakravarthy, K.; Chelvane, A. The improved galvanic corrosion resistance of a eutectic high entropy alloy through heat treatment. J. Mater. Res. 2022, 37, 4211–4221. [Google Scholar] [CrossRef]
- Song, L.F.; Hu, W.B.; Li, X.X.; Cheng, Z.; Liao, B.K.; Shan, W.; Guo, X.P. Comparison of electrochemical characteristics and passive behavior of as-cast and heat-treated AlCoCrFeNi2.1 eutectic high-entropy alloys in Cl-containing sulfuric acid solution. J. Mater. Eng. Perform. 2025. [Google Scholar] [CrossRef]
- Gao, X.Z.; Lu, Y.P.; Zhang, B.; Liang, N.N.; Wu, G.Z.; Sha, G.; Liu, J.Z.; Zhao, Y.H. Microstructural origins of high strength and high ductility in an AlCoCrFeNi2.1 eutectic high-entropy alloy. Acta Mater. 2017, 141, 59–66. [Google Scholar] [CrossRef]
- Wang, L.; Yao, C.L.; Shen, J.; Zhang, Y.P.; Wang, T.; Ge, Y.H.; Gao, L.H.; Zhang, G.J. Microstructures and room temperature tensile properties of as-cast and directionally solidified AlCoCrFeNi2.1 eutectic high-entropy alloy. Intermetallics 2020, 118, 106681. [Google Scholar] [CrossRef]
- Wu, W.; Hong, Y.; Xu, X.; Cheng, H.; Liu, Z. Effect of annealing time on the microstructure and SCC behavior of an austenite-based low-density steel in a marine atmosphere. Corros. Sci. 2022, 205, 110466. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Hou, Q.; Li, C.T.; Li, X.G.; Shao, J.M. Correlation between grain boundaries, carbides and stress corrosion cracking of Alloy 690TT in a high temperature caustic solution with lead. Corros. Sci. 2018, 144, 97–106. [Google Scholar] [CrossRef]
- Wang, Z.B.; Hu, H.X.; Liu, C.B.; Zheng, Y.G. The effect of fluoride ions on the corrosion behavior of pure titanium in 0.05 M sulfuric acid. Electrochim. Acta 2014, 135, 526–535. [Google Scholar] [CrossRef]
- Yang, Z.; Li, L.; Qiao, Y.; Li, C.; Zhang, L.; Cui, J.; Ren, D.; Ji, H.; Zheng, Y. Cavitation erosion-corrosion properties of as-cast TC4 and LPBF TC4 in 0.6 mol/L NaCl solution: A comparison investigation. Ultrason. Sonochem. 2024, 108, 106947. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, H.J.; Zhou, W.H.; Wang, Z.B.; Sun, J.; Li, Y. Effect of enthalpy relaxation rejuvenation on microstructure and corrosion resistance of a Zr-based bulk metallic glass. J. Non-Cryst. Solids 2024, 646, 123259. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.B.; Pang, S.J.; Zheng, Y.G.; Li, Y. Oxygen impurity improving corrosion resistance of a Zr-based bulk metallic glass in 3.5 wt% NaCl solution. Corros. Sci. 2021, 192, 109867. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Luo, H.; Zou, S.W.; Chen, Y.H.; Li, Z.M.; Du, C.W.; Li, X.G. Influence of carbon on the corrosion behaviour of interstitial equiatomic CoCrFeMnNi high-entropy alloys in a chlorinated concrete solution. Corros. Sci. 2020, 163, 108287. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of AlxCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]

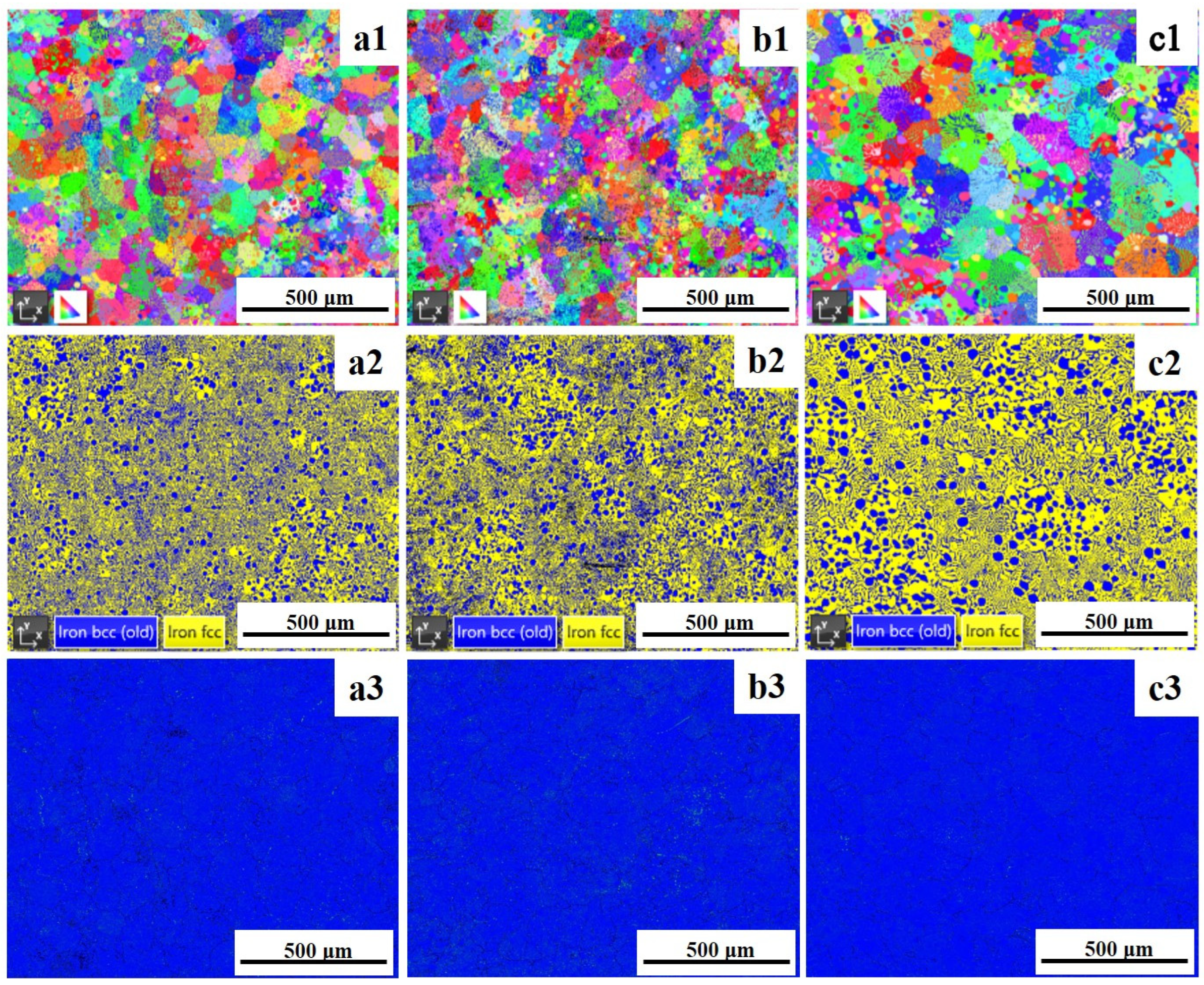
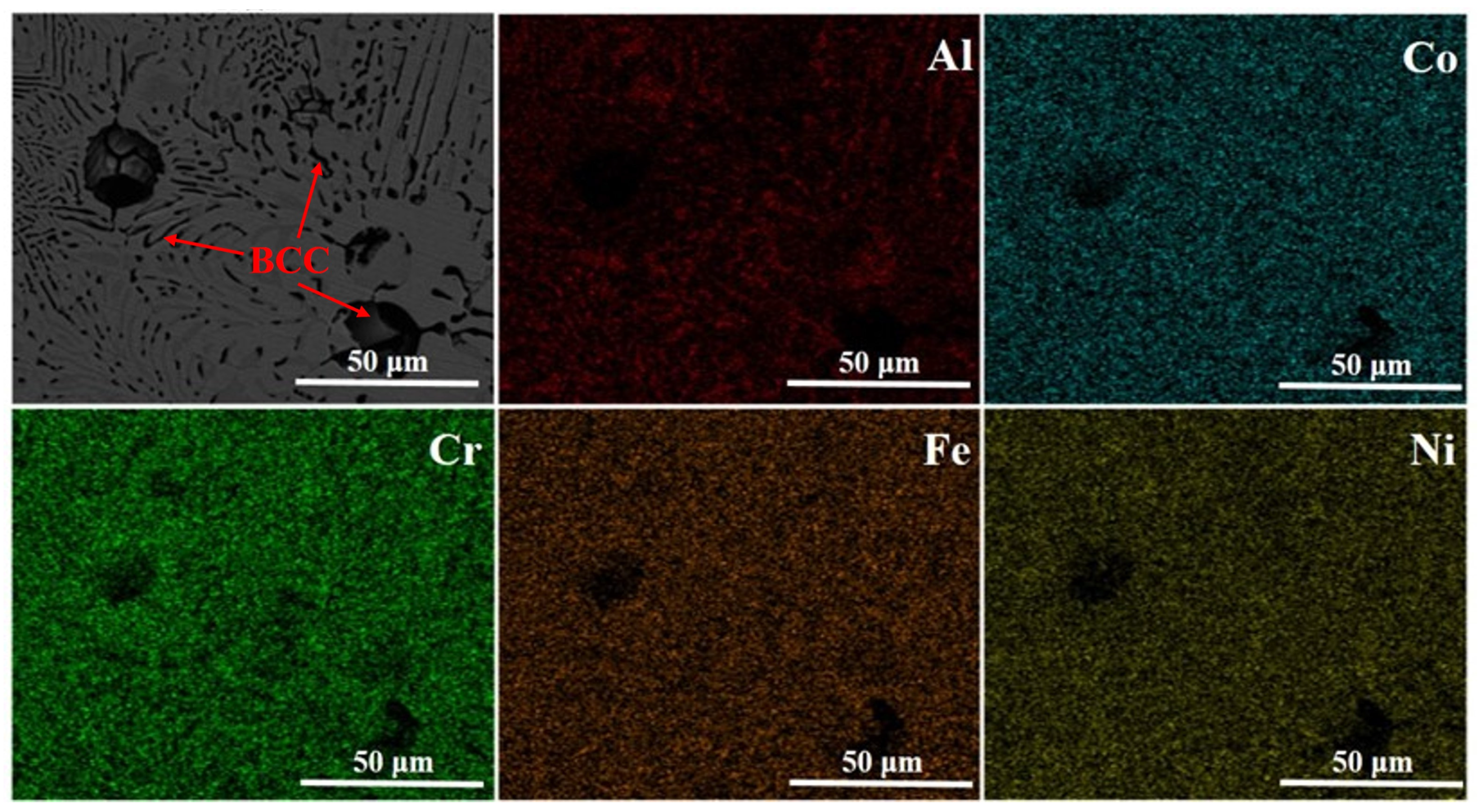

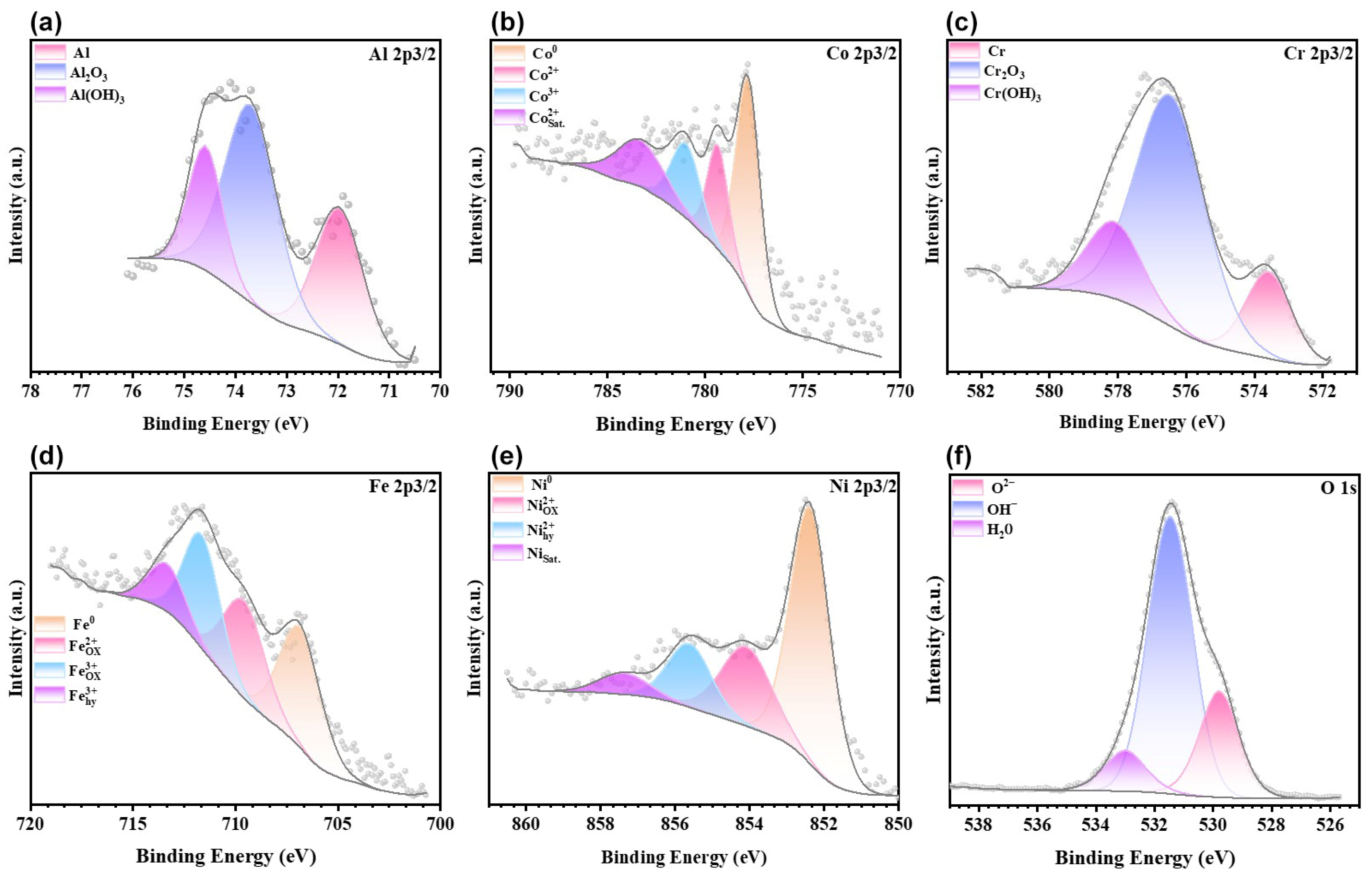

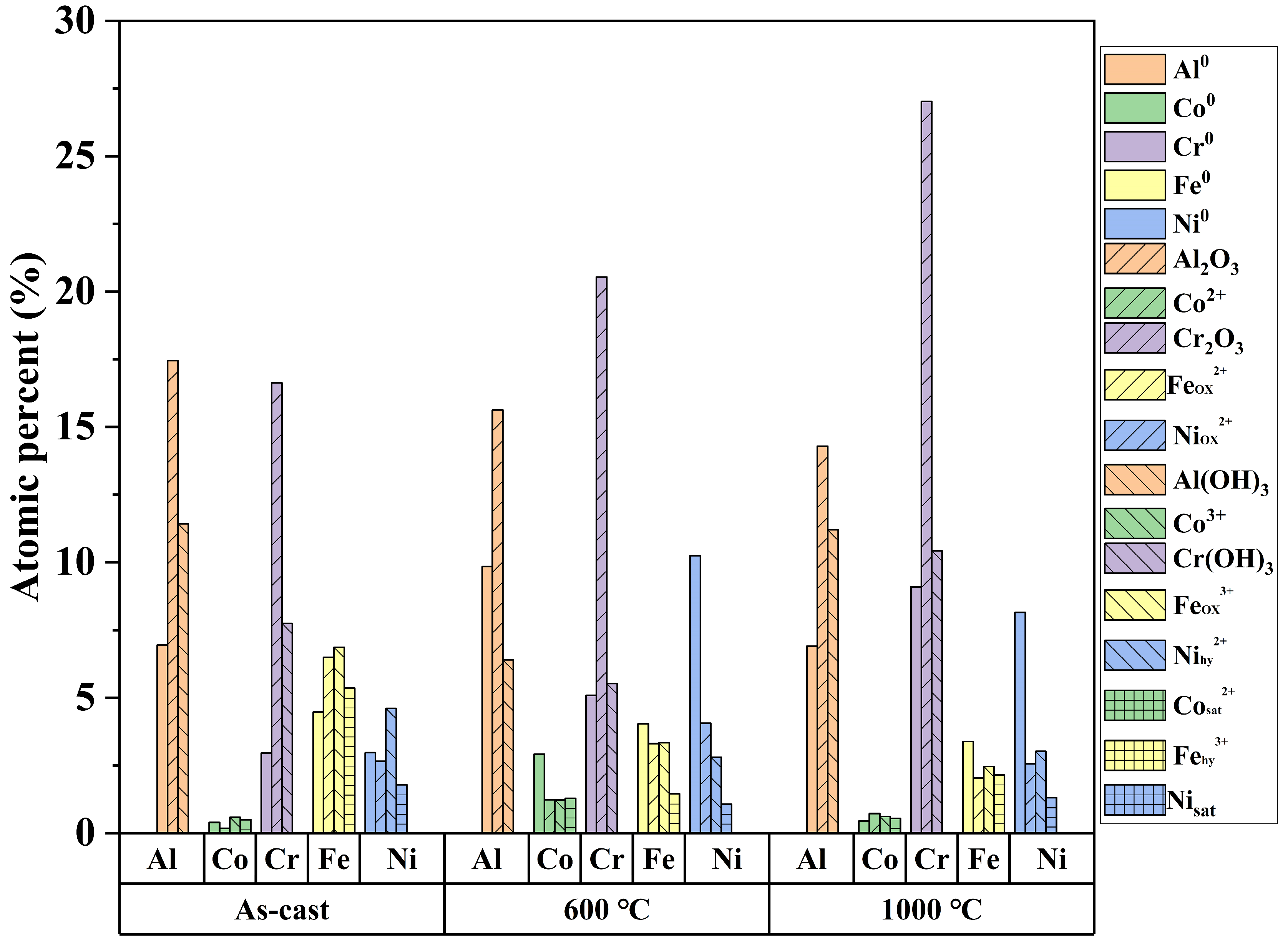
| Samples | BCC (B2) | FCC (L12) |
|---|---|---|
| as-cast | 42 | 58 |
| 600 °C | 38 | 62 |
| 1000 °C | 33 | 67 |
| Samples | Ecorr (mVSCE) | Epit (mVSCE) | Epit − Ecorr (mVSCE) | Icorr (μA/cm2) | −βc (mV/dec) | ipass at −0.1 VSCE (μA/cm2) |
|---|---|---|---|---|---|---|
| As-cast | −242 ± 7 | 135 ± 15 | 377 ± 47 | 0.28 ± 0.01 | 174 ± 5 | 0.34 ± 0.03 |
| 600 °C | −236 ± 9 | 171 ± 21 | 407 ± 42 | 0.29 ± 0.02 | 172 ± 8 | 0.35 ± 0.02 |
| 1000 °C | −228 ± 6 | 210 ± 27 | 438 ± 53 | 0.28 ± 0.04 | 180 ± 4 | 0.33 ± 0.02 |
| Samples | Rs (Ω cm2) | Qf (μF cm−2 S−n) | Rf (kΩ cm2) | n | χ2 |
|---|---|---|---|---|---|
| As-cast | 3.2 ± 0.2 | 36.9 ± 5.2 | 540 ± 14 | 0.91 ± 0.01 | 5 ± 3 × 10−4 |
| 600 °C | 2.6 ± 0.2 | 34.7 ± 1.5 | 520 ± 9 | 0.92 ± 0.01 | 8 ± 2 × 10−4 |
| 1000 °C | 3.2 ± 0.1 | 39.1 ± 4.9 | 510 ± 23 | 0.94 ± 0.01 | 3 ± 2 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Sun, H.; Sun, J. Effect of Heat Treatment on Corrosion of an AlCoCrFeNi2.1 Eutectic High-Entropy Alloy in 3.5 wt% NaCl Solution. Metals 2025, 15, 681. https://doi.org/10.3390/met15060681
Jiang J, Sun H, Sun J. Effect of Heat Treatment on Corrosion of an AlCoCrFeNi2.1 Eutectic High-Entropy Alloy in 3.5 wt% NaCl Solution. Metals. 2025; 15(6):681. https://doi.org/10.3390/met15060681
Chicago/Turabian StyleJiang, Jun, Haijing Sun, and Jie Sun. 2025. "Effect of Heat Treatment on Corrosion of an AlCoCrFeNi2.1 Eutectic High-Entropy Alloy in 3.5 wt% NaCl Solution" Metals 15, no. 6: 681. https://doi.org/10.3390/met15060681
APA StyleJiang, J., Sun, H., & Sun, J. (2025). Effect of Heat Treatment on Corrosion of an AlCoCrFeNi2.1 Eutectic High-Entropy Alloy in 3.5 wt% NaCl Solution. Metals, 15(6), 681. https://doi.org/10.3390/met15060681





