Plasma–Chemical Low-Temperature Reduction of Aluminum with Methane Activated in Microwave Plasma Discharge
Abstract
1. Introduction
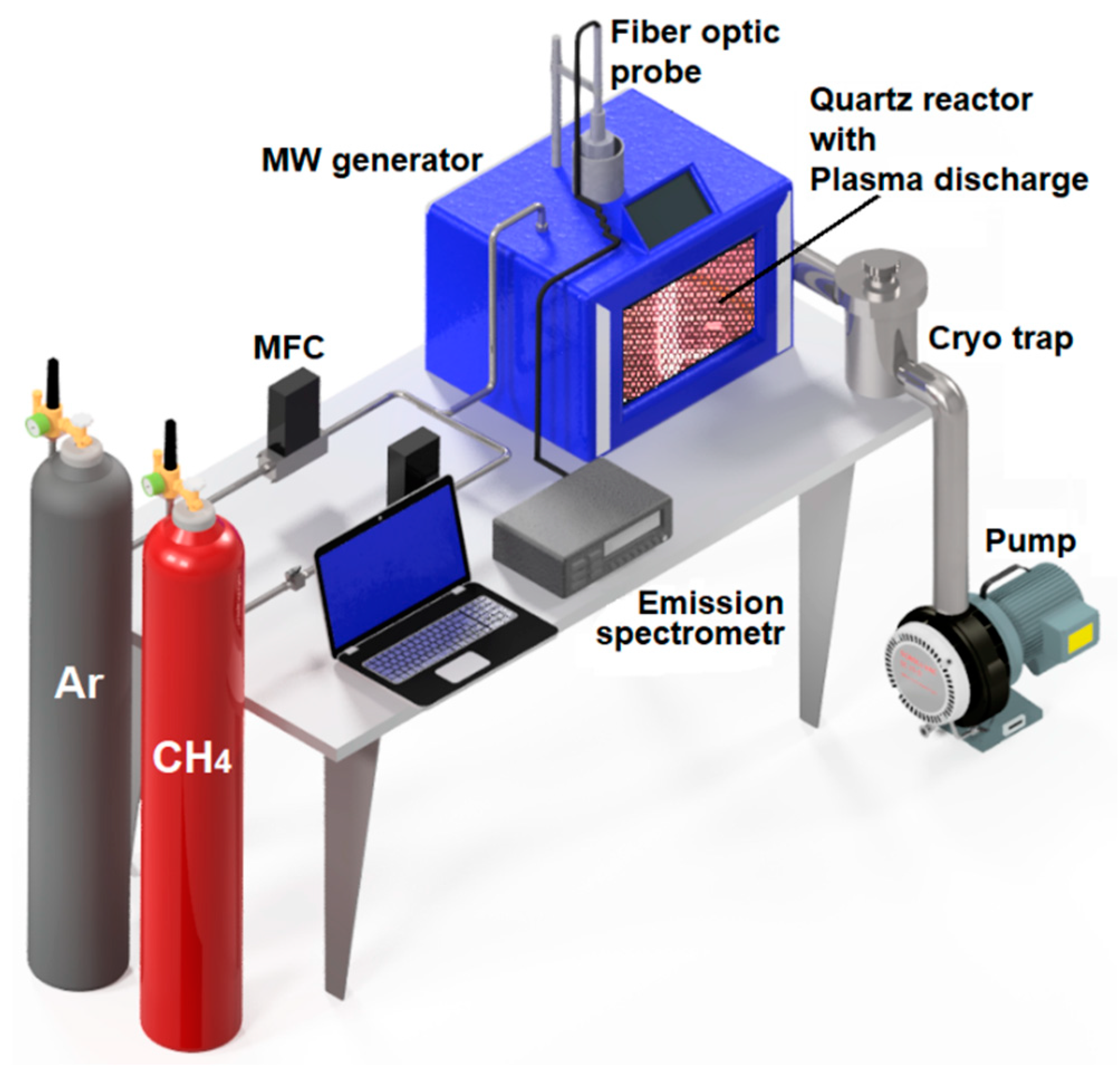
2. Materials and Methods
3. Results and Discussions
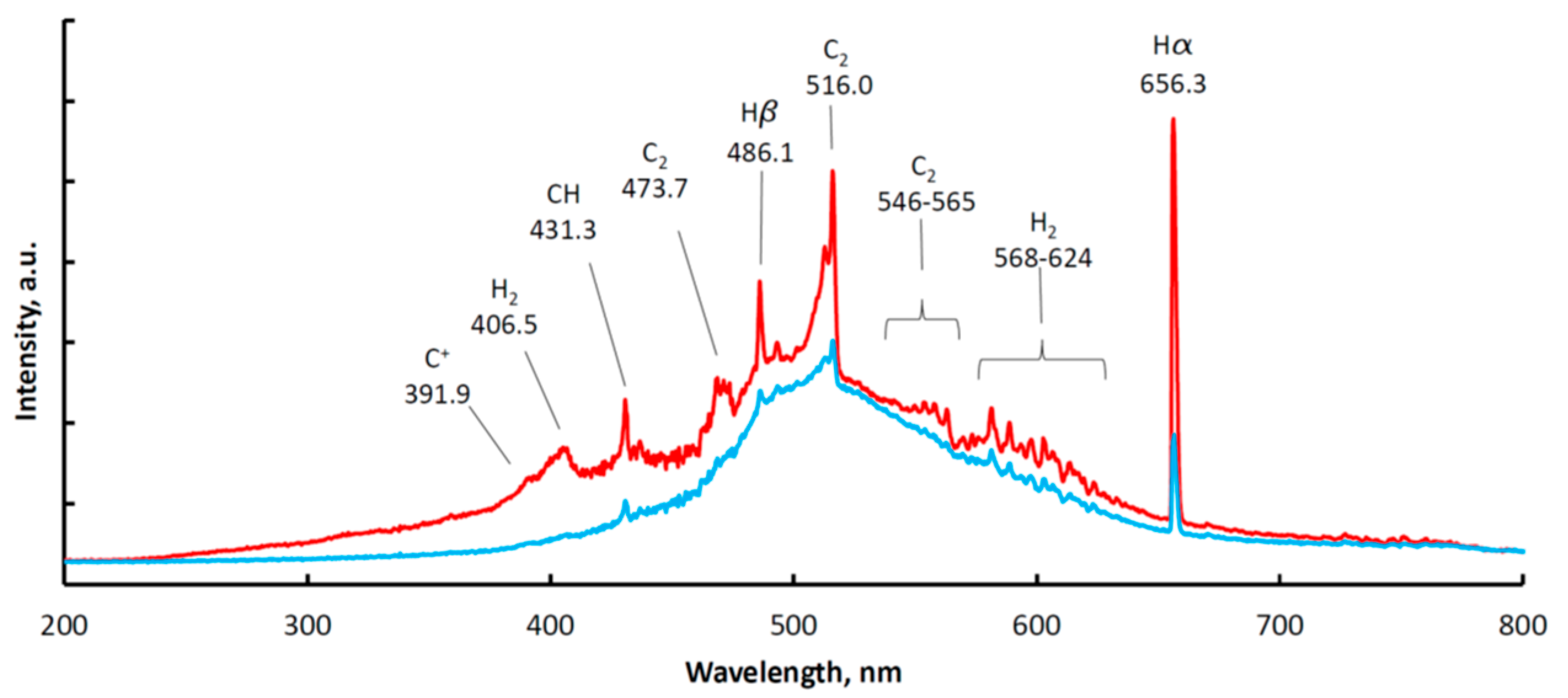
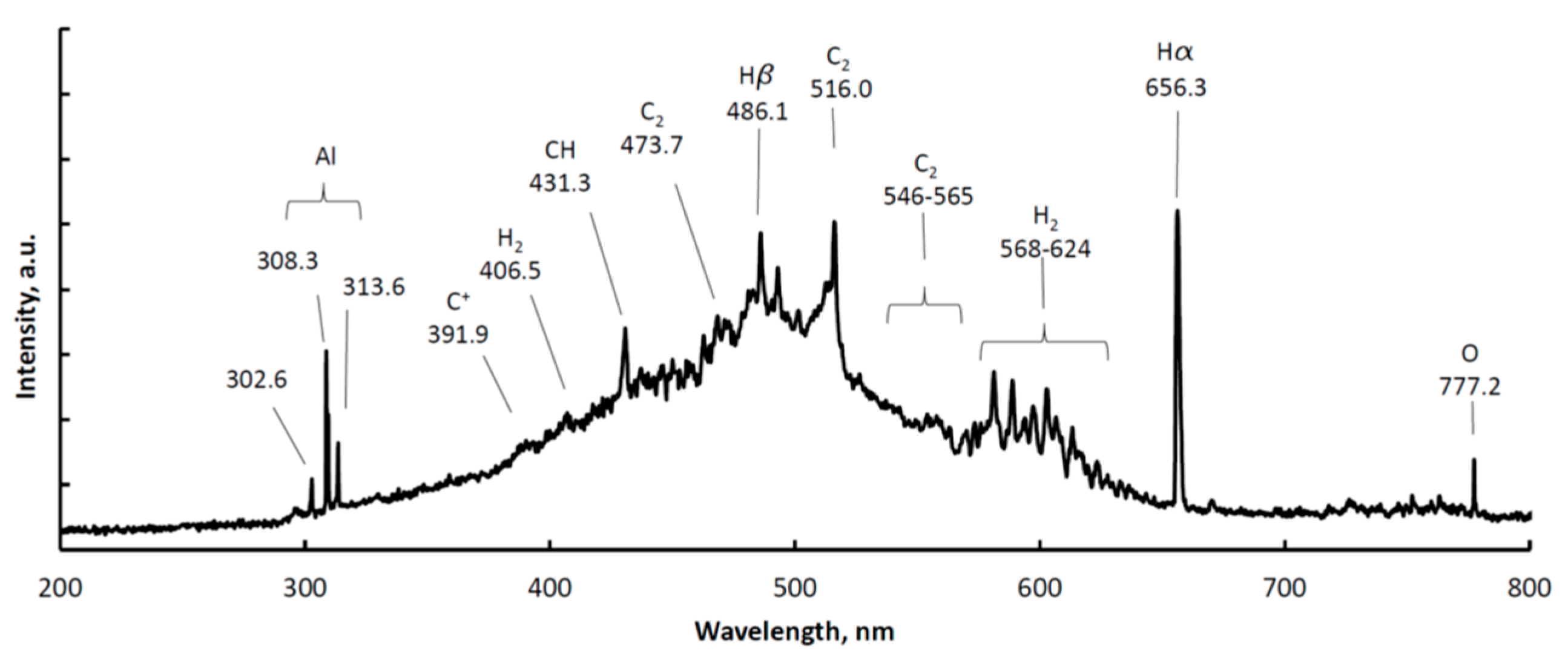
3.1. Optical Emission Spectroscopy (OES)
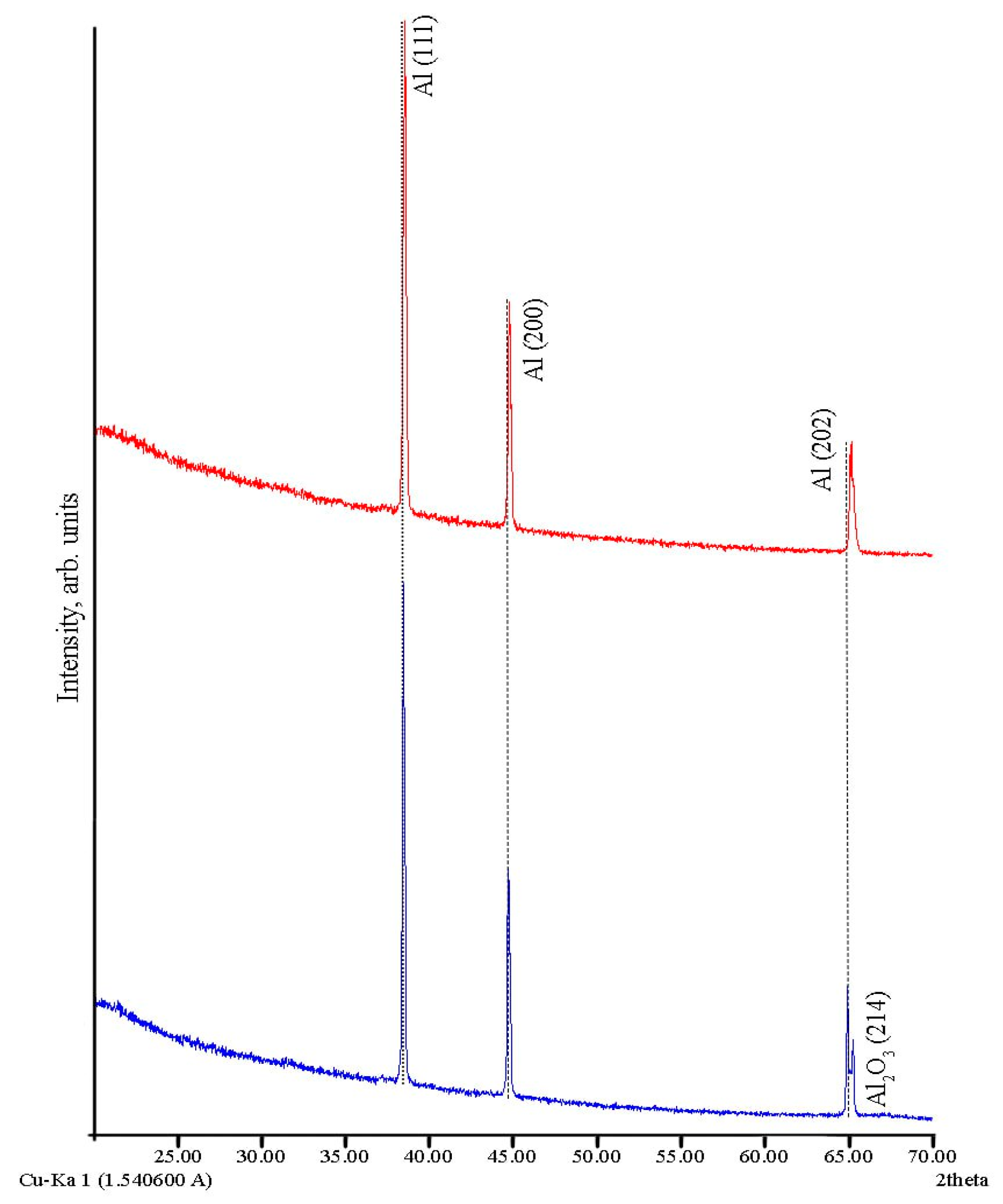
3.2. X-Ray Diffraction (XRD) Study of Al Powder
3.3. Scanning Electron Microscopy (SEM EDX) Study of Al
3.4. ICP-MS Study of the Behavior of Impurities in Aluminum During Plasma–Chemical Reduction
3.5. Study of Gaseous Products of the Plasma–Chemical Reduction Process
3.6. Synthesis of Trimethylaluminum Using Methane Plasma-Processed Aluminum
3.7. Gas Chromatography Mass Spectrometry of Trimethylaluminum
3.8. ICP-MS of Trimethylaluminum
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICP-MS | Inductively coupled plasma mass spectrometry |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| MW | Microwave |
| SIMS | Secondary ion mass spectrometry |
| ALD | Atomic layer deposition |
| MOCVD | Metalorganic chemical vapor deposition |
| TMA | Trimethylaluminum |
References
- Qi, M.; Chen, C.; Wei, J.; Mei, X.; Sun, C.; Su, G.; Zhang, C.; Yan, M.; Yang, F.; Guo, Z. Superior Mechanical Properties and Microstructural Evolution of Powder Metallurgy 2195 Al-Li Alloy Subjected to Hot Extrusion. J. Alloys Compd. 2023, 962, 171184. [Google Scholar] [CrossRef]
- Kumar, K.B.; Ingole, S.B.; Saeedjassim, A.; YatikaGori; Kaushik, A.; Kumar, D.; Jain, A. Aluminum-Foam by Powder Metallurgy: A Review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Dunkley, J. Advances in Atomisation Techniques for the Formation of Metal Powders. In Advances in Powder Metallurgy: Properties, Processing and Applications; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Kawasaki, A. Advances in Powder and Powder Metallurgy. J. Jpn. Soc. Powder Powder Metall. 2015. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, H.; Wu, L.; Hu, C.; Gao, W.; Huang, L.; Li, L. Research Progress in Preparation of Metal Powders by Pressurized Hydrogen Reduction. Int. J. Hydrog. Energy 2021, 46, 35102–35120. [Google Scholar] [CrossRef]
- Halmann, M.; Steinfeld, A.; Epstein, M.; Vishnevetsky, I. Vacuum Carbothermic Reduction of Alumina. Miner. Process. Extr. Met. Rev. 2013, 35, 126–135. [Google Scholar] [CrossRef]
- Khanna, R.; Ikram-Ul-Haq, M.; Seetharaman, S.; Sahajwalla, V. Carbothermic Reduction of Alumina at 1823 K: On the Role of Molten Iron and Reaction Mechanisms. ISIJ Int. 2016, 56, 1300–1302. [Google Scholar] [CrossRef]
- Yu, Q.; Yuan, H.; Zhu, F.; Zhang, H.; Wang, C.; Liu, D.; Yang, B. Carbothermic Reduction of Alumina with Carbon in Vacuum. J. Cent. South Univ. 2012, 19, 1813–1816. [Google Scholar] [CrossRef]
- Halmann, M.; Epstein, M.; Steinfeld, A. Carbothermic Reduction of Alumina by Natural Gas to Aluminum and Syngas: A Thermodynamic Study. Miner. Process. Extr. Metall. Rev. 2012, 33, 352–361. [Google Scholar] [CrossRef]
- Zhu, F.L.; Yang, B.; Li, Y.; Yuan, H.B.; Yu, Q.C.; Xu, B.Q.; Dai, Y.N. Behavior Analysis of Silica in Aluminum Production by Alumina Carbothermic Reduction-Chlorination Process in Vacuum. Vacuum 2011, 85, 968–971. [Google Scholar] [CrossRef]
- Frank, R.A.; Finn, C.W.; Elliott, J.F. Physical Chemistry of the Carbothermic Reduction of Alumina in the Presence of a Metallic Solvent: Part II. Measurements of Kinetics of Reaction. Metall. Mater. Trans. B 1989, 20, 161–173. [Google Scholar] [CrossRef]
- Worrell, W.L. Carbothermic Reduction of Alumina: A Thermodynamic Analysis. Can. Metall. Q. 1965, 4, 87–95. [Google Scholar] [CrossRef]
- Shimpo, R.; Ogawa, O.; Shimizu, H.; Goto, S. A Basic Study on the Carbothermic Reduction of Alumina. J. Min. Inst. Jpn. 1987, 103, 325–330. [Google Scholar] [CrossRef][Green Version]
- Palmer, R.; Doan, T.; Lloyd, P.; Jarvis, B.; Ahmed, N. Reduction of TiO2 with Hydrogen Plasma. Plasma Chem. Plasma Process. 2002, 22, 335–350. [Google Scholar] [CrossRef]
- Ramachandran, M.; Reddy, R.G. Direct reduction of magnesium oxide to magnesium using thermal plasma technology. Min. Metall. Explor. 2015, 32, 30–37. [Google Scholar] [CrossRef]
- Rains, R.K.; Kadlec, R.H. The reduction of Al2O3 to aluminum in a plasma. Metall. Trans. 1970, 1, 1501–1506. [Google Scholar] [CrossRef]
- Halmann, M.; Frei, A.; Steinfeld, A. Carbothermal Reduction of Alumina: Thermochemical Equilibrium Calculations and Experimental Investigation. Energy 2007, 32, 2420–2427. [Google Scholar] [CrossRef]
- Gallon, H.J.; Tu, X.; Twigg, M.V.; Whitehead, J.C. Plasma-assisted methane reduction of a NiO catalyst—Low temperature activation of methane and formation of carbon nanofibres. Appl. Catal. B Environ. 2011, 106, 616–620. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Peng, J.; Xue, L.; He, G. Cold plasma activated Ni0/Ni2+ interface catalysts for efficient electrocatalytic methane oxidation to low-carbon alcohols. Green Chem. 2024, 26, 7091–7100. [Google Scholar] [CrossRef]
- Song, L.; Kong, Y.; Li, X. Hydrogen production from partial oxidation of methane over dielectric barrier discharge plasma and NiO/γ-Al2O3 catalyst. Int. J. Hydrogen Energy 2017, 42, 19869–19876. [Google Scholar] [CrossRef]
- Ahasan, R.; Hossain, M.; Wang, R. Non-thermal plasma enabled catalytic dry reforming of methane over a ceria nanorod supported NiO catalyst: The role of Ru as a coke resistant active site. J. Mater. Chem. A 2024, 12, 29708–29725. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. In-Liquid Plasma: A Novel Tool in the Fabrication of Nanomaterials and in the Treatment of Wastewaters. RSC Adv. 2017, 7, 47196–47218. [Google Scholar] [CrossRef]
- Lee, Y.; DuMont, J.W.; George, S.M. Trimethylaluminum as the Metal Precursor for the Atomic Layer Etching of Al2O3 Using Sequential, Self-Limiting Thermal Reactions. Chem. Mater. 2016, 28, 2994–3003. [Google Scholar] [CrossRef]
- Smith, L.M.; Rushworth, S.A.; Jones, A.C.; Roberts, J.S.; Chew, A.; Sykes, D.E. Oxygen Incorporation in Aluminium-Based Semiconductors Grown by Metalorganic Vapour Phase Epitaxy. J. Cryst. Growth 1993, 134, 140–146. [Google Scholar] [CrossRef]
- Aarik, L.; Piller, C.-T.; Raud, J.; Talviste, R.; Jõgi, I.; Aarik, J. Atomic layer deposition of α-Al2O3 from trimethylaluminum and H2O: Effect of process parameters and plasma excitation on structure development. J. Cryst. Growth 2023, 609, 27148. [Google Scholar] [CrossRef]
- Hata, M.; Takata, H.; Yako, T.; Fukuhara, N.; Maeda, T.; Uemura, Y. The Effects of Oxygen Impurity in TMA on AlGaAs Layers Grown by MOVPE. J. Cryst. Growth 1992, 124, 427–432. [Google Scholar] [CrossRef]
- Yamashita, S.; Watanuki, K.; Ishii, H.; Shiba, Y.; Kitano, M.; Shirai, Y.; Sugawa, S.; Ohmi, T. Dependence of the Decomposition of Trimethylaluminum on Oxygen Concentration. J. Electrochem. Soc. 2011, 158, H93–H96. [Google Scholar] [CrossRef]
- Mochalov, L.; Logunov, A.; Vorotyntsev, A.; Vorotyntsev, V.; Mashin, A. Purification of Tellurium through Thermal Decomposition of Plasma Prepared Tellurium Hydride. Sep. Purif. Technol. 2018, 204, 276–280. [Google Scholar] [CrossRef]
- Mohanta, A.; Lanfant, B.; Asfaha, M.; Leparoux, M. Optical Emission Spectroscopic Study of Ar/H2/CH4 Plasma during the Production of Graphene Nano-Flakes by Induction Plasma Synthesis. J. Phys. Conf. Ser. 2017, 825, 012010. [Google Scholar] [CrossRef]
- Heintze, M.; Magureanu, M.; Kettlitz, M. Mechanism of C2 Hydrocarbon Formation from Methane in a Pulsed Microwave Plasma. J. Appl. Phys. 2002, 92, 7022–7031. [Google Scholar] [CrossRef]
- Goriuc, A.; Jităreanu, A.; Mârțu, I.; Dascălu, C.; Kappenberg-Niţescu, D.; Solomon, S.-M.; Mârțu, A.; Foia, L.; Țapu, I.; Istrate, B.; et al. Experimental EDX Analysis of Different Periodontal Splinting Systems. Exp. Ther. Med. 2021, 22, 1384. [Google Scholar] [CrossRef]
- Yeoh, L.S.; Chong, K.C.; Li, S.; Kaeppel, A. Advanced EDX Analysis for Memory Devices. In Proceedings of the International Symposium on the Physical and Failure Analysis of Integrated Circuits, IPFA, Singapore, 20–23 July 2020. [Google Scholar] [CrossRef]
- Ziegler, K.; Gellert, H.G.; Lehmkuhl, H.; Pfohl, W.; Zosel, K. Metallorganische Verbindungen, XXVI Aluminiumtrialkyle und Dialkyl-Aluminiumhydride aus Olefinen, Wasserstoff und Aluminium. Liebigs Ann. Chem. 1960, 629, 1–13. [Google Scholar] [CrossRef]
- Grosse, A.V.; Mavity, J.M. Organoaluminum Compounds: I. Methods of Preparation1. J. Org. Chem. 1940, 5, 106–121. [Google Scholar] [CrossRef]
- Wesslau, H. Das Problem der Kettenlängenverteilung Bei Der Stufenweisen Metallorganischen Synthese. Eur. J. Org. Chem. 1960, 629, 198–206. [Google Scholar] [CrossRef]
- Lehmkuhl, H.; Kintopf, S.; Janssen, E. Elektrochemische Synthese Metallorganischer Verbindungen. J. Organomet. Chem. 1973, 56, 41–52. [Google Scholar] [CrossRef]


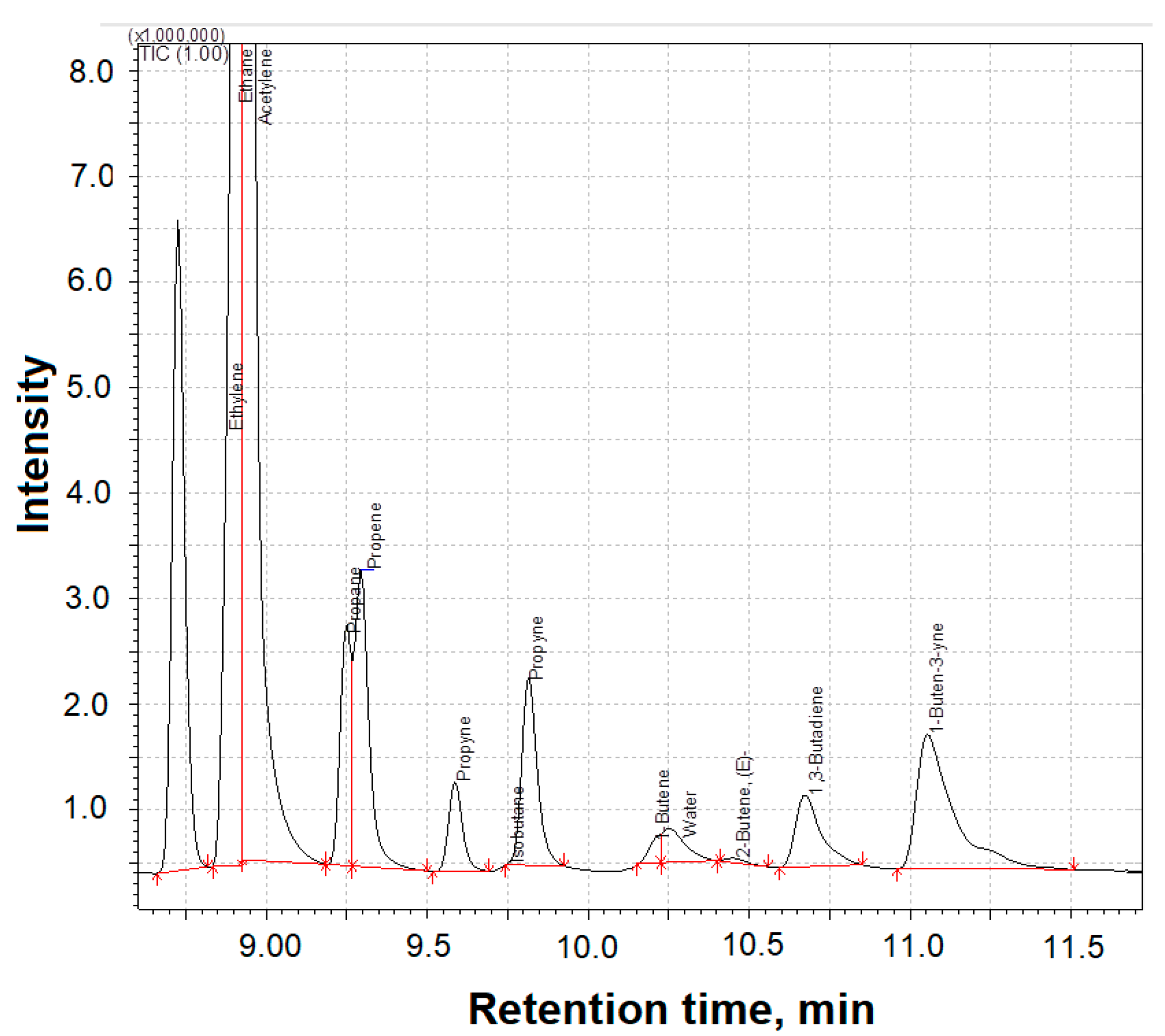
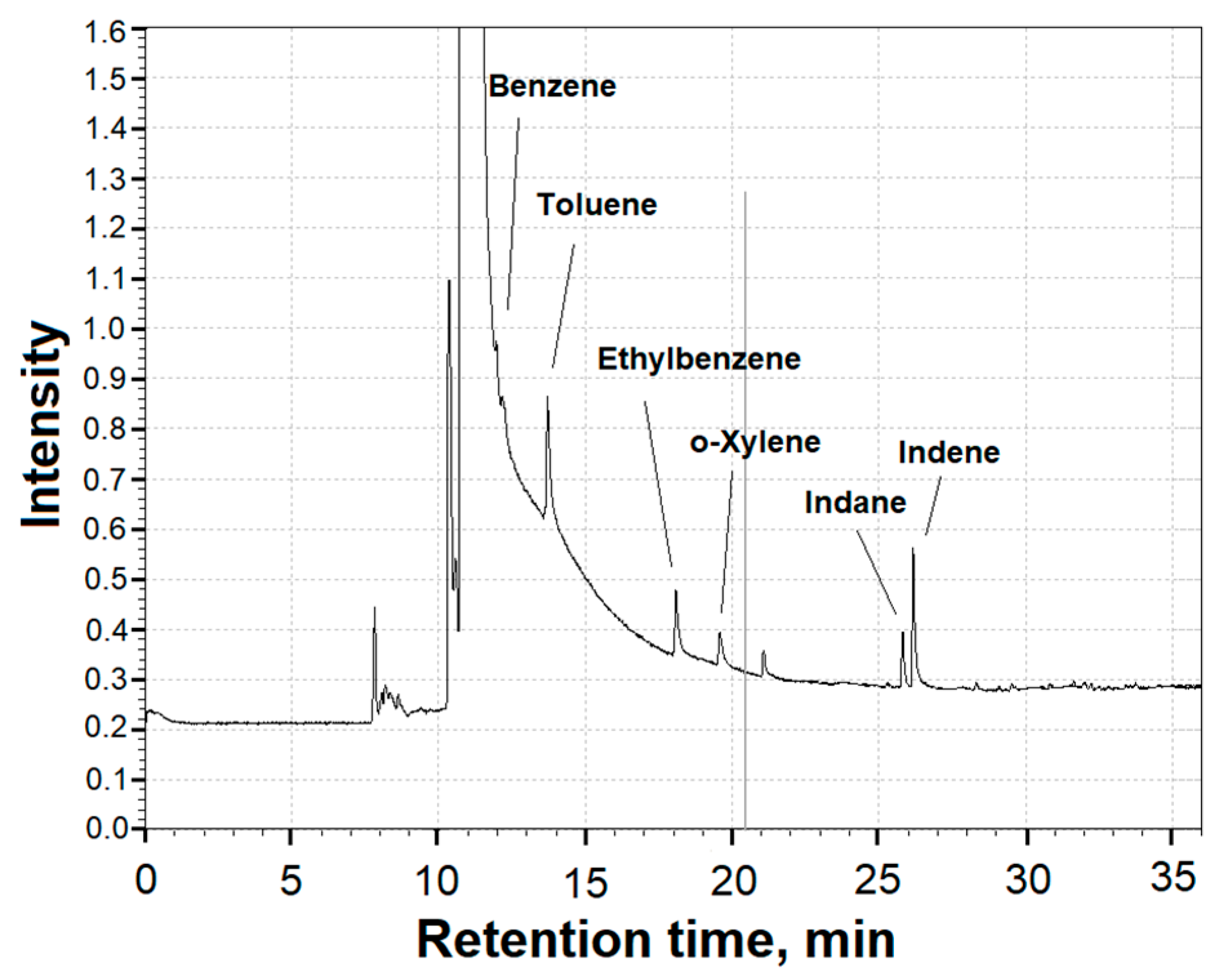
| Element | Before Plasma Treatment, at.% | After Plasma Treatment, at.% |
|---|---|---|
| Al | 97 | 100 |
| O | 3 | - |
| Element | Units | Before Plasma Treatment | After Plasma Treatment |
|---|---|---|---|
| Fe | ppm | 8.06 | 8.04 |
| Si | ppm | 15.74 | 16.05 |
| Cu | ppm | 5.54 | 5.51 |
| Mg | ppm | 15.09 | 15.07 |
| Ca | ppm | 11.63 | 13.05 |
| Substance | Unit of Measurement | Concentration |
|---|---|---|
| Trimethylaluminum (TMA), content of the main substance by weight | % | ≥99.99 |
| Ba | ppm | ≤0.02 |
| Ca | ppm | ≤0.05 |
| Cd | ppm | ≤0.03 |
| Cr | ppm | ≤0.01 |
| Cu | ppm | ≤0.03 |
| Fe | ppm | ≤0.04 |
| Mg | ppm | ≤0,04 |
| Mn | ppm | ≤0,04 |
| Ni | ppm | ≤0,03 |
| Pb | ppm | ≤0.02 |
| Si | ppm | ≤0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logunov, A.; Vorotyntsev, A.; Prokhorov, I.; Maslov, A.; Belousov, A.; Zanozin, I.; Logunova, E.; Zelentsov, S.; Petukhov, A.; Suvorov, S. Plasma–Chemical Low-Temperature Reduction of Aluminum with Methane Activated in Microwave Plasma Discharge. Metals 2025, 15, 514. https://doi.org/10.3390/met15050514
Logunov A, Vorotyntsev A, Prokhorov I, Maslov A, Belousov A, Zanozin I, Logunova E, Zelentsov S, Petukhov A, Suvorov S. Plasma–Chemical Low-Temperature Reduction of Aluminum with Methane Activated in Microwave Plasma Discharge. Metals. 2025; 15(5):514. https://doi.org/10.3390/met15050514
Chicago/Turabian StyleLogunov, Alexander, Andrey Vorotyntsev, Igor Prokhorov, Alexey Maslov, Artem Belousov, Ivan Zanozin, Evgeniya Logunova, Sergei Zelentsov, Anton Petukhov, and Sergey Suvorov. 2025. "Plasma–Chemical Low-Temperature Reduction of Aluminum with Methane Activated in Microwave Plasma Discharge" Metals 15, no. 5: 514. https://doi.org/10.3390/met15050514
APA StyleLogunov, A., Vorotyntsev, A., Prokhorov, I., Maslov, A., Belousov, A., Zanozin, I., Logunova, E., Zelentsov, S., Petukhov, A., & Suvorov, S. (2025). Plasma–Chemical Low-Temperature Reduction of Aluminum with Methane Activated in Microwave Plasma Discharge. Metals, 15(5), 514. https://doi.org/10.3390/met15050514







