Abstract
Manganese-based oxides, particularly β-MnO2, have emerged as promising cathode materials for aqueous zinc-ion batteries (ZIBs) due to their high theoretical capacity, low cost, and intrinsic safety. However, their sluggish reaction kinetics, limited active sites, and poor conductivity often lead to suboptimal electrochemical performance. To address these limitations, we propose a facile ethanol-mediated hydrothermal strategy to engineer rod-like β-MnO2 nanostructures with tailored oxygen vacancies. By precisely adjusting ethanol addition (3–5 mL) during synthesis, oxygen vacancy concentrations were optimized to enhance electronic conductivity and active site exposure. The experimental results demonstrate that β-MnOx-2-5 synthesized with 5 mL of ethanol delivers an exceptional areal capacity of 4.87 mAh cm−2 (348 mAh g−1, 469.8 Wh kg−1) at 200 mA cm−2 under a high mass loading of 14 mg cm−2. Further, a hybrid electrode combining oxygen-deficient β-MnO2-x-3 (air-calcined) and structurally stable β-Mn5O8-y-3 (Ar-calcined) achieves a retained capacity of 3.9 mAh cm−2 with stable cycling performance, achieving an optimal equilibrium between high capacity and long-term operational durability. Systematic characterizations (XPS, ESR, XANES, FT-EXAFS) confirm vacancy-induced electronic structure modulation, accelerating ion diffusion and redox kinetics. This scalable vacancy engineering approach, requiring only ethanol dosage control, presents a viable pathway toward industrial-scale ZIB applications.
1. Introduction
The growing demand for large-scale renewable energy integration underscores the urgent need for energy storage systems that combine a low cost, intrinsic safety, and high energy density [1]. Although commercial lithium-ion batteries dominate the market with their mature energy density and cycle life, critical challenges, including limited lithium reserves, escalating costs, and safety risks (e.g., thermal runaway), impede their sustainable deployment in grid-scale storage as well as specialized applications across other fields [2,3,4,5]. In contrast, aqueous zinc-ion batteries (ZIBs) have emerged as a promising alternative, leveraging abundant zinc resources, non-flammable aqueous electrolytes, and competitive energy density (theoretical: 820 mAh g−1) while maintaining environmental compatibility [6,7,8,9]. The cathode material design critically determines ZIBs performance by coordinating structural integrity maintenance and tailored ion diffusion pathways, thereby synergistically stabilizing cycling durability and enhancing capacity retention [10,11,12].
Among transition metal oxide cathodes for Zn-ion batteries, MnO2 polymorphs have become principal contenders owing to their ultrahigh theoretical capacity (~308 mAh g−1, C = mAh g−1), originating from multivalent Mn redox couples (Mn3+/Mn4+), structural versatility across various crystalline phases, and intrinsic compatibility with defect engineering protocols [13,14,15,16,17]. Nevertheless, intrinsic limitations remain in standard MnO2 cathodes, manifesting as rapid capacity fade, insufficient high-rate capability, and significantly underutilized theoretical capacities (<40% achieved experimentally)—deficiencies primarily ascribed to poor ionic diffusivity (σ_ion ~10−11 S cm−1) and Jahn–Teller distortion-induced structural collapse [18,19,20]. While conventional optimization strategies including morphological nanoarchitecturing (e.g., nanowires, nanospheres), conductive matrix hybridization (graphene, CNTs), and heteroatom substitution doping (Co, Fe, Ni) have demonstrated partial success in enhancing capacity metrics [21,22,23,24,25,26], these modified systems still fail to address the fundamental bottlenecks of sluggish ion transfer kinetics and irreversible phase transitions under prolonged cycling. This persistent performance gap underscores the necessity for paradigm-shifting modification approaches.
Vacancy engineering presents an innovative pathway for revolutionizing MnO2 cathodes through controlled oxygen vacancy (VO) incorporation [27,28]. The strategic creation of VO defects induces substantial electronic structure reorganization: (i) redistributing localized charge density via d-band center modulation, (ii) narrowing intrinsic bandgaps from 1.3 eV to ~0.8 eV, and (iii) establishing in-gap states near the Fermi level for improved electronic conductivity [29,30]. Concurrently, VO sites significantly optimize ionic transport by (i) weakening Zn2+ adsorption energy (ΔE_ads reduced by ~0.35 eV), (ii) creating low-energy diffusion pathways (activation energy lowered from 0.68 to 0.23 eV), and (iii) buffering structural stress during (de)intercalation via expanded interlayer spacing (d(001) expanded by ~1.8 Å) [31,32,33,34,35,36].
The practical efficacy of VO-enriched MnO2 has been validated in energy systems beyond ZIBs. Fu et al. [37] developed hierarchical MnO2-x nanosheets through a three-step hydrothermal annealing process, achieving superb supercapacitor performance: specific capacitance of 452.4 F g−1 at 1 A g−1 with remarkable 69.8% retention at 50 A g−1, significantly outperforming pristine MnO2 (58.4% retention at 30 A g−1). In Na-ion battery applications, Chae’s group [38] synthesized Ca/Na-co-doped MnO2 (CNMO) with vacancy-enriched frameworks via a solid-state reaction, delivering a 152 mAh g−1 initial capacity with an ultrahigh 98.8% capacity retention after 1000 cycles; a breakthrough attributed to VO-facilitated Na+ diffusion kinetics. These cross-system successes corroborate the universal enhancement mechanism of oxygen vacancies in charge storage materials.
In this research, we devised an ethanol-modulated hydrothermal reduction approach to fabricate β-MnO2 nanorod cathodes featuring tunable oxygen vacancy concentrations (VO ≈ 8.3–15.7 at%). By accurately controlling the ethanol/oxidizer molar ratios within the range of 1:2 to 3:1 during the reaction–diffusion process, we successfully achieved optimal vacancy defect engineering without the need for intricate post-treatment procedures.
The optimized, oxygen vacancy-rich β-MnO2 demonstrates outstanding Zn2+ storage capabilities. At a current density of 0.2 A g−1, it can deliver an areal capacity of 3.9 mAh cm−2 (equivalent to a gravimetric capacity of 279 mAh g−1 and an energy density of 377 Wh kg−1), and maintains a remarkable 94.3% capacity retention after 150 cycles. This study stands out with its distinctive preparation strategy and remarkable performance optimization. Unlike the conventional methods of forming oxygen defects via the Ostwald ripening mechanism and complex heat treatment processes, our adopted ethanol-mediated hydrothermal strategy offers numerous advantages [39]. It is not only more efficient to execute but also more cost-effective. Significantly, this strategy enables precise regulation of the oxygen vacancy concentration without the requirement for additional complex post-treatment steps, thereby enhancing its practicality and scalability in actual industrial production. This ethanol-dosage-controlled defect engineering model holds great potential in providing a scalable solution for the development of high-loading aqueous battery cathodes, paving the way for more efficient and advanced energy storage systems. The innovation of our present study compared to previous works is summarized in Table 1.

Table 1.
Novel contributions of the present study.
2. Materials and Methods
2.1. Material Preparation
2.1.1. Preparation of MnOOH Precursors
An aqueous solution of potassium permanganate was mixed with various concentrations of anhydrous ethanol, and the mixture underwent a hydrothermal reaction at 140–160 °C. After the reaction, the product was washed and dried to obtain the MnOOH precursor, with a potassium permanganate solution-to-anhydrous ethanol volume ratio ranging from 11:1 to 35:1.
2.1.2. Synthesis of β-MnO2
The precursors were sintered at 350–450 °C to obtain the β-MnO2 cathode materials. The Mn-containing precursor was synthesized through a hydrothermal reaction involving potassium permanganate and varying concentrations of anhydrous ethanol as raw materials. This reaction produced a precursor that contained Mn-based compounds, such as γ-MnOOH. The aqueous solution of potassium permanganate used in this process was prepared by dissolving potassium permanganate in deionized water to achieve a concentration range of 35–45 mg/mL. The optimal duration for the hydrothermal reaction was 18–24 h. The resulting precursor was utilized as a nanorod template for the subsequent synthesis of the target product β-MnO2. The hydrothermal reaction was cooled to room temperature with a furnace at the end of the hydrothermal reaction, after which the products were washed several times using anhydrous ethanol and deionized water. The washed product underwent an initial drying process in a low-temperature oven at a temperature range of 60–80 °C for 6–12 h. Subsequently, it was transferred to a vacuum drying oven, where it was dried at a vacuum level of 0.01–0.1 MPa and a temperature of 100–150 °C for an additional 6–8 h. Following these drying steps, the Mn-containing precursor was obtained. It should be noted that the precursor was crushed before sintering to ensure the uniformity of calcination. During the sintering process, the temperature was firstly raised to 350–450 °C in an air atmosphere at 3–5 °C/min and held for 3–5 h, after which it was cooled to room temperature with the furnace, and the oxygen-deficient β-MnO2 zinc battery cathode materials could be obtained. According to the amount of ethanol added (5 mL, 4 mL, and 3 mL), the fabricated cathode materials were named as β-MnO2-x-5, β-MnO2-x-4, and β-MnO2-x-3 (abbreviated as Air5, Air4, Air3 in the figures, respectively). Meanwhile, Mn5O8-y-3 (referred to as Ar3 in the figures), obtained by sintering and cooling in a tube furnace with the same heating mode in an argon atmosphere, was used as a control. Meanwhile, commercially available β-MnO2 (Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China) was utilized, synthesized via the thermal decomposition of manganese carbonate. This commercial product served as the baseline material for comparative analysis.
2.2. Material Characterization
The morphology and microstructural information of the samples were analyzed by FESEM (Hitachi, SU8010, Hitachi Ltd., Tokyo, Japan) and TEM (Hitachi, H-8100, Hitachi Ltd., Tokyo, Japan). The valence changes of the generated substances and the intensity of oxygen vacancies were detected by X-ray photoelectron spectrometry (Thermo Kα, Thermo Fisher Scientific, Inc., Waltham, MA, USA). Carbon and sulfur analyses of the substances were carried out before and after the calcination (Wuxi High Speed HIR944, Wuxi High Speed Analytical Instruments Co., Ltd., Wuxi, China), while the carbon content of the material before and after calcination was analyzed. The phase composition of the resulting material was analyzed using XRD (D8 X-ray diffractometer, Bruker Corporation, Billerica, MA, USA). The introduction of oxygen vacancies and the intensity of the ESR of oxygen vacancies with the addition of different amounts of ethanol were then demonstrated by electron spin resonance (ESR) testing of the material with a Bruker EMXplus device (Bruker Corporation, Billerica, MA, USA). An X-ray absorption fine structure spectroscopy (XANES and FT-EXAFS Synchrotron radiation facility, Shanghai Synchrotron Radiation Laboratory, a large-scale public scientific research platform) analysis was performed to further confirm the introduction of oxygen vacancies.
2.3. Battery Preparation and Testing
2.3.1. Preparation of Cathode Electrode
Different β-MnO2 cathode materials with varying ethanol contents (β-MnO2-x-5, β-MnO2-x-4, β-MnO2-x-3, and Mn5O8-y-3) were ball-milled for 10 min to create a powder. This powder, serving as the cathode active material, was then mixed with PTFE granular powders and conductive carbon in a mass ratio of 80:10:10 to form a homogeneous mixture. PTFE has good chemical stability. During battery preparation and use, it does not release harmful chemical substances and meets the current requirements for green and environmentally friendly materials. Compared with PVDF (polyvinylidene fluoride), PVDF may produce fluorine-containing organic pollutants during synthesis and processing, imposing a certain pressure on the environment. Then, the mixture was fibrillated using high-speed dry air accelerated through Laval nozzles. This allowed the PTFE molecular chain to extend and unfold, forming fibers that bound other materials together [40]. Subsequently, the candy-like powders were hot-rolled twice at 180 °C using a horizontal-type roller to form a free-standing β-MnO2 powder film. Finally, the free-standing films and the graphite-protected stainless-steel current collectors were combined through another horizontal-type hot roller at 160 °C to form the solvent-free (SF) electrodes for the Zn-Mn batteries. The graphite-protected stainless-steel current collectors were prepared by printing graphite ink on stainless-steel foils (20 μm, TISCO, Taiyuan Iron & Steel Co., Ltd., Taiyuan, China), as reported in our previous work.
For the Zn-Mn batteries using 2025 coin cells, their components were assembled in the following order: a β-MnO2 cathode, a PPS solid-state separator (90 μm, supplied by Tongling Electric Co., Ltd., Zhenjiang, China), a zinc–indium powder solvent-free anode, and 3 M Zn(OTf)2 + 0.1 M MnSO4 as the electrolyte. In our previous studies, a sandwich-structured solid-electrolyte separator (designated as Paper-PPS-PE) has been developed [41,42,43]. The special structural design helps to enhance the wettability of the separator in aqueous solutions, its electrolyte absorption capacity, and its zinc-ion transport ability. These aspects serve as important structural evidence for the suitability of the PPS separator in zinc-ion battery systems.
2.3.2. Battery Performance Test
The assembled zinc-ion battery was left to stand for 10 h, and then the battery was subjected to a charge/discharge cycling test using a Sunway instrument. Constant-current charge/discharge experiments were carried out in the voltage range of 0.8–1.9 V at current densities of 25 mA cm−2, 50 mA cm−2, 100 mA cm−2, and 200 mA cm−2. An electrochemical workstation was used to obtain the capacity–voltage diagram and cyclic voltammetry curve, and the results of electrochemical performance were obtained by testing the multiplicity performance and cycling performance of the fabricated anode.
3. Results and Discussion
3.1. Structure, Phase, Valence, and Environmental Characterization
The main principles used in the hydrothermal synthesis of rod-like β-MnO2 with oxygen vacancies are as follows. In the process, potassium permanganate (KMnO4) and varying concentrations of ethanol were combined and thoroughly mixed in an aqueous solution. This solution was then transferred to a reactor where it underwent a hydrothermal reaction at 150 °C. During this reaction, the MnO4− ions from the potassium permanganate and the C2H5+ from the ethanol interacted, leading to the formation of MnOOH, which contained carbon functional groups and exhibited a Mn3+ oxidation state.
4MnO4− + 3CH3CH2OH → 4MnO2 + 4H2O+ 3CH3COO− + OH
MnO2 + H2O + e → MnOOH + OH−
Carbon and sulfur analyses revealed significant differences in carbon content among the samples, depending on the concentration of ethanol used. These variations influenced the amount of residual H+ and C removed from oxygen during the subsequent calcination process, which transformed the MnOOH into β-MnO2 [44]. By adjusting the volume of anhydrous ethanol to either 3 mL, 4 mL, or 5 mL, and performing the calcination under an oxygen-containing atmosphere, three distinct samples were created, β-MnO2-x-5, β-MnO2-x-4, and β-MnO2-x-3, each with a unique level of oxygen vacancies. Additionally, a control sample, β-MnO2-y-3, was prepared by calcining under an oxygen-free atmosphere, allowing for a comparison of the effects of oxygen presence during the calcination step.
Figure 1 displays the scanning electron microscope (SEM), transmission electron microscope (TEM), and energy-dispersive spectroscopy (EDS) mappings of the β-MnO2 materials. From the SEM images shown in Figure 1a and Figure S1, it is evident that β-MnO2 exhibits a rod-like nanorod structure consisting of crossed nanorods. The microscopic morphology obtained after the introduction of oxygen vacancies into the synthesized β-MnO2 is essentially similar. The unique structure can maintain a free and open gap between neighboring nanosheets, which can provide various ion transport channels, effectively accelerating ion migration and ensuring fast diffusion kinetics of ions. In addition, this unique rod-like structure helps the electrochemical reaction on the particle surface. Its (101) crystalline facet is very stable and can still be recovered after complete charging without obvious structural distortion, which greatly mitigates the volume change in the active material caused by the electrochemical reaction during charging and discharging. In addition, the analysis of magnified transmission electron microscopy (TEM) images (Figure 1b–d) shows that the layer spacing of the (111) crystalline facet of the fabricated β-MnO2-x-4 is 0.246 nm, which is close to that of the standard β-MnO2. Figure 1e–g show the energy-dispersive spectral mapping (EDS) images of β-MnO2-x-4, and it can be observed that the elements of Mn and O on the fabricated substance have a uniform distribution.
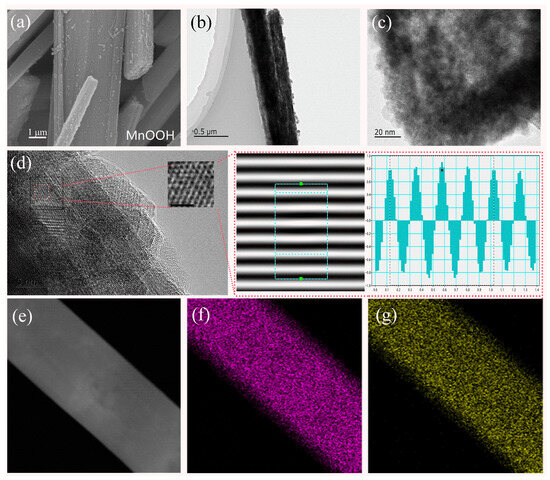
Figure 1.
Characterization of before (MnOOH) and after (β-MnO2) calcination with the addition of 4 mL of ethanol to the reaction: (a) SEM image of MnOOH; (b,c) TEM images at different magnifications of β-MnO2; (d) images analyzed in terms of crystallite spacing; (e) EDS image of β-MnO2; (f) EDS image of the element Mn in β-MnO2; (g) EDS image of the element O in β-MnO2.
Figure 2 shows the X-ray diffraction (XRD) images of MnOOH and MnO2 before and after the experimental calcination, along with the X-ray photoelectron spectroscopy (XPS) images of the corresponding materials. In Figure 2a,b, it can be clearly seen that the diffraction peaks of MnOOH prior to calcination are in substantial agreement with its respective JCPDS standard pattern (MnOOH #88-0649), which validates the successful synthesis of MnOOH precursors. After calcination, the diffraction peaks of β-MnO2 match almost perfectly with the standard peaks of β-MnO2 (β-MnO2 #72-1984), indicating the formation of a highly pure β-MnO2 phase. No discernible impurity peaks are detected, providing strong evidence for the high purity of the β-MnO2 obtained following calcination. When the XRD pattern of the experimentally measured β-MnO2 sample is meticulously compared with its JCPDS standard pattern, all the diffraction peaks in the sample align precisely with those of β-MnO2 in the standard pattern. There are no extraneous peaks that cannot be attributed to β-MnO2, further confirming the absence of significant impurities in the sample. In addition, it can be observed that the Mn oxide calcined in an argon atmosphere is in agreement with the standard card (Mn5O8 #72-1427). This indicates that during the calcination process in an oxygen-free atmosphere, the oxidation reaction is incomplete. As a result, some Mn3+ cannot be fully converted into Mn4+, leading to the formation of Mn5O8. The O-1s XPS spectra of the samples are shown in Figure 2c–f. The peaks are simulated by the Gaussian–Lorentzian hybrid function [44,45]. Subsequently, parameters such as peak position, peak width, and peak area are continuously adjusted to minimize the difference between the fitting curve and the actual measurement data. The peaks located at 529.8, 531.20, and 532.55 eV belong to Mn-O(MnO2) bonds, Mn-O(MnOOH) bonds, and oxygen vacancies, respectively. It can be found that the intensity of the Mn-O(MnO2) bond peaks increases significantly after calcination, while the intensity of the Mn-O(MnOOH) bond peaks decreases significantly. The intensity of the oxygen vacancy peaks is obviously low for the material calcined under an Ar gas atmosphere relative to the material calcined in an air atmosphere. Moreover, the intensity of the oxygen vacancy peaks of the materials calcined under an Ar gas atmosphere is significantly lower after calcination compared to those calcined under the air atmosphere. The addition of 4 mL and 5 mL of β-MnO2-x-4 and β-MnO2-x-5 resulted in relatively greater oxygen vacancy peak intensities than that of β-MnO2-x-3, which further confirms that the samples with higher amounts of added alcohols have higher oxygen vacancy concentrations. The Mn-2p XPS spectra of the sample with 3 mL of ethanol before and after calcination are shown in Figure 2g–i. It can be observed that after calcination, the peak value of Mn3+ decreases significantly, while that of Mn4+ increases significantly. This indicates that the trivalent manganese in MnOOH is converted to tetravalent manganese in MnO2 after the reaction. Moreover, by comparing the peak values after calcination in argon and air, it can be found that the peak value of Mn4+ after calcination in air is higher, suggesting a higher degree of oxidation.
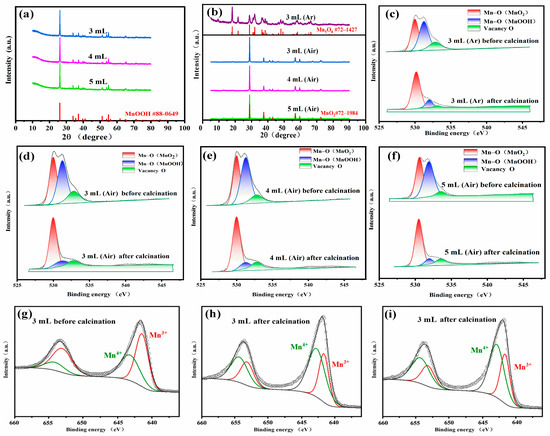
Figure 2.
Characterization of MnOOH (before calcination) and β-MnO2 (after calcination in air atmosphere) and Mn5O8 (after calcination in Ar gas atmosphere) produced by adding different concentrations of C2H5OH: (a,b) XRD images before and after calcination; (c) XPS images of Mn5O8-y-3 (3 mL; Ar); (d) O-1s, XPS images of β-MnO2-x-3 (3 mL; air); (e,f) O-1s, XPS images of β-MnO2-x-4 (4 mL; air) and β-MnO2-x-5 (5 mL; air); (g) Mn-2p, XPS images of MnOOH, black solid lines represent the experimental XPS data; circle lines represent the fitted values; (h,i) Mn-2p, XPS images of β-MnO2-x-3 and Mn5O8-y-3, lines correspond those in (g).
Figure 3 displays the ESR images and XPS images of the samples, along with Raman mapping, CS-analyzed images (spectral data visualizations generated from carbon–sulfur determination tests), XANES spectra, and FT-EXAFS mapping. It can be found from the ESR pattern in Figure 3a that they all have electron spin resonance (ESR) signals at g = 2.0 due to the electrons being trapped in vacancies [46]. The sample with the most amount of ethanol added, β-MnO2-x-5, has the highest ESR intensity, which suggests that the present sample produces more oxygen vacancies during calcination as compared to the other samples with a lower ethanol content. In addition, the chemical composition and valence states of the above products were further investigated by XPS characterization, as shown in Figure 3b. In the Mn-2p XPS spectra, a pair of peaks at 642.5 and 654.8 eV are observed, belonging to Mn-2p3/2 and Mn-2p1/2, respectively. A spin-energy difference of 12.3 eV confirms the characterization of the manganese dioxide phase [47]. Raman spectroscopy was performed on these samples, as shown in Figure 3c. The characteristic peaks located at 503, 572, and 625 cm−1 correspond to the stretching vibration modes of the Mn-O bonds in the MnO6 octahedrons. The MnO6 octahedrons are an important component of the MnO2 structure. It can be observed that the positions of the peaks in the curve obtained by calcination in air shift to higher wavenumbers compared with those in the curve obtained by calcination in argon. This indicates that after the introduction of oxygen vacancies, a certain distortion occurs, leading to an increase in the force constant of the Mn-O bonds. Furthermore, by observing the images of the three samples calcined in air, it can be found that as the amount of ethanol increases, the intensity of the peaks related to the stretching vibration mode of the Mn-O bonds gradually decreases. That is to say, the covalent interaction between the Mn-O layers is more affected by the oxygen vacancies. It can be analyzed and concluded that the samples with a higher amount of ethanol added have more oxygen vacancies. Figure 3d displays the CS analysis of the precursors with varying amounts of anhydrous ethanol and the MnO2 generated after calcination. Before calcination, it was observed that the C content in MnOOH was generally proportional to the amount of ethanol added during the reaction. Specifically, the C content increased with the addition of more ethanol, reaching a maximum of 3.02% when 5 mL of ethanol was used. However, after calcination, the C content dropped to less than 0.05%, indicating that calcination had a significant impact on the C content. This indicates that nearly all of the C is consumed during the calcination process. In the K-edge X-ray absorption fine structure (XANES) spectra of Mn shown in Figure 3e, the Mn K-edge binding energy of the resulting samples shifted gradually towards lower values as the vacancy concentration increased. This shift indicates that the structural symmetry of β-MnO2 was partially disrupted, and some Mn4+ ions were reduced to Mn3+ following the formation of oxygen vacancies in the product. In order to better understand the coordination environment of the samples, the Mn k-edge in β-MnO2-x-3, Mn5O8-y-3, was analyzed by Fourier transform extended X-ray absorption fine structure (FT-EXAFS) spectroscopy, as shown in Figure 3f. The strong peaks centered at ~2.2 and 2.5 Å in the Mn k-edge spectra are inferred to be due to the Mn-O and Mn-Mn coordination states, respectively. Apparently, the Mn-O peak intensity of Mn5O8-y-3 is relatively higher than that of the other samples. When oxygen vacancies are generated in the product, the corresponding Mn-O peak intensity decreases gradually from Mn5O8-y-3 to β-MnO2-x-3, which can be attributed to the removal of more oxygen vacancies, which greatly increases the degree of structural distortion. The analysis of these results proves that the oxygen vacancies in the samples can be controlled through the adjustment of the reaction time and the amount of methanol added.

Figure 3.
Characterization of β-MnO2 and Mn5O8 generated after calcination with different C2H5OH additions: (a) ESR images of β-MnO2-x-5, β-MnO2-x-4, β-MnO2-x-3, and Mn5O8-y-3; (b) XPS images of β-MnO2-x-5, β-MnO2-x-4, β-MnO2-x-3, and Mn5O8-y-3; (c) Raman images of β-MnO2-x-5, β-MnO2-x-4, β-MnO2-x-3, and Mn5O8-y-3; (d) CS–analyzed images; (e) XANES spectra of β-MnO2-x-3 and Mn5O8-y-3; (f) the corresponding FT–EXAFS plot spectra of β-MnO2-x-3 and Mn5O8-y-3.
3.2. Electrochemical Performance Analysis
In comparing β-MnO2-y-3, β-MnO2-x-3, β-MnO2-x-4, and β-MnO2-x-5 as anodes for zinc-ion batteries, the electrochemical performance of the button-type zinc-ion battery was determined by using dry Zn powder as the anode and β -MnO2-y or β-MnO2-x as the cathode, and a 3 M Zn(CF3SO3)2/0.1 M MnSO4 mixed solution as the electrolyte (the loading of all batteries was uniformly 14 mg cm−2). Figure 4 displays the charge/discharge profiles, rate performance, and cycling stability of zinc-ion batteries with various electrodes.
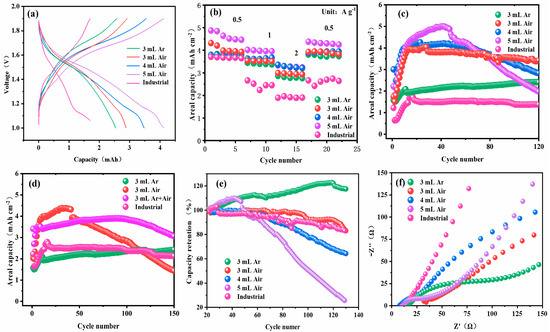
Figure 4.
Performance testing of aqueous zinc-ion batteries with Mn-based cathodes by introducing different amounts of oxygen vacancies (adding different ethanol amounts under different calcination atmospheres), with the loading of the batteries uniformly around 14 mg cm−2: (a) the charge/discharge profiles of industrial MnO2, β-MnO2-x-5, β-MnO2-x-4, β-MnO2-x-3, and β-MnO2-y-3; (b) rate performance of industrial MnO2, β-MnO2-x-5, β-MnO2-x-4, β-MnO2-x-3, and β-MnO2-y-3; (c,d) cycling number–specific areal capacity diagram of industrial MnO2, β-MnO2-x-5, β-MnO2-x-4, β-MnO2-x-3, β-MnO2-y-3, and β-MnO2-x–3 + β-Mn5O8-y-3. (e) Capacity retention; (f) impedance diagram (EIS).
According to previous experimental experience [48,49,50,51], the addition of sufficient Mn2+ to the electrolyte during the charging process contributes to the formation of MnO2, which effectively prevents the dissolution of Mn2+ and significantly improves the efficiency of galvanization and stripping. Therefore, the electrolyte structure plays a crucial role in the electrochemical performance of the energy storage system [52]. When the battery was cycled for 20 circles, a constant-current charge–discharge experiment was carried out within the voltage range of 0.8–1.9 V (vs. Zn2+/Zn) for the fabricated battery, as shown in Figure 4a. The multiplicative performance tests were also performed for batteries with different contents of ethanol added in the range of 0.5–2 A g−1. By referring to a large number of studies on MnO2-based electrode materials, the current density is often selected to be in the range of 0.05–0.2 C. The current density chosen in this study was within the conventional testing range of this field, which facilitates comparative analysis with other research results. As shown in Figure 4b and Figure S2, it can be found that the best multiplicity performance was measured for the anode with the most oxygen vacancies, i.e., with 5 mL of ethanol added and calcined in air, and the area capacities of the β-MnO2-x-5 anode at current densities of 0.5, 1.0, and 2.0 A g−1 were 4.87, 4.01, and 3.33 mAh cm−2. The capacity retention rate of the β-MnO2-x-5 anode was 64% at 2 A g−1, corresponding to a gravimetric capacity of 348 mAh g−1 (at 0.5 A g−1). Based on voltage and capacity calculations, with a charge–discharge range of 0.8–1.9 V and an average voltage of 1.4 V, the energy density was 469.8 Wh kg−1 (i.e., capacity (mAh/g) × voltage (V)/1000 = 348 mAh/g × 1.35 V/1000 = 469.8 Wh kg−1), which is a significant improvement over the industrial β-MnO2 multiplicity performance. Moreover, through the comprehensive analysis of β-MnO2-x-3, β-MnO2-x-4, and Mn5O8-y-3 with different ethanol amounts added and the characterization experiments performed before, it was found that the introduction of oxygen vacancies leads to a higher specific capacity compared with industrial β-MnO2. The analysis shows that, compared to industrial β-MnO2, β-MnO2 with introduced oxygen vacancies exhibits a higher specific capacity. Moreover, the more oxygen vacancies introduced, the better the battery’s rate capability and the higher the maximum specific capacity achieved. This is because oxygen vacancies optimize the ion transport path: on the one hand, they weaken the adsorption energy of Zn2+, making it easier for ions to insert into and extract from the electrode material; on the other hand, they create a low-energy path for ion diffusion, reducing the activation energy of ion diffusion, accelerating the diffusion rate of ions in the electrode material, and enabling the battery to maintain good performance even at high current densities.
Cycling performance tests were conducted on batteries made with these cathodes, as shown in Figure 4c and Figure S3. It can be observed that the β-MnO2-x-5 cathode has the highest maximum areal capacity of approximately 4.87 mAh cm−2, but there is significant capacity fade after about 50 cycles. However, other samples, compared to the most oxygen-deficient β-MnO2-x-5, with the highest number of oxygen vacancies, demonstrate better cycling performance and capacity retention. When calcined under argon and used as the cathode, Mn5O8-y-3 can maintain a high areal capacity retention rate for up to 150 cycles. Correspondingly, β-MnO2-x-3 and MnO2-y-3 also exhibit good cycling stability and capacity retention. The introduction of a higher number of oxygen vacancies enhances the specific capacity but reduces battery stability and accelerates battery decay. MnO2 materials prepared by calcination without introducing oxygen vacancies during the process can achieve a longer cycle life at a certain areal capacity. Although excessive oxygen vacancies increase the specific capacity, they lead to an unstable material structure. From the perspective of crystal structure, the introduction of oxygen vacancies disrupts the original crystal structure symmetry of β-MnO2 and changes the bond length and bond angle of the Mn-O bond. During the charge–discharge process, the insertion and extraction of Zn2+ exacerbates this structural distortion. As the number of cycles increases, the structural distortion accumulates continuously, ultimately resulting in the collapse of the material structure and thus a rapid decline in capacity. To explore better battery cathode performance, β-MnO2-x-3 and Mn5O8-y-3 were mixed in a 1:1 mass ratio as the cathode. The electrochemical performance data obtained showed that the specific capacity could reach about 3.9 mAh cm−2 (279 mAh g−1, 376.6 Wh Kg−1 (279 mAh/g × 1.35 V/1000 = 376.6 Wh kg−1)). Ultimately, the electrode combining β-MnO2-x-3 with a higher oxygen defect content and Mn5O8-y-3 with a more stable structure exhibits excellent comprehensive performance, with both areal capacity and cycling performance at a high overall level. The capacity retention rate is shown in Figure 4e and the coulombic efficiency is shown in Figure S4. Since the battery generally reaches its optimal capacity after about 20 cycles, the highest capacity achieved at the 20th cycle is used for plotting. Although the coulombic efficiency is maintained at a relatively high level, it can be found that the more oxygen vacancies are introduced, the lower the capacity retention rate is after 100 cycles. The samples calcined under argon basically show a slow upward trend, indicating that their structure is stable, and they have a higher retention rate compared to industrial materials. Subsequently, to further explore the electrochemical properties of the battery, impedance spectroscopy (EIS) was carried out, as shown in Figure 4f. The results show that for the battery with the manganese-based oxide synthesized by the method described in this paper as the cathode, its impedance is significantly lower than that of industrial manganese dioxide. This is attributed to the fact that its unique rod-like structure provides a more efficient path for electron transfer, allowing electrons to move more freely within the material, reducing the resistance encountered during the charging and discharging process, and thus reducing the overall impedance of the battery. At the same time, it can be found that the 3Ar sample with the least structural distortion has the lowest impedance. This is because the minimum degree of distortion ensures a relatively complete internal structure of the material, enabling electrons and ions to flow smoothly. This well-preserved structure has less resistance to charge transfer, thus minimizing the impedance. Conversely, the 5Air sample, with the highest oxygen vacancy concentration, has a high impedance. Although oxygen vacancies can enhance its capacity, excessive oxygen vacancies will damage the crystal structure of the material. The destruction of the structure leads to a disordered atomic arrangement, which in turn hinders the movement of electrons and ions, ultimately making the charge transfer process more difficult and increasing the impedance of the battery.
4. Conclusions
In summary, in this study we successfully synthesized β-MnO2 zinc-ion battery cathode materials with oxygen vacancies through a direct hydrothermal method using ethanol and potassium permanganate. By simply adjusting the amount of ethanol added during the synthesis process, the introduction of oxygen vacancies can be effectively controlled. Various characterization analyses were conducted to confirm the presence and quantity of these oxygen vacancies. The results indicate that compared to industrial β-MnO2, this cathode material not only exhibits a higher specific capacity but also demonstrates superior cycling performance. When β-MnO2-x-5 is used as the cathode material, the battery achieves an areal capacity of 5 mAh cm−2 (357 mAh g−1, 469.8 Wh kg−1) at a loading of 14 mg/cm2. On the other hand, when β-Mn5O8-y-3, which has a more stable structure and the same loading, is used as the cathode material, the battery maintains a high areal capacity of 2 mAh cm−2, with virtually no capacity fade after 150 cycles. When electrodes are fabricated by mixing the two materials in a 1:1 mass ratio, they achieve an areal capacity of 3.9 mAh cm−2 (279 mAh g−1, 376.6 Wh kg−1) after approximately 150 cycles, ensuring good cycling performance while achieving a high areal capacity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/met15050526/s1, Figure S1: SEM images of MnOOH; Figure S2: Cycling number–specific capacity diagram of industrial MnO2, β-MnO2-x-5, β-MnO2-x-4, β-MnO2-x-3, and Mn5O8-y-3 at different current densities; Figure S3: Cycling number–specific capacity diagram of industrial MnO2, β-MnO2-x-5, β-MnO2-x-4, β-MnO2-x-3, and Mn5O8-y-3; Figure S4: Diagram of charge–discharge, specific capacity, and coulombic efficiency of the materials calcined in an argon atmosphere.
Author Contributions
J.-C.W.: writing—review and editing, writing—original draft, supervision, investigation, formal analysis, data curation. Y.Y.: methodology, writing—original draft, investigation, formal analysis, data curation. H.Z.: writing—review and editing, validation, supervision, resources, project administration, methodology, investigation, funding acquisition, formal analysis, data curation, conceptualization. X.S.: validation, resources, investigation, formal analysis, data curation. H.G.: supervision, investigation, funding acquisition, formal analysis. Z.L.: methodology, investigation, formal analysis, data curation. X.L.: resources, funding acquisition, formal analysis. Y.D.: validation, methodology, investigation, formal analysis, data curation. Y.Q.: writing—review and editing, supervision, resources, project administration, investigation, funding acquisition, formal analysis, data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (NSFC) (Grants 51702131, 22208130), the Natural Science Foundation of Jiangsu Province, China (Grant SBK2017041705), the Senior Talents Fund of Jiangsu University (5501220011), the China Postdoctoral Science Foundation (2023M730482). The authors acknowledge S. Yao, H. Zhu, R. Yan from Jiangsu Tongling Electric Co., Ltd. for the pilot line support (Grant HX230002).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that this study received funding from Jiangsu Tongling Electric Co., Ltd. (Grant HX230002). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Selvakumaran, D.; Pan, A.; Liang, S.; Cao, G. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J. Mater. Chem. A 2019, 7, 18209–18236. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Zhu, Z.; Chai, X.; Xu, L.; Quan, L.; Yuan, C.; Tian, S. Design and performance of a distributed electric drive system for a series hybrid electric combine harvester. Biosyst. Eng. 2023, 236, 160–174. [Google Scholar] [CrossRef]
- Jiangyi, H.; Fan, W. Densign and testing of a small orchard tractor driven by a power battery. Eng. Agrícola 2023, 43, e20220195. [Google Scholar]
- Lu, Q.; Wu, D.; Jiang, Y.; Liu, Z.; Chen, B.; Zhu, M.; Schmidt, O. Flexible MXene films for batteries and beyond. Carbon Energy 2022, 4, 598–620. [Google Scholar]
- Zhang, H.; Li, S.; Xu, L.; Momen, R.; Deng, W.; Hu, J.; Zou, G.; Hou, H.; Ji, X. High-yield carbon dots interlayer for ultra-stable zinc batteries. Adv. Energy Mater. 2022, 12, 2200665. [Google Scholar] [CrossRef]
- Liu, C.; Xie, X.; Lu, B.; Zhou, J.; Liang, S. Electrolyte strategies toward better zinc-ion batteries. ACS Energy Lett. 2021, 6, 1015–1033. [Google Scholar] [CrossRef]
- Wang, N.; Guo, Z.; Ni, Z.; Xu, J.; Qiu, X.; Ma, J.; Wei, P.; Wang, Y. Molecular tailoring of an n/p-type phenothiazine organic scaffold for zinc batteries. Angew. Chem. Int. Ed. 2021, 60, 20826–20832. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Y.; Ni, Z.; Zhang, Y.; Xu, J.; Kong, T.; Huang, J.; Li, W.; Ma, J.; Wang, Y. Chemically self-charging aqueous zinc-organic battery. J. Am. Chem. Soc. 2021, 143, 15369–15377. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Wang, X.; Zhang, J.; Hu, Y.; Wang, D.; Zuin, L.; Zhou, T.; Wu, Y.; et al. High-performance reversible aqueous Zn-ion battery based on porous MnOx nanorods coated by MOF-derived N-doped carbon. Adv. Energy Mater. 2018, 8, 1801445. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, H.; Song, B.; Xiong, D.; Tao, S.; Deng, W.; Hu, J.; Hou, H.; Zou, G.; Ji, X. Angstrom-level ionic sieve 2D-MOF membrane for high power aqueous zinc anode. Adv. Funct. Mater. 2023, 33, 2300339. [Google Scholar] [CrossRef]
- Zong, Q.; Wu, Y.; Liu, C.; Wang, Q.; Zhuang, Y.; Wang, J.; Tao, D.; Zhang, Q.; Cao, G. Tailoring layered transition metal compounds for high-performance aqueous zinc-ion batteries. Energy Storage Mater. 2022, 52, 250–283. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Wang, X.; Meng, J.; Liu, X.; Wu, B.; Han, C.; Mai, L. Comprehensive understanding of the roles of water molecules in aqueous Zn-ion batteries: From electrolytes to electrode materials. Energy Environ. Sci. 2021, 14, 3796–3839. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, S.; Fang, G.; Yang, Y.; Zhou, J. Ultra-high mass-loading cathode for aqueous zinc-ion battery based on graphene-wrapped aluminum vanadate nanobelts. Nano-Micro Lett. 2019, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific challenges for the implementation of Zn-ion batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Gao, J.; Xie, X.; Liang, S.; Lu, B.; Zhou, J. Inorganic colloidal electrolyte for highly robust zinc-ion batteries. Nano-Micro Lett. 2021, 13, 69. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Q.; Wang, D.; Liang, G.; Zhu, Y.; Mo, F.; Huang, Z.; Li, X.; Ma, L.; Tang, T.; et al. A flexible solid-state aqueous zinc hybrid battery with flat and high-voltage discharge plateau. Adv. Energy Mater. 2019, 9, 1902473. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Cheng, Q.; Liao, F.; Yang, G.; Lu, X.; Chen, L. Interlayer engineering of preintercalated layered oxides as cathode for emerging multivalent metal-ion batteries: Zinc and beyond. Energy Storage Mater. 2021, 38, 397–437. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, X.; Li, Y.; Chen, Q.; Chen, M. Challenges and design strategies for high performance aqueous zinc ion batteries. Energy Storage Mater. 2021, 42, 533–569. [Google Scholar] [CrossRef]
- Cui, Y.; Zhuang, Z.; Xie, Z.; Cao, R.; Hao, Q.; Zhang, N.; Liu, W.; Zhu, Y.; Huang, G. High-energy and long-lived Zn–MnO2 battery enabled by a hydrophobic-ion-conducting membrane. ACS Nano 2022, 16, 20730–20738. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Liang, G.; Li, H.; Liu, Z.; Tang, Z.; Liang, J.; Zhi, C. A Superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery. ACS Nano 2019, 13, 10643–10652. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guan, S.; Weng, Y.; Xu, S.M.; Lu, H.; Meng, X.; Zhou, S. Highly efficient vacancy-driven photothermal therapy mediated by ultrathin MnO2 nanosheets. ACS Appl. Mater. Interfaces 2019, 11, 6267–6275. [Google Scholar] [CrossRef]
- Zhao, R.; Wu, Y.; Liang, Z.; Gao, L.; Xia, W.; Zhao, Y.; Zou, R. Metal–organic frameworks for solid-state electrolytes. Energy Environ. Sci. 2020, 13, 2386–2403. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Cheng, S.; Jiang, K. An Ultrastable presodiated titanium disulfide anode for aqueous “rocking-chair” zinc ion battery. Adv. Energy Mater. 2019, 9, 1900993. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, H.; Choi, J.H.; Choi, J.-C.W. Crystal water for high performance layered manganese oxide cathodes in aqueous rechargeable zinc batteries. Energy Environ. Sci. 2019, 12, 1999–2009. [Google Scholar] [CrossRef]
- Xiao, X.; Duan, X.; Song, Z.; Deng, X.; Deng, W.; Hou, H.; Zheng, R.; Zou, G.; Ji, X. High-throughput production of cheap mineral-based heterostructures for high power sodium ion capacitors. Adv. Funct. Mater. 2022, 32, 2110476. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, T.; Lu, J.; Sun, L.; Ni, Z. Defect engineering in 2D materials: Precise manipulation and improved functionalities. Research 2019, 2019, 4641739. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Chen, W.; Zou, Y.; Chen, R.; Zang, S.; Wang, Y.; Yao, X.; Wang, S. Insight into the design of defect electrocatalysts: From electronic structure to adsorption energy. Mater. Today 2019, 31, 47–68. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Z.; Shadike, Z.; Lei, M.; Liu, J.; Li, C. Defect-concentration-mediated T-Nb2O5 anodes for durable and fast-charging Li-ion batteries. Adv. Funct. Mater. 2022, 32, 2107060. [Google Scholar] [CrossRef]
- Xiong, D.; Yang, L.; Cao, Z.; Li, F.; Deng, W.; Hu, J.; Hou, H.; Zou, G.; Ji, X. In situ construction of high-density solid electrolyte interphase from MOFs for advanced Zn metal anodes. Adv. Funct. Mater. 2023, 33, 2301530. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Xu, L.; Zou, K.; Deng, W.; Hou, H.; Zou, G.; Ji, X. Novel nonstoichiometric niobium oxide anode material with rich oxygen vacancies for advanced lithium-ion capacitors. ACS Appl. Mater. Interfaces 2023, 15, 5387–5398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect engineering on electrode materials for rechargeable batteries. Adv. Mater. 2020, 32, 1905923. [Google Scholar] [CrossRef]
- Deng, X.; Zou, K.; Momen, R.; Cai, P.; Chen, J.; Hou, H.; Zou, G.; Ji, X. High content anion (S/Se/P) doping assisted by defect engineering with fast charge transfer kinetics for high-performance sodium ion capacitors. Sci. Bull. 2021, 66, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Z.; Foster, C.W.; Banks, C.E.; Qiu, X.; Ji, X. Oxygen vacancies evoked blue TiO2(B) nanobelts with efficiency enhancement in sodium storage behaviors. Adv. Funct. Mater. 2017, 27, 1700856. [Google Scholar] [CrossRef]
- Li, Y.; Qian, J.; Zhang, M.; Wang, S.; Wang, Z.; Li, M.; Bai, Y.; An, Q.; Xu, H.; Wu, F.; et al. Co-construction of sulfur vacancies and heterojunctions in tungsten disulfide to induce fast electronic/ionic diffusion kinetics for sodium-ion batteries. Adv. Mater. 2020, 32, 2005802. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Tian, C.; Yang, C.; Xu, J.; Meng, Y.; Manke, I.; Chen, N.; Wu, Z.; Zhan, L.; Wang, Y.; et al. P-doped NiTe2 with Te-vacancies in lithium–sulfur batteries prevents shuttling and promotes polysulfide conversion. Adv. Mater. 2022, 34, 2106370. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, X.; Zha, D.; Zhu, J.; Ouyang, X.; Wang, X. Yolk–shell-structured MnO2 microspheres with oxygen vacancies for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 1601–1611. [Google Scholar] [CrossRef]
- Chae, M.S.; Chakraborty, A.; Kunnikuruvan, S.; Attias, R.; Maddukuri, S.; Gofer, Y.; Major, D.T.; Aurbach, D. Vacancy-driven high rate capabilities in calcium-doped Na0.4MnO2 cathodes for aqueous sodium-ion batteries. Adv. Energy Mater. 2020, 10, 2002077. [Google Scholar] [CrossRef]
- Xiong, T.; Yu, Z.G.; Wu, H.; Du, Y.; Xie, Q.; Chen, J.; Zhang, Y.-W.; Pennycook, S.J.; Lee, W.S.V.; Xue, J. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous zinc ion battery. Adv. Energy Mater. 2019, 9, 1803815. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, H.; Zhang, Z.; Liu, Y.; Song, J.; Liu, T.; He, Y.; Meng, A.; Sun, C.; Hu, M.; et al. The semicoherent interface and vacancy engineering for constructing Ni(Co)Se2@Co(Ni)Se2 heterojunction as ultrahigh-rate battery-type supercapacitor cathode. Adv. Funct. Mater. 2022, 32, 2202063. [Google Scholar] [CrossRef]
- Wu, J.-C.; Shen, X.; Zhou, H.; Li, X.; Gao, H.; Ge, J.; Xu, T.; Zhou, H. Zn-In Alloying Powder Solvent Free Electrode Toward High-Load Ampere-Hour Aqueous Zn-Mn Secondary Batteries. Small 2024, 20, 2308541. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Wu, J.-C.; Gao, H.; Zhou, H.; Shi, Y.; Gu, J. Boosting the Mechanical and Electrochemical Performance of MnO2 Dry Electrode with Bentonite for Ampere-Hour Aqueous Zn-ion Batteries. Batter. Supercaps, 2025; e202400757, (EarlyView). [Google Scholar] [CrossRef]
- Wu, J.-C.; Liu, Z.; Zhou, H.; Gao, H.; Zhou, H.; Li, X.; Yin, Y.; Shen, X. Preformation of Zn based layered double hydroxides on Zn powder solvent-free electrode for low temperature soft-package Zn-MnO2 battery. J. Power Sources 2025, 640, 236778. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Chen, L.; Yarley, O.P.N.; Zhou, C. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crops Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Qiang, S.; Li, Z.; He, S.; Zhou, H.; Zhang, Y.; Cao, X.; Yuan, A.; Zou, J.; Wu, J.; Qiao, Y. Modulating electronic structure of CoS2 nanorods by Fe doping for efficient electrocatalytic overall water splitting. Nano Energy 2025, 134, 110564. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Zhang, Z.; Wu, Y.; Yang, G.; Chen, B.; Xiong, W. Preparation of beta-MnO2 and alpha-Mn2O3 nanorods via a self-sacrificing template route and their characterization and application. Gaodeng Xuexiao Huaxue Xuebao/Chem. J. Chin. Univ. 2015, 36, 436–441. [Google Scholar]
- Wang, S.; Li, L.; He, W.; Shao, Y.; Li, Y.; Wu, Y.; Hao, X. Oxygen vacancy modulation of bimetallic oxynitride anodes toward advanced Li-ion capacitors. Adv. Funct. Mater. 2020, 30, 2000350. [Google Scholar] [CrossRef]
- Zhang, J.; He, T.; Zhang, W.; Sheng, J.; Amiinu, I.S.; Kou, Z.; Yang, J.; Mai, L.; Mu, S. Na-Mn-O nanocrystals as a high capacity and long life anode material for Li-ion batteries. Adv. Energy Mater. 2017, 7, 1062092. [Google Scholar] [CrossRef]
- Zhai, X.-Z.; Qu, J.; Hao, S.-M.; Jing, Y.-Q.; Chang, W.; Wang, J.; Li, W.; Abdelkrim, Y.; Yuan, H.; Yu, Z.-Z. Layered birnessite cathode with a displacement/intercalation mechanism for high-performance aqueous zinc-ion batteries. Nano-Micro Lett. 2020, 12, 56. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Ge, J.; Fan, L.; Rao, A.M.; Zhou, J.; Lu, B. Surface-substituted prussian blue analogue cathode for sustainable potassium-ion batteries. Nat. Sustain. 2022, 5, 225–234. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Mathew, V.; Song, J.; Kim, S.; Kim, S.; Jo, J.; Baboo, J.P.; Pham, D.T.; Putro, D.Y.; et al. Facile synthesis and the exploration of the zinc storage mechanism of β-MnO2 nanorods with exposed (101) planes as a novel cathode material for high performance eco-friendly zinc-ion batteries. J. Mater. Chem. A 2017, 5, 23299–23309. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).