Effect of Leaching on Particle Migration and Pore Structure of Ionic Rare Earth Ores with Different Fine Particle Contents
Abstract
1. Introduction
2. Theoretical Basis and Experimental Methods
2.1. Theoretical Basis
2.1.1. Fractal Theory
2.1.2. Relaxation Theory
2.2. Experimental Methods
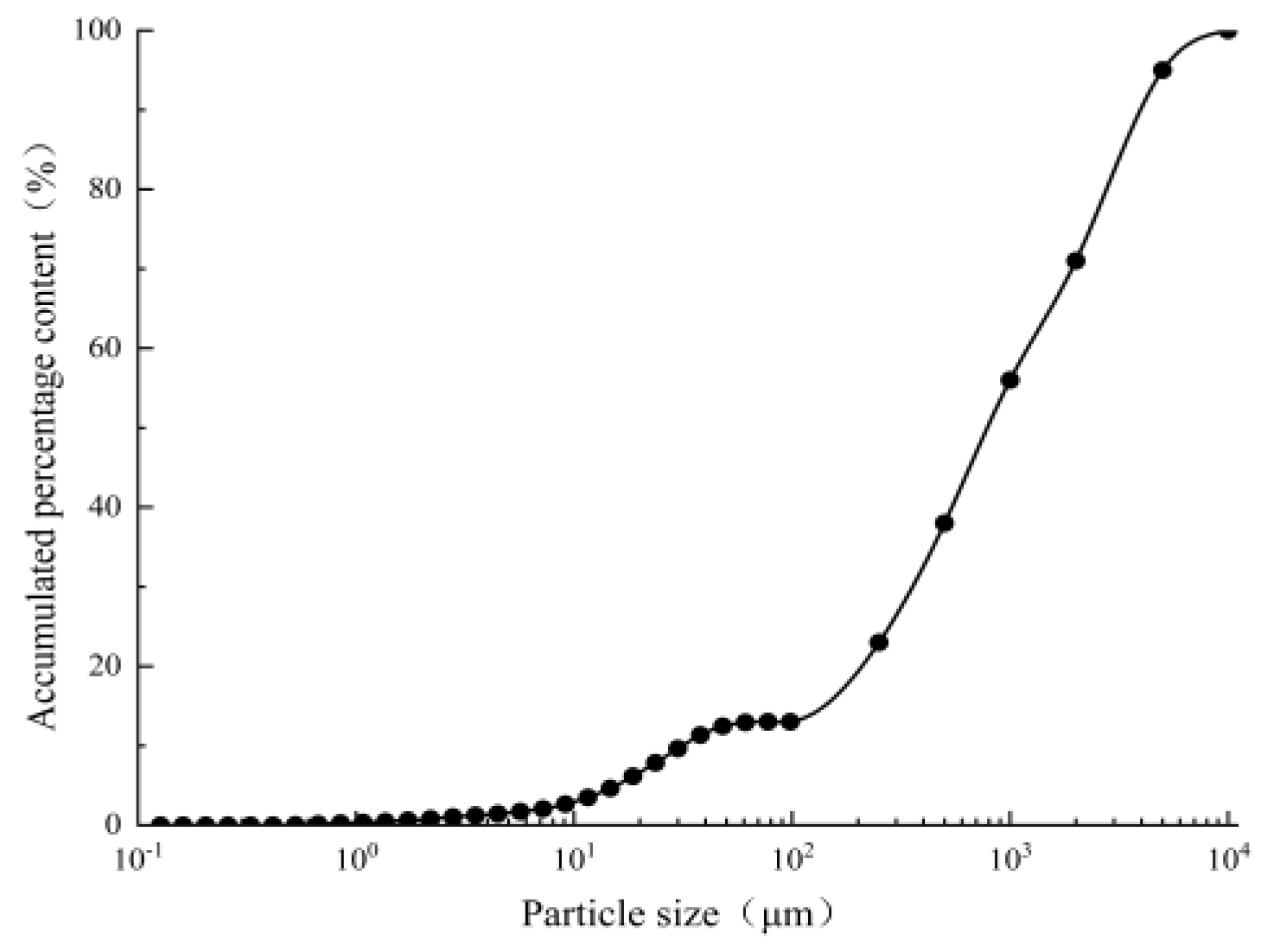
2.2.1. Experimental Materials
2.2.2. Column Leaching Experiment
2.2.3. Particle Gradation Experiment
2.2.4. Pore Structure Experiment
3. Results and Discussion
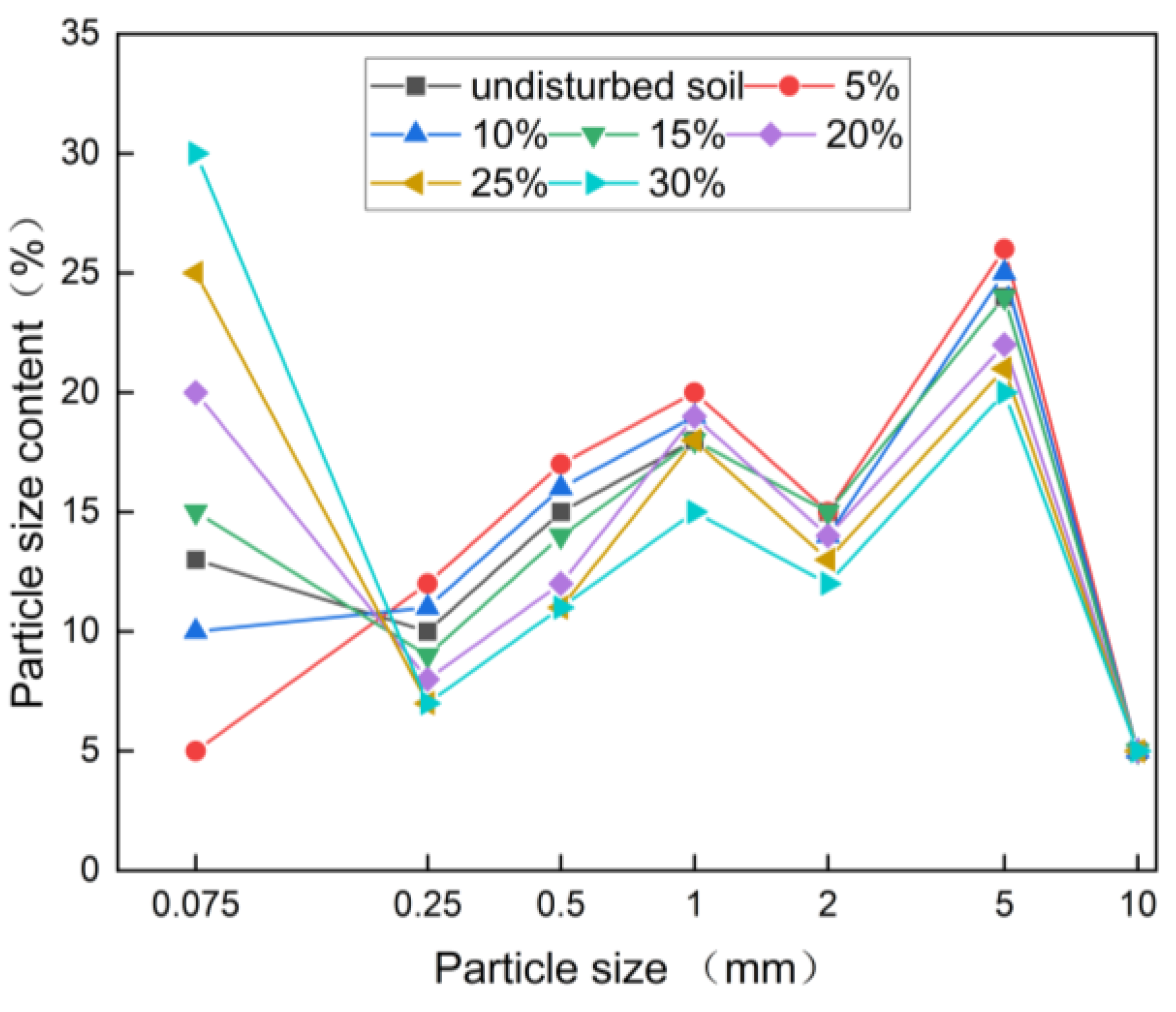
3.1. Rare Earth Ore Column Leaching Full Particle Size Gradation Analysis
3.1.1. The Change Rule of the Full Particle Size Gradation of Deionized Water Leaching Ore Soil
3.1.2. The Change Rule of the Full Particle Size Gradation of Magnesium Sulfate Leaching Ore Soil
3.2. The Change in Behavior of Fine Ionic Rare Earth Ore Particles During In Situ Column Leaching
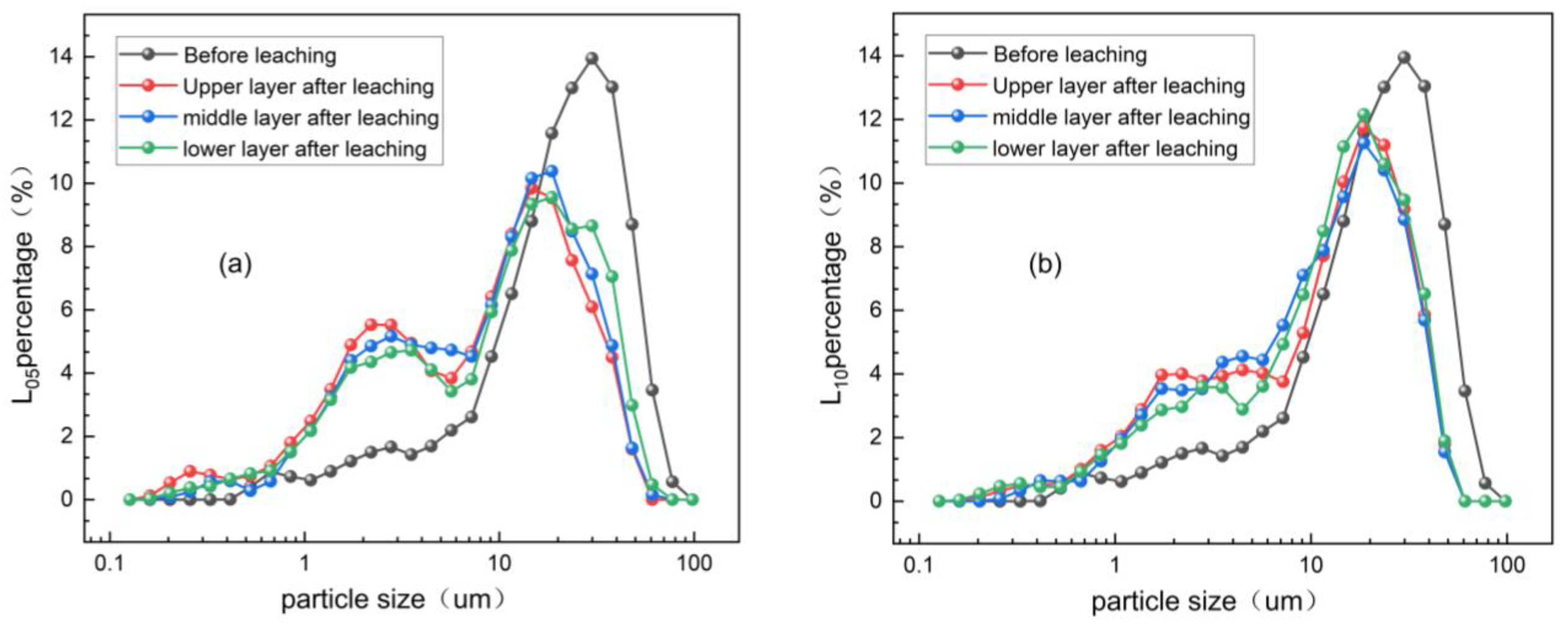
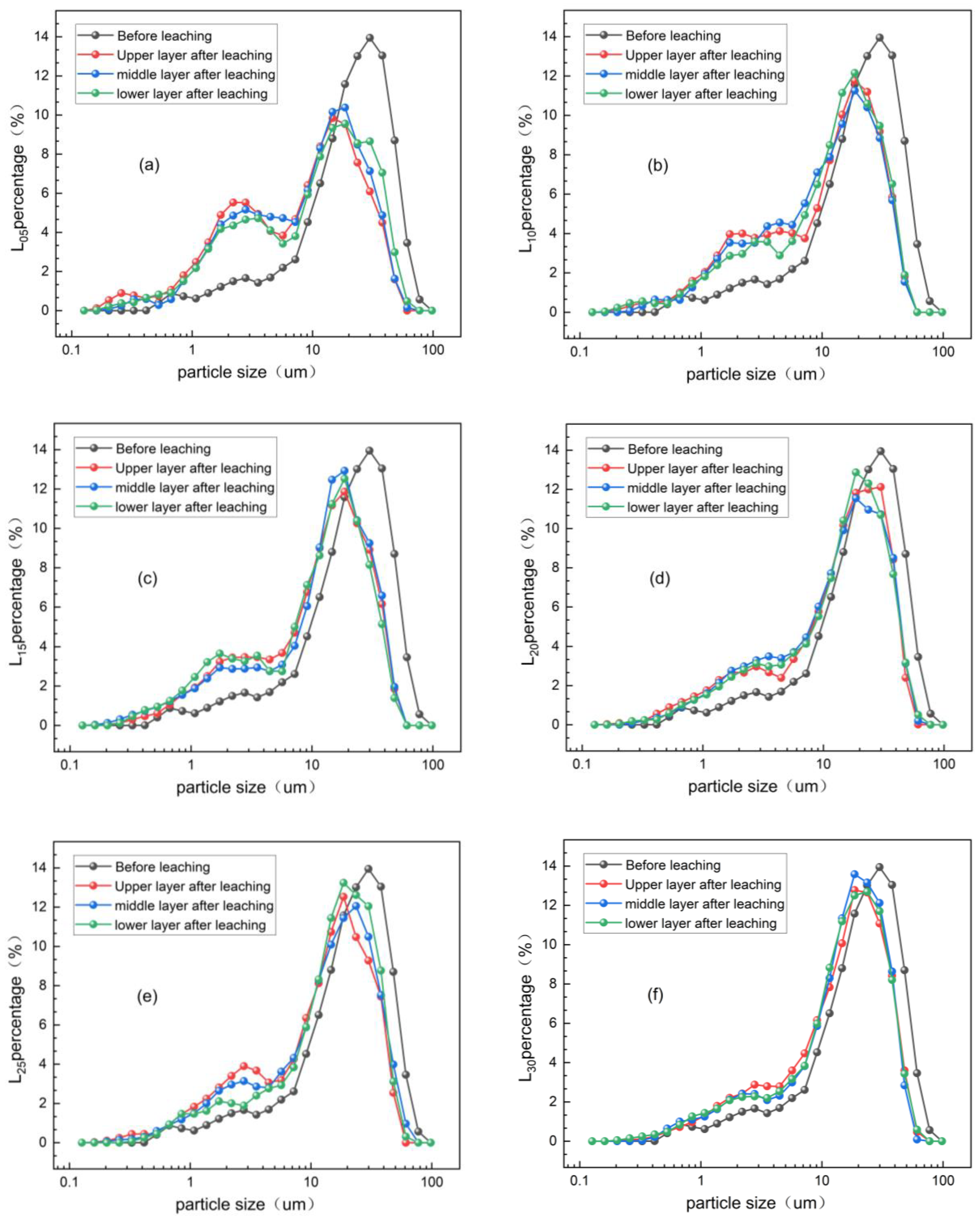
3.2.1. Fine Particle Size Distribution of Rare Earth Ore After Water Leaching
3.2.2. Fine Particle Size Gradation of Magnesium Sulfate Leaching Ore Soil
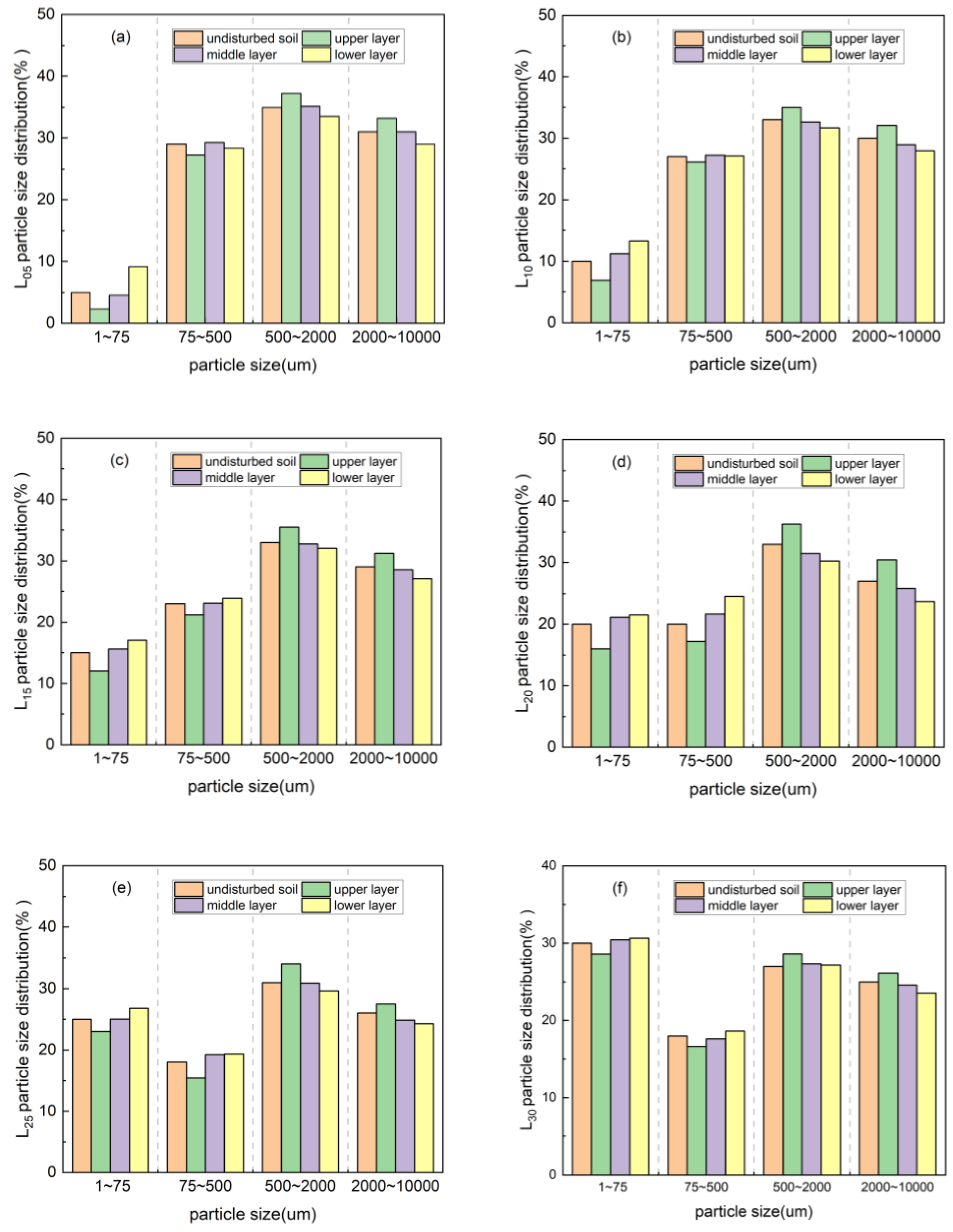
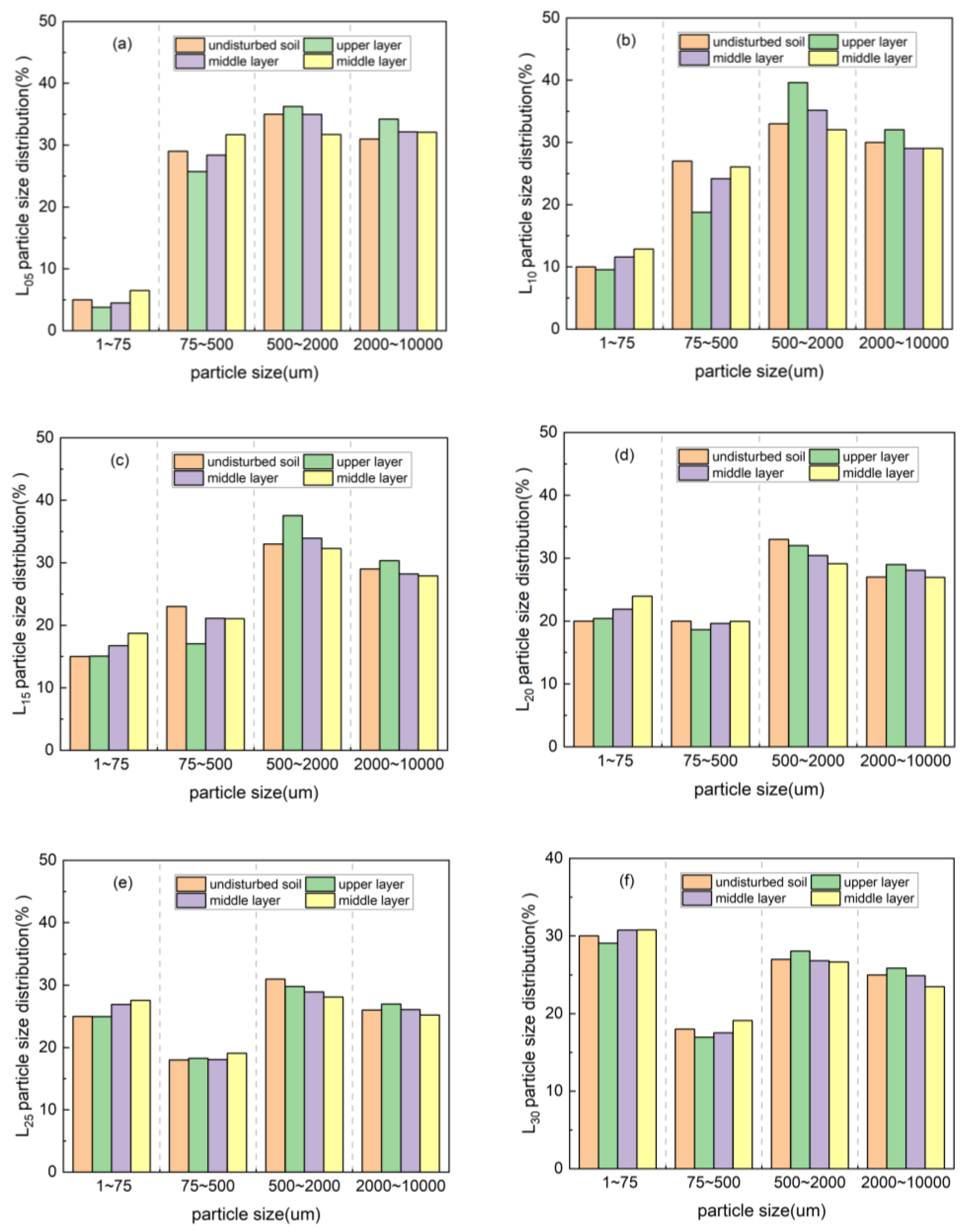
3.3. Porosity Variation of Rare Earth Ore During the Column Leaching Process
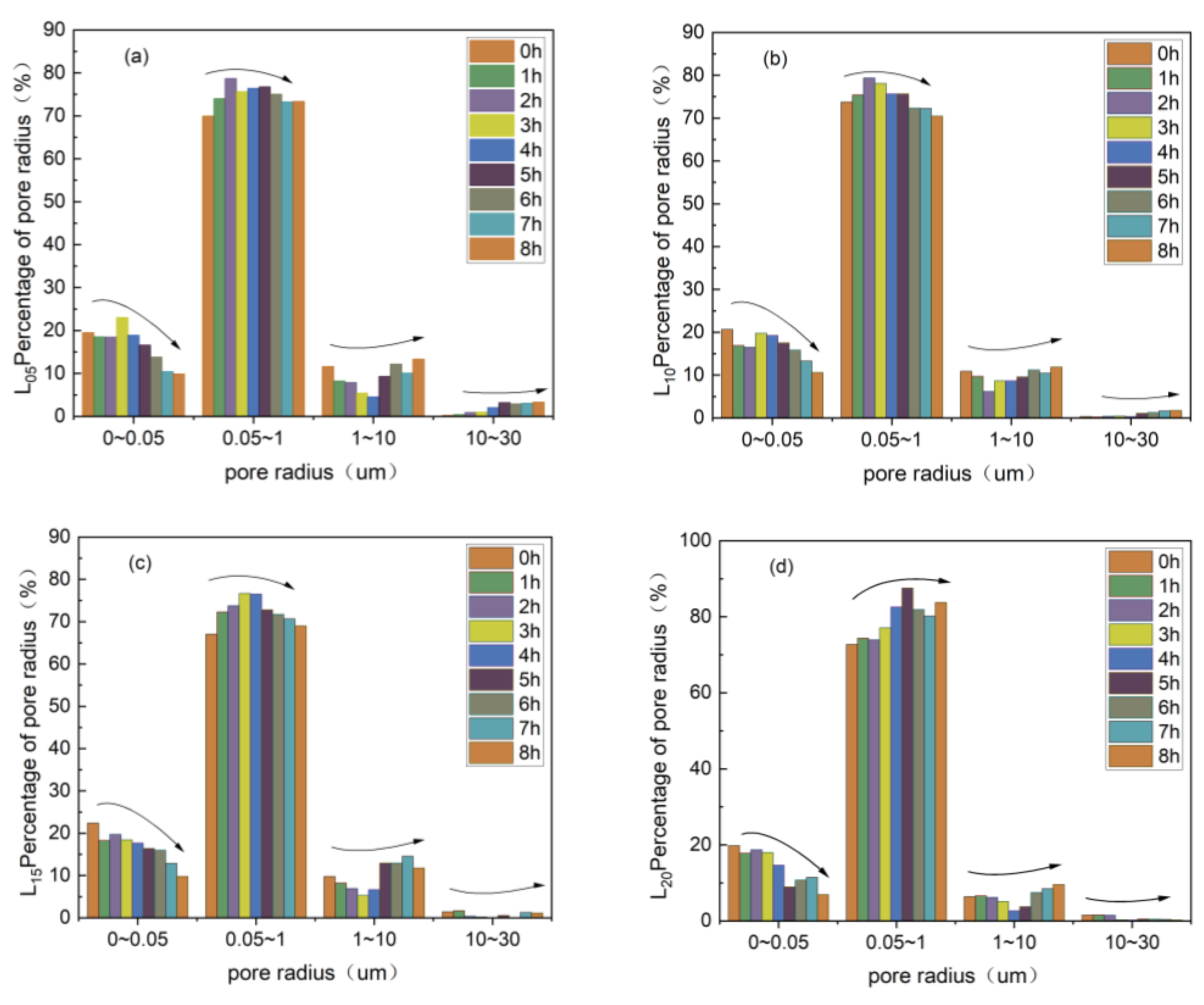
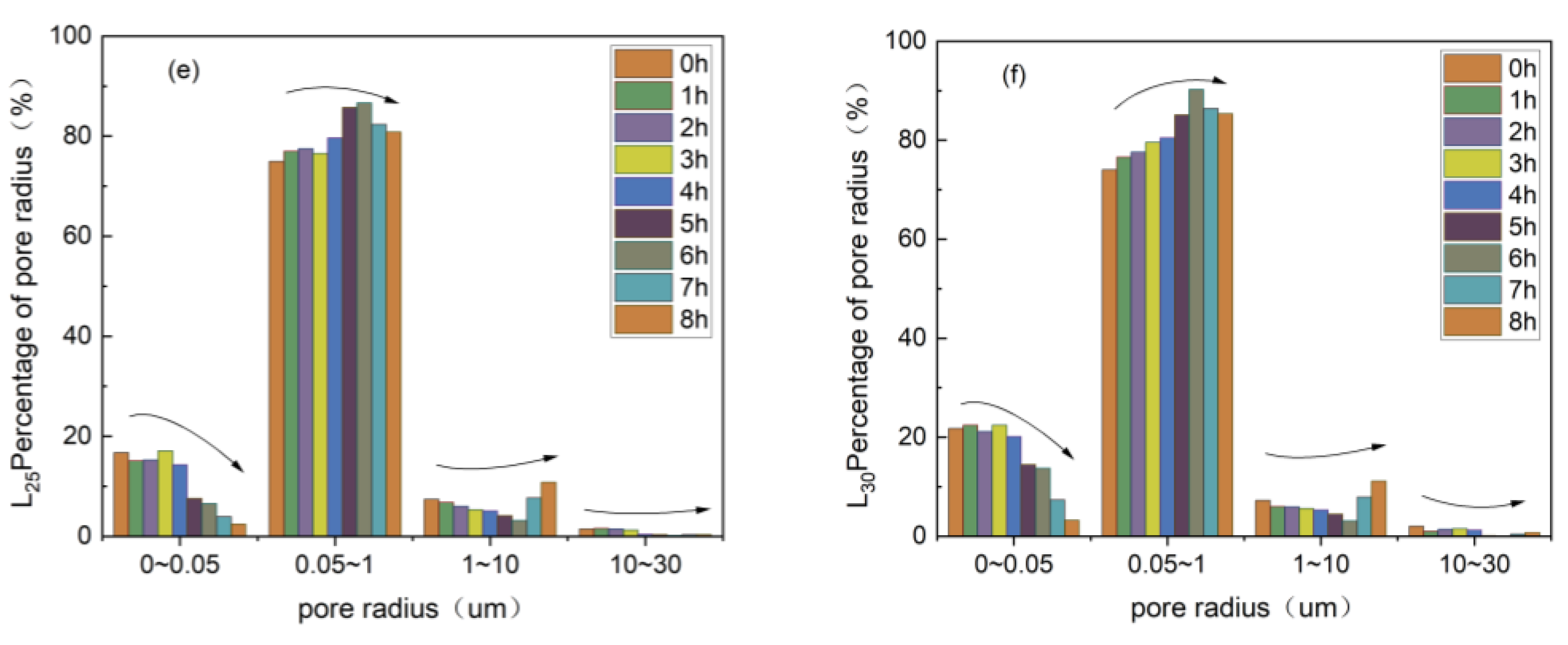
3.3.1. Change in the Pore Size Distribution of Rare Earth Ores in Deionized Water Column Leaching
3.3.2. Change in Pore Size Distribution of Rare Earth Ores in Magnesium Sulfate Column Leaching
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, J.; Chi, R.; Yin, J. Leaching Process of Rare Earths from Weathered Crust Elution-Deposited Rare Earth Ore. Trans. Nonferrous Met. Soc. China 2010, 20, 892–896. [Google Scholar]
- Dong, Y.; Feng, M.; Liu, Z.; Wu, Y.; Li, Y.; Wu, W. Numerical Simulation of Coupled Bilayer-Temperature Field in Rare Earth Electrolyzer. Rare Earths 2019, 40, 88–94. [Google Scholar]
- Huang, X.; Long, Z.; Wang, L.; Feng, Z. Technology Development for Rare Earth Cleaner Hydrometallurgy in China. Rare Met. 2015, 34, 215–222. [Google Scholar]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The Story of Rare Earth Elements (REEs): Occurrences, Global Distribution, Genesis, Geology, Mineralogy and Global Production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar]
- Wang, Z.; Chen, Z.; Zhao, Z.; Chen, B.; Zou, X. REE Mineral and Geochemical Characteristics of Neoproterozoic Metamorphic Rocks in South Jiangxi Province. Mineral. Depos. 2019, 38, 837–850. [Google Scholar]
- Li, S.; Zhou, H.; Shi, Q.; Meng, X.; Zhao, Y.; Qiu, G.; Zhang, X.; Yu, H.; He, X.; He, H.; et al. Comparative Chemical and Non-Contact Bioleaching of Ion-Adsorption Type Rare Earth Ore Using Ammonium Sulfate and Metabolites of Aspergillus niger and Yarrowia lipolytica. Hydrometallurgy 2023, 216, 105987. [Google Scholar]
- Chen, Z.; Zhang, Z.; Liu, D.; Chi, X.; Chen, W.; Chi, R. Swelling of Clay Minerals during the Leaching Process of Weathered Crust Elution-Deposited Rare Earth Ores by Magnesium Salts. Powder Technol. 2020, 367, 889–900. [Google Scholar]
- Wang, D.; Rao, Y.; Shi, L.; Xu, W.; Huang, T. Relationship between Permeability Coefficient and Fractal Dimension of Pore in Ionic Rare Earth Magnesium Salt Leaching Ore. Geofluids 2022, 2022, 2794446. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Feng, L.; Yang, M. Rare Earth Element Migration and Impact of Dicranopteris dichotoma at Mines in South China. Chemosphere 2021, 278, 130433. [Google Scholar]
- Fan, X.; Xue, Q.; Liu, S.; Tang, J.; Qiao, J.; Huang, Y.; Sun, J.; Liu, N. The Influence of Soil Particle Size Distribution and Clay Minerals on Ammonium Nitrogen in Weathered Crust Elution-Deposited Rare Earth Tailing. Ecotoxicol. Environ. Saf. 2021, 208, 111663. [Google Scholar]
- Guo, Z.; Lai, Y.; Jin, J. Effect of Particle Size and Grain Composition on Two-Dimensional Infiltration Process of Weathered Crust Elution-Deposited Rare Earth Ores. Trans. Nonferrous Met. Soc. China 2020, 30, 1647–1661. [Google Scholar]
- Ahfir, N.; Hammadi, A.; Alem, A.; Wang, H.; Le Bras, G.; Ouahbi, T. Porous Media Grain Size Distribution and Hydrodynamic Forces Effects on Transport and Deposition of Suspended Particles. J. Environ. Sci. 2017, 53, 161–172. [Google Scholar]
- Jiang, S.; Bai, B. Influence of Particle Shape on the Suspended Particle Transport and Deposition in Porous Media. Rock Soil Mech. 2018, 39, 2043–2051. [Google Scholar]
- Bennacer, L.; Ahfir, N.; Alem, A.; Wang, H. Coupled Effects of Ionic Strength, Particle Size, and Flow Velocity on Transport and Deposition of Suspended Particles in Saturated Porous Media. Transp. Porous Media 2017, 118, 265–283. [Google Scholar]
- Rao, Y.; Zhang, X.; Gao, Z.; Xiang, R.; Zhang, L. Experimental Study on Pore Structure and Soil-Water Characteristic Curve of Ionic Rare Earth Ore under Seepage. Minerals 2023, 13, 733. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Wang, Y.; Wang, D.; Hu, K.; Zhong, W.; Guo, Z. Influence of Ammonium Sulfate Leaching Agent on Engineering Properties of Weathered Crust Elution-Deposited Rare Earth Ore. Acta Geotech. 2023, 19, 2041–2062. [Google Scholar]
- Wang, G.; Wang, X.; Hu, S.; Hong, B. Experimental Study on the Effect of Particle Transport on the Structure of Ionic Rare Earth Ores. Mining Res. Dev. 2015, 35, 37–42. [Google Scholar]
- Zhang, L.; Wen, B.; Chen, L.; Wang, L. Variations in Pore Structures and Permeabilities of the Ion-Adsorption Rare Earth Ores in the Zones with Different Weathering Degree Before and After Leaching. Hydrogeol. Eng. Geol. 2024, 51, 117–124. [Google Scholar]
- Zhou, N.; Matsumoto, T.; Hosokawa, T.; Suekane, T. Pore-Scale Visualization of Gas Trapping in Porous Media by X-Ray CT Scanning. Flow Meas. Instrum. 2010, 21, 262–267. [Google Scholar]
- Liu, D.; Yan, W.; Zhang, Z.; Chi, R. Effect of Particle Gradation on Pore Structure and Seepage Law of Solution in Weathered Crust Elution-Deposited Rare Earth Ores. Int. J. Min. Sci. Technol. 2023, 33, 1261–1272. [Google Scholar]
- Luo, X.; Zhang, Y.; Zhou, H.; He, K.; Zhang, B.; Zhang, D.; Xiao, W. Pore Structure Characterization and Seepage Analysis of Ionic Rare Earth Orebodies Based on Computed Tomography Images. Int. J. Min. Sci. Technol. 2022, 32, 411–421. [Google Scholar] [CrossRef]
- Wu, X.; Feng, J.; Zhou, F.; Liu, C.; Chi, R. High Sedimentation Efficiency and Enhanced Rare Earth Recovery in the Impurity Removal Process of Rare Earth Leachate by Flocculation System. J. Environ. Chem. Eng. 2024, 12, 112626. [Google Scholar] [CrossRef]
- Knyazev, V.; Morozov, M. To the Origins of Fractal Theory. Vopr. Filos. 2022, 2022, 116–127. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Pan, W.; Guo, L.; Shan, Y. A Unimodal Soil-Water Characteristic Curve Model for Gap-Graded Soil Based on Bimodal Grain-Size Distribution and Fractal Theory. Transp. Geotech. 2025, 50, 101465. [Google Scholar] [CrossRef]
- Bird, N.; Preston, A.; Randall, E.; Whalley, W.; Whitmore, A. Measurement of the Size Distribution of Water-Filled Pores at Different Matric Potentials by Stray Field Nuclear Magnetic Resonance. Eur. J. Soil Sci. 2005, 56, 135–143. [Google Scholar]
- Ni, H.; Liu, J.; Wang, Z.; Sa, Q.; Zhang, X. NMR-Based Comparative Study of Gas Permeability and Pore Structure of GMZ Bentonite. Prog. Nucl. Energy 2024, 169, 105096. [Google Scholar] [CrossRef]
- Yu, Y.; Rong, K.; Cui, W.; Cheng, W.; Chen, Y.; Wei, W. Pore Structure Dynamic Evolution of Coal During Hydraulic Intrusion Based on NMR. Measurement 2024, 227, 114247. [Google Scholar]
- Sun, S.; Wei, G.; Wang, Z.; Jia, T.; Liu, X. Fractal Characteristics of the Microstructure of Loaded Coal Based on NMR and μ-CT. Energy Sources Part A 2025, 47, 4703–4720. [Google Scholar] [CrossRef]
- Zhang, M. Simulation Study on Seepage-Exchange-Migration Process of In-Situ Leaching Ore of Ionic Rare Earths. Ph.D. Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2022. [Google Scholar]
- GB/T 50123-2019; Standard for Geotechnical Testing Methods. Standards Press of China: Beijing, China, 2019.
- Yang, J.; Yin, Z.; Laouafa, F.; Hicher, P. Modeling Coupled Erosion and Filtration of Fine Particles in Granular Media. Acta Geotech. 2019, 14, 1615–1627. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, K.; Zhou, J.; Jin, J.; Zhou, K. Predicting Soil-Water Characteristic Curve from Fractal Particle-Size Distribution of Ion-Adsorption Rare Earth Ores. Chin. Rare Earths 2022, 43, 56. [Google Scholar]
- Zhang, L.; Wen, B.; Chen, L.; Chen, H.; Wu, K. Variations in Pore Structures and Permeabilities of Ion Adsorption Rare Earth Ores During Simulated In-Situ Leaching: Effect of Newly Formed Clay Particles and Their Swelling. Hydrometallurgy 2024, 228, 106357. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Z.; Rao, Y.; Shi, L.; Xu, W. Evolutionary Law of Pore Structure of Ion-Adsorbed Rare Earth Ore Leaching Process. Minerals 2023, 13, 322. [Google Scholar] [CrossRef]
- Peng, S.; Fu, G.; Zhao, X. Integration of USEPA WASP Model in a GIS Platform. J. Zhejiang Univ. Sci. A 2010, 11, 1015–1024. [Google Scholar] [CrossRef]





| Parameter | Natural Density (g/cm3) | Dry Density (g/cm3) | Moisture Content (%) | Specific Gravity of Soil Particles | Porosity (%) |
|---|---|---|---|---|---|
| Value | 1.63 | 1.35 | 20.47 | 2.70 | 49.87 |
| Particle Diameter (mm) | <0.075 | 0.075–0.25 | 0.25–0.5 | 0.5–1 | 1–2 | 2–5 | >5 |
|---|---|---|---|---|---|---|---|
| Percentage of interval (%) | 13 | 10 | 15 | 18 | 15 | 24 | 5 |
| Cumulative percentage (%) | 13 | 23 | 38 | 56 | 71 | 95 | 100 |
| Sample | Before Ore Leaching | Upper Layer | Middle Layer | Lower Layer |
|---|---|---|---|---|
| L05 | 2.21 | 2.17 | 2.16 | 2.21 |
| L10 | 2.32 | 2.28 | 2.29 | 2.31 |
| L15 | 2.39 | 2.34 | 2.35 | 2.36 |
| L20 | 2.42 | 2.35 | 2.38 | 2.41 |
| L25 | 2.45 | 2.37 | 2.41 | 2.44 |
| L30 | 2.50 | 2.40 | 2.42 | 2.44 |
| Sample | Before Ore Leaching | Upper Layer | Middle Layer | Lower Layer |
|---|---|---|---|---|
| L05 | 2.21 | 2.34 | 2.37 | 2.39 |
| L10 | 2.32 | 2.43 | 2.44 | 2.45 |
| L15 | 2.39 | 2.47 | 2.50 | 2.52 |
| L20 | 2.42 | 2.50 | 2.49 | 2.50 |
| L25 | 2.45 | 2.48 | 2.51 | 2.52 |
| L30 | 2.50 | 2.49 | 2.49 | 2.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, Y.; Wan, J.; Tan, S.; Yang, Z.; Rao, G.; Huang, Q.; Xie, Y.; Lai, Q. Effect of Leaching on Particle Migration and Pore Structure of Ionic Rare Earth Ores with Different Fine Particle Contents. Metals 2025, 15, 396. https://doi.org/10.3390/met15040396
Rao Y, Wan J, Tan S, Yang Z, Rao G, Huang Q, Xie Y, Lai Q. Effect of Leaching on Particle Migration and Pore Structure of Ionic Rare Earth Ores with Different Fine Particle Contents. Metals. 2025; 15(4):396. https://doi.org/10.3390/met15040396
Chicago/Turabian StyleRao, Yunzhang, Jiazheng Wan, Shujun Tan, Zhihua Yang, Guozhu Rao, Qiang Huang, Yangjun Xie, and Qiande Lai. 2025. "Effect of Leaching on Particle Migration and Pore Structure of Ionic Rare Earth Ores with Different Fine Particle Contents" Metals 15, no. 4: 396. https://doi.org/10.3390/met15040396
APA StyleRao, Y., Wan, J., Tan, S., Yang, Z., Rao, G., Huang, Q., Xie, Y., & Lai, Q. (2025). Effect of Leaching on Particle Migration and Pore Structure of Ionic Rare Earth Ores with Different Fine Particle Contents. Metals, 15(4), 396. https://doi.org/10.3390/met15040396





