Influence of Heat Treatment Temperature on the Electrochemical Properties of Cold-Rolled 0.2%C–3%Al–6/8.5%Mn–Fe Medium-Manganese Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results
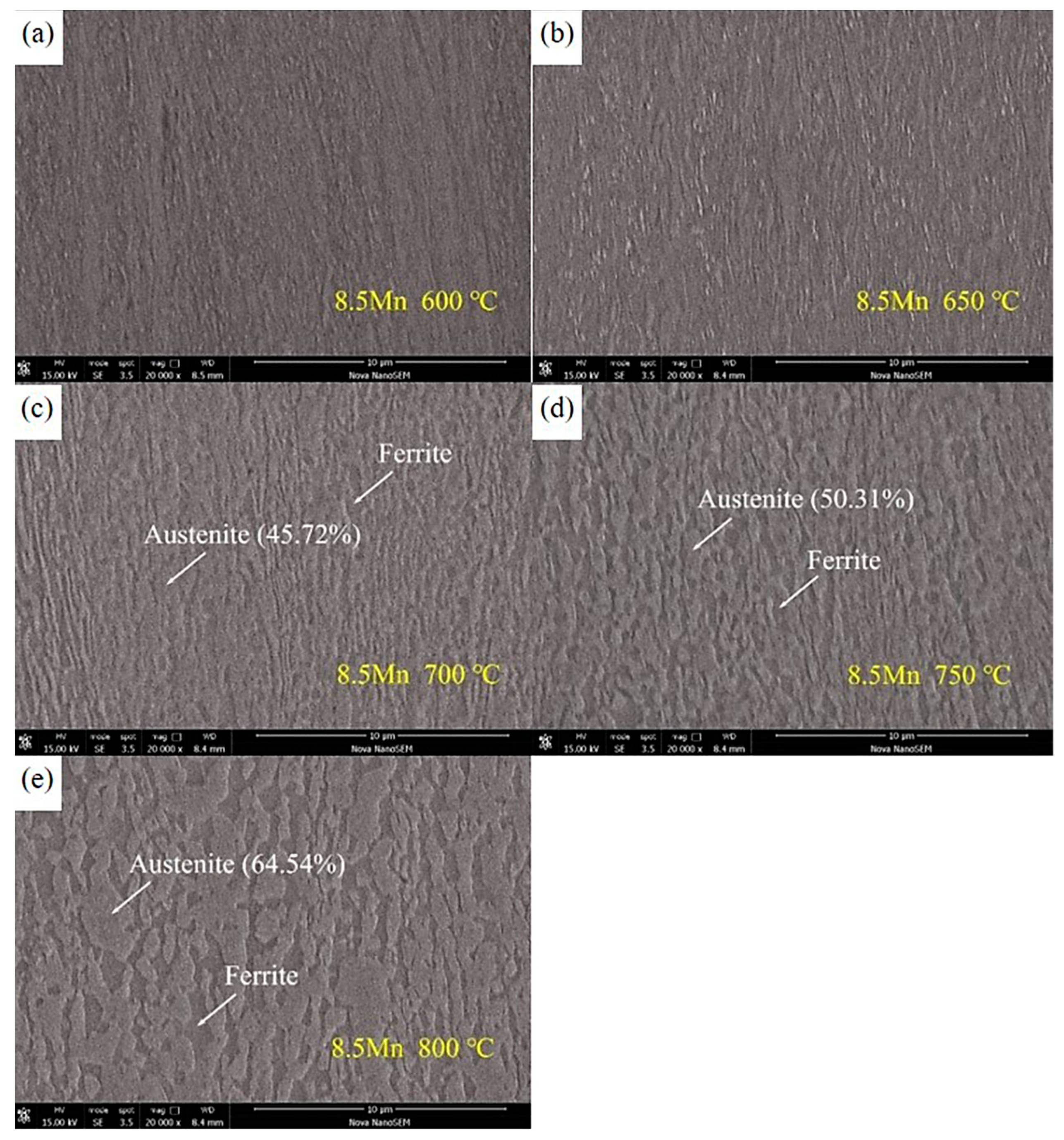
3.1. Microstructure Evolution
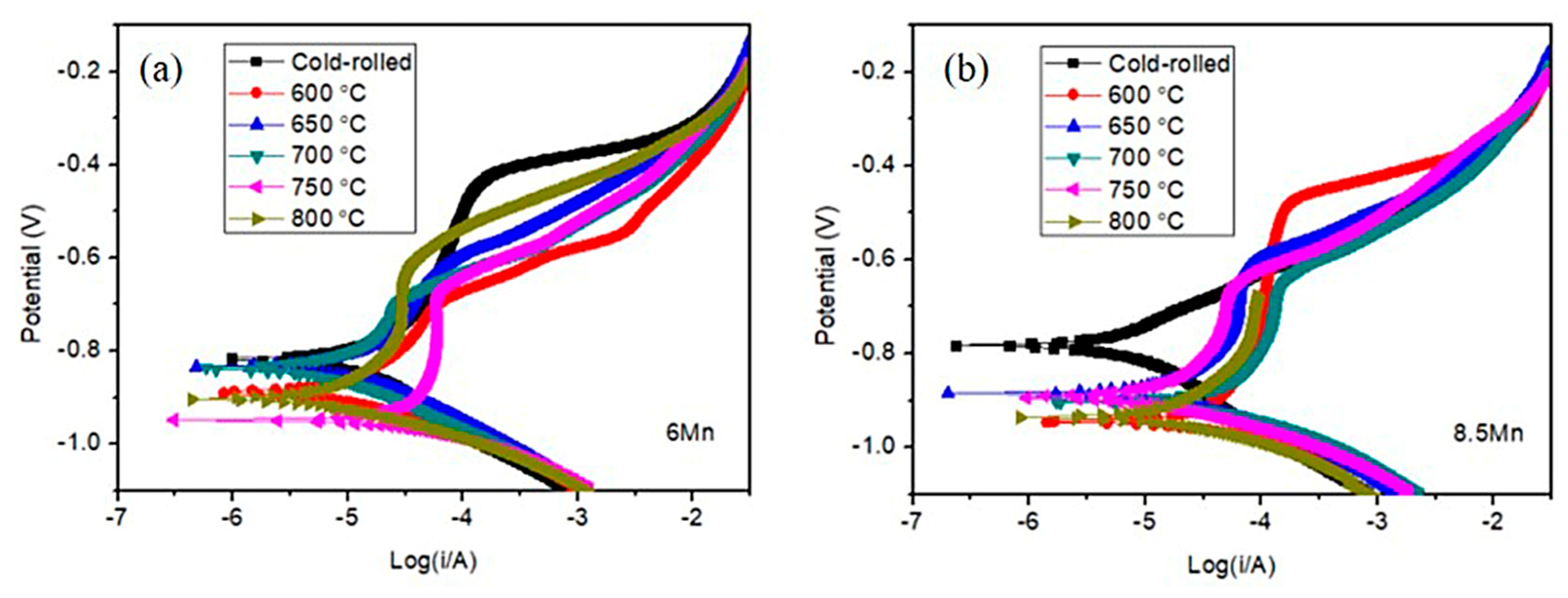
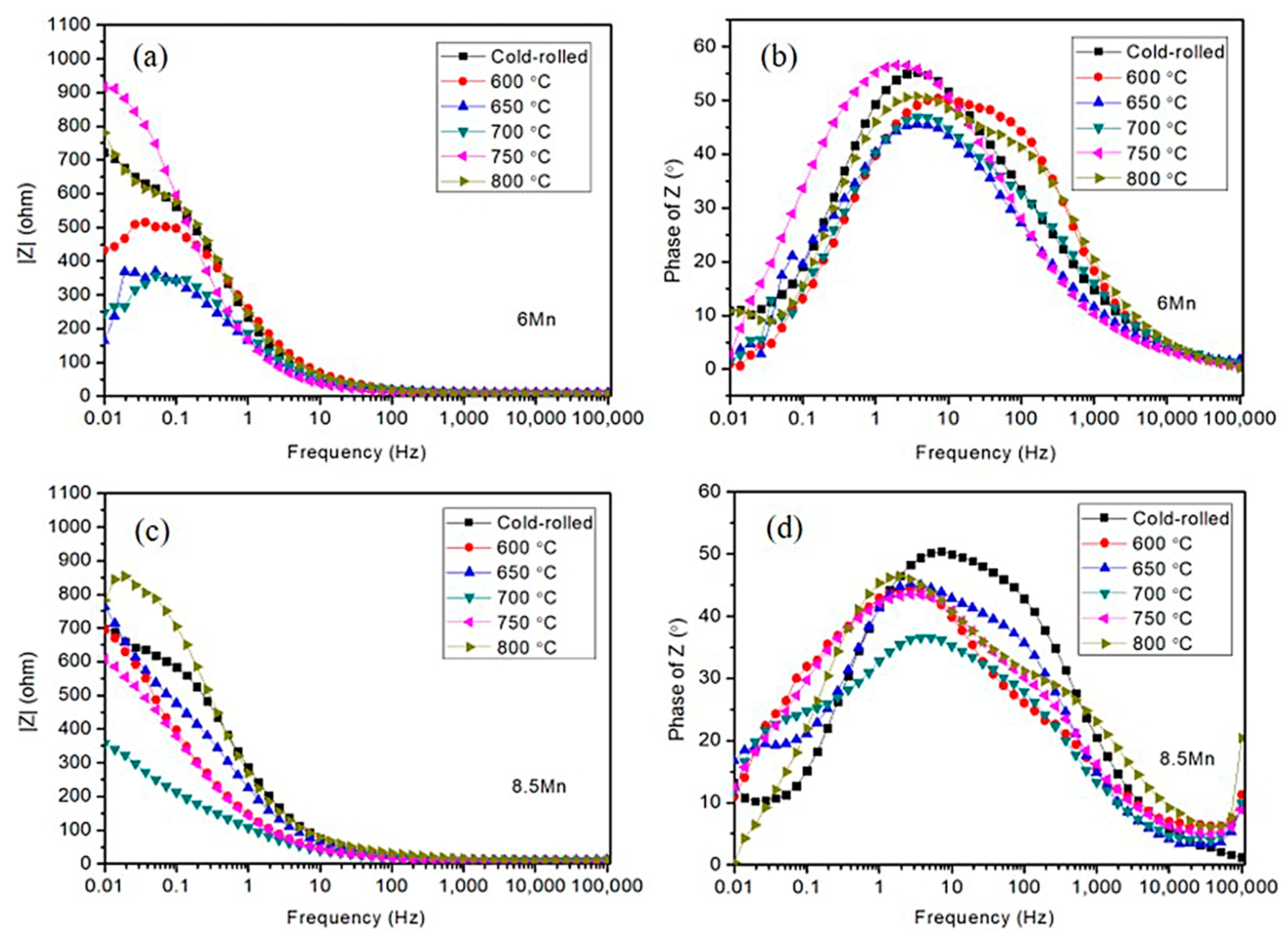
3.2. Electrochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohapatra, S.; Poojari, G.; Das, S.; Das, K. Insights into the dynamic impact behavior of intercritically annealed automotive-grade Fe–7Mn–4Al−0.18C steel. Mat. Sci. Eng. A 2023, 887, 145769. [Google Scholar] [CrossRef]
- Leták, R.; Jirková, H.; Kučerová, L.; Jeníček, Š.; Volák, J. Effect of forming and heat treatment parameters on the mechanical properties of medium manganese steel with 5% Mn. Materials 2023, 16, 4340. [Google Scholar] [CrossRef]
- Dykas, J.; Samek, L.; Grajcar, A.; Kozłowska, A. Modelling of phase diagrams and continuous cooling transformation diagrams of medium manganese steels. Symmetry 2023, 15, 381. [Google Scholar] [CrossRef]
- Liu, T.; Dong, Y.; Qin, D.; Wu, H.; Gao, X.; Du, L. Effect of rolling temperature on microstructure and mechanical properties of medium manganese steel. Mat. Sci. Eng. A 2023, 863, 144547. [Google Scholar] [CrossRef]
- Mou, Y.; Li, Z.; Zhang, X.; Misra, D.; He, L.; Li, H. Design of an effective heat treatment involving intercritical hardening for high strength/high elongation of 0.2C–3Al–(6–8.5)Mn–Fe TRIP steels: Microstructural evolution and deformation behavior. Metals 2019, 9, 1275. [Google Scholar] [CrossRef]
- Hu, Z.; Fu, H. Effect of Si Content on Microstructure and Properties of Low-Carbon Medium-Manganese Steel after Intercritical Heat Treatment. Metals 2024, 14, 675. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Mou, Y.; Cai, Z.; Misra, D.; Zhang, X.; Li, H. The significance of microstructural evolution on governing impact toughness of Fe–0.2C–6Mn–3Al medium-Mn TRIP steel studied by a novel heat treatment. Int. J. Mater. Res. 2021, 112, 271–279. [Google Scholar] [CrossRef]
- Qiao, Y.; Zheng, Z.; Yang, H.; Long, J.; Han, P. Recent progress in microstructural evolution, mechanical and corrosion properties of medium-Mn steel. J. Iron Steel Res. Int. 2023, 30, 1463–1476. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Mou, Y.; Cai, Z.; Misra, D.; He, L.; Li, H.; Ding, H. Design of an effective heat treatment involving intercritical hardening for high-strength–high elongation of 0.2C–1.5Al–(6–8.5)Mn-Fe TRIP steels: Microstructural evolution and deformation behavior. Mater. Sci. Tech. 2020, 36, 500–510. [Google Scholar] [CrossRef]
- Yan, X.; Kang, S.; Xu, M.; Li, P. Corrosion Product Film of a Medium-Mn Steel Exposed to Simulated Marine Splash Zone Environment. Materials 2021, 14, 5652. [Google Scholar] [CrossRef]
- Mohapatra, S.; Palai, D.; Satpathy, B.; Das, S.; Das, K. Electrochemical study of intercritically annealed Fe–0.18C–7Mn–4Al steel. Mater. Today Commun. 2023, 34, 105282. [Google Scholar] [CrossRef]
- Su, G.; Yu, C.; Zheng, H.; Gao, X.; Xie, H.; Huo, M.; Wu, H.; Xu, J.; Du, L.; Jiang, Z. The wet–dry cycling corrosion behavior of low-carbon medium manganese steel exposed to a 3.5% NaCl solution environment. J. Mater. Eng. Perform. 2022, 31, 7856–7869. [Google Scholar] [CrossRef]
- Choudhary, S.; Nanda, V.; Shekhar, S.; Garg, A.; Mondal, K. Effect of microstructural anisotropy on the electrochemical behavior of rolled mild steel. J. Mater. Eng. Perform. 2017, 26, 185–194. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L. Effects of cold deformation on electrochemical corrosion behaviors of 304 stainless steel. Anti-Corros. Methods Mater. 2017, 64, 252–262. [Google Scholar] [CrossRef]
- Su, G.; Gao, X.; Du, L.; Zhang, D.; Hu, J.; Liu, Z. Influence of Mn on the corrosion behaviour of medium manganese steels in a simulated seawater environment. Int. J. Electrochem. Sci. 2016, 11, 9447–9461. [Google Scholar] [CrossRef]
- Bara, M.; Niedźwiedź, M.; Skoneczny, W. Influence of anodizing parameters on surface morphology and surface-free energy of Al2O3 layers produced on EN AW-5251 Alloy. Materials 2019, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Poling, W.A.; Moor, D.E.; Speer, J.G.; Findley, K.O. Temperature effects on tensile deformation behavior of a medium manganese TRIP Steel and a quenched and partitioned steel. Metals 2021, 11, 375. [Google Scholar] [CrossRef]
- Arafin, M.A.; Szpunar, J.A. A new understanding of intergranular stress corrosion cracking resistance of pipeline steel through grain boundary character and crystallographic texture studies. Corros. Sci. 2009, 51, 119–128. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, N.; Zou, J.; Lin, X.; Liu, Z.; Yuan, S.; Yu, Y.; Wang, Z.; Zeng, Q.; Chen, W.; et al. Super-hydrophobicity and corrosion resistance of laser surface textured AISI 304 stainless steel decorated with Hexadecyltrimethoxysilane (HDTMS). Opt. Laser. Technol. 2020, 127, 106146. [Google Scholar] [CrossRef]
- Shkatulyak, N.M.; Tkachuk, O.M. A role played by the crystallographic texture in the process of corrosion of hot-rolled rods made of carbon steel. Mater. Sci. 2012, 48, 153–161. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.; Cheng, X.; Li, X. Comparative effect of (111) and (110) crystallographic orientation on the passive behavior of low alloy steels in bicarbonate solution. Appl. Surf. Sci. 2021, 561, 150066. [Google Scholar] [CrossRef]
- Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Effect of grain size on the corrosion resistance of low carbon steel. Mater. Res. Express 2020, 7, 016522. [Google Scholar] [CrossRef]
- Mishra, R.; Balasubramaniam, R. Effect of nanocrystalline grain size on the electrochemical and corrosion behavior of nickel. Corros. Sci. 2004, 46, 3019–3029. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Y.; Zuo, H.; Feng, J.; Li, Z.; Cai, Z.; He, L.; Li, H. Microstructure evolution and mechanical properties of light medium manganese steel: Different rolling directions during warm stamping. J. Mater. Eng. Perform. 2024, 33, 14119–14133. [Google Scholar] [CrossRef]
- Li, Z.; Ding, H.; Misra, R.D.K.; Cai, Z.; Li, H. Microstructural evolution and deformation behavior in the Fe–(6,8.5)Mn–3Al–0.2C TRIP steels. Mat. Sci. Eng. A 2016, 672, 161–169. [Google Scholar] [CrossRef]
- Tsai, W.; Chen, J. Galvanic corrosion between the constituent phases in duplex stainless steel. Corros. Sci. 2007, 49, 3659–3668. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, W.; Dai, X.; Zhang, P.; Wang, Y. Prominent role of reversed austenite on corrosion property of super 13Cr martensitic stainless steel. J. Mater. Res. Technol. 2023, 22, 1753–1767. [Google Scholar] [CrossRef]
- Hu, B.; Zheng, Q.; Lu, Y.; Jia, C.; Liang, T.; Zheng, C. Stabilizing austenite via intercritical Mn partitioning in a medium Mn steel. Scr. Mater. 2023, 225, 115162. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, C.; Wang, Y.; Liu, C.; Yao, Y.; Zhang, J.; Yang, Z.; Zhang, C.; Liu, Y.; Chen, H. Mechanistic role of Mn heterogeneity in austenite decomposition and stabilization in a commercial quenching and partitioning steel. Acta Mater. 2023, 250, 118869. [Google Scholar] [CrossRef]
- Bernard, M.; Golf, A.H.; Thi, B.V. Electrochromic reactions in manganese oxides. J. Electrochem. Soc. 1993, 140, 3065–3070. [Google Scholar] [CrossRef]


| Steel | C | Al | Mn | Fe |
|---|---|---|---|---|
| 6 Mn | 0.20 | 3.20 | 6.0 | 90.60 |
| 8.5 Mn | 0.19 | 3.11 | 8.4 | 88.30 |
| Samples | Ecorr (V) (Mode) | Icorr (A/cm2) | Epit (V) (Mode) |
|---|---|---|---|
| Cold-rolled | −0.82 | 1.349 × 10−5 | −0.44 |
| 600 °C | −0.89 | 1.0 × 10−5 | −0.70 |
| 650 °C | −0.84 | 1.288 × 10−5 | −0.65 |
| 700 °C | −0.86 | 1.023 × 10−5 | −0.70 |
| 750 °C | −0.93 | 3.548 × 10−5 | −0.68 |
| 800 °C | −0.90 | 9.120 × 10−6 | −0.64 |
| Samples | Ecorr (V) (Mode) | Icorr (A/cm2) | Epit (V) (Mode) |
|---|---|---|---|
| Cold-rolled | −0.76 | 6.025 × 10−6 | - |
| 600 °C | −0.92 | 7.943 × 10−5 | −0.49 |
| 650 °C | −0.88 | 1.995 × 10−5 | −0.62 |
| 700 °C | −0.90 | 4.786 × 10−5 | −0.65 |
| 750 °C | −0.89 | 1.445 × 10−5 | −0.65 |
| 800 °C | −0.92 | 2.238 × 10−5 | - |
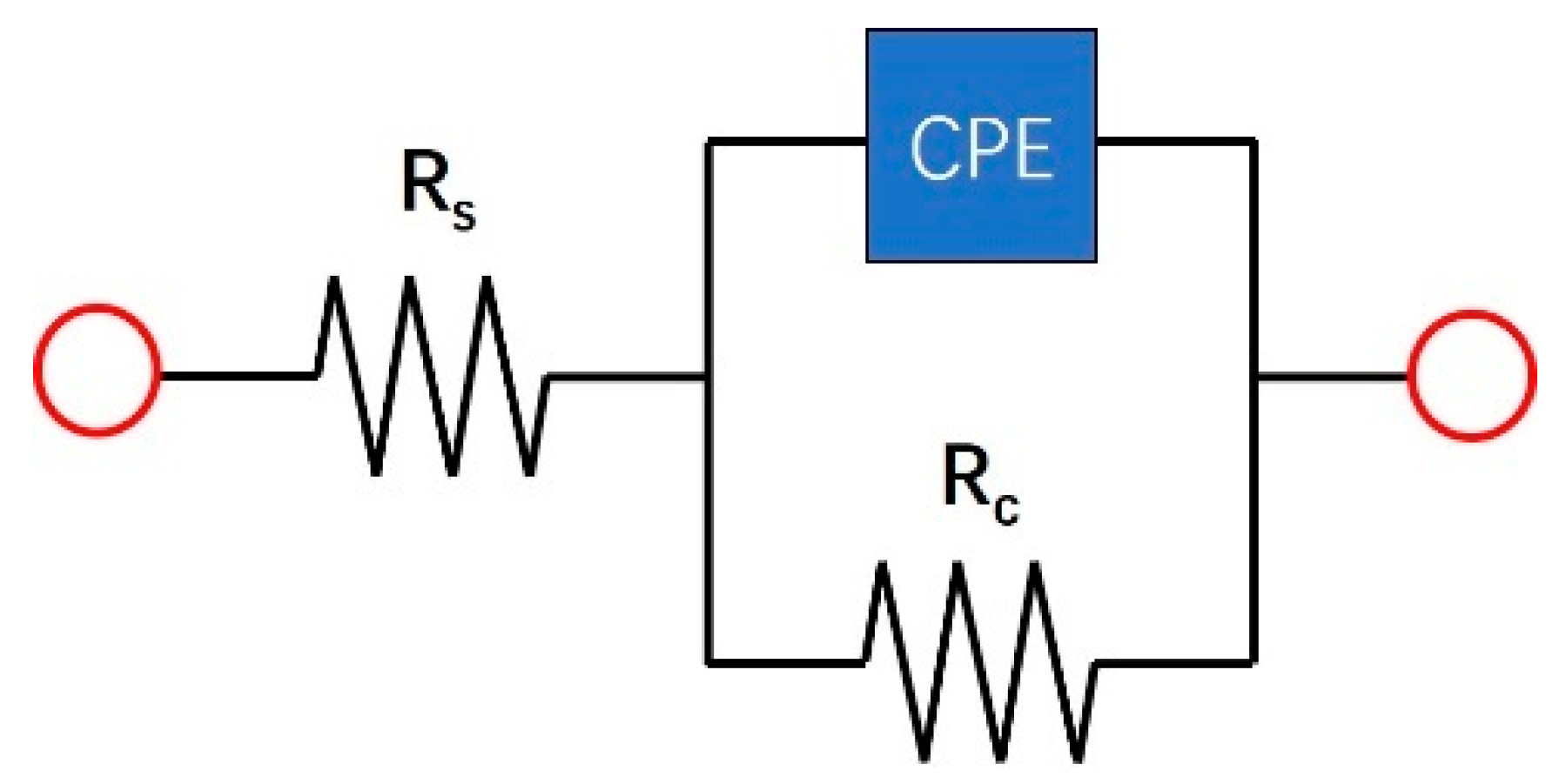
| Samples | Rs (Ω·cm2) | CPE | Rc (Ω·cm2) | % Error | |
|---|---|---|---|---|---|
| P (Ω−1·s−n·cm−2) | n | ||||
| Cold-rolled | 7.63 | 1.09 × 10−3 | 0.69 | 760.51 | ≤4.66 |
| 600 °C | 6.55 | 7.58 × 10−4 | 0.70 | 521.97 | ≤3.67 |
| 650 °C | 9.91 | 1.58 × 10−3 | 0.64 | 433.78 | ≤4.22 |
| 700 °C | 8.09 | 1.20 × 10−3 | 0.65 | 338.74 | ≤7.51 |
| 750 °C | 7.55 | 1.52 × 10−3 | 0.71 | 1041.10 | ≤4.31 |
| 800 °C | 6.17 | 1.02 × 10−3 | 0.65 | 766.51 | ≤4.44 |
| Samples | Rs (Ω·cm2) | CPE | Rc (Ω·cm2) | % Error | |
|---|---|---|---|---|---|
| P (Ω−1·s−n·cm−2) | n | ||||
| Cold-rolled | 6.79 | 8.13 × 10−4 | 0.66 | 730.95 | ≤2.68 |
| 600 °C | 8.81 | 2.38 × 10−3 | 0.73 | 974.33 | ≤7.99 |
| 650 °C | 9.62 | 1.31 × 10−3 | 0.60 | 772.31 | ≤6.27 |
| 700 °C | 7.91 | 3.42 × 10−3 | 0.51 | 436.55 | ≤5.52 |
| 750 °C | 7.27 | 2.34 × 10−3 | 0.56 | 813.37 | ≤4.94 |
| 800 °C | 9.86 | 1.13 × 10−3 | 0.57 | 1073.41 | ≤10.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Zuo, H. Influence of Heat Treatment Temperature on the Electrochemical Properties of Cold-Rolled 0.2%C–3%Al–6/8.5%Mn–Fe Medium-Manganese Steel. Metals 2025, 15, 275. https://doi.org/10.3390/met15030275
Luo J, Zuo H. Influence of Heat Treatment Temperature on the Electrochemical Properties of Cold-Rolled 0.2%C–3%Al–6/8.5%Mn–Fe Medium-Manganese Steel. Metals. 2025; 15(3):275. https://doi.org/10.3390/met15030275
Chicago/Turabian StyleLuo, Jihui, and Huixin Zuo. 2025. "Influence of Heat Treatment Temperature on the Electrochemical Properties of Cold-Rolled 0.2%C–3%Al–6/8.5%Mn–Fe Medium-Manganese Steel" Metals 15, no. 3: 275. https://doi.org/10.3390/met15030275
APA StyleLuo, J., & Zuo, H. (2025). Influence of Heat Treatment Temperature on the Electrochemical Properties of Cold-Rolled 0.2%C–3%Al–6/8.5%Mn–Fe Medium-Manganese Steel. Metals, 15(3), 275. https://doi.org/10.3390/met15030275





