Microstructure Evolution During Preparation of Semi-Solid Billet for 7075 Aluminum Alloy by EASSIT Process
Abstract
1. Introduction
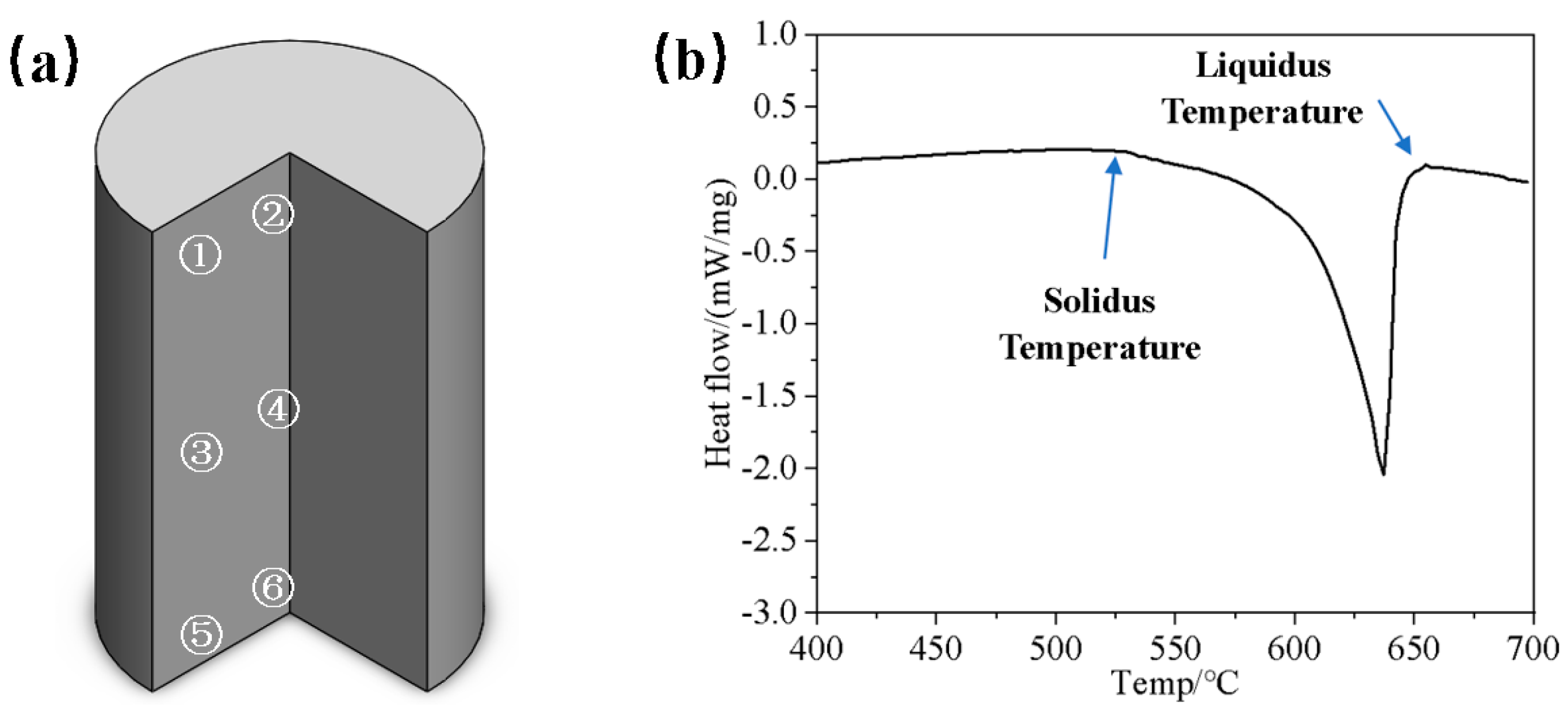
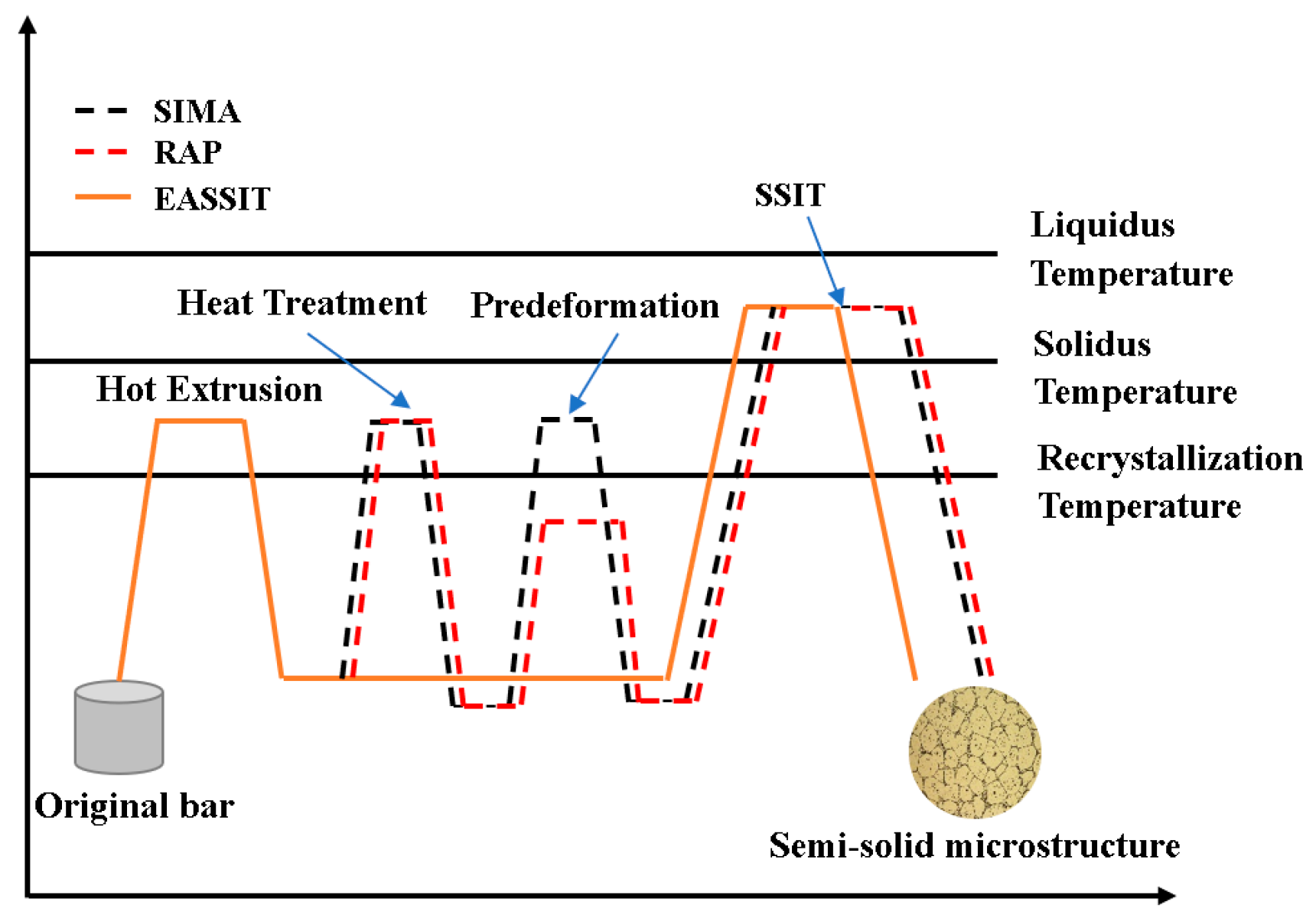
2. Materials and Methods
3. Results and Discussion
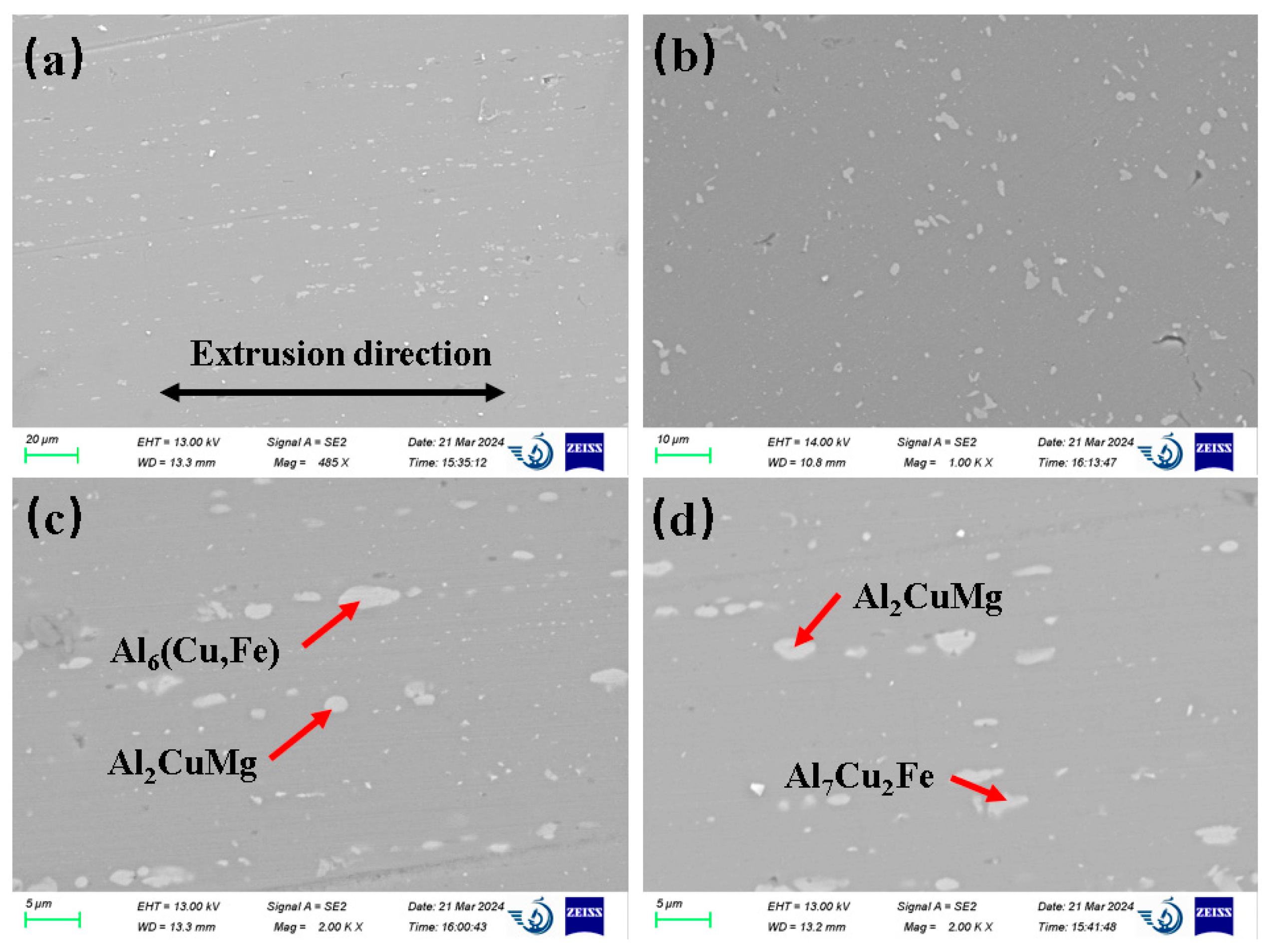
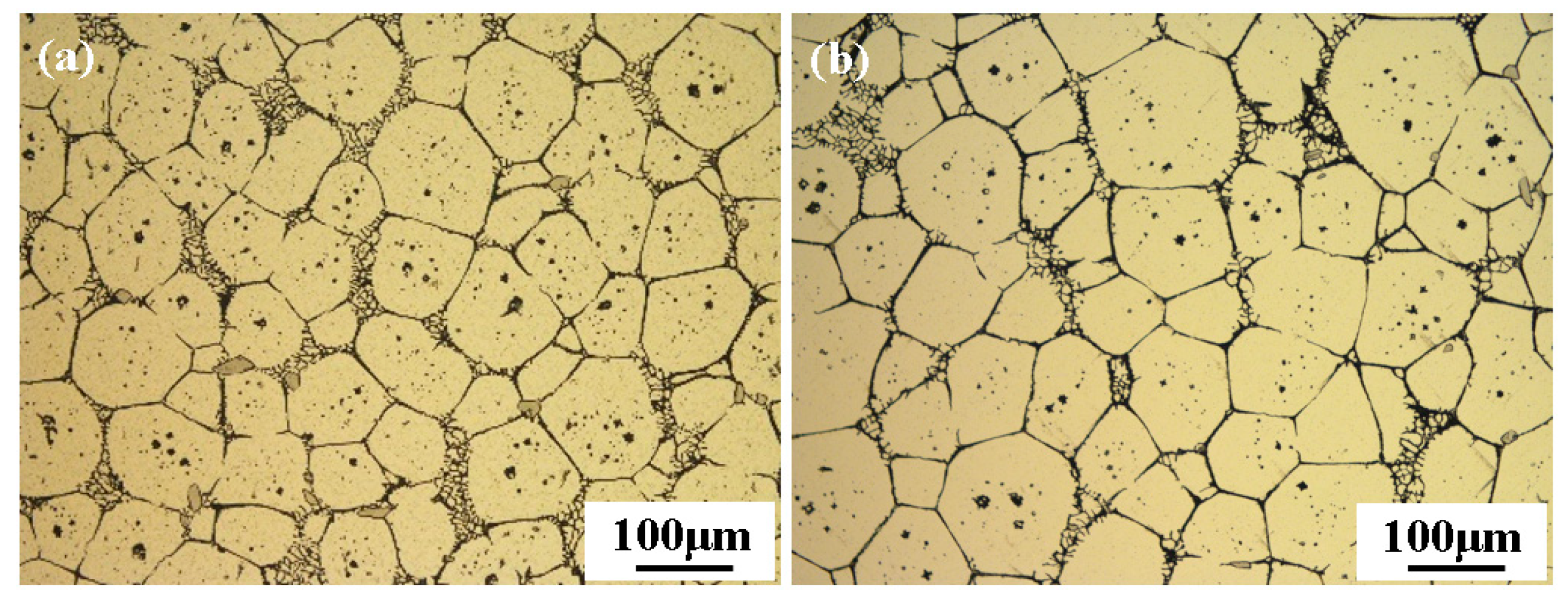
3.1. Microstructure of the Original Sample
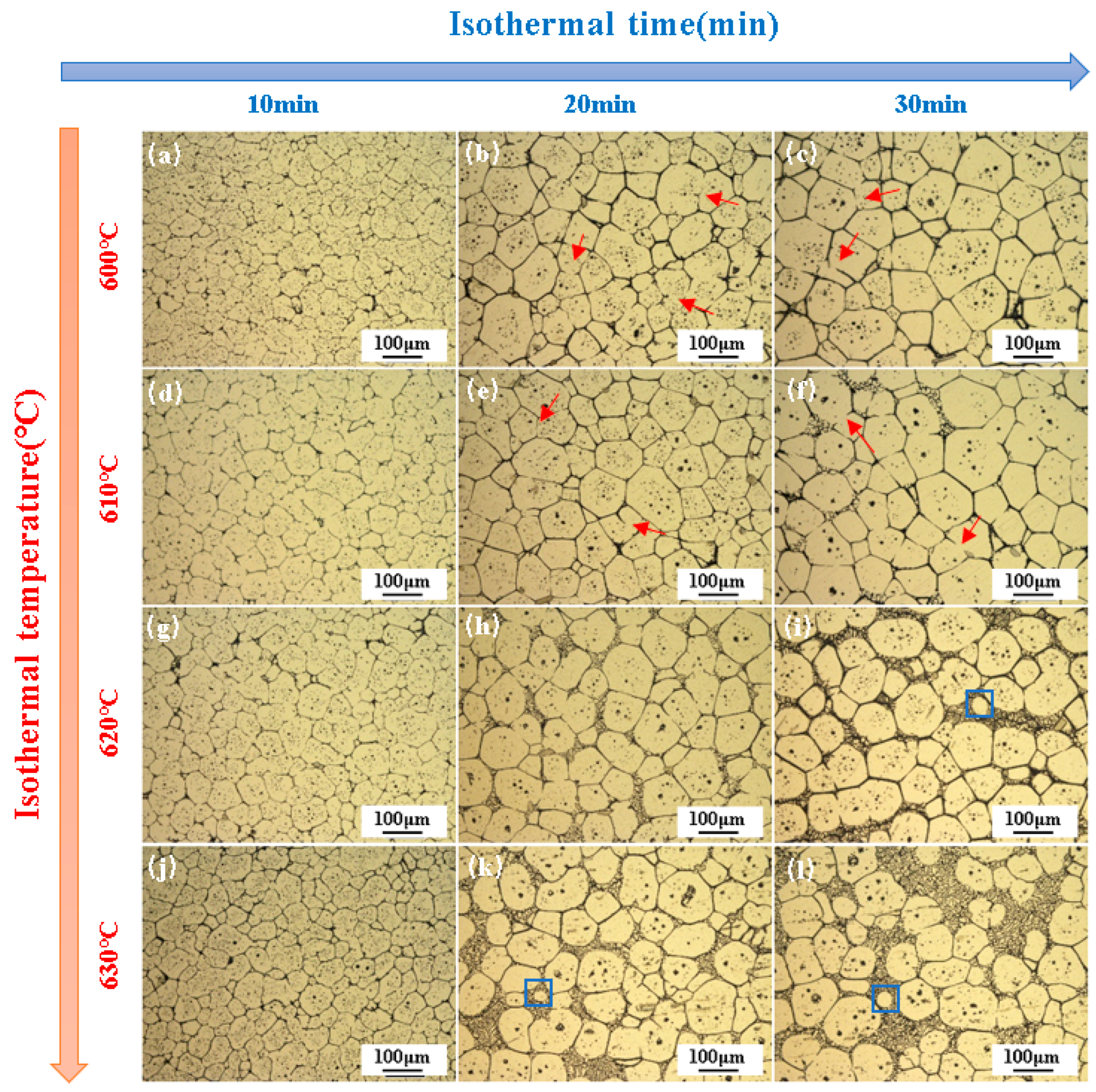
3.2. Semi-Solid Microstructure Evolution
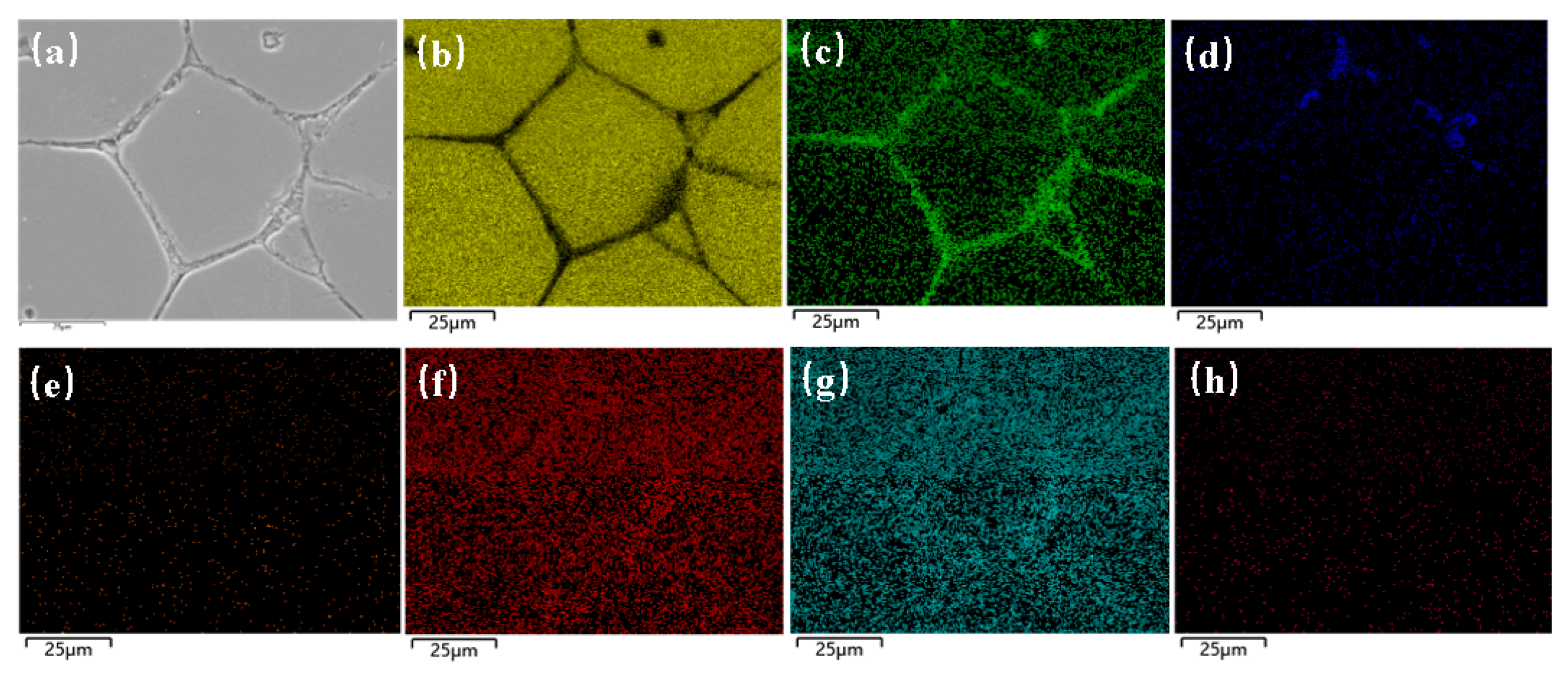
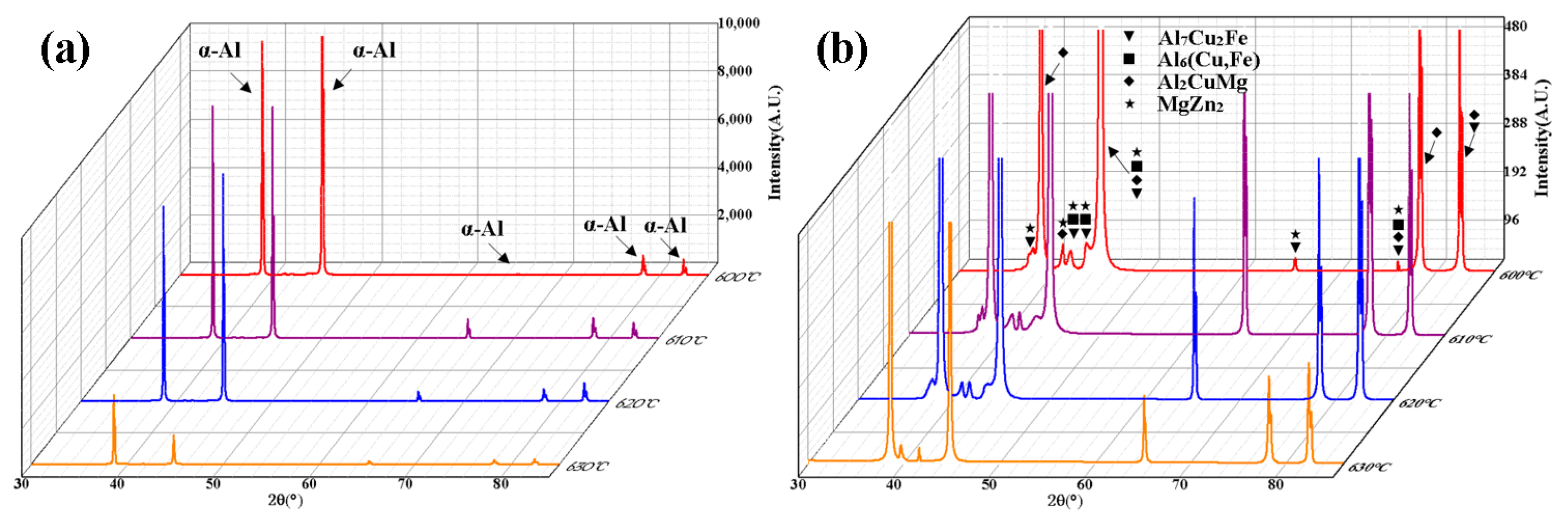
3.3. Phase Evolution and Alloying Element Distribution During Semi-Solid Isothermal Treatment
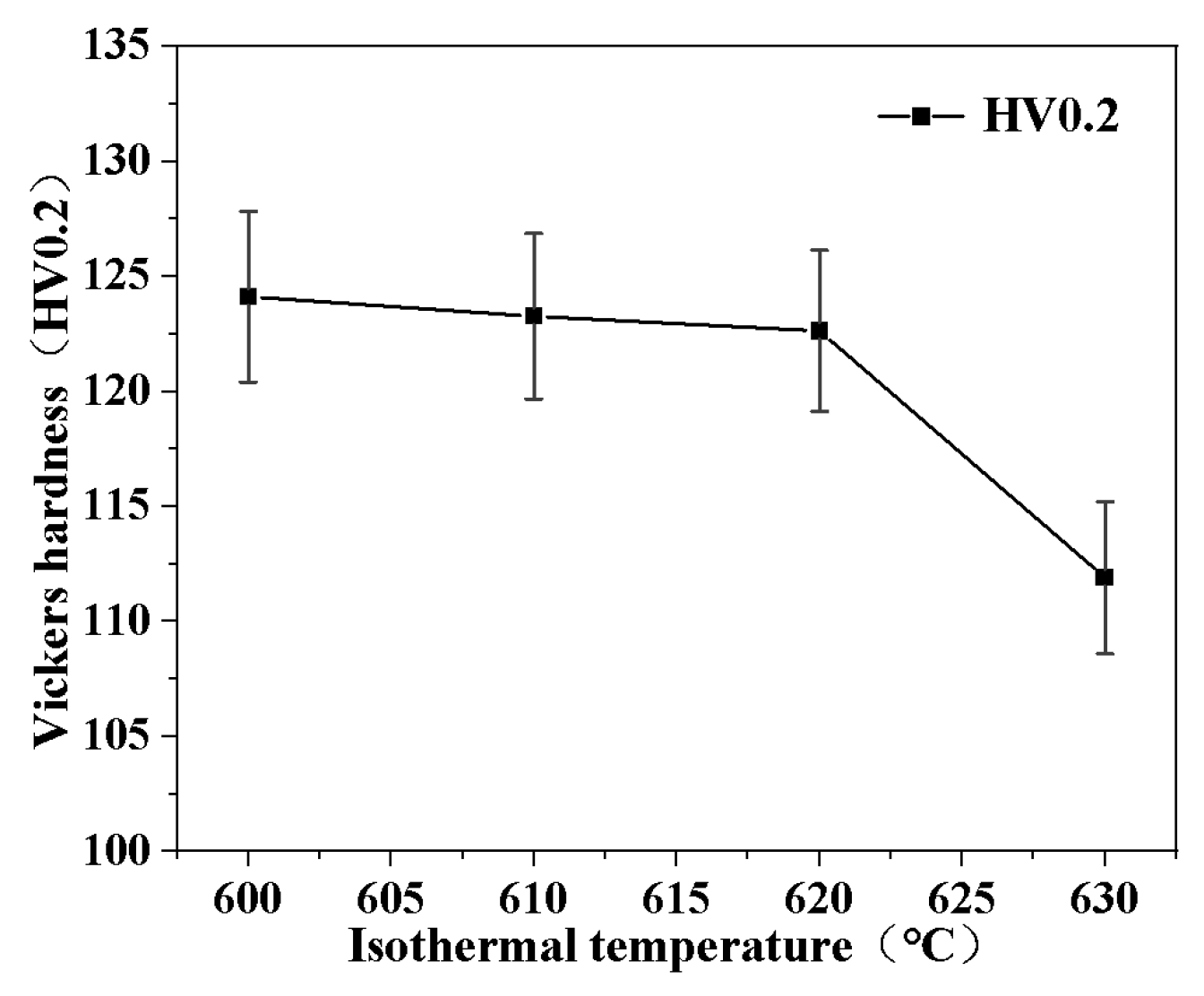
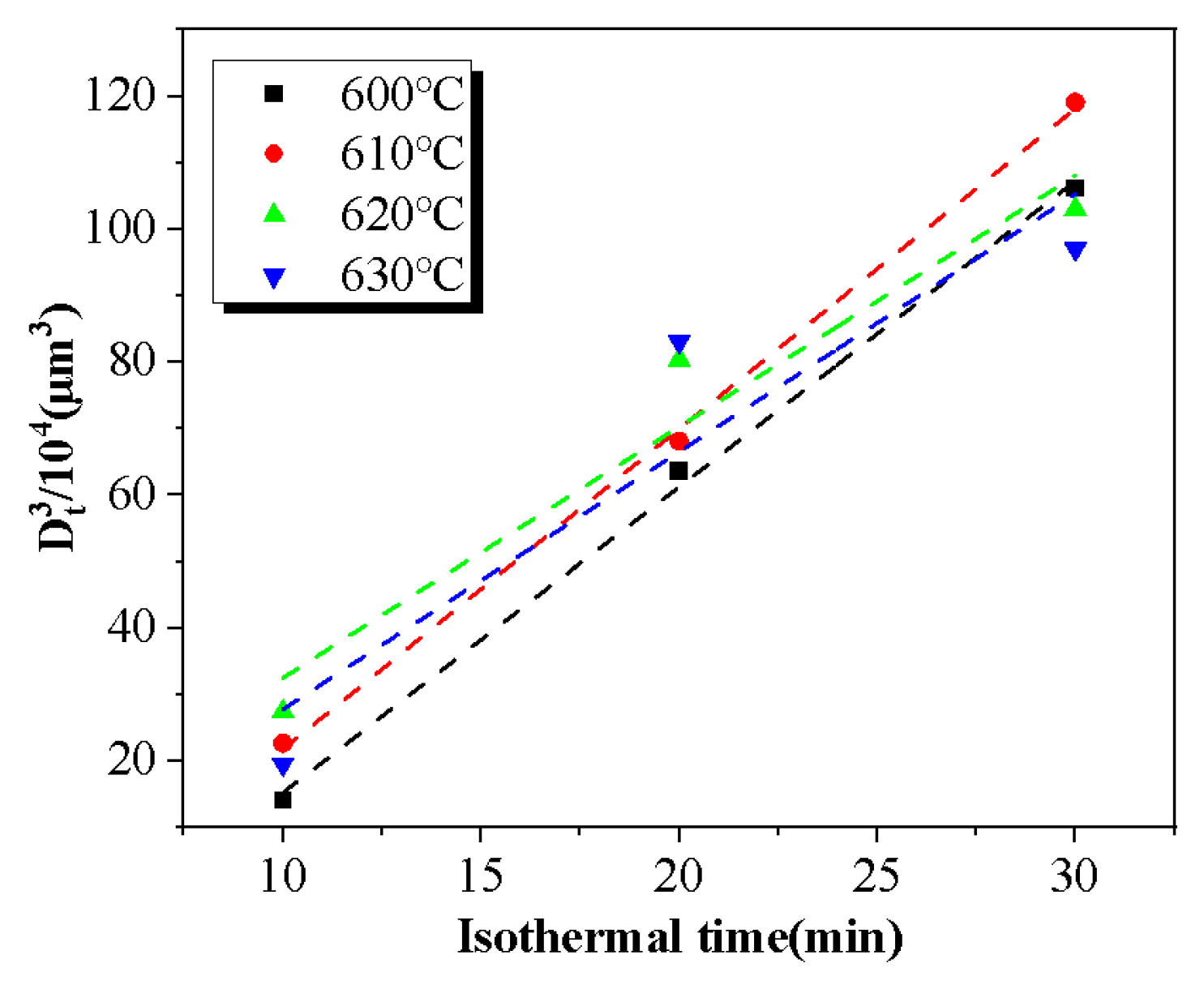
3.4. Coarsening Kinetics in Semi-Solid Isothermal Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EASSIT | Extrusion alloy semi-solid isothermal treatment |
| AGS | Average grain size |
| SF | Shape factor |
| LSW | Lifshitz–Slyozov–Wagner |
| SIMA | Strain-induced melting activation |
| RAP | Recrystallization remelting |
References
- Ma, Z.Y.; Xiao, B.L.; Zhang, J.F.; Zhu, S.Z.; Wang, D. Overview of Research and development for aluminum matrix composites driven by aerospace equipment demand. Acta Metall. Sin. 2023, 59, 457–466. [Google Scholar]
- Kalsar, R.; Ma, X.; Darsell, J.; Zhang, D.; Kappagantula, K.; Herling, D.R.; Joshi, V.V. Microstructure evolution, enhanced aging kinetics, and mechanical properties of AA7075 alloy after friction extrusion. Mater. Sci. Eng. A 2022, 833, 142575. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, X.; Zhang, Z.; Song, G.; Liu, L. Microstructure and mechanical properties of 7075-T6 aluminum alloy plates by welding with weld reinforcement rolling. Mater. Sci. Eng. A 2024, 889, 145854. [Google Scholar] [CrossRef]
- Dash, S.; Biswas, S.; Peng, H.; Jiang, X.; Li, D.; Chen, D. Deformation behavior of dissimilar ultrasonic spot-welded joints of a clad 7075 aluminum alloy to galvanized high-strength low-alloy steel. Mater. Sci. Eng. A 2024, 894, 146179. [Google Scholar] [CrossRef]
- Fu, J.L.; Wang, K.K. Formation of spheroidal microstructure of semisolid Al-Zn-Mg-Cu alloy prepared by RAP and modified SIMA. Rare Met. 2023, 42, 3150–3160. [Google Scholar] [CrossRef]
- Neag, A.; Becker, E. Aluminiums for thixoforging and preparatory conditions and parameters necessary before forming: A review. Heliyon 2023, 9, e22332. [Google Scholar] [CrossRef]
- Binesh, B.; Aghaie-Khafri, M. RUE-based semi-solid processing: Microstructure evolution and effective parameters. Mater. Des. 2016, 95, 268–286. [Google Scholar] [CrossRef]
- Bolouri, A.; Shahmiri, M.; Cheshmeh, E. Microstructural evolution during semisolid state strain induced melt activation process of aluminum 7075 alloy. Trans. Nonferrous Met. Soc. China 2010, 20, 1663–1671. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Xiao, G.; Nie, X. Comparison of microstructural evolution of 7075 aluminum alloy fabricated by SIMA and RAP. J. Mech. Work. Technol. 2016, 238, 361–372. [Google Scholar] [CrossRef]
- Chang, Z.; Wu, Y.; Su, N.; Deng, Q.; Wu, Q.; Xue, Y.; Peng, L.; Ding, W. Microstructural evolution of Mg-10Gd-3Y-1Zn-0.4Zr (wt%) alloy prepared by strain-induced melt activation process. Mater. Charact. 2021, 171, 110831. [Google Scholar] [CrossRef]
- Binesh, B.; Aghaie-Khafri, M. Microstructure and texture characterization of 7075 Al alloy during the SIMA process. Mater. Charact. 2015, 106, 390–403. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Guo, Y.; Zhao, S.-D.; Fan, X.-G. Direct preparation of semi-solid billets by the semi-solid isothermal heat treatment for commercial cold-rolled ZL104 aluminum alloy. Int. J. Miner. Met. Mater. 2021, 28, 1164–1173. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Jiang, J.-F.; Xiao, G.-F.; Zhang, Y.; Huang, M.-J.; Wang, Y. Effects of temperature and time on three-dimensional microstructural evolution of semi-solid 2A14 aluminum alloy during short process preparation of semi-solid billets. Trans. Nonferrous Met. Soc. China 2022, 32, 2091–2109. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, G.; Wang, Y.; Qi, Y. Microstructure evolution of wrought nickel based superalloy GH4037 in the semi-solid state. Mater. Charact. 2018, 141, 229–237. [Google Scholar] [CrossRef]
- Jiang, J.; Tang, S.; Zhang, Y.; Chen, Q.; Wang, Y.; Li, H. Microstructure Evolution of 7005 Alloy Semisolid Billets Fabricated by Large Deformation-Induced Isothermal Spheroidization. J. Mater. Eng. Perform. 2023, 33, 12343–12354. [Google Scholar] [CrossRef]
- Binesh, B.; Aghaie-Khafri, M. Phase Evolution and Mechanical Behavior of the Semi-Solid SIMA Processed 7075 Aluminum Alloy. Metals 2016, 6, 42. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Li, M.; Tang, S.; Huang, Y.; Liu, Y.; Huang, C. Achieving enhanced mechanical properties of extruded Mg-Gd-Y-Zn-Zr alloy by regulating the initial LPSO phases. Mater. Sci. Eng. A 2024, 917, 147411. [Google Scholar] [CrossRef]
- Czerwinski, F. strain induced melt activation (SIMA): Original concept, its impact and present understanding. Int. J. Cast Met. Res. 2020, 33, 157–164. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, M.; Wang, Y.; Liu, Y.; Zhang, Y. Microstructure evolution and formation mechanism of CoCrCu1.2FeNi high entropy alloy during the whole process of semi-solid billet preparation. J. Mater. Sci. Technol. 2022, 120, 172–185. [Google Scholar] [CrossRef]
- Gashti, A.B.; Abedi, H.; Salehi, M. Microstructure evolution and constitutive modeling of as-cast A356 aluminum alloy in semi-solid deformation regime. J. Mater. Res. Technol. 2023, 24, 7720–7731. [Google Scholar] [CrossRef]
- Das, P. Isothermal coarsening of cooling slope processed semi-solid A380 Al alloy slurry. Int. J. Cast Met. Res. 2023, 36, 162–184. [Google Scholar] [CrossRef]
- Takajo, S.; Kaysser, W.; Petzow, G. Analysis of particle growth by coalescence during liquid phase sintering. Acta Met. 1984, 32, 107–113. [Google Scholar] [CrossRef]
- Gan, X.L.; Deng, H.Q.; Xiao, S.F.; Li, X.F.; Hu, W.Y. The alloying processes in solid-solid and liquid-solid Li-Pb interfaces with atomistic simulations. J Alloys Compd. 2015, 632, 467–472. [Google Scholar] [CrossRef]
- Agrawal, S.; Heilmaier, M.; Skrotzki, W.; Suwas, S. Strengthening mechanisms in Ni and Ni-5Fe alloy. Mater. Sci. Eng. 2025, 924, 147752. [Google Scholar] [CrossRef]
- ASM Handbook. Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1992; Volume 3. [Google Scholar]
- Zhang, L.; Liu, Y.; Cao, Z.; Zhang, Y.; Zhang, Q. Effects of isothermal process parameters on the microstructure of semisolid AZ91D alloy produced by SIMA. J. Mech. Work. Technol. 2009, 209, 792–797. [Google Scholar] [CrossRef]
- Hu, B.-L.; Wang, K.-S.; Hu, P.; Xing, H.-R.; Li, S.-L.; Ge, S.-W.; Han, J.-Y.; Hua, X.-J.; Fu, J.-B.; Volinsky, A.A. Secondary phases effects on microstructure and mechanical properties of lanthanum-doped titanium-zirconium-molybdenum alloy. Int. J. Refract. Met. Hard Mater. 2021, 95, 105439. [Google Scholar] [CrossRef]
- Li, S.-L.; Hu, P.; Han, J.-Y.; Ge, S.-W.; Hua, X.-J.; Xing, H.-R.; Deng, J.; Hu, B.-L.; Yang, F.; Wang, K.-S. The formation mechanism of micro-nano secondary phase in solid-liquid doped TZM alloy. Mater. Charact. 2022, 186, 111800. [Google Scholar] [CrossRef]
- Fan, X.-G.; Jiang, D.-M.; Meng, Q.-C.; Zhang, B.-Y.; Wang, T. Evolution of eutectic structures in Al-Zn-Mg-Cu alloys during heat treatment. Trans. Nonferrous Met. Soc. China 2006, 16, 577–581. [Google Scholar] [CrossRef]
- Boettinger, W.J.; Kattner, U.R.; Moon, K.W.; Perepezko, J.H. DTA and heat-flux DSC measurements of alloy melting and freezing. In Methods for Phase Diagram Determination, 1st ed.; Zhao, J.C., Ed.; Elsevier: Ames, IA, USA, 2007; pp. 194–200. [Google Scholar]
- Jiang, J.; Zhang, Y.; Wang, Y.; Xiao, G.; Liu, Y.; Zeng, L. Spheroidizing Process of 2A12 Aluminum Alloy Grains during Heating up and Semisolid Isothermal Treatment Stages. J. Mater. Eng. Perform. 2021, 30, 5974–5986. [Google Scholar] [CrossRef]
- Jin, L.; Kai, K.; Wang, W.; Xiao, K.; Hai, Z. Microstructure evolution and thixoforming behavior of 7075 aluminum alloy in the semi-solid state prepared by RAP method. Int. J. Miner. Metall. Mater. 2016, 23, 1404–1415. [Google Scholar]
- Chen, Q.; Zhao, Z.; Chen, G.; Wang, B. Effect of accumulative plastic deformation on generation of spheroidal structure, thixoformability and mechanical properties of large-size AM60 magnesium alloy. J. Alloys Compd. 2015, 632, 190–200. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Wen, H.; Xia, D.; Sun, W. Microstructural evolution of a fine-grained 7075Al alloy processed by friction stir process during partial remelting. Mater. Charact. 2016, 121, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, L.; Jia, J.; Xu, B. Microstructure evolution of a SIMA processed AZ91D magnesium alloy based on repetitive upsetting-extrusion (RUE) process. Mater. Charact. 2016, 118, 309–323. [Google Scholar] [CrossRef]
- Eberl, D. Crystal growth according to the law of proportionate effect. Am. Miner. 2024, 109, 2–7. [Google Scholar] [CrossRef]


| Zn | Mg | Cu | Si | Fe | Mn | Al |
|---|---|---|---|---|---|---|
| 5.20 | 2.50 | 1.20 | 0.04 | 0.29 | 0.11 | Bal |
| Element | A (at.%) | B (at.%) | C (at.%) | D (at.%) | α-Al Solid Grain (at.%) |
|---|---|---|---|---|---|
| Al | 95.18 | 94.76 | 90.12 | 94.37 | 92.72 |
| Zn | 3.41 | 4.01 | 4.13 | 3.85 | 4.81 |
| Mg | 0.08 | 1.10 | 2.82 | 0.10 | 1.20 |
| Cu | 1.26 | 0.08 | 2.80 | 1.30 | 1.10 |
| Si | 0.05 | 0.02 | 0.09 | 0.01 | 0.06 |
| Fe | 0.02 | 0.03 | 0.04 | 0.37 | 0.11 |
| Temperature (°C) | K (μm3 × s−1) | R2 |
|---|---|---|
| 600 | 767.17 | 0.9981 |
| 610 | 803.35 | 0.9989 |
| 620 | 629.73 | 0.9489 |
| 630 | 645.98 | 0.8800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Chang, M.; Fan, S.; Liu, B.; Wang, Y.; Li, S.; Zhang, C.; Zhang, P.; Zhao, S. Microstructure Evolution During Preparation of Semi-Solid Billet for 7075 Aluminum Alloy by EASSIT Process. Metals 2025, 15, 452. https://doi.org/10.3390/met15040452
Hu Y, Chang M, Fan S, Liu B, Wang Y, Li S, Zhang C, Zhang P, Zhao S. Microstructure Evolution During Preparation of Semi-Solid Billet for 7075 Aluminum Alloy by EASSIT Process. Metals. 2025; 15(4):452. https://doi.org/10.3390/met15040452
Chicago/Turabian StyleHu, Yanghu, Ming Chang, Shuqin Fan, Boyang Liu, Yongfei Wang, Shuangjiang Li, Chao Zhang, Peng Zhang, and Shengdun Zhao. 2025. "Microstructure Evolution During Preparation of Semi-Solid Billet for 7075 Aluminum Alloy by EASSIT Process" Metals 15, no. 4: 452. https://doi.org/10.3390/met15040452
APA StyleHu, Y., Chang, M., Fan, S., Liu, B., Wang, Y., Li, S., Zhang, C., Zhang, P., & Zhao, S. (2025). Microstructure Evolution During Preparation of Semi-Solid Billet for 7075 Aluminum Alloy by EASSIT Process. Metals, 15(4), 452. https://doi.org/10.3390/met15040452







