Abstract
In the process of continuous casting, especially high-speed continuous casting, the inflow state of the mold flux is particularly important. The fluxing agent is one of the most important factors affecting the flow state. The influence of the typical fluxing agent B2O3 on the viscous characteristics and melt structure of the fluorine-free CaO-SiO2-M2O (M = Li, Na) system was analyzed. The following conclusions were drawn. In the CaO-SiO2-Na2O slags, with the increasing addition of B2O3, the viscosity, breaking temperature, and polymerization degree of the slag show a gradually decreasing trend. When the mass fraction of B2O3 increased from 0 to 10%, the increase in two-dimensional [BO3] structural units played a dominant role. When the mass fraction of B2O3 reached 15%, the network was affected by the increase in [BO3] and the low-polymerized [SiO4] tetrahedrons. The CaO-SiO2-Li2O slag system had a lower breaking temperature due to the formation of phases such as Li2O·2B2O3, of a low melting temperature. The initial degree of depolymerization of the network was high. Upon increasing the addition of B2O3, the relative proportion of the network modifier structural units significantly increased, resulting in the enhanced instability of the network structure. As a result, the effect of [SiO4]-polymerization was stronger than that of [BO3]-depolymerization in maintaining the stability of the network structure.
1. Introduction
Since mold flux technology’s advent, it has played an important and positive role in improving the quality of slabs. According to the latest report of the World Steel Association, the continuous casting ratio of global steel production has reached 96.7% [1]. With the development of continuous casting technology, mold fluxes, as an important auxiliary material, have an increasingly prominent influence on slab quality. As we know, mold fluxes’ main roles are in heat insulation, preventing oxidation, absorbing inclusions, the lubrication of slabs, controlling heat transfer in mold, and so on, in the continuous casting process [2,3,4]. In essence, the melting and inflow state of mold fluxes is one of the most important factors affecting whether the above metallurgical functions can be effectively exerted. By analyzing the basic composition and corresponding functions of mold fluxes, it can be seen that the fluxing agent is the main component in adjusting the melting temperature, viscosity, and other indexes of mold fluxes, and furthermore, that it can directly affect the inflow state of mold fluxes and the effective play of the metallurgical functions [4,5,6,7,8].
Fluxing agents in mold fluxes include CaF2, NaF, Na2O, K2O, Li2O, and B2O3, etc. [9,10,11,12,13,14,15,16]. Among them, CaF2 and Na2O are the most common in mold fluxes. The addition of CaF2 and other fluorine-containing components in mold fluxes is about 12~20%, which can reduce the melting temperature and adjust the viscosity. Moreover, CaF2 and other fluorine-containing components can also participate in the formation of cuspidine, which precipitates during the cooling process and plays a role in controlling heat transfer as the main crystallization phase in conventional mold fluxes. However, with the improvement of the environmental protection requirements, the design and development of a fluorine-free continuous casting mold flux has become one of the research hotspots of the relevant scholars [17,18,19,20,21,22,23,24]. For example, Zhao, Chen, and Wang, etc., carried out the research and development of a fluorine-free mold flux for peritectic steel billets, low-carbon steel slabs, and titanium-containing steel, and characterized its properties, respectively [19,20,21,22]. Although fluorine-free mold fluxes have become one of the main development directions of continuous casting mold fluxes, how to find a suitable substitute for F, design a new type of mold flux, and obtain the physical and chemical properties similar to those of conventional fluorine-containing mold fluxes are still some of the problems that researchers are committed to solving at present. As far as the role of F in conventional mold fluxes is concerned, adopting a suitable new flux to replace it is the first solution that researchers think of. Zhao et al. carried out a fluorine-containing material substitution test by adding materials containing Li2O, B2O3, BaO, MnO, and other components to mold fluxes [19,20]. Chen et al. added low-melting point fluxing material containing B2O3 to a mold flux for a low-carbon steel slab continuous casting mold to replace fluorine and sodium in mold fluxes, thus reducing the harm of fluoride in the continuous casting process [21]. Wang et al. used B2O3 and TiO2 as substitutes for CaF2, and measured the viscosity of mold fluxes with different contents of CaF2, TiO2, and B2O3 [22].
Although the authors have carried out a large number of experimental studies on the design and development of fluorine-free mold fluxes [25,26,27,28,29,30,31], there is still a lack of basic theoretical support on how to design the slag system and how to optimize the composition of components. Moreover, as far as fluxes such as lithium oxide and boron oxide are concerned, they are only added in a small amount in conventional mold fluxes, and their action rules on fluorine-free mold fluxes need to be further explored. Furthermore, within the research framework addressing fluxing agent functionality, slag systems exhibit inherent compositional complexity. The current theoretical models face limitations in establishing mechanistic frameworks for fluxing effects with reduced component systems. To address this constraint, the present study developed a targeted investigation employing simplified silicate-based slag systems, enabling the systematic exploration of prototypical fluxing agent interactions. This methodological approach facilitates the precise elucidation of fluxing mechanisms while maintaining experimental controllability. Through controlled parametric variation in fluxing agent concentrations, the research establishes quantitative correlations between structural modifications and thermophysical property evolution, thereby advancing the fundamental understanding of fluxing agent operational principles in complex slag systems.
In this paper, a new type of fluorine-free CaO-SiO2-Na2O-based slag system is designed based on the conventional mold flux system. At the same time, the fluxing effect of the same group of oxides is considered, and the CaO-SiO2-Li2O-based slag system is compared. However, there are many kinds of alkaline components in the above slag system, and suitable components for regulating the glass properties need to be introduced. Therefore, B2O3 is introduced in this paper. The effect of B2O3 on the melt structure and viscosity of the CaO-SiO2-M2O (M=Li, Na) slag system was investigated. The research results can provide a basis for the performance optimization of conventional mold fluxes and the design and development of mold fluxes with a high casting speed and no fluorination.
2. Design of Experimental Slag System
In order to ensure the proper melting temperature of the experimental slag, the basic composition of the slag system should be designed based on the proper region with low melting temperature. In industrial continuous casting operations, mold fluxes typically exhibit a basicity range (CaO/SiO2 ratio) of 0.8~1.2. Phase diagram analysis via FactSage thermodynamic simulations for the CaO-SiO2-Na2O (Figure 1) and CaO-SiO2-Li2O (Figure 2) systems reveals that the theoretical melting temperatures can be depressed below 1300 °C when either the Na2O or Li2O content approaches 15% within this basicity range. Based on these findings, the experimental flux basicity was standardized at 1.0 (mid-range value of 0.8~1.2) with equivalent 15% additions of Na2O or Li2O. To systematically evaluate the influence of B2O3 on slag properties, on the basis of keeping the proportion of CaO, SiO2, and M2O (M = Na or Li) unchanged, supplementary B2O3 was introduced through gradient increments of the target mass fraction of 0%, 5%, 10%, and 15%, establishing comparative frameworks for both alkali-modified silicate systems. The specific composition is shown in Table 1 and Table 2.
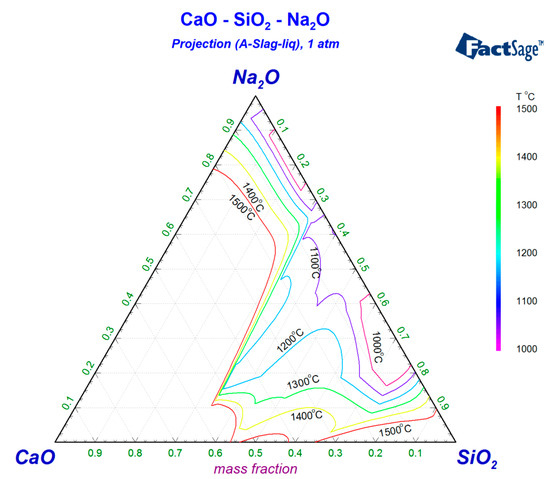
Figure 1.
Isotherms of CaO-SiO2-Na2O slag.
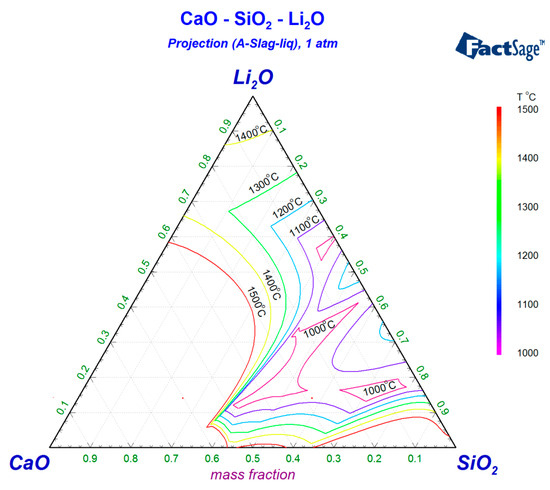
Figure 2.
Isotherms of CaO-SiO2-Li2O slag.

Table 1.
Composition of CaO-SiO2-Na2O-B2O3 slag (wt.%).

Table 2.
Composition of CaO-SiO2-Li2O-B2O3 slag (wt.%).
3. Experimental Process
3.1. Preparation of Experimental Slag
The experimental slag was prepared using analytically pure (purity more than 99.9%) chemical reagents. In order to remove water and impurities, all reagents were pretreated with high temperature calcination in a muffle furnace, and the calcination time was more than 1 h. The calcination temperature of each reagent is shown in Table 3. The treated chemical reagents were mixed evenly according to the components shown in Table 1 and Table 2, then pre-melted in a high temperature resistance furnace, and then pre-melted slag samples were prepared after water quenching, crushing, and grinding.

Table 3.
Calcination temperature of each reagent.
3.2. Melting Temperature Measurement
The melting temperature of the slag was measured using the hemispherical point method. First, the slag sample was ground to a particle size within 0.074 mm. An appropriate amount of the slag sample was taken and pressed into a cylinder with a diameter of 3 mm and a height of 3 mm. During the test process, the temperature was heated at 10 °C/min, and the corresponding temperature when the sample height reaches half of the initial height is the melting temperature. The details are presented in the previous work [32].
3.3. Viscosity Measurement
The viscosity of molten slag during the continuous cooling process was measured via a RTW-16 melt physical property comprehensive tester. The temperature was automatically controlled and measured by the controlling system through two thermal couples. The measuring temperature error was within ±5 °C. The initial calibration procedures involved determining the viscometer’s viscosity constant in ambient conditions using standardized castor oil (viscosity certified) through torque measurements at predetermined rotational speeds. Subsequent instrument calibration coefficients were established through comparative analysis with certified viscosity references (castor oil, with a viscosity of 1.514, 0.986, 0.651, and 0.451 and Pa·s at 10, 15, 20, and 25 °C, respectively). Validation tests confirmed measurement accuracy with relative errors constrained to ±1%.
In the experiment, about 140 g of pre-melting slag was placed in a graphite crucible lined with a molybdenum sheet (Figure 3) to realize the isolation of the slag and graphite crucible. The furnace was heated at 10 °C/min until the slag was completely melted and the temperature was kept for 30 min. During viscosity testing, the molybdenum hammer rotates at a constant speed of 200 revolutions/min and the furnace was cooled at −3 °C/min. The testing process is protected by Ar and the viscosity changes in the cooling process are automatically recorded by the computer-control system.
Figure 3.
Diagram of crucible structure.
The breaking temperature is defined as the critical point where viscosity exhibits abrupt changes during continuous cooling. The tangent method [33] was employed to identify the breaking temperature. At elevated temperatures, the viscosity–temperature relationship follows the Arrhenius equation closely. However, this correlation becomes invalid below a characteristic temperature threshold (the breaking temperature), resulting in distinct slope differences between the high-temperature and low-temperature regions of the viscosity–temperature curve. By constructing tangent lines for these thermally distinct zones and identifying their intersection point, the breaking temperature of the slag can be precisely determined.
3.4. Melt Structure Test
Raman spectroscopy was used to test the melt structure of mold fluxes, and a Raman spectrometer was used as the main equipment. All Raman spectra were measured at room temperature, and all the samples were the quenched pre-melted slags shown in the Section 3.1. The region of the recorded wave number is 800~1500 cm−1, which is generally related to the metallurgical slags. The wavelength of the laser is 532 nm, and the resolution is 0.65 cm−1. After the Raman spectra were obtained, different structural units were obtained using software Origin 8.5. The raw Raman spectra were processed through smoothing and baseline correction, followed by deconvolution using the Gaussian fitting methodology, with the goodness-of-fit rigorously maintained at R2 ≥ 0.99. In addition, the polymerization degree of the slag can be further calculated and analyzed by means of the Raman scattering coefficient. The Raman scattering coefficients of various (superscript i denotes the number of bridging oxygen groups, i = 0, 1, 2, 3, 4) [34,35,36] in actual silicate melt are different. By studying the Raman spectra of the silicate system, Mysen et al. defined the scattering coefficient of with reference to the NMR data, as shown in Table 4. The mole fraction of each group is calculated according to Equation (1) [37,38,39]:
where Xi is the mole fraction of , θi is the Raman scattering coefficient, and Ai is the characteristic peak area fraction. The compactness of the melt structure is reflected by the content of Xi in the different structural units.
Xi = θi Ai

Table 4.
Scattering coefficient of .
4. Results and Discussion
4.1. Change in Viscous Characteristics
Figure 4 shows the viscosity–temperature curve of the CaO-SiO2-Na2O slag with different B2O3 additions, and Figure 5 shows the breaking temperature of the CaO-SiO2-Na2O slag system and the viscosity of the mold flux at 1300 °C with different B2O3 additions. When there is no B2O3 in the slag, the viscosity and breaking temperature are higher; the breaking temperature of the mold flux is 1361 °C and the viscosity at 1300 °C is about 1.96 Pa s. With the increase in B2O3 content, the viscosity and breaking temperature of the mold flux decreased gradually. When the B2O3 content was 15%, the viscosity reached the lowest value at 1300 °C, which was 0.14 Pa·s, and the breaking temperature dropped to the lowest value, which was 1024 °C.
Figure 4.
Viscosity curves of CaO-SiO2-Na2O slags with different B2O3 additions.
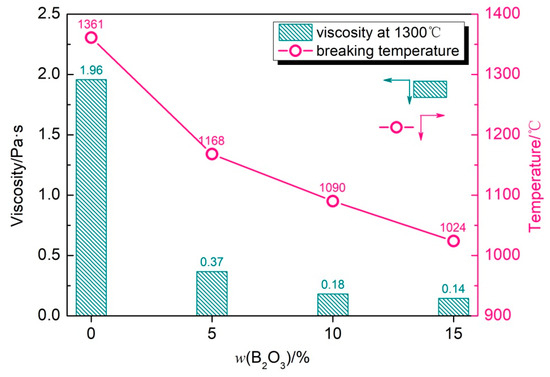
Figure 5.
Viscosity at 1300 °C and breaking temperature of CaO-SiO2-Na2O slags with different B2O3 additions.
The comparative analysis reveals that the variation pattern of the breaking temperature with the B2O3 content aligns with the trend observed for melting temperature. The hemisphere point test results demonstrate a progressive decrease in the slag melting temperature with increasing B2O3 content. Specifically, with B2O3 additions of 0%, 5%, 10%, and 15%, the corresponding melting temperatures measure 1291 °C, 1126 °C, 1026 °C, and 968 °C, respectively. Notably, all slag samples exhibit melting temperatures below their respective breaking temperatures. This inverse correlation indicates enhanced thermal stability in slags with reduced melting temperatures. In the B2O3-free CaO-SiO2-Na2O system, the pronounced crystallization tendency correlates with a relatively elevated breaking temperature.
Figure 6 shows the viscosity–temperature curve of the CaO-SiO2-Li2O slag system with different B2O3 additions, and Figure 7 shows the breaking temperature of the CaO-SiO2-Li2O slag system with different B2O3 additions and the viscosity of the mold flux at 1300 °C. From the above results, it can be concluded that the breaking temperature of the mold flux decreases obviously after adding B2O3 to the slag. When adding 5% B2O3, the breaking temperature decreases from 1050 °C to 895 °C, with a decrease range of 155 °C. However, when the content of B2O3 is further increased, the breaking temperature of the mold flux can be kept relatively stable. When the content of B2O3 is 5~15%, the breaking temperature can be stabilized in the range of 890~900 °C without significant fluctuation. The precipitation of phase in the slag is one of the main factors leading to the non-Newtonian transformation of the slag, which shows an obvious turning point on the viscosity curve. According to the phase diagram of Li2O-SiO2-B2O3, when the addition of B2O3 increases from 0 to 15%, B2O3 can combine with Li2O to form crystalline phases with a low melting point, such as Li2O·2B2O3. During the process of slag cooling, the precipitation temperature and precipitation trend of the phases decrease further, which leads to the decrease in the breaking temperature of the slag. In contrast with the changing trend at the breaking temperature, when the addition of B2O3 increases from 0 to 15%, the viscosity of the mold flux continues to increase, and the viscosity increases from 0.021 Pa·s to about 0.14 Pa·s at 1300 °C. Additionally, as can be seen from the enlarged local view in Figure 6, above 1000 °C, the viscosity of the slag as a whole shows an increasing trend with the increase in the B2O3 content. However, the degree of increase in viscosity gradually decreases.
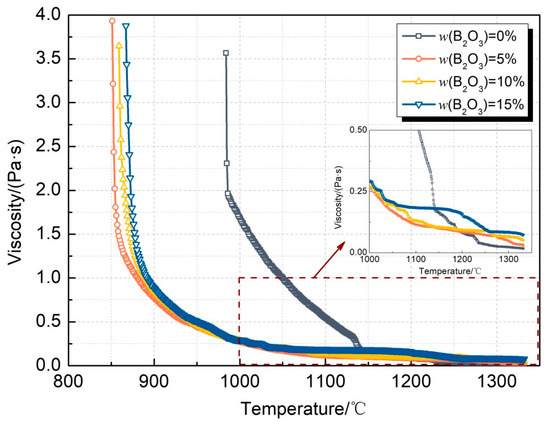
Figure 6.
Viscosity curves of CaO-SiO2-Li2O slags with different B2O3 additions.
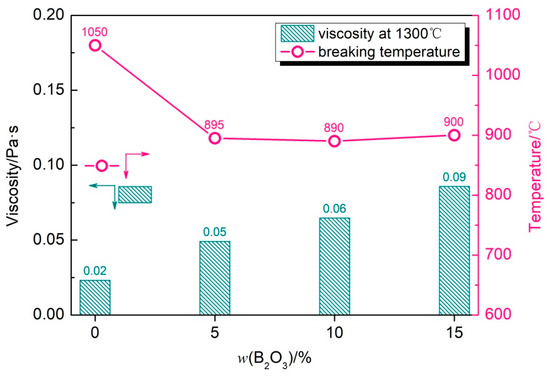
Figure 7.
Viscosity at 1300 °C and breaking temperature of CaO-SiO2-Li2O slags with different B2O3 additions.
Comparative analysis reveals that the addition of B2O3 to the CaO-SiO2-Li2O slag significantly reduces the melting temperature, mirroring the behavior observed in the CaO-SiO2-Na2O system. A coherent correlation exists between the melting temperature and breaking temperature variation trends. Progressive increases in the B2O3 content induce systematic decreases in the melting temperature, with measured values of 982 °C, 881 °C, 876 °C, and 849 °C corresponding to 0%, 5%, 10%, and 15% B2O3 additions, respectively. All tested slag compositions exhibit melting temperatures consistently below their characteristic breaking temperatures. This inverse thermal relationship demonstrates enhanced slag stability at lower melting temperatures. Notably, the B2O3-free CaO-SiO2-Li2O composition displays particularly pronounced thermal stability, despite its relatively elevated breaking temperature.
From comparing the above results, it can be seen that B2O3 has obviously different effects in the CaO-SiO2-Na2O and CaO-SiO2-Li2O slag systems. In the CaO-SiO2-Na2O slag system, B2O3 plays a significant role in reducing the viscosity and breaking temperature. When the addition of B2O3 is not higher than 10%, it can significantly reduce the viscosity. In the range of 0~15%, with the increase in B2O3 content, the viscosity of mold flux decreases first and then increases slightly, and the breaking temperature decreases continuously. When the addition of B2O3 is 10%, the viscosity reaches the lowest value at 1300 °C, which is 0.178 Pa·s. However, in the CaO-SiO2-Li2O slag system, B2O3 has an obvious effect on viscosity. The reasons for the above different effects will be analyzed in combination with the melt structure analysis.
4.2. Microscopic Analysis of Slag Structure
Figure 8 shows the Raman spectra curves of the CaO-SiO2-Na2O slag system with different B2O3 contents. From the test curves, it can be seen that there are different absorption peaks in different wave numbers. Among them, there is SiO4 tetrahedron in the range of 800~1200 cm−1 and a two-dimensional BO3 unit in the range of 1150~1450 cm−1. SiO4 tetrahedron is the main network former of slags in the above-mentioned structural units, and the increase in the proportion of the structural units will lead to an increase in the complexity of the slag network structure. The two-dimensional BO3 unit is the main network modifier, and an increase in the proportion of this structural unit will depolymerize the network structure and reduce the degree of network aggregation. According to the experimental results, with the addition of B2O3 in slags, two-dimensional BO3 units are formed in each slag. Moreover, with the increase in B2O3 content, the characteristic peak height and relative peak area of the two-dimensional BO3 unit increase. When the content of B2O3 reaches 15%, the relative area of the BO3 unit gradually tends to be stable. In addition, with the addition of B2O3 and the increase in B2O3 content, the highest wave peak of the SiO4 tetrahedral unit gradually migrates to the lower wave number region. Based on the above results, it can be preliminarily determined that the complexity of the slag network structure decreases gradually with the addition of B2O3.
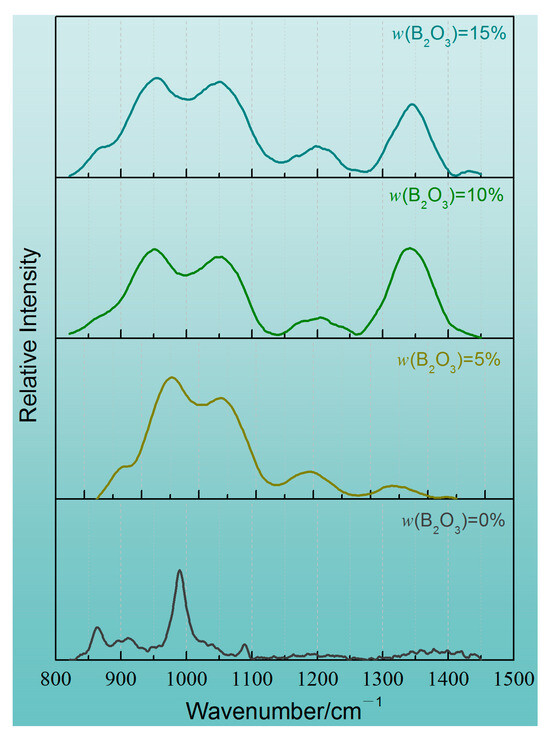
Figure 8.
Raman spectra of CaO-SiO2-Na2O slags with different B2O3 additions after smoothing to baseline.
The aggregation degree of SiO4 tetrahedron can show the change law of the network structure more directly. Figure 9 shows the Gaussian spectrum results of the SiO4 tetrahedral units in the CaO-SiO2-Na2O slag system with different B2O3 contents in the range of 800~1200 cm−1, Table 5 shows the distribution of structural units in different polymerization forms, and Figure 10 shows the mole fractions of different polymers. From the analysis of the test results and spectrum results, it can be seen that with the addition of B2O3, the proportion of structural units with a low degree of polymerization is higher. After adding B2O3, both and change to in the structural units with a lower polymerization degree. With the increase in B2O3 content, the number of free O2− ions in slags decreases due to the formation of two-dimensional BO3 units, and some change to . However, the depolymerization effect of the two-dimensional BO3 units is stronger. With the increase in two-dimensional BO3 units, a large number of structural units with a high degree of polymerization are depolymerized and is generated.
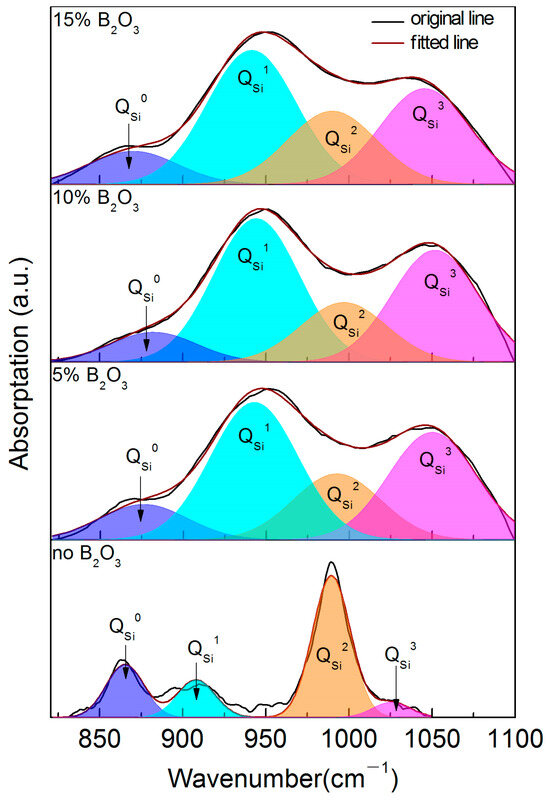
Figure 9.
Gaussian spectral solution results of CaO-SiO2-Na2O-B2O3 slag system.

Table 5.
Statistical results of different structural units of CaO-SiO2-Na2O-B2O3 slags.
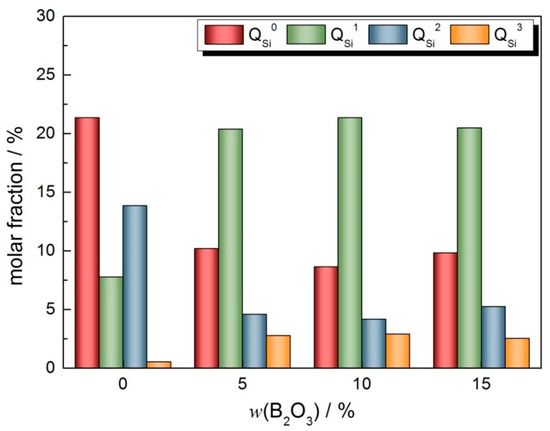
Figure 10.
Variation in mole fraction of in CaO-SiO2-Na2O-B2O3 slag system.
Combined with the change trend of viscosity, it can be seen that the degree of polymerization of the mold flux decreases gradually with the increase in the B2O3 addition. But the main reasons for this are different. When the amount of B2O3 increased from 0 to 10%, the area fraction of the BO3 units corresponding to 1150~1450 cm−1 increased, and B2O3 combined preferentially with O2- ions to form BO3 structural units. The distribution of the BO3 structure units is two-dimensional lamellar, which can effectively reduce the complexity of the three-dimensional network structure of the slag, and the peak value of spectral lines moves to the low-frequency region as a whole, thus reducing the degree of polymerization of the slag. When the addition of B2O3 is continuously increased to 15%, except for the two-dimensional BO3 structural units, the low degree of polymerization units is mainly composed of in different polymers of SiO4 tetrahedron, which lead to the solution of the slag network structure and the decrease in viscosity.
The Raman spectra and analysis results of the CaO-SiO2-Li2O slag system with different B2O3 addition amounts are shown in Figure 11, Figure 12 and Figure 13 and Table 6. From the test results, it can be seen that the main structural elements of the slag are still SiO4 tetrahedron and two-dimensional BO3 units. In the CaO-SiO2-Li2O slag system without any B2O3 addition, the envelope peaks in the spectral test curves are mainly concentrated in the low-wave number region around 850 cm−1. It can be concluded that, unlike in the CaO-SiO2-Na2O slag system, when the same mass fraction of Li2O is added to the slag, more free oxygen ions will be produced in the slag, and the network of the slag will be depolymerized in large quantities. The SiO4 tetrahedron is mainly composed of the unit with the lowest polymerization degree, and its mole fraction is close to 70%.
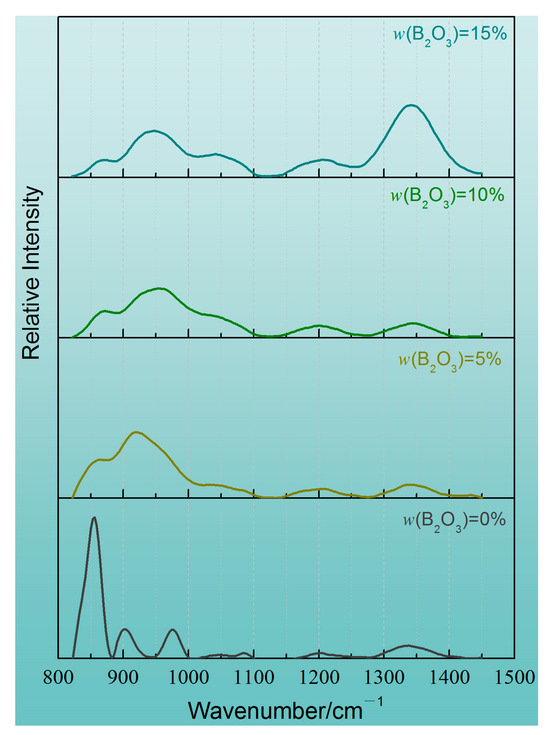
Figure 11.
Raman spectra of CaO-SiO2-Li2O slags with different B2O3 additions after smoothing to baseline.
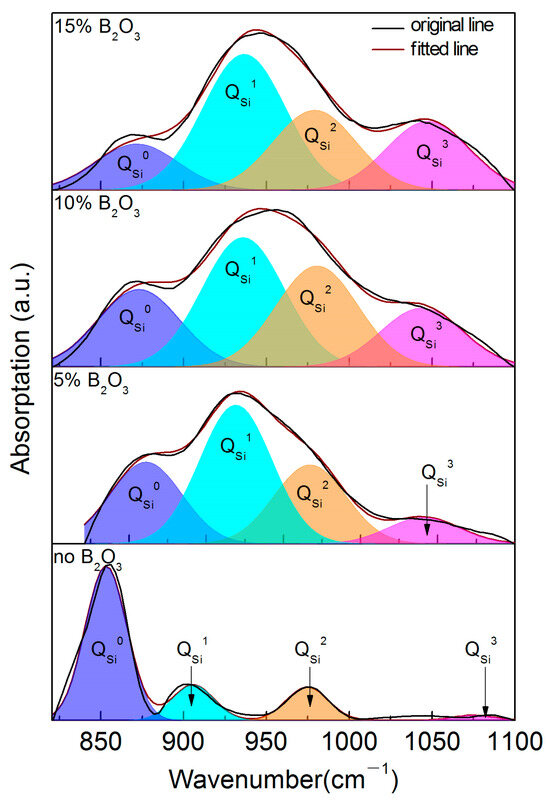
Figure 12.
Gaussian spectral solution results of CaO-SiO2-Li2O-B2O3 slag system.
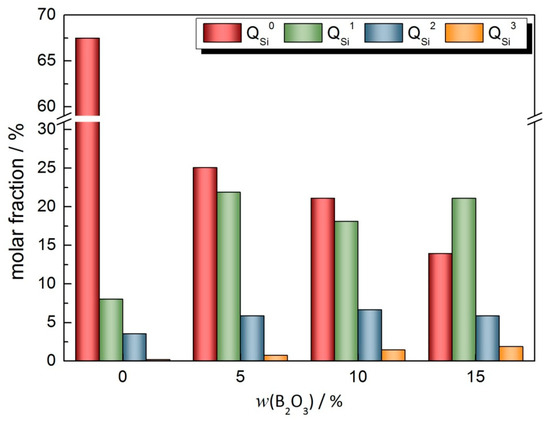
Figure 13.
Variation in mole fraction of in CaO-SiO2-Li2O-B2O3 slag system.

Table 6.
Statistical results of different structural units of CaO-SiO2-Li2O-B2O3 slags.
Because the network polymer is depolymerized in large quantities, the instability of the slag network structure is enhanced. In this case, with the addition of B2O3 in the slag, B2O3 combined with a large number of free O2− ions in the slag to form a two-dimensional BO3 unit. Moreover, with the increase in the B2O3 addition in the slag, the proportion of the BO3 unit increases greatly. However, the two-dimensional structure of the BO3 unit weakens the three-dimensional structure of the slag network. The further increase in two-dimensional BO3 units will affect the instability of the network structure. At this time, from the point of view of maintaining the dynamic balance of the network structure, SiO4 tetrahedral structural units with different degrees of polymerization will undergo dynamic recombination. At this time, a large number of structural units with a low degree of polymerization will polymerize, and the proportion of structural units with a high degree of polymerization will gradually increase. In this process, with the increase in the B2O3 content, the mole fraction of the structural units will decrease significantly. When the content of B2O3 reaches 15%, the proportion of BO3 structural units increases obviously with the increase in the B2O3 content in the slag and O2− ions produced by the polymerization of units with a low degree of polymerization, such as .
It can be seen from the above results that with the increase in the B2O3 addition in the CaO-SiO2-Li2O slag system, the two-dimensional depolymerization network and tetrahedral polymerization of SiO4 exist simultaneously in the slag. Combined with the viscosity change law, it can be seen that the viscosity of the mold flux increases as a whole. Therefore, in order to maintain the stability of the network structure, when the addition of the B2O3 is increased from 0 to 15%, the relative proportion of network modifiers in the mold flux will increase obviously, which will lead to the instability of the slag network structure. Combined with the spectral results, it can be seen that the relative proportions of structural units with different degrees of polymerization in the network former of slag change, which effectively balances the instability of the network structure caused by an excessive increase in the proportion of network modifiers. The polymerization of the SiO4 tetrahedral structure is stronger than the depolymerization of the two-dimensional BO3 unit.
As shown in Figure 13 and Table 6, when the addition of the B2O3 is increased from 0 to 15%, the mole fraction of the unit in the slag reaches about 15%, and its proportion decreases by about 80%. The proportion of the units with a higher degree of polymerization reaches about 23%, which is about three times higher. Under the comprehensive action of the above changes, the network structure tends to be tight, thus effectively improving the degree of polymerization of the mold flux. Furthermore, the viscosity of the mold flux increases with the increase in the B2O3 addition.
By comparing the above results, it can be concluded that B2O3 plays different roles in the CaO-SiO2-Na2O slag system and CaO-SiO2-Li2O slag system. In the CaO-SiO2-Na2O slag system, the proper addition of B2O3 can effectively increase the relative proportion of network modifiers and low degree of polymerization network-forming unit, and then reduce the degree of polymerization and viscosity of the slag. In the CaO-SiO2-Li2O slag system, increasing the amount of B2O3 will increase the instability of the network structure because of the large increase in two-dimensional structural units, which can effectively promote the formation of a high degree of polymerization network-former structure, and then increase the degree of polymerization of the mold flux and the viscosity of the slag.
5. Conclusions
The effect of B2O3 on the viscous characteristics of the CaO-SiO2-M2O (M = Li, Na) slag system was studied experimentally, and the melt structure was experimentally determined using Raman spectral analysis technology. The spectral curve was analyzed via the Gaussian fitting method, and the effect of B2O3 on the melt structures of different slag systems was discussed. Under the conditions of the slag systems in this experiment, the following conclusions are obtained:
- (1)
- In the CaO-SiO2-Na2O slag system, with the increase in the B2O3 addition, the viscosity and breaking temperature of the slag show a gradually decreasing trend. When the B2O3 addition is 15%, the viscosity reaches the lowest value at 1300 °C, which is 0.14 Pa·s. The breaking temperature drops to the lowest value, 1024 °C;
- (2)
- In the CaO-SiO2-Na2O slag system, the polymerization degree of the mold flux decreases gradually with the increase in the addition of B2O3. When the addition of B2O3 increases from 0 to 10%, the increase in two-dimensional BO3 structural units plays a dominant role. When the addition of B2O3 is increased to 15%, the network depolymerization is influenced by the increase in BO3 structure units and the low degree of polymerization units of different polymers of SiO4 tetrahedron;
- (3)
- In the CaO-SiO2-Li2O slag system, the inflection point temperature of the slag after adding B2O3 is significantly reduced, and the inflection point temperature is reduced by 155 °C when 5% B2O3 is added. However, with the further increase in the B2O3 content, the breaking temperature of the slag can remain relatively stable, without any significant fluctuation. The CaO-SiO2-Li2O slag system has a lower breaking temperature, due to the formation of phases such as low-melting-point Li2O·2B2O3;
- (4)
- In the CaO-SiO2-Li2O slag system, the initial degree of depolymerization of the network is high. In order to maintain the stability of the network structure, when the addition of B2O3 increases from 0 to 15%, the relative proportion of the network modifier structural units in the protection slag will significantly increase, resulting in the enhanced instability of the network structure. The effect of SiO4 tetrahedral structure polymerization is stronger than that of two-dimensional BO3 unit depolymerization.
Author Contributions
Conceptualization, J.Q.; data curation, Y.S. and Y.D.; investigation, J.W., J.Q. and Y.S.; software, J.W. and Y.D.; supervision, C.L.; writing—original draft, J.W. and J.Q.; writing—review and editing, J.Q. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support of the Science and Technology Project of Liaoning Province (2023JH2/101700347) and the National Natural Science Foundation of China (51904064).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 2024 World Steel in Figures. Available online: https://worldsteel.org/wp-content/uploads/World-Steel-in-Figures-2024.pdf (accessed on 1 February 2025).
- Mills, K.C.; Däcker, C.-Å. The Casting Powders Book; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Zheng, Y.; Qi, J.; Zheng, X.; Liu, C.; Jiang, M. Dissolution behavior of CeAlO3 in mold fluxes with different w(CaO)/w(Al2O3). J. Iron Steel Res. 2023, 35, 1092–1099. [Google Scholar]
- Mills, K.C. A short history of mould powders. Ironmak. Steelmak. 2017, 44, 326–332. [Google Scholar] [CrossRef]
- Brandaleze, E.; Di Gresia, G.; Santini, L.; Martín, A.; Benavidez, E. Mould Fluxes in the Steel Continuous Casting Process; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef][Green Version]
- Dong, Y.N.; Bai, Y.; Shen, Y.; Wang, P.; Chen, B.; Wang, P. Mechanism and control measures of tail surface cracks near corners of peritectic steel slab castings. J. Iron Steel Res. 2024, 36, 736–742. [Google Scholar]
- Chen, S.J.; Shen, Y.Y.; Yan, W.; Wang, Y.; Li, D. Prediction of viscosity and melting temperature for mold fluxes based on machine learing algorithms. Contin. Cast. 2024, 43, 63–72. [Google Scholar]
- Wang, R.R.; Wang, M.; Wang, L.H.; Zhao, J.; Wang, Q.; Wang, Z. Research progress on mechanism and prediction model of continuous casting sticker breakout of automotive steel. Contin. Cast. 2024, 1–13. [Google Scholar] [CrossRef]
- Gu, S.; Yu, L.; Wen, G.; Tang, P.; Wang, Z.; Gao, Z. Qualitative, Quantitative and Mechanism Research of Volatiles in the Most Commonly Used CaO–SiO2–CaF2–Na2O Slag During Casting Process. Trans. Indian Inst. Met. 2021, 74, 775–782. [Google Scholar] [CrossRef]
- Long, X.; Long, S.; Luo, W.; Li, X.; Tu, C.; Na, Y.; Xu, J. Crystallization of Slag Films of CaO-Al2O3-BaO-CaF2-Li2O- Based Mold Fluxes for High-Aluminum Steels’ Continuous Casting. Materials 2023, 16, 1903. [Google Scholar] [CrossRef]
- Han, F.; Yu, L.; Wen, G.; Guo, J.; Ran, C.; Gu, S. Effect of Na2O on the Sintering and Melting Behavior of CaO-SiO2-CaF2 Slag. J. Mater. Res. Technol. 2022, 19, 866–876. [Google Scholar] [CrossRef]
- Mo, R.; He, W.; Li, Z.; Ren, Y.; Zhang, L. Influence of BaO and MgO on viscosity, crystalline phase, and structure of CaO–Al2O3–10%SiO2–20%CaF2-based system. Ceram. Int. 2023, 49, 27311–27326. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Jiang, M. Effect of Fluorine on Melt Structure for CaO-SiO2-CaF2 and CaO-Al2O3-CaF2 by Molecular Dynamics Simulations. ISIJ Int. 2020, 60, 2176–2182. [Google Scholar] [CrossRef]
- Long, X.; Luo, W.; Li, X.; Long, S.; Ma, H.; Luo, D.; Zheng, C. Structure and Heat Transfer Characteristic Evolution of CaO-SiO2 -CaF2 -Based Solid Mold Flux Film upon Solidification. Metals 2024, 14, 1. [Google Scholar] [CrossRef]
- Lao, Y.; Gao, Y.; Wang, Q.; Li, G. Investigation on surface tension of CaF2–CaO–SiO2 (–MgO–Al2O3) melts. Metall. Res. Technol. 2024, 121, 518. [Google Scholar] [CrossRef]
- Seo, M.S.; Sohn, I. Volatilization of fluorine in a CaO–SiO2–CaF2-based system with the substitution of Na2O with K2O. J. Am. Ceram. Soc. 2022, 105, 6320–6334. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zhang, D.; Feng, W.H.; Liu, L.; Han, X.; Liu, Z. Research progress on crystallization properties of protectine slag for fluorin free continuous casting mold. Gansu Metall. 2023, 45, 56–61. [Google Scholar]
- Yan, X.P.; Liu, L.; Han, X.L.; Wang, W.; Zhang, D. Effect of boron on microstructure of fluorine-free mold fluxes. Iron Steel 2022, 57, 72–80. [Google Scholar]
- Zhao, K. Effect of Borax on Properties of Fluorine-Free Mold Flux and Mineralogical Constitution of Slag Film. Master’s Thesis, North China University of Science and Technology, Qinhuangdao, China, 2021. [Google Scholar]
- Zhao, C.B.; Li, X.Y.; Xu, J.Y.; Chen, Y.; Li, D. Development and Application of Fluorine—Free Mould Fluxes for Peritectic Steel Billet Continuous Casting. Wide Heavy Plate 2020, 26, 15–20. [Google Scholar]
- Chen, Y.Y.; Li, X.Y.; Xu, J.Y.; Zhao, C.; Qin, J. Development and Application of Fluorine Free Mould Powder for Low Carbon Steel Slab Continuous Casting. Wide Heavy Plate 2019, 25, 26–29. [Google Scholar]
- Wang, X.J.; Wu, B.B.; Zhu, L.G.; Fan, Y.; Tian, K. Study on Rheological Properties of Fluorine-free Continuous Casting Mould Powder. Iron Steel Vanadium Titan. 2017, 38, 135–139. [Google Scholar]
- Ding, M.T. Development of fluoride-free mold powder. Steelmaking 2013, 29, 57–59. [Google Scholar]
- Cao, J.; Li, Y.; Lin, W.; Che, J.; Zhou, F.; Tan, Y.; Li, D.; Dang, J.; Chen, C. Assessment of Inclusion Removal Ability in Refining Slags Containing Ce2O3. Crystals 2023, 13, 202. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Cui, Y.; Zhang, X.; He, S. Viscosity and structure of CaO–BaO–SiO2–Al2O3-based mold slags for continuous casting of high titanium steel with different TiO2 absorption. J. Iron Steel Res. Int. 2024, 31, 1936–1946. [Google Scholar]
- Sun, R.; Wu, J.; Wang, H.; Zhang, W.; Liu, C.; Gu, S.; Xing, W.; Yu, L. Reaction mechanism between carbon and CaO–SiO2–Al2O3–Na2O slag system during continuous casting process. J. Mater. Res. Technol. 2024, 30, 8882–8893. [Google Scholar] [CrossRef]
- Hruška, B.; Svoboda, R.; Nowicka, A.; Michálková, J.; Pecušová, B.; Michalík, J.; Chromčíková, M. Systematic assessment of the compositional dominance over physico-chemical properties in the SiO2-Al2O3-B2O3 based glassy networks. J. Non-Cryst. Solids 2025, 652, 123414. [Google Scholar] [CrossRef]
- Bi, Z.; Li, K.; Jiang, C.; Zhang, J.; Ma, S.; Sun, M.; Wang, Z.; Li, H. Performance and Transition Mechanism from Acidity to Basicity of Amphoteric Oxides (Al2O3 and B2O3) in SiO2-CaO-Al2O3-B2O3 system: A Molecular Dynamics Study. Ceram. Int. 2021, 47, 12252–12260. [Google Scholar] [CrossRef]
- Wu, X.X.; Xi, Z.H.; He, S.P.; Wang, Q.; Zhang, X. Exploration of Li2O reduction by mold flux for high quality low carbon steel slab continuous casting. Iron Steel 2023, 58, 110–119. [Google Scholar]
- Wang, Z.C.; Zeng, J.; Dou, K.; Wang, W.; Lin, H.; Liu, X. Analysis of comprehensive physical and chemical properties of medium and high carbon steel mold flux in thin slab. Contin. Cast. 2023, 27–32. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, K.; Chen, B.; Qian, J.; Wang, X.; Zhu, L. Prediction of viscosity of mold fluid-free protective slag based on improved whale optimization algorithm-extreme learning machine. Sci. Technol. Eng. 2024, 24, 14614–14622. [Google Scholar]
- Qi, J.; Liu, C.J.; Yang, D.P.; Zhang, C.; Jiang, M.F. Study of a new mold flux for heat-resistant steel containing cerium continuous casting. Steel Res. Int. 2016, 87, 890–898. [Google Scholar] [CrossRef]
- Jie, Q.I.; Chengjun, L.I.U.; Chunlong, L.I.; Jiang, M. Viscous properties of new mould flux based on aluminate system with CeO2 for continuous casting of RE alloyed heat resistant steel. J. Rare Earths 2016, 34, 328–335. [Google Scholar]
- Mysen, B.O.; Finger, L.W.; Seifert, F.A.; Virgo, D. Curve-fitting of Raman spectra of amorphous materials. Am. Miner. 1982, 67, 686. [Google Scholar]
- McMillan, P.F.; Wolf, G.H.; Poe, B.T. Vibrational spectroscopy of silicate liquids and glasses. Chem. Geol. 1992, 96, 351. [Google Scholar] [CrossRef]
- Mysen, B.O. Structure and properties of magmatic liquids: From haplobasalt to haploandesite. Geochim. Cosmochim. Acta 1999, 63, 95. [Google Scholar] [CrossRef]
- Mysen, B.O.; Frantz, J.D. Silicate melts at magmatic temperatures: In-situ structure determination to 1651 C and effect of temperature and bulk composition on the mixing behavior of structural units. Contrib. Mineral. Petrol. 1994, 117, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Jiang, G.C.; You, J.L.; Hou, H.Y.; Chen, H. Raman scattering coefficients of symmetrical stretching modes of microstructural units in sodium silicate melts. Acta Phys. Sin. 2005, 54, 961. [Google Scholar]
- Min, Y.; Zhong, M.; Huang, J.; Liu, C.; Jiang, M. Structural behavior of Al3+ in mould flux glasses of CaO–SiO2–Al2O3–Na2O–CaF2 system. Steel Res. Int. 2014, 85, 1194–1199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).