Failure Analysis and Corrosion Resistance of Carbon Steel Pipelines in Concentrated Sulfuric Acid
Abstract
1. Introduction
2. Corrosion Failure Analysis
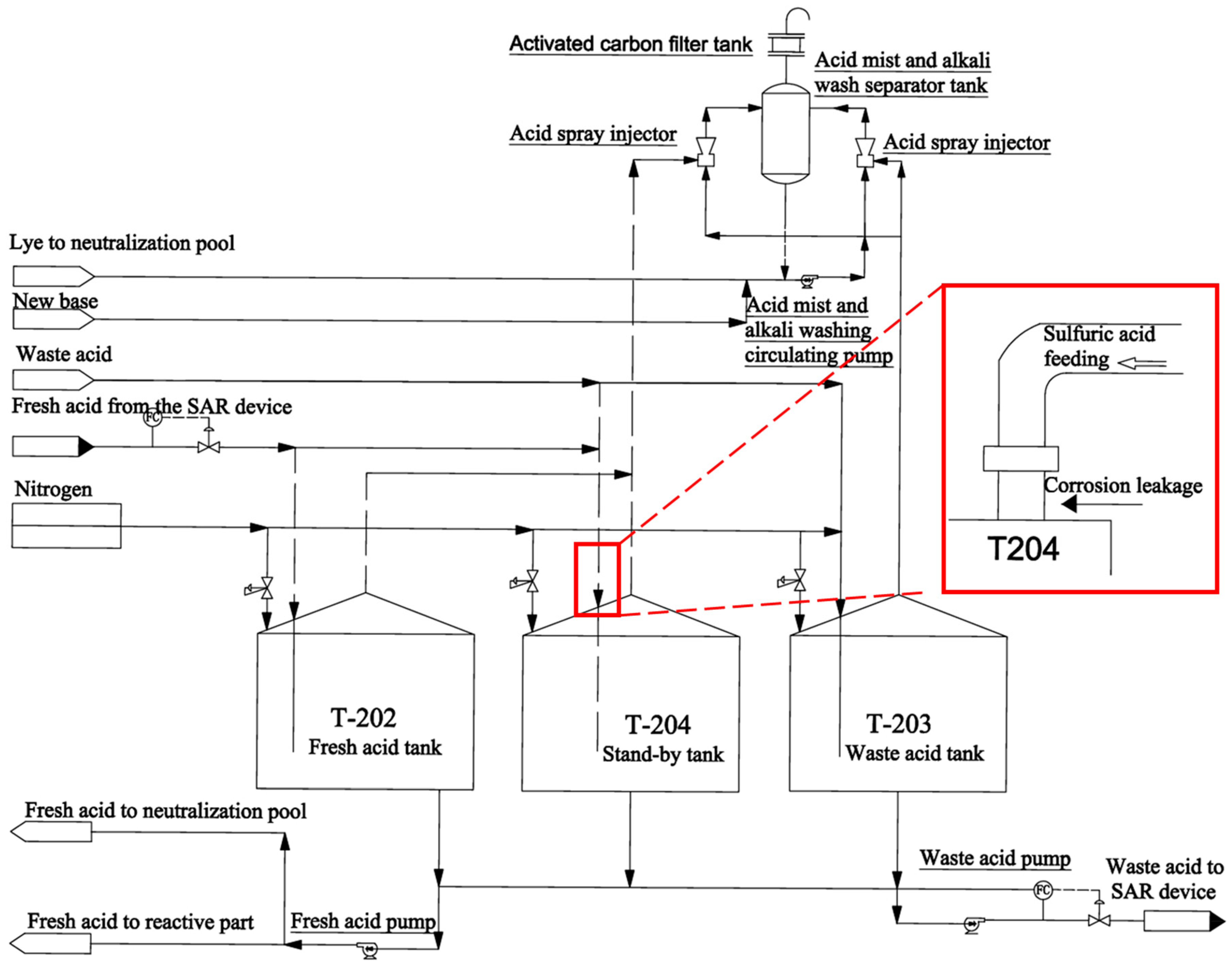
2.1. Process Flow
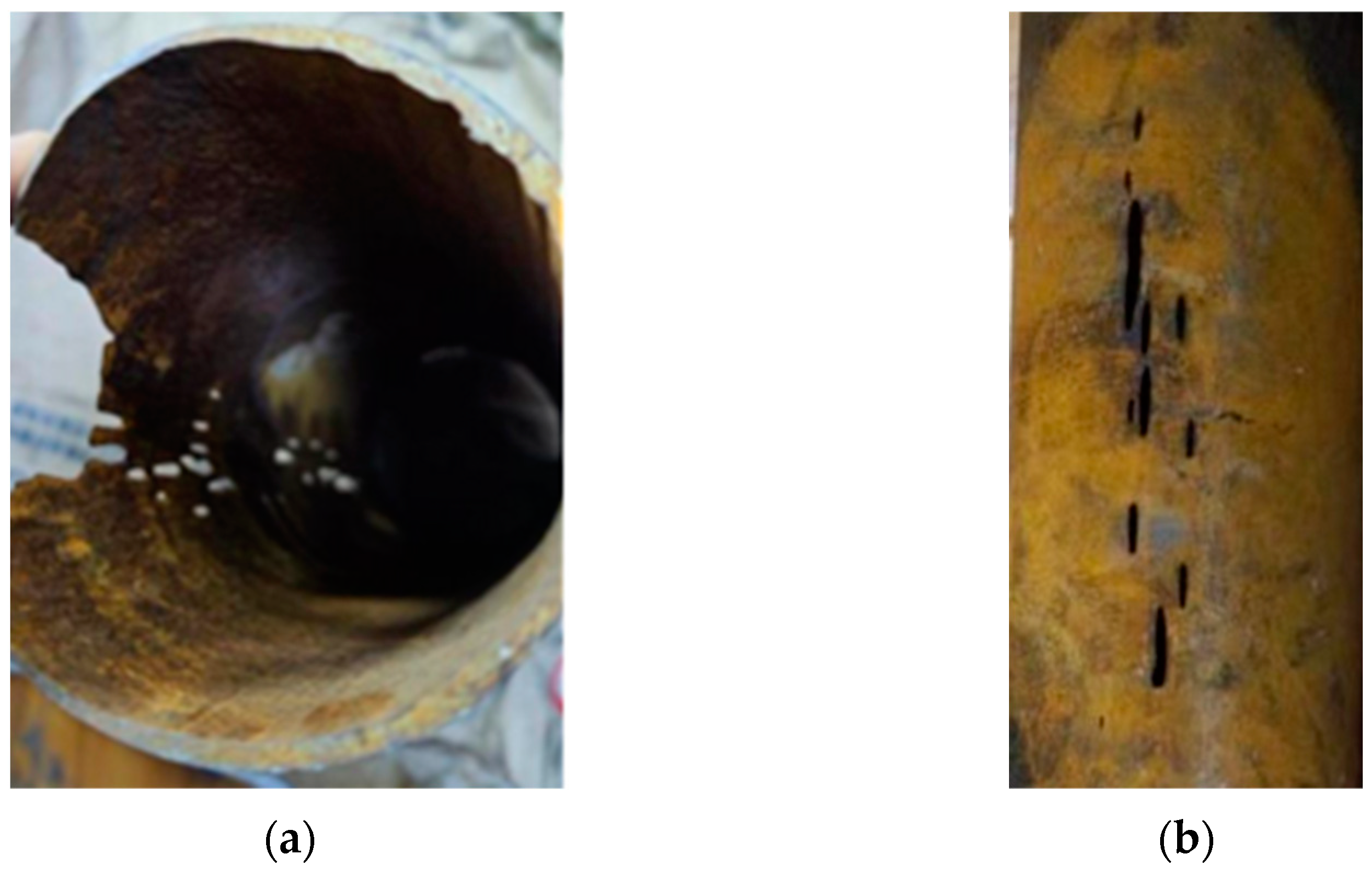
2.2. Analysis of Failed Components
2.3. Immersion Testing Method
2.4. Electrochemical Testing Method
2.5. Characterization
3. Results
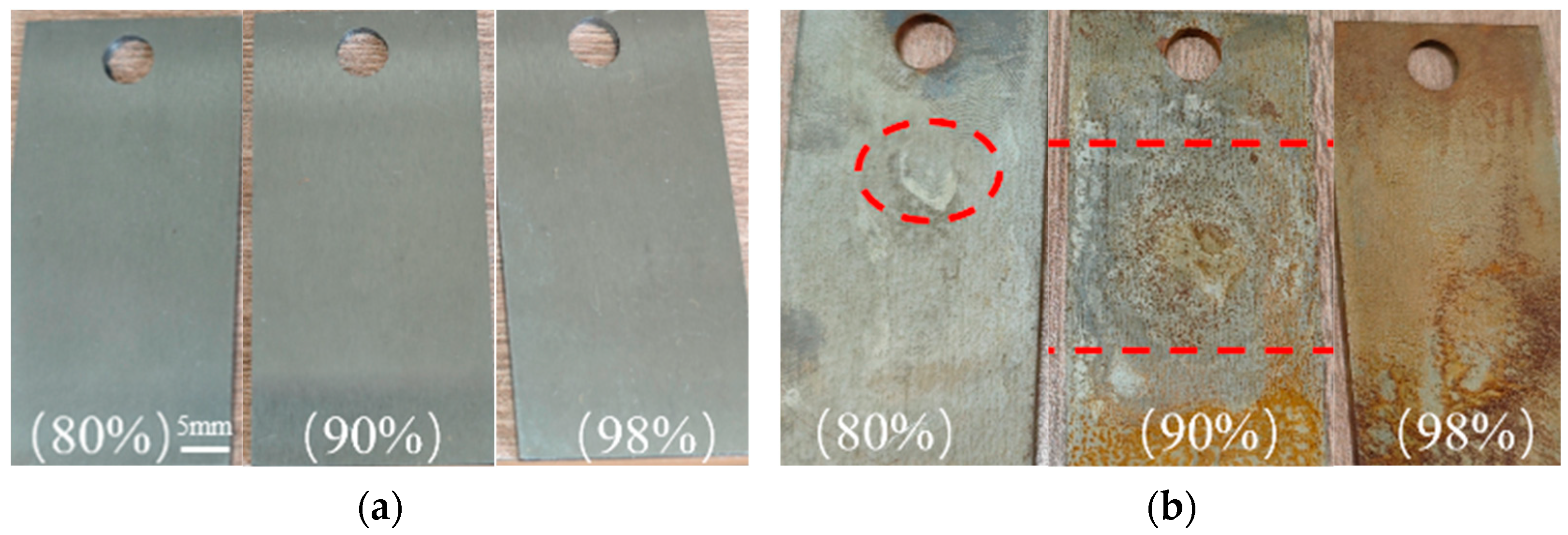
3.1. Macroscopic Morphology Analysis
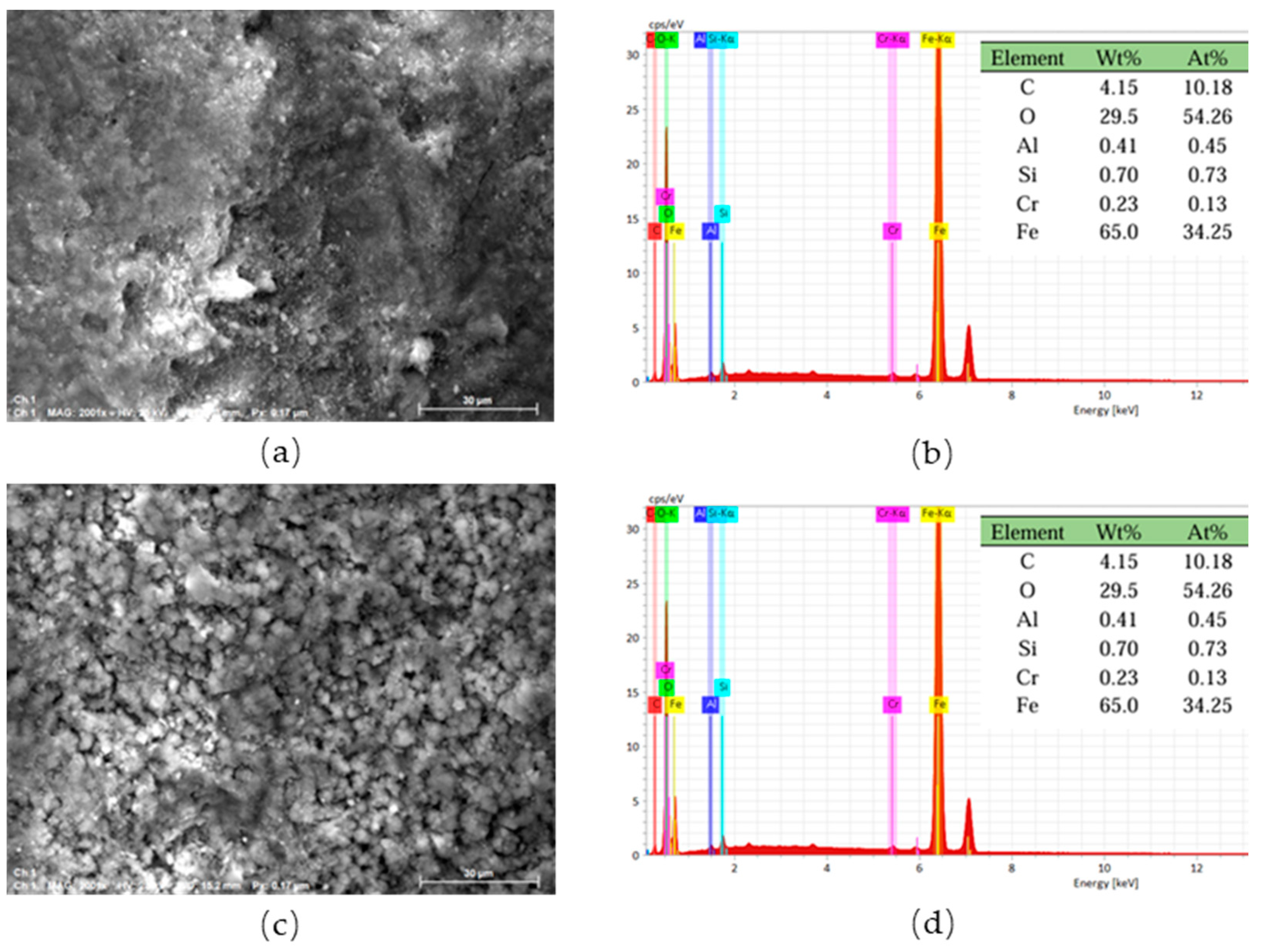
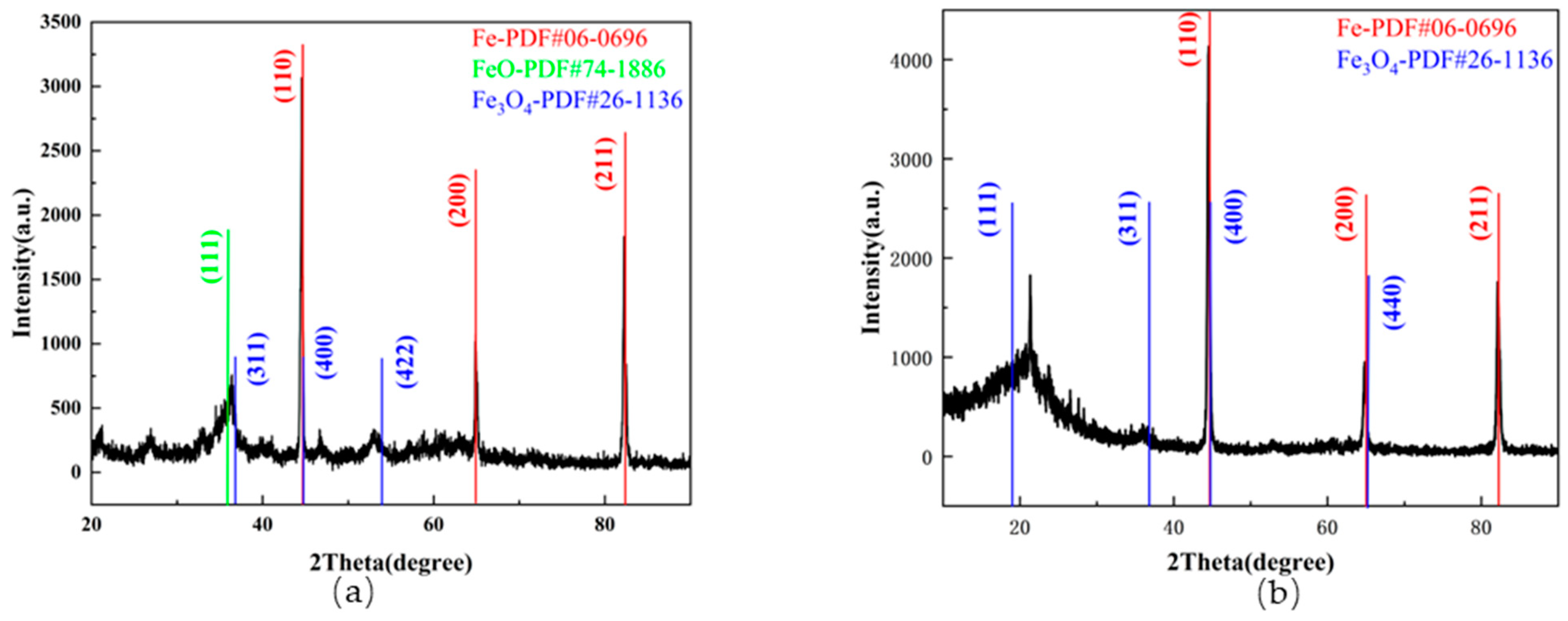
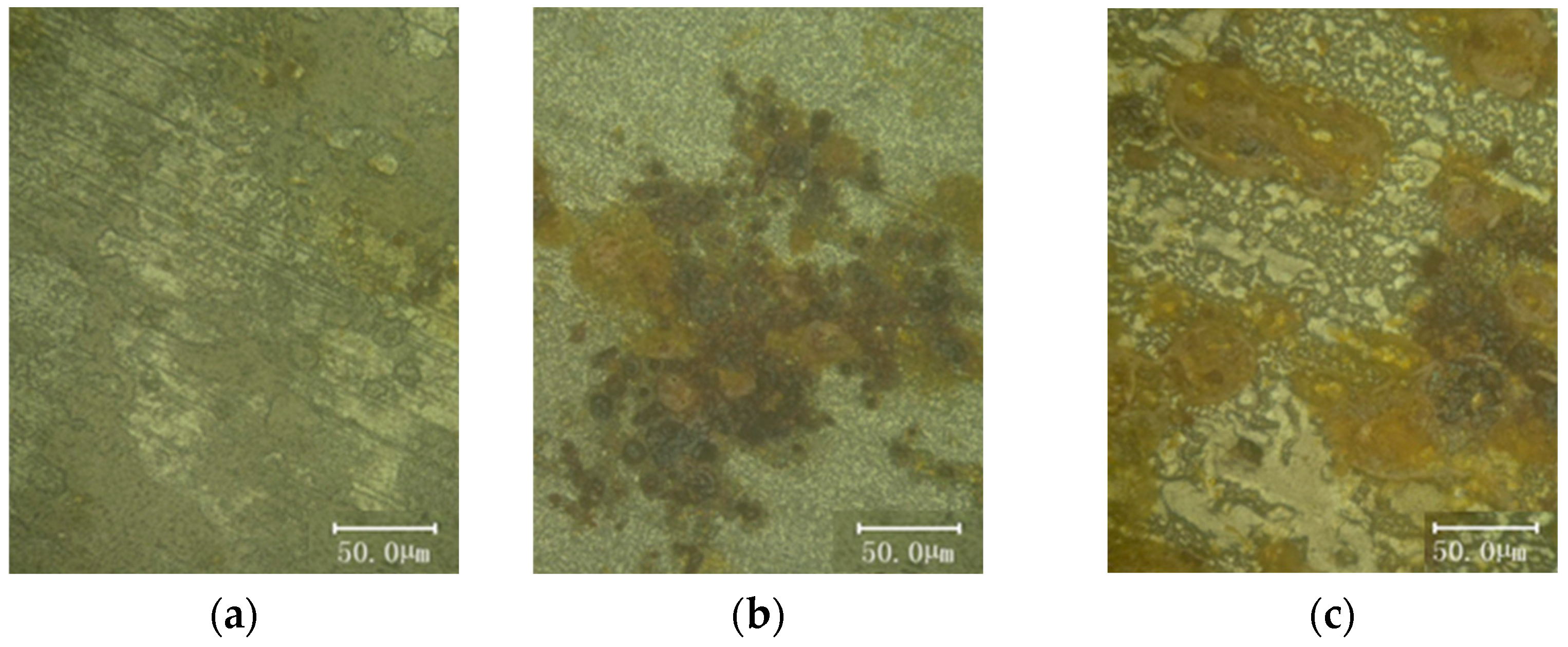
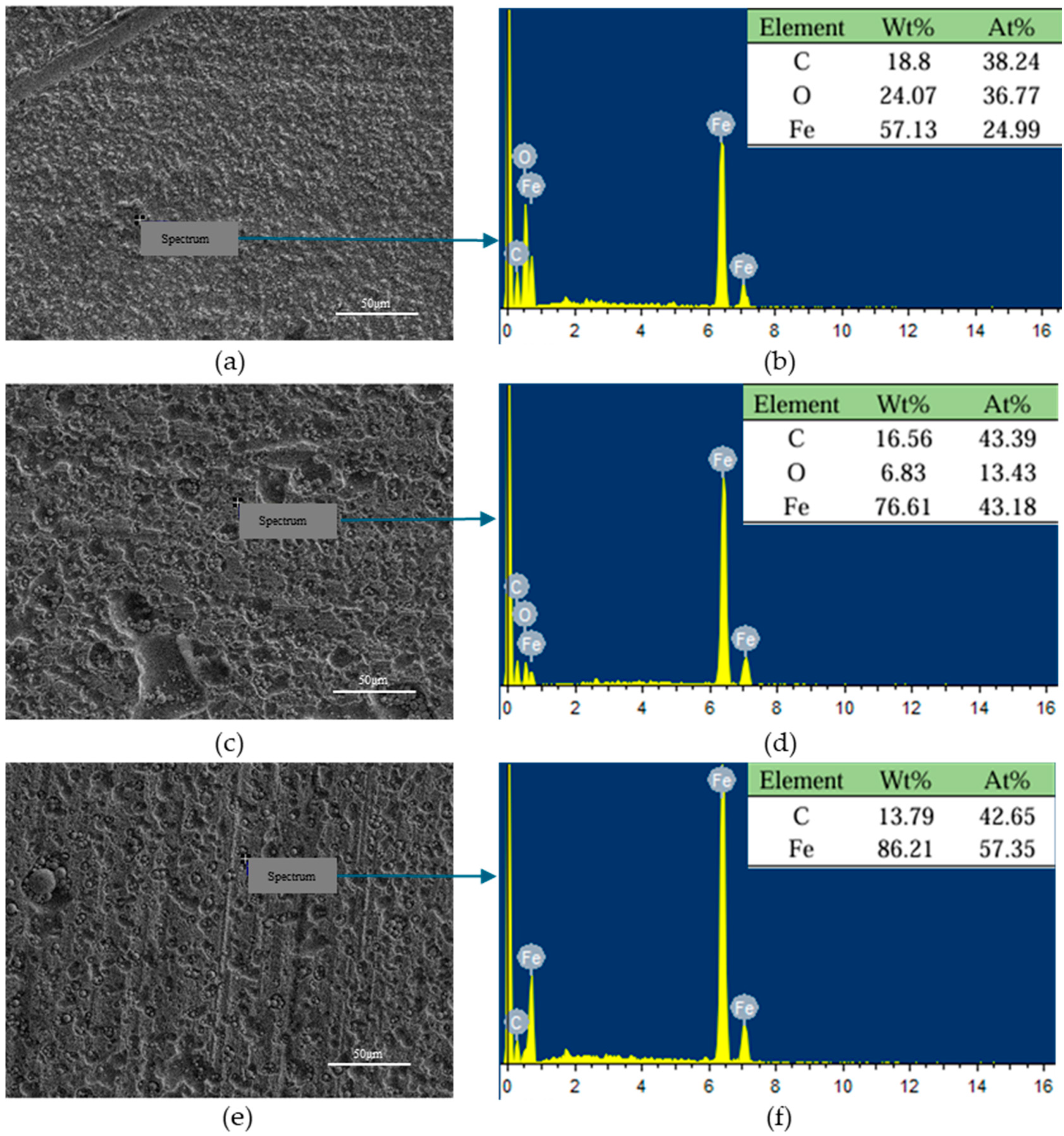
3.2. Microscopic Morphological and Chemical Composition Analysis
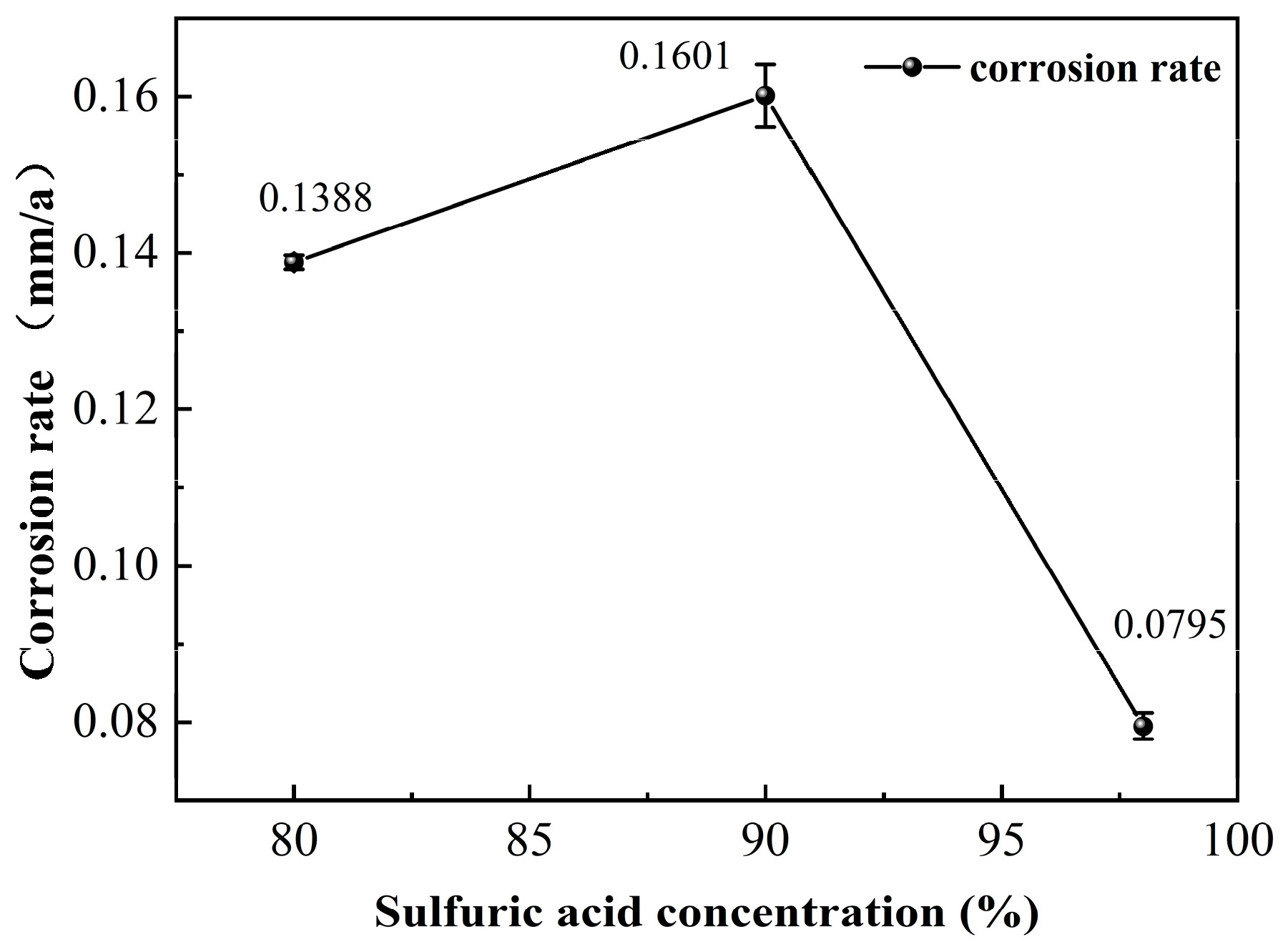
3.3. Immersion Corrosion Results
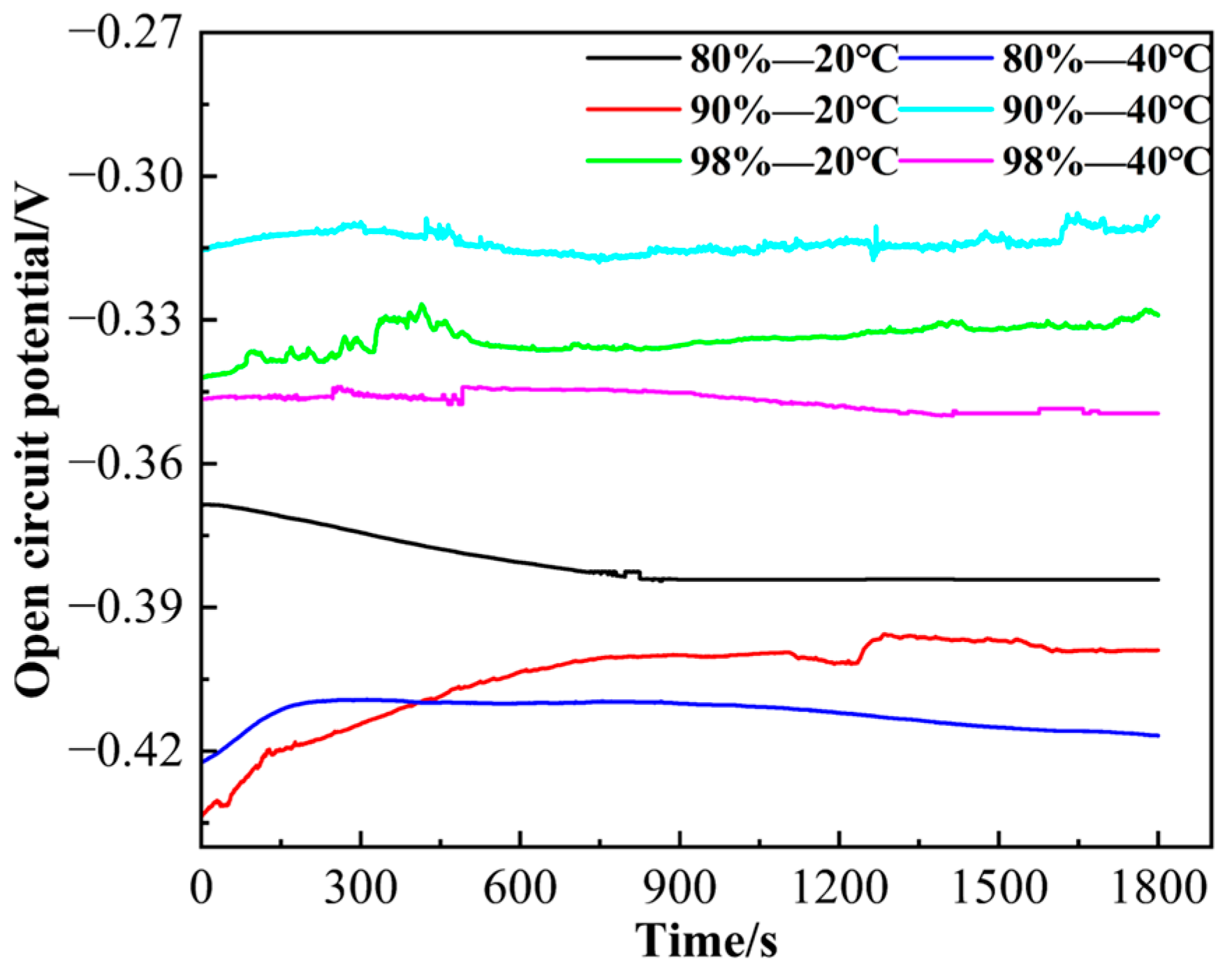
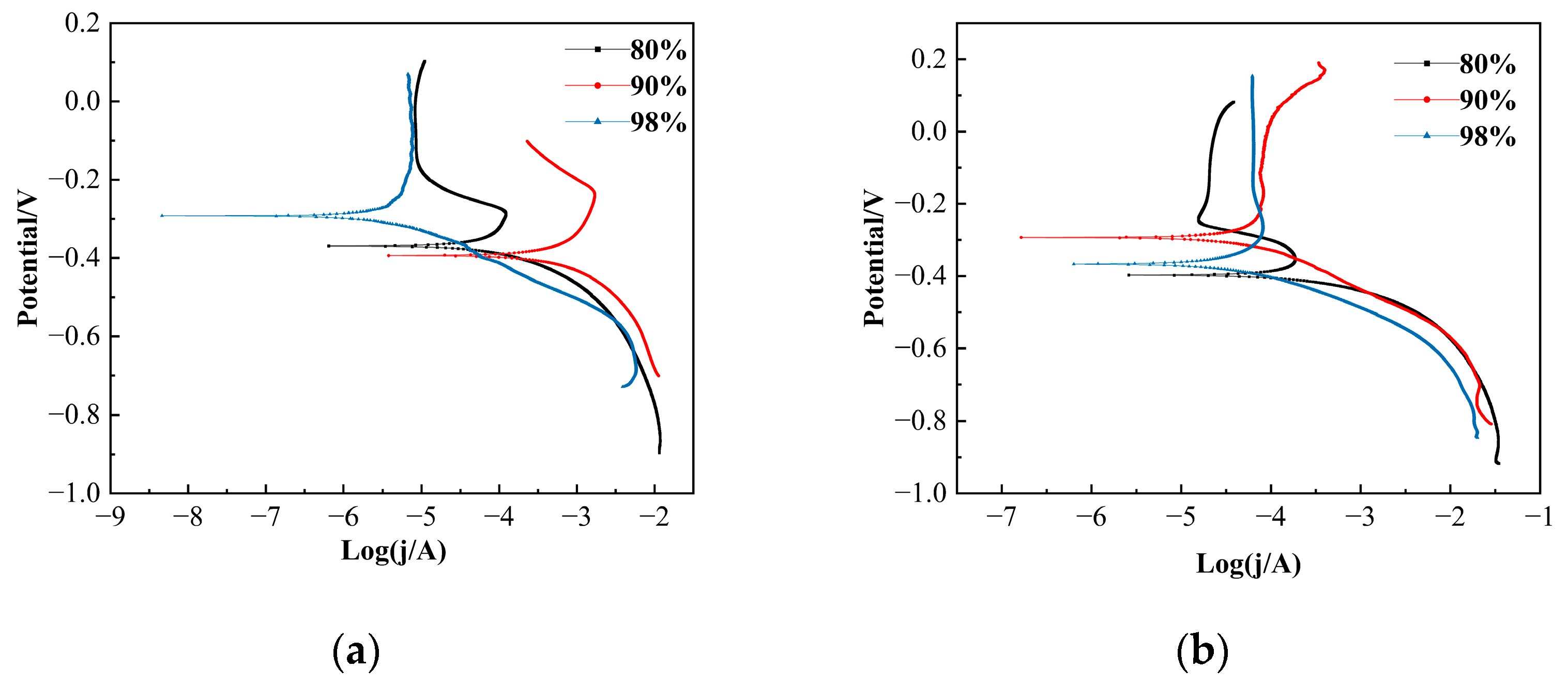
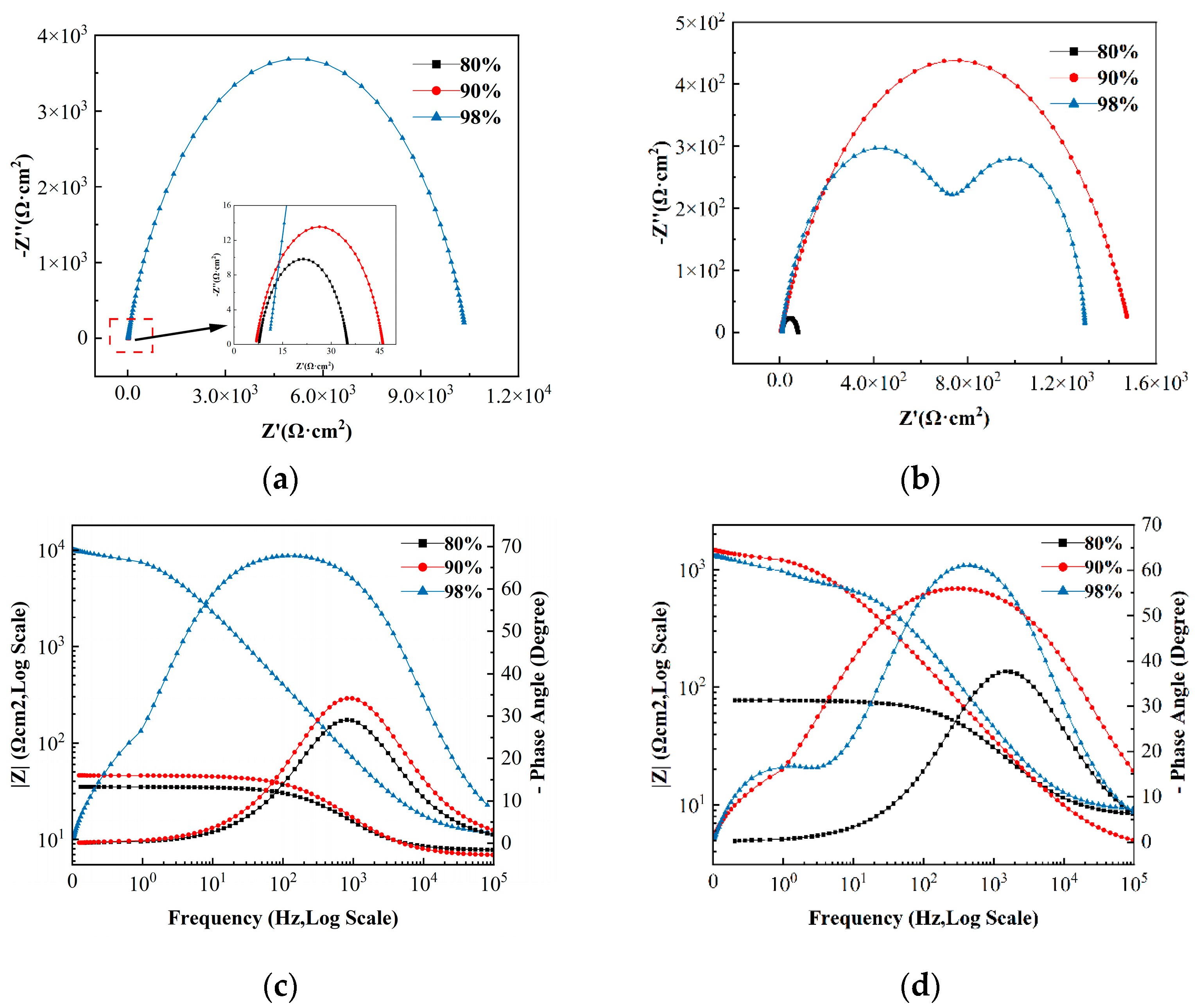
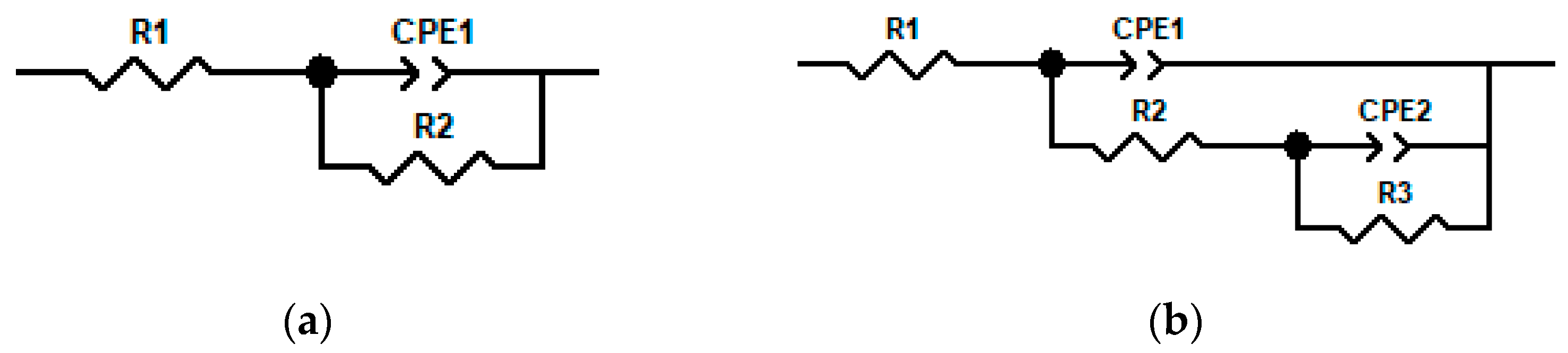
3.4. Electrochemical Corrosion Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.H.; Kang, S.J.; Kim, W.C.; Kim, J.G. Failure analysis of low carbon steel pipeline for district heating and cooling systems: Case studies. Eng. Fail. Anal. 2024, 166, 108826. [Google Scholar] [CrossRef]
- Chen, T.; Sun, L.; Li, Q.; Liu, C. Comprehensive analysis of corrosion failure of blast furnace gas pipeline in a steel plant. Eng. Fail. Anal. 2023, 154, 107651. [Google Scholar] [CrossRef]
- Al-Mazeedi, H.A.; Al-Wakaa, B.; Ravindranath, K. Window-type rupture of carbon steel pipe in a hydroprocessing plant of a petroleum refinery due to ammonium bisulfide corrosion. Eng. Fail. Anal. 2021, 120, 105089. [Google Scholar] [CrossRef]
- Ellison, B.T.; Schmeal, W.R. Corrosion of steel in concentrated sulfuric acid. J. Electrochem. Soc. 1978, 125, 524. [Google Scholar] [CrossRef]
- Panossian, Z.; de Almeida, N.L.; de Sousa, R.M.F.; de Souza Pimenta, G.; Marques, L.B.S. Corrosion of carbon steel pipes and tanks by concentrated sulfuric acid: A review. Corros. Sci. 2012, 58, 1–11. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, C.; Asselin, E.; Shi, P.; Jiang, M. Kinetics of passive film growth on 304 stainless steel in H2SO4 pickling solution under chemical oxidation. Corrosion 2018, 74, 705–714. [Google Scholar] [CrossRef]
- Javidi, M.; Abadeh, H.K.; Namazi, F.; Yazdanpanah, H.R.; Shiri, N.S. A new perspective on the corrosion of carbon steels in H2SO4 acid environments: Statistical analysis of corrosion mechanisms by response surface modeling. Mater. Chem. Phys. 2024, 312, 128608. [Google Scholar] [CrossRef]
- Burstein, G.T.; Daymond, B.T. The remarkable passivity of austenitic stainless steel in sulphuric acid solution and the effect of repetitive temperature cycling. Corros. Sci. 2009, 51, 2249–2252. [Google Scholar] [CrossRef]
- Sato, N. An overview on the passivity of metals. Corros. Sci. 1990, 31, 1–19. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Wu, Q.; Gu, J.; Baker, I.; Song, M. Enhanced corrosion resistance of FeCoCrNiMn high-entropy alloy in aqueous H2SO4 solutions by N and Si alloying. J. Mater. Eng. Perform. 2025, 1–12. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.; Chen, L.; Zhang, T.; Li, H.; Zhang, B.; Yang, W.; Cui, J.; Hou, B.; Wang, F.; et al. The dual nature of Cu content on the corrosion resistance of low alloy steels in sulfuric acid: A time-dependent reversal of corrosion resistance. Corros. Sci. 2023, 224, 111486. [Google Scholar] [CrossRef]
- Fujimura, A.; Abe, M.; Kawano, A.; Iwai, M.; Kitano, S.; Habazaki, H.; Fushimi, K. Effect of Al and Si addition on passivation of stainless steels in H2SO4 solution. Corros. Sci. 2024, 227, 111756. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Cabral-Miramontes, N.; Nieves-Mendoza, D.; Lara-Banda, M.; Maldonado-Bandala, E.; Olguín-Coca, J.; Lopez-Leon, L.D.; Estupiñan-Lopez, F.; Calderon, F.A.; Gaona Tiburcio, C. Anodizing of AA2024 aluminum–copper alloy in citric-sulfuric acid solution: Effect of current density on corrosion resistance. Coatings 2024, 14, 816. [Google Scholar] [CrossRef]
- Trentin, A.; Mardoukhi, A.; Lambai, A.; Pohjanne, P.; Huttunen-Saarivirta, E. Pitting corrosion of austenitic and duplex stainless steels in dilute acids at elevated temperature: Effect of electrolyte chemistry and material microstructure. Corros. Sci. 2025, 247, 112769. [Google Scholar] [CrossRef]
- Hayashida, S.; Takahashi, M.; Deguchi, H.; Tsuchiya, H.; Hanaki, K.; Yamashita, M.; Fujimoto, S. Structure of corrosion product formed on carbon steel covered with NiSO4-added resin coating under sulfuric acid mist environment containing chloride. Mater. Trans. 2021, 62, 781–787. [Google Scholar] [CrossRef]
- Gola, K.; Ledwig, P.; Dubiel, B. Effect of microstructure of additively manufactured Inconel 625 on long-term corrosion behaviour in sulfuric acid media. JOM 2023, 75, 1242–1250. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Reza, M.S.; Hossain, N.; Ali, M.R.; Kowser, M.A.; Shuvho, M.B.A. Improvement of corrosion resistance of galvanization/nickel/chrome plating low carbon steels in H2SO4 under dynamic condition. Surf. Topogr. Metrol. Prop. 2021, 9, 035018. [Google Scholar] [CrossRef]
- Javidi, M.; Abadeh, H.K.; Yazdanpanah, H.R.; Namazi, F.; Shiri, N.S. Synergistic effect of temperature, concentration and solution flow on corrosion and passive film of austenitic SS 304L and 316L in concentrated sulfuric acid. Corros. Sci. 2024, 237, 112306. [Google Scholar] [CrossRef]
- Ouarga, A.; Zirari, T.; Fashu, S.; Lahcini, M.; Youcef, H.B.; Trabadelo, V. Corrosion of iron and nickel based alloys in sulphuric acid: Challenges and prevention strategies. J. Mater. Res. Technol. 2023, 26, 5105–5125. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Isotahdon, E.; Lindgren, M.; Mardoukhi, A.; Mocnik, P.; Kosec, T.; Jorcin, J.B.; Mameng, S.H.; El Ouazari, Y.; Wegrelius, L. Corrosion of stainless steels S31603, S31655, and S32101 in sulfuric acid solutions: Effects of concentration, chlorides, and temperature. Corrosion 2022, 78, 943–962. [Google Scholar] [CrossRef]
- Abdelfatah, A.; Raslan, A.M.; Mohamed, L.Z. Corrosion characteristics of 304 stainless steel in sodium chloride and sulfuric acid solutions. Int. J. Electrochem. Sci. 2022, 17, 220417. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Golozar, M.A.; Saatchi, A.; Raeissi, K. Effect of solution concentration on semiconducting properties of passive films formed on austenitic stainless steels. Corros. Sci. 2010, 52, 205–209. [Google Scholar] [CrossRef]
- Boissy, C.; Alemany-Dumont, C.; Normand, B. EIS evaluation of steady-state characteristic of 316L stainless steel passive film grown in acidic solution. Electrochem. Commun. 2013, 26, 10–12. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Z.; Wang, Y.; Ju, P.; Tang, Y.; Zuo, Y. Corrosion resistance mechanism of palladium film-plated stainless steel in boiling H2SO4 solution. Corros. Sci. 2018, 135, 222–232. [Google Scholar] [CrossRef]
- Huang, K.; Zhou, X.; Guo, H.; Chen, Y. Thermal annealing effect on the corrosion resistance of AlCuNiTiZr high entropy alloy films in sulfuric acid solution. Thin Solid Film. 2021, 730, 138707. [Google Scholar] [CrossRef]
- Tao, S. 1-Phenyl-1H-tetrazol as corrosion inhibitor for pipeline steel in sulfuric acid solution. Int. J. Electrochem. Sci. 2021, 16, 210335. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; He, K.H.; Han, G.-C.; Lv, Y.; Han, J.; Wei, X. Two diamine Schiff base as a corrosion inhibitors for carbon steel in sulfuric acid solution: Electrochemical assessment and theoretical calculation. Int. J. Electrochem. Sci. 2021, 16, 210559. [Google Scholar] [CrossRef]
- Gapsari, F.; Setyarini, P.H.; Anam, K.; Hadisaputra, S.; Hidayatullah, S.; Purnami; Sulaiman, A.M.; Lai, C.W. Efficacy of Andrographis paniculata leaf extract as a green corrosion inhibitor for mild steel in concentrated sulfuric acid: Experimental and computational insights. Results Surf. Interfaces 2025, 18, 100361. [Google Scholar] [CrossRef]
- Peng, W.; Hou, J.; Ding, K.; Guo, W.; Qiu, R.; Xu, L. Corrosion Behavior of 304 Stainless Steel in Deep Sea Environment. J. Chin. Soc. Corros. Prot. 2019, 39, 145–151. (In Chinese) [Google Scholar]
- Chenghui, Y.; Jinshuo, L.I.; Qiang, Y.U.; Wang, H.; Li, B.; Wu, J.; Chaofang, D.; Xiao, K. Corrosion behaviour and mechanism of acid-resistant steel in acidic solutions with different Cl− concentrations. J. Mater. Res. Technol. 2024, 30, 7242–7255. [Google Scholar] [CrossRef]
- Richardson, J.A. Corrosion in sulfuric acid. Shreir’s Corros. 2010, 2, 1226–1249. [Google Scholar]
- Dean, S.W.J.; Grab, G.D. Corrosion of carbon steel tanks in concentrated sulfuric acid service. Mater. Perform. 1986, 25, 48–52. [Google Scholar]
- Gilli, G.; Zucchi, F. Diffractometric analysis of the products of anodic oxidation of Fe in concentrated H2SO4. Corros. Sci. 1968, 8, 801–808. [Google Scholar] [CrossRef]

| C | Si | Mn | P | S | Cr | Ni | Cu |
|---|---|---|---|---|---|---|---|
| 0.21 | 0.31 | 0.58 | 0.018 | 0.003 | 0.12 | 0.001 | 0.002 |
| H2SO4 (wt.%) | T (°C) | ba (V/dec) | bc (V/dec) | Ecorr (V) | jcorr (μA/cm2) |
|---|---|---|---|---|---|
| 80 | 20 | 0.1531 | 0.1195 | −0.3695 | 817.8 |
| 90 | 20 | 0.1670 | 0.3557 | −0.3950 | 2200 |
| 98 | 20 | 0.00011 | 0.5571 | −0.292 | 12.742 |
| 80 | 40 | 0.1190 | 0.0927 | −0.397 | 489.2 |
| 90 | 40 | 0.1193 | 1.0989 | −0.293 | 212 |
| 98 | 40 | 0.09145 | 0.9478 | −0.367 | 134.3 |
| T/°C | wt.% | EC | R1/Ω·cm2 | R2/Ω·cm2 | R3/Ω·cm2 | n1 | n2 | Q1/μF·cm2 | Q2/μF·cm2 | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 80 | (a) | 7.706 | 27.15 | —— | 0.796 | — | 86.45 | —— | 2.76 × 10−3 |
| 40 | 80 | (a) | 7.695 | 27.35 | —— | 0.793 | — | 89.13 | —— | 3.86 × 10−3 |
| 20 | 90 | (a) | 6.773 | 39.21 | —— | 0.769 | — | 87.38 | —— | 1.43 × 10−3 |
| 40 | 90 | (a) | 4.057 | 1487 | —— | 0.679 | — | 76.19 | —— | 6.47 × 10−3 |
| 20 | 98 | (a) | 10.62 | 10394 | —— | 0.786 | — | 15.73 | —— | 9.40 × 10−3 |
| 40 | 98 | (b) | 7.09 | 877.3 | 429.5 | 0.759 | 1.0 | 28.19 | 580.4 | 3.02 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, G.; Cao, X.; Mohammed, Y.M.; Wu, W. Failure Analysis and Corrosion Resistance of Carbon Steel Pipelines in Concentrated Sulfuric Acid. Metals 2025, 15, 506. https://doi.org/10.3390/met15050506
Ou G, Cao X, Mohammed YM, Wu W. Failure Analysis and Corrosion Resistance of Carbon Steel Pipelines in Concentrated Sulfuric Acid. Metals. 2025; 15(5):506. https://doi.org/10.3390/met15050506
Chicago/Turabian StyleOu, Guofu, Xiaomin Cao, Yusif Mukhtar Mohammed, and Wangping Wu. 2025. "Failure Analysis and Corrosion Resistance of Carbon Steel Pipelines in Concentrated Sulfuric Acid" Metals 15, no. 5: 506. https://doi.org/10.3390/met15050506
APA StyleOu, G., Cao, X., Mohammed, Y. M., & Wu, W. (2025). Failure Analysis and Corrosion Resistance of Carbon Steel Pipelines in Concentrated Sulfuric Acid. Metals, 15(5), 506. https://doi.org/10.3390/met15050506







