1. Introduction
Nanocrystalline ferromagnetic alloys, renowned for their superior soft magnetic properties (SMPs) and energy-saving advantages, are essential in transformers, inductors, and other critical components, advancing energy conversion and signal transmission technologies [
1,
2,
3]. The prevalence of third-generation semiconductor devices demands power electronic equipment with higher frequencies, increased power density, and improved efficiency, driving the need for wider, ultra-thin, and lower-loss nanocrystalline ribbons. These ultra-thin ribbons are crucial for high permeability (
μ) and minimizing core loss at high frequencies [
4]. Since Y. Yoshizawa et al. developed FINEMET (Fe-Si-B-Nb-Cu) nanocrystalline alloys in 1988 [
5], researchers have created nearly a thousand alloys by adjusting compositions. Despite laboratory advancements incorporating Co, Mo, and other elements for superior SMPs [
6,
7], most alloys face industrial production challenges. The NANOPERM (Fe-Zr(V/Ta)-B-Cu) and HITPERM (Fe-Co-B-Zr(Ta/Hf)-Cu) alloys are easy to be oxidized and have poor AFA, while NANOMET (Fe-Si-B-P-Cu) and Cu-free alloys require high heating rate during annealing [
8,
9,
10,
11]. Only FINEMET and its modified alloys have achieved industrial-scale production. On the other hand, heat treatment techniques, including magnetic field, stress, and rapid annealing methods, have been explored to optimize SMPs [
12,
13,
14,
15]. These treatments regulate microstructure and magnetic domains, with magnetic field and stress annealing effectively inducing magnetic anisotropy, significantly enhancing SMPs at high frequencies. Innovative approaches, such as using quartz sand for flexible annealing, have been developed to improve both SMPs and plasticity in nanocrystalline alloys [
16]. Despite these advancements, the preparation of ultra-thin precursor ribbons often encounters clogging issues due to the high viscosity of the alloy, which is closely related to the viscosity and structural characteristics of the melt [
17,
18].
Reduction in ribbon thickness is needed to shorten the distance between the bottom of the nozzle and the outer surface of a copper wheel, known as the nozzle wheel gap [
19]. This method usually leads to clogging at the nozzle orifice, primarily due to the poor fluidity of the commercial alloy melts, which hampers their ability to spread out into the melt puddle between the nozzle and copper wheel, thereby limiting solidify rapidly into ribbons [
20]. Moreover, better fluidity typically corresponds to lower viscosity (
η) of the melt, which improves the migration ability of atoms and, in turn, accelerates the crystallization, thereby reducing the amorphous formation ability (AFA) of the precursor [
21]. Consequently, achieving a balance between viscosity and fluidity remains a key challenge in fabricating ultra-thin ribbons with amorphous precursors.
Viscosity not only reflects the fluidity and AFA of the melt but also indicates structural changes in the alloy melt as a function of temperature [
22]. When the viscosity of a metal melt decreases with the increase in temperature, a sudden transition zone in viscosity often appears, which has been observed in pure component elemental metals such as Sb and In, as well as binary and multicomponent alloys such as CuZr, CuSn, and CoFeSiB [
23]. This abrupt change in viscosity reflects a liquid-liquid phase (LLP) transition, which indicates a reorganization of metal atoms in the melt [
24]. The LLP transition exists widely in many alloys such as CuZr-based alloys and FeB-based alloys [
25,
26], which points to the inhomogeneity of the internal structure of the melt and can affect the precursor structure of solidified alloys [
27,
28,
29]. L. Li et al. observed an abnormal decrease in viscosity within a specific range during the cooling process of ZrCuNiAl alloy melt. This abnormal decrease is related to the promotion of icosahedral cluster formation through LLP transition, which makes the melt form a more stable liquid phase during the cooling process, thereby optimizing the disorder of the ribbon obtained via rapid quenching [
25]. W. Chu et al. found that in CuZrAl and CuZrTiNi alloy systems, increasing the doping contents of Al, Ti, or Ni weakens the structural characteristics and restrains the abnormal increase in viscosity; in addition, during the LLP transition, the formation of more icosahedral clusters enhances the AFA of the amorphous precursor ribbons [
30]. On the other hand, this discontinuous viscosity transition, which takes place during the cooling of Zr-based alloy melts, also occurs during the heating of FeB-based alloy melts [
31]. It has been found that the weakening of the viscosity mutation region in the melt, where LLP transition is weakened, is related to the enhancement in AFA of amorphous alloys. For instance, Y.W. Bai et al. discovered that the addition of rare earth elements greatly weakens the discontinuity of viscosity changes in FeB-based alloy melts, forms more stable polyhedral structures with rare earth elements as coordination atoms, and optimizes AFA in FeBY amorphous bulk alloys [
32]. Similarly, Y.B. Zhao et al. revealed that the viscosity mutation region of FeB-based alloys containing Y weakens, and the primitive clusters tend to decompose into short-range ordered clusters at high temperatures, which leads to more amorphous regions in the bulk alloys [
33]. However, most current studies on viscosity primarily focus on CuZr multicomponent alloys and Fe-based bulk alloys. The viscosity changes of Fe-based nanocrystalline alloy masters remain underexplored, and the relationship between these viscosity changes and the AFA of precursor soft magnetic ribbon is also vague. Therefore, investigating the links between viscosity, precursor structure, nanocrystalline structure, and SMPs is essential for advancing the development of nanocrystalline materials with superior properties.
Herein, to balance the viscosity and fluidity, the FeSiCuBNbAlP alloy was chosen for its enhanced SMPs and AFA due to the addition of Al and P elements [
5,
34,
35,
36]. Sn was added to reduce viscosity and enhance the fluidity of the alloy melt [
37]. On this basis, the influence of the change of viscosity on the structure of the precursor and its reasons, as well as the changes in the process of nanocrystallization were studied. To study structural and property changes in ribbons prepared by LLP transition, high-temperature-preservation (HTP) treatment was applied to some master alloys. HTP involves maintaining the alloy melt at a temperature that exceeds the sudden change zone of viscosity for a prolonged time to complete the LLP transition. Therefore, we study the effect of melt LLP transition on the structure of precursors and nanocrystals. The study examined alloys with varying Sn content and the presence or absence of HTP treatment, exploring the relationship between viscosity, precursor structure, and nanocrystallized microstructure, as well as their impact on the SMPs of the nanocrystalline ribbons. The purpose of the study is to first obtain highly disordered precursors by adjusting melting viscosity, fragility parameter ratio (
F), and HTP approach, and then to achieve fine uniform nanocrystalline structure and improved magnetic properties in Fe-based nanocrystalline alloys.
3. Results and Discussion
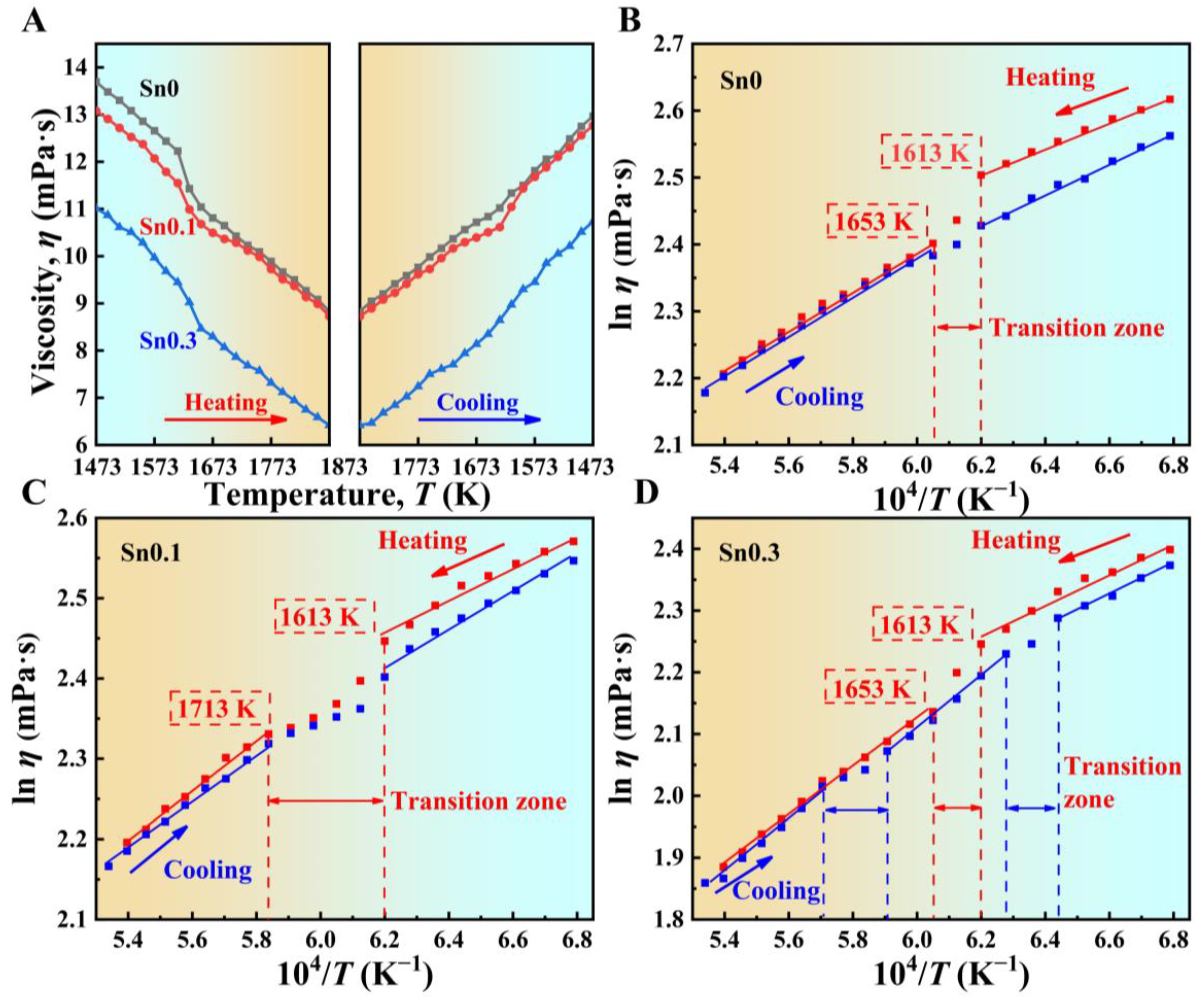
Figure 1A shows the viscosity of Sn0, Sn0.1, and Sn0.3 melts changes as a function of temperature during heating from 1473 K to 1873 K and then cooling back to 1473 K. For all three alloy melts, viscosity decreases with the increasing of temperature and increases again during cooling. The addition of Sn lowers the viscosity of the melt and 0.3 at.% Sn exhibits the most significant decrease, which indicates incorporating low-melting-point metal effectively reduces the viscosity of the melts. Meanwhile, there is at least a discontinuous viscosity change for all three melts during heating and cooling, which is consistent with that previously reported in Fe-based alloy melts [
39]. The presence of several sections on the dependencies ln
η =
f(1/T) reflects the change of cluster structure at different temperature regions. Because of the wide fitting temperature range of the Arrhenius formula, the model is suitable for analyzing the activation energy of clusters. To provide a more intuitive analysis, we use an Arrhenius equation to fit the viscosity changes in the melts as follows [
40,
41]:
where
η0 is the high-temperature limit of viscosity,
Ea is the activation energy of fluid clusters, R is the gas constant, and
T is the absolute temperature. The measured viscosity for the three alloys in various temperature ranges fitted with Equation (1) are shown in
Figure 1B–D, where solid lines denote fitting curves.
During heating, a sharp drop in viscosity is observed in all three alloys as the temperature increases to near 1613 K. The viscosity of melts transitions from continuous to discontinuous change with increasing temperature, indicating the occurrence of LLP transition characterized by a sudden drop in viscosity. This LLP transition is likely driven by the dissociation and rearrangement of clusters [
42]. The Sn0.1 melt shows a longer range of sudden change in viscosity, spanning from 1613 K to 1713 K, compared to that of the Sn0 and Sn0.3 melts. The extended transition range observed for Sn0.1 may result from the dissociation of certain clusters in the melt at higher temperatures, thus prolonging the region of the sudden viscosity change. This phenomenon indicates that higher activation energy is required for cluster transformation in Sn0.1 melt and more types of clusters are involved in the decomposition into smaller clusters [
21]. After heating to 1653 K for Sn0 and Sn0.3, as well as 1713 K for Sn0.1, viscosity returns to a linear trend, suggesting that the energy required for cluster activation increases and the melt structure stabilizes [
17,
33].
During the cooling process, in contrast to how the viscosity of Sn0 melt changes almost linearly with the decrease in temperature, the Sn0.1 melt exhibits an obvious sudden change in viscosity, and the Sn0.3 melt exhibits two sudden changes in viscosity during cooling. The observed discontinuous change in viscosity of the Sn0.1 and Sn0.3 melt during cooling may be attributed to the rearrangement of atoms [
43]. The relevant parameters (
Ea,
η0) obtained by fitting are listed in
Table 1. Sn0.1 exhibits a similar value of
Ea within the same temperature range during both the heating and cooling stages, indicating that the melt requires approximately the same amount of energy to activate clusters. By comparing the
Ea values of several stages in Sn0.3 melt, the structural evolution in the high-temperature region is relatively weak, and the structure of Sn0.3 melt tends to be stable when it decreases to 1553 K.
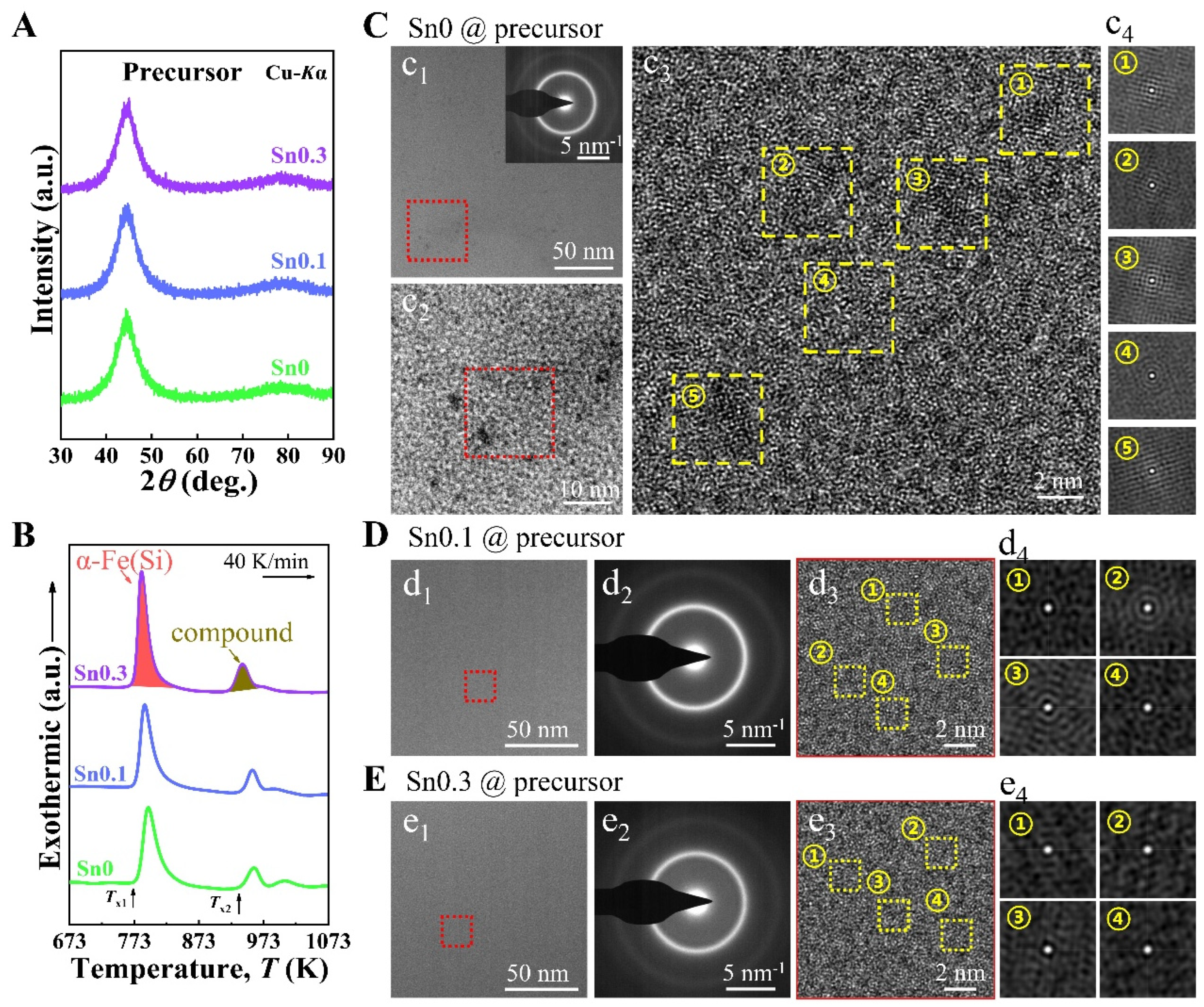
To clarify the relationship between the viscosity of melts and the microstructure of the precursor ribbons, the phases of Sn0, Sn0.1, and Sn0.3 precursor ribbons were identified by XRD, as shown in
Figure 2A. Each alloy precursor shows an obvious broad peak at 2
θ ≈ 45°, which indicates the characteristic of an amorphous nature. The full width at half maximum (FWHM) of the 2
θ ≈ 45° peak is 5.82 ± 0.04°. The characteristic of an amorphous nature is further verified by a disordered maze, as shown in subsequent TEM images (
Figure 2). The absence of crystalline structure is evidenced by the halo rings observed in the SAED patterns (inset of
Figure 2c
1;
Figure 2(d
2,e
2)). Furthermore, no contrast change is observed in the TEM images of Sn0.1 (
Figure 2d
1) and Sn0.3 (
Figure 2e
1) precursors. Additionally, fringes are absent in their HRTEM images (
Figure 2(d
3,e
3)), and disordered structure is observed in the 2D auto-correlation patterns (
Figure 2(d
4,e
4)). These observations indicate that the Sn0.1 and Sn0.3 precursors exhibit a completely amorphous structure, with no evidence of local crystallization [
29]. However, the Sn0 precursor exhibits a contrast change in TEM images (
Figure 2(c
1,c
2)). The HRTEM image of Sn0 displays lattice fringe-like structures, approximately 2 nm in size (marked by yellow dotted squares), which are randomly dispersed within the amorphous matrix (
Figure 2c
3). Through the auto-correlation analysis of the regions marked by the yellow dotted squares in
Figure 2c
3, it is found that the relevant 2D auto-correlation patterns (
Figure 2c
4) show fringes indicative of atomic arrangements resembling crystal-like structures, suggesting local crystallization occurs in the amorphous matrix of the Sn0 precursor [
44]. Consequently, the Sn0 alloy has relatively poorer AFA, compared to the Sn0.1 and Sn0.3 alloys. This result contradicts the principle that higher viscosity of melts correlates with better AFA. However, the addition of Sn alters the viscosity behavior of melts during cooling, transiting from a linear change in Sn0 melt to a discontinuous transition in Sn0.1 and Sn0.3 melts (
Figure 1). This discontinuous viscosity is related to the rearrangement and stabilization of clusters, and the change in melt stability can be used to explain why the AFA of the alloys is promoted [
45].
On the other hand, considering that the AFA of the alloy may be related to its LLP transition at high temperature, we use the superheated fragility parameter (
M) to describe the dynamic behavior of the melt at high temperature, and the smaller
M value indicates that the melt is more stable at this temperature, which is defined as [
46]:
where
TL is the liquidus temperature. The difference in melt stability in various temperature regions usually reflects the degree to which the melt is affected by temperature. In order to quantify the degree of dynamic transition of the melt from high temperature to low temperature, the parameter
F can be defined to calculate the ratio of
M in the high-temperature (HT) region to low-temperature (LT) region [
33]:
The corresponding
M and
F values for each alloy are listed in
Table 1. When the
F value of the melt is greater than 1, the fragility of the melt decreases during the cooling from the HT region to the LT region, which means that the stability of the melt is improved, and the cluster structure in the HT region is maintained stably [
46]. Specifically, the locally favored structures (LFSs) in the HT region are retained in the form of crystal-like structures (CLSs) of Fe
3B cluster structure, which share vertices, edges, or faces in the process of further cooling to form crystal phase, which is not conducive to the formation of amorphous structure [
33]. Therefore, the lower the
F value, the weaker the tendency of CLSs to retain from the HT region, the more it can promote the formation of an amorphous phase in the precursor structure, and the better the AFA [
21,
32]. The
F value of the Sn0 melt is higher than those of the Sn0.1 and Sn0.3 melts, indicating a greater tendency for the Sn0 melt to precipitate a crystalline phase during the transition from liquid phase to solid phase, resulting in lower AFA of precursors. Consequently, a small number of crystal-like regions can be observed in the TEM images of the Sn0 precursor (
Figure 2C).
Figure 2B shows the DSC curves of three alloy precursors, which mainly show two obvious exothermic peaks corresponding to different crystallization processes. The first exothermic peak corresponds to the precipitation of the α-Fe(Si) crystal phase and the second exothermic peak corresponds to the precipitation of Fe-B compounds [
47]. The heat treatment temperature range is determined according to the two onset crystallization temperatures of the precursors. The primary crystallization peak temperatures of Sn0, Sn0.1, and Sn0.3 are 794, 788, and 784 K, respectively. Compared to Sn0, the peak positions of Sn0.1 and Sn0.3 are shifted by −6 K and −10 K, respectively. This shift indicates that the addition of Sn slightly reduces the primary crystallization temperature. These Sn0, Sn0.1, and Sn0.3 precursor ribbons were subjected to heat treatment at intervals of 20 K in the range of 793–893 K for 10 min to produce nanocrystalline ribbons. The samples heat-treated at 873 K were found to exhibit the best SMPs (
Figure S1, Supporting Information). Therefore, we have selected the Sn0, Sn0.1, and Sn0.3 nanocrystalline ribbons obtained under the optimal heat-treatment condition (873 K) for the observation and analysis of their phase and microstructures. The XRD patterns (
Figure S2, Supporting Information) of three alloy nanocrystalline ribbons exhibit sharp crystallization peaks at 2
θ ≈ 45°, 65°, and 85°, corresponding to (110), (200), and (211) diffractions, respectively. These peaks represent the formation of α-Fe(Si) grains. The TEM image (
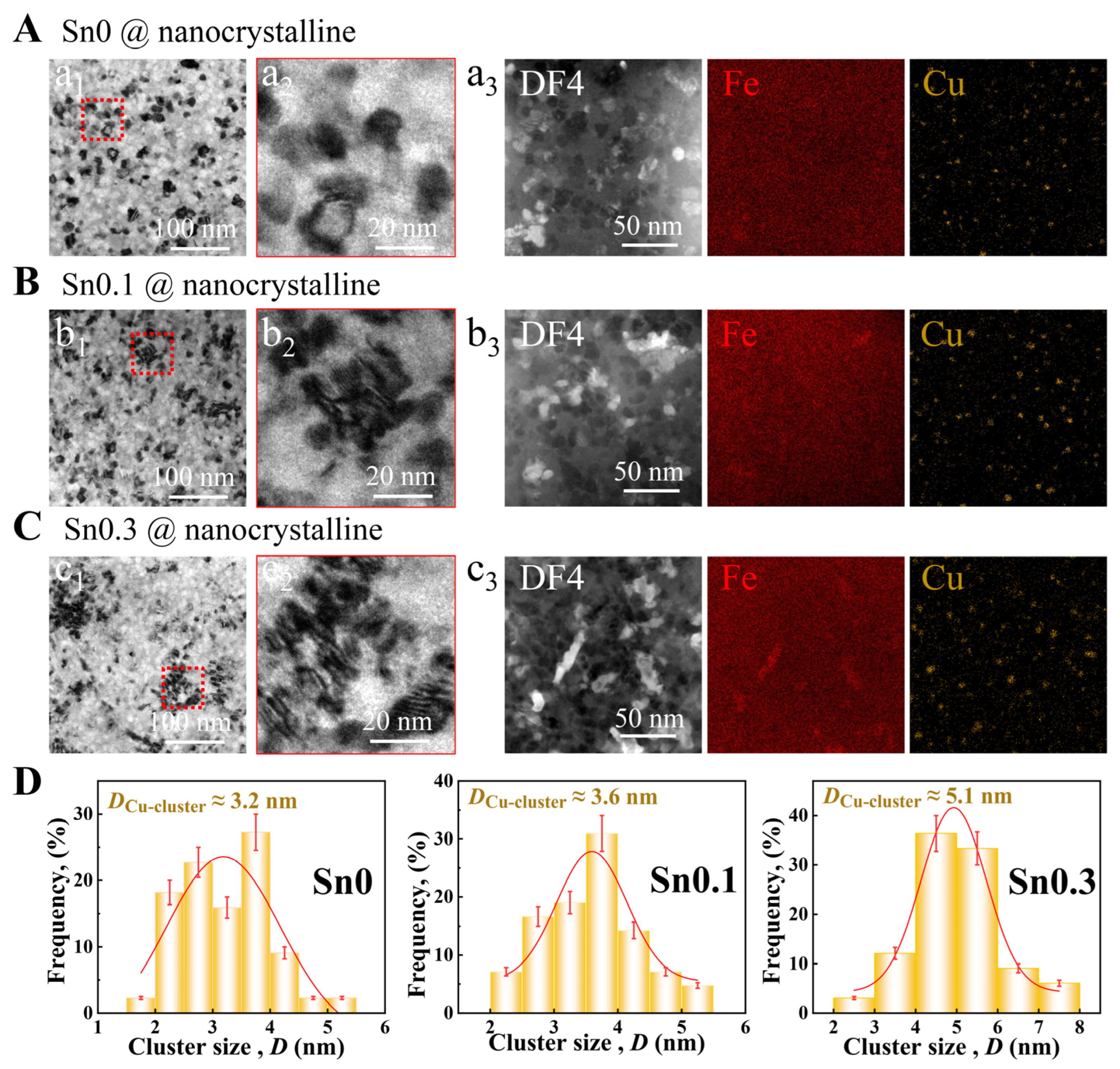
Figure 3a
1) reveals that the optimally heat-treated Sn0 ribbon exhibits a nanocrystalline-amorphous dual-phase structure. The magnified image (
Figure 3a
2) shows precipitated nanocrystals of similar sizes are uniformly and randomly distributed within the amorphous matrix. However, the microstructure of the Sn0.1 and Sn0.3 ribbons is different from that of the Sn0 ribbon. The nanocrystals in the Sn0.1 and Sn0.3 ribbons are irregularly distributed in an amorphous matrix, exhibiting local aggregation of some nanocrystals (
Figure 3(b
1,c
1)). This aggregation with the same orientation becomes more pronounced with increasing Sn content (
Figure 3(b
2,c
2)). As the size of Cu clusters may affect the crystallization process, we calculate the size of Cu clusters in the samples. We perform Gaussian fitting to the size distribution, and the position of the fitting peak represents the mean cluster size. With increasing Sn content, the size of Cu clusters increases (
Figure 3(a
3,b
3,c
3)), and the average size increases from 3.2 to 5.1 nm (
Figure 3D). As nucleation sites, Cu clusters tend to precipitate more grains around them with the increase in their size; at the same time, Sn clusters are easy to agglomerate with the increase in content, and the distribution is similar to that of Cu clusters (
Figure S3, Supporting Information), which can promote the nucleation and growth of grains and interact with Cu clusters to cause local aggregation of grains [
48].
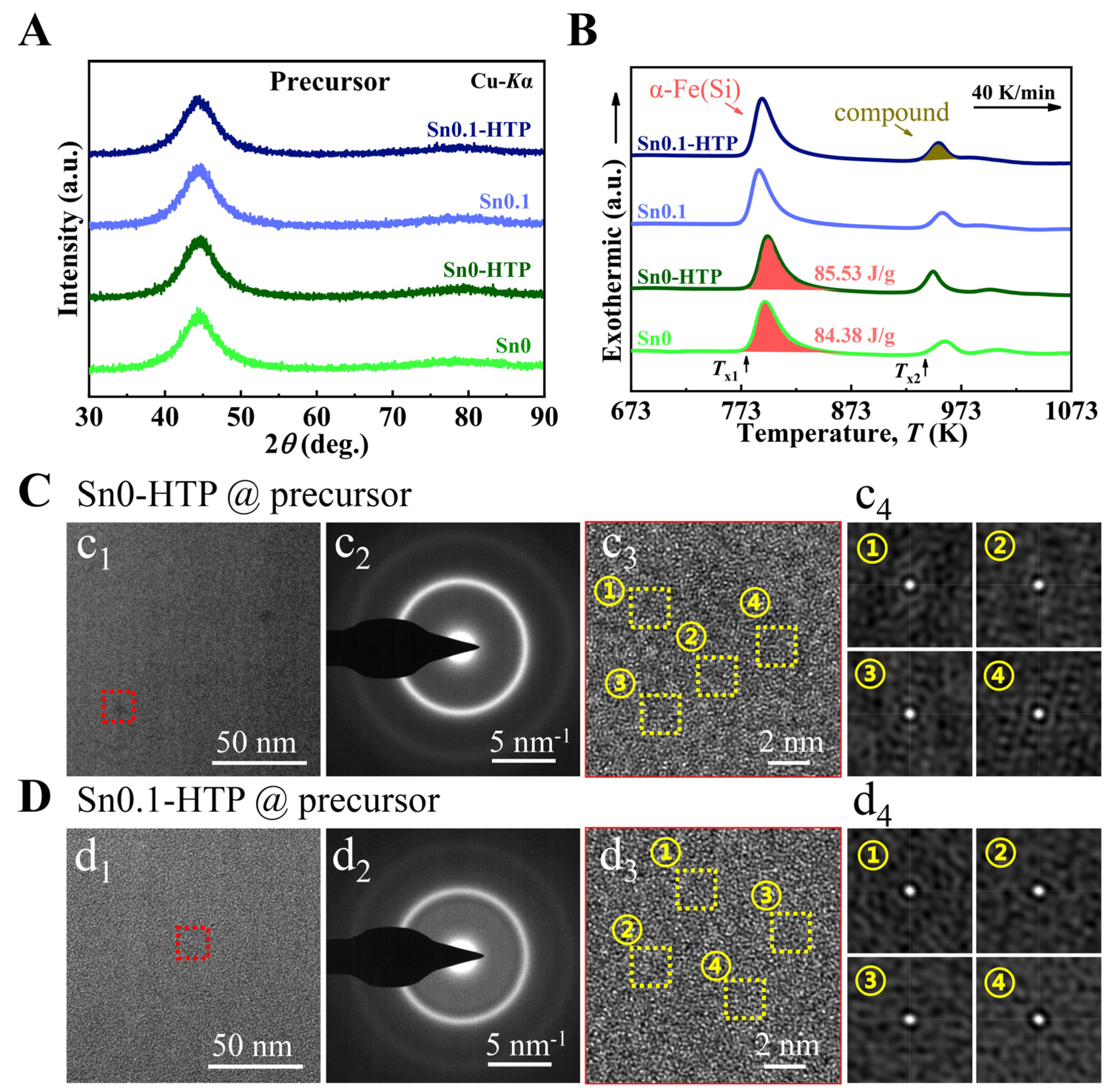
HTP treatment reportedly affects the chemical distribution of master alloys [
49] and, consequently, could influence the fragility parameter of melts and AFA of the precursor ribbons. To achieve a superior nanocrystalline-amorphous dual-phase structure, the Sn0 and Sn0.1 melts were treated through HTP to produce Sn0-HTP and Sn0.1-HTP precursor ribbons. The XRD patterns (
Figure 4A) reveal the HTP treatment has a negligible effect on the amorphous nature of precursors. However, as shown in DSC curves (
Figure 4B), for Sn0, the HTP treatment resulted in an increase of 4 K in
Tx1 and an increase of 1.15 J/g in the primary crystallization enthalpy (Δ
H). The differences can be attributed to the presence of quenched nanograins in Sn0. It is worth noting that there is no crystallization region or crystal-like region in the TEM image (
Figure 4(c
1,c
3)) of the Sn0-HTP precursor, and the 2D image of the local region after auto-correlation analysis also exhibits a disordered structure (
Figure 4c
4), which contrasts with that of Sn0 precursor, indicating the effectiveness of HTP treatment in enhancing AFA of precursor. The Sn0.1-HTP precursors also show a completely disordered structure (
Figure 4D).
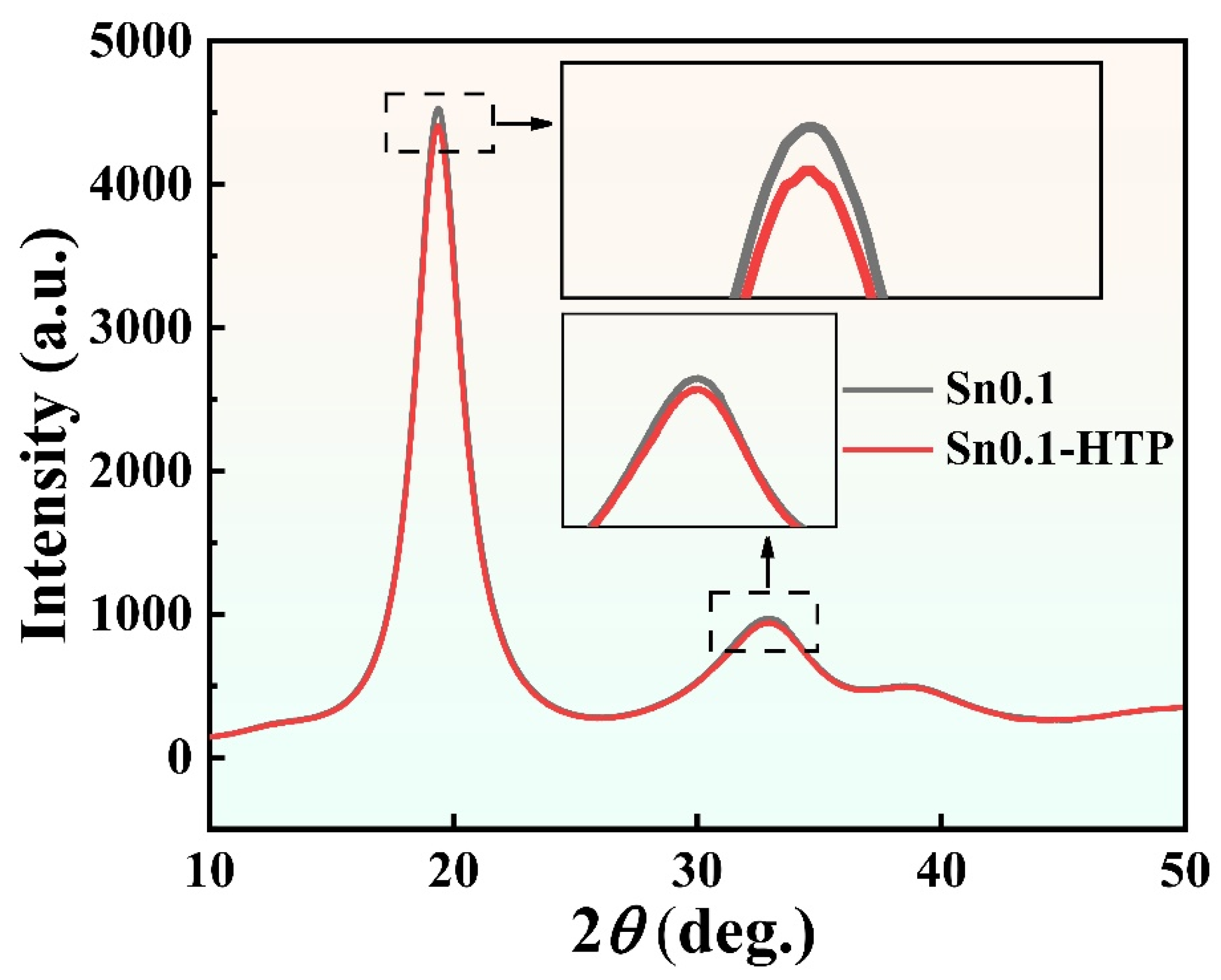
As shown in the synchrotron radiation XRD image in
Figure 5, the diffraction intensity of the Sn0.1 precursor is higher than that of Sn0.1-HTP at 2
θ ≈ 20° and 32°, which indicates that the Sn0.1-HTP precursor has a relatively high degree of disorder [
17]. Therefore, the HTP treatment in melts effectively enhances the AFA of precursor ribbons. Furthermore, by comparing the
F values of melts with and without HTP treatments (
Table 1), it is found that after HTP treatment, the
F values of Sn0 and Sn0.1 melts decrease from 1.53 to 1.14 and from 1.50 to 1.27, respectively. Consequently, the AFA of Sn0-HTP and Sn0.1-HTP is improved and the disorder of microstructure is enhanced. However, after HTP treatment, the
F value of Sn0.3 increases from 1.48 to 1.76, which leads to worse AFA, so it is difficult to obtain precursor ribbons with good surface quality and amorphous structure.
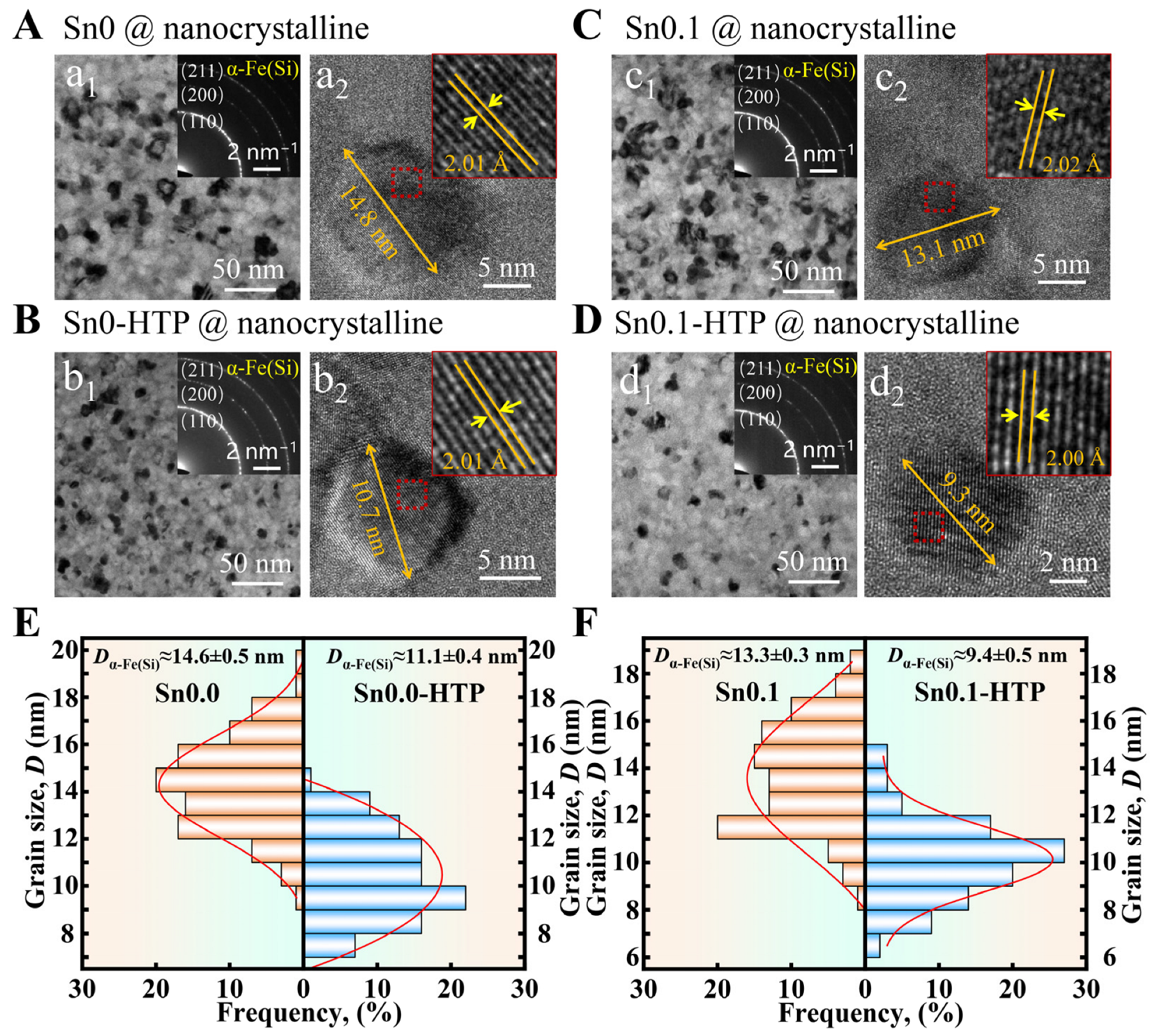
Figure 6 shows the TEM images and grain size distribution of the nanocrystalline ribbons obtained after the optimal heat treatment of the above samples. Except for a small amount of grain aggregation in Sn0.1, other nanocrystalline ribbons exhibit nanocrystals uniformly distributed in the amorphous matrix. The (110), (200), and (211) diffractions observed in the SAED patterns (inset of
Figure 6(a
1,b
1,c
1,d
1)) and the interplanar distance of approximating 2 Å (inset of
Figure 6(a
2,b
2,c
2,d
2)) confirm that the precipitated grains are α-Fe(Si), which is consistent with the results in
Figure S4. After comparing the grain size of HRTEM images, we found that the grains in Sn0-HTP and Sn0.1-HTP samples were 3–4 nm smaller than those in Sn0 and Sn0.1, respectively. The statistical results of grain size, as shown in
Figure 6E,F, display that the average grain sizes of Sn0 and Sn0.1 are approximately 14.6 nm and 13.3 nm, respectively. In contrast, the average sizes of Sn0-HTP and Sn0.1-HTP are around 11.1 nm and 9.4 nm, respectively. Debye–Scherrer formula as
D =
Kλ/(
βcos
θ) was used to calculate the grain size of each sample in the XRD patterns. The average grain sizes of Sn0 and Sn0.1 are 14.2 ± 0.3 nm and 13.1 ± 0.5 nm, respectively, and those of Sn0-HTP and Sn0.1-HTP are 11.6 ± 0.4 nm and 10.2 ± 0.4 nm, respectively. The average grain size calculated from XRD results is close to the grain size estimated by our statistics from TEM observations. These findings clearly demonstrate that the HTP treatment is effective in refining the grain size of the obtained nanocrystalline ribbons. The reason may be that the heterogeneous nucleation points in the master alloy decompose at high temperatures for a long time or decrease due to material exchange in the process of thermal convection, which is helpful in optimizing the microstructure of the alloy [
50]. This HTP process ensures that the precursors possess a homogeneous microstructure, which is crucial for refining their nanocrystallized structure.
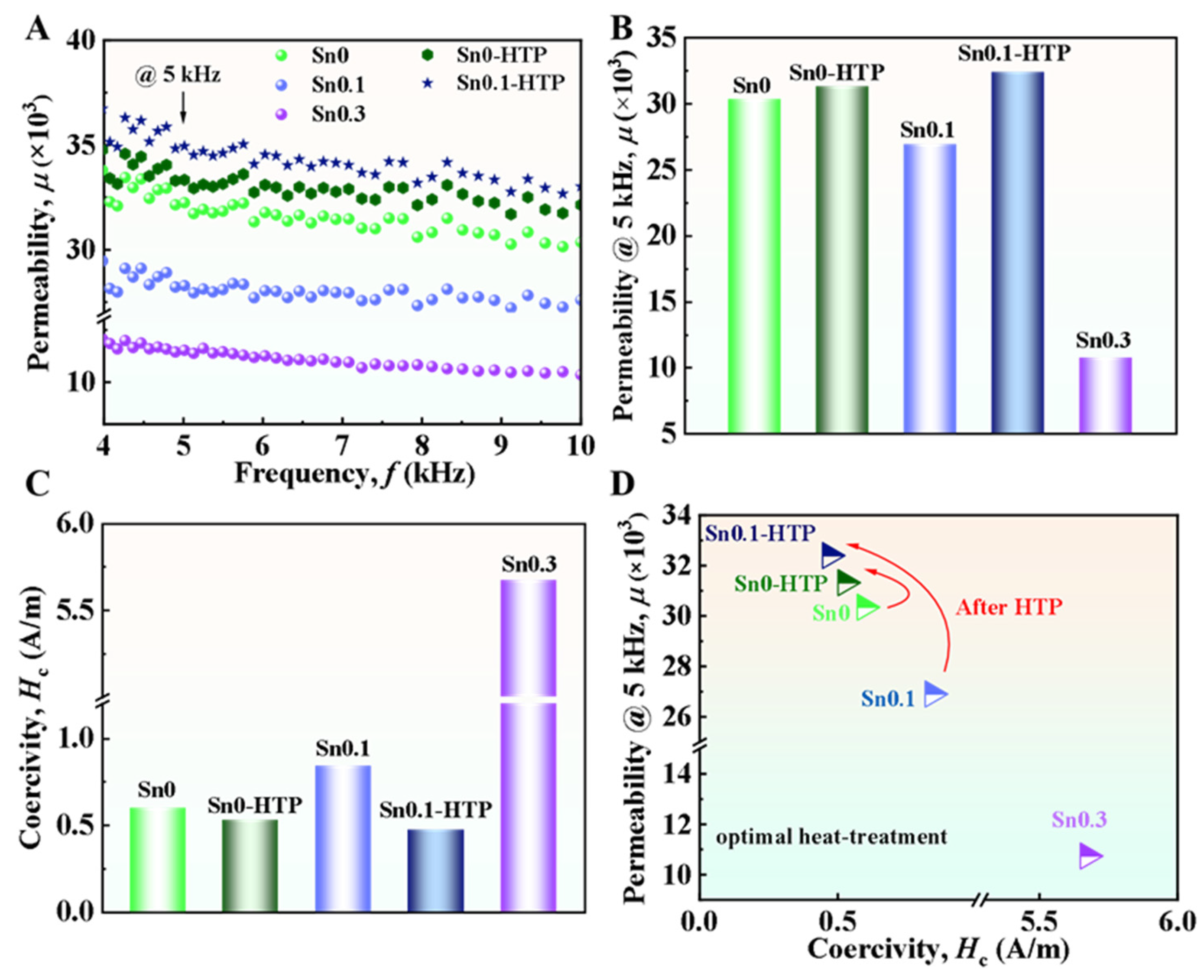
We further performed an investigation on the SMPs of Sn0, Sn0.1, Sn0.3, Sn0-HTP, and Sn0.1-HTP alloys. The precursor ribbons were heat-treated at 873 K for 10 min to yield nanocrystalline ribbons. As shown in
Figure 7A,B, the
μ of Sn0-HTP and Sn0.1-HTP nanocrystalline ribbons, measured at frequencies ranging from 4 to 10 kHz, is higher than that of Sn0 and Sn0.1, indicating that the effectiveness of HTP in enhancing permeability of nanocrystalline materials. The
μ at 5 kHz of Sn0-HTP and Sn0.1-HTP reaches 31,000 and 32,400, respectively. Accordingly, the
Hc of Sn0-HTP and Sn0.1-HTP nanocrystalline ribbons are as low as 0.5 and 0.4 A/m, respectively, which is smaller than those of Sn0 and Sn0.1 (
Figure 7C,D). This is mainly attributed to the fact that HTP treatment can refine the nanocrystals obtained after nanocrystallization. Correspondingly,
Hc and
μ vary with the average size of nanocrystals (
D) as
Hc∝
D6 and
μ∝1/
D6, respectively [
51]. However, the Sn0.3 nanocrystalline ribbon exhibits the lowest
μ of 10,700 and the highest
Hc of 5.7 A/m, which is ascribed to the weakening of averaging effect of random magnetocrystalline anisotropy induced by its inhomogeneous nanocrystalline structure with aggregation of some nanocrystals having the same orientation [
52]. As shown in
Figure 3, when the content of Sn increases from 0.1 at% to 0.3 at%, the average size of Cu clusters increases by 1.5 nm, and the phenomenon of grain aggregation is more obvious. We calculated the average grain size by Scherrer’s equation and found that the average grain size of Sn0.1 and Sn0.3 is 13.1 nm and 12.9 nm, respectively, and the change is not obvious, so grain aggregation is an important reason for the significant difference in SMPs between them. In contrast to the Sn0.3 alloy with obvious grain aggregation in the same orientation, Sn0.1 exhibits relatively uniform fine nanograins with weak aggregation, which induces weak anisotropy and results in a low
Hc of 0.8 A/m. The precursor of Sn0 alloy shows local crystallization regions characterized by approximately 2 nm lattice fringe-like structures, dispersed in the amorphous matrix, indicating relatively poor AFA. Adding 0.1 at% Sn leads to a discontinuous change in viscosity during cooling. This addition also reduces the value of
F, thereby enhancing the AFA, resulting in the precursor of Sn0.1 alloy having a completely amorphous structure. Although the addition of 0.3 at.% Sn also leads to a completely amorphous structure in the precursor, it increases the size of Cu clusters and forms Sn clusters, which causes grain aggregation during the nanocrystallization process, As a result, the magnetic softness deteriorates, the Sn0.3 alloy has a high
Hc of 5.7 A/m due to the increase in effective magnetic anisotropy caused by grain aggregation In contrast, the Sn0.1 alloy has a low
Hc of 0.8 A/m due to its ultrafine nanograins with weak local aggregation. HTP treatment promotes the AFA and disorder in the precursor ribbons, refining the grain size of the nanocrystalline ribbons. This grain refinement leads to a decrease in the effective magnetic anisotropy and the optimization of SMPs.
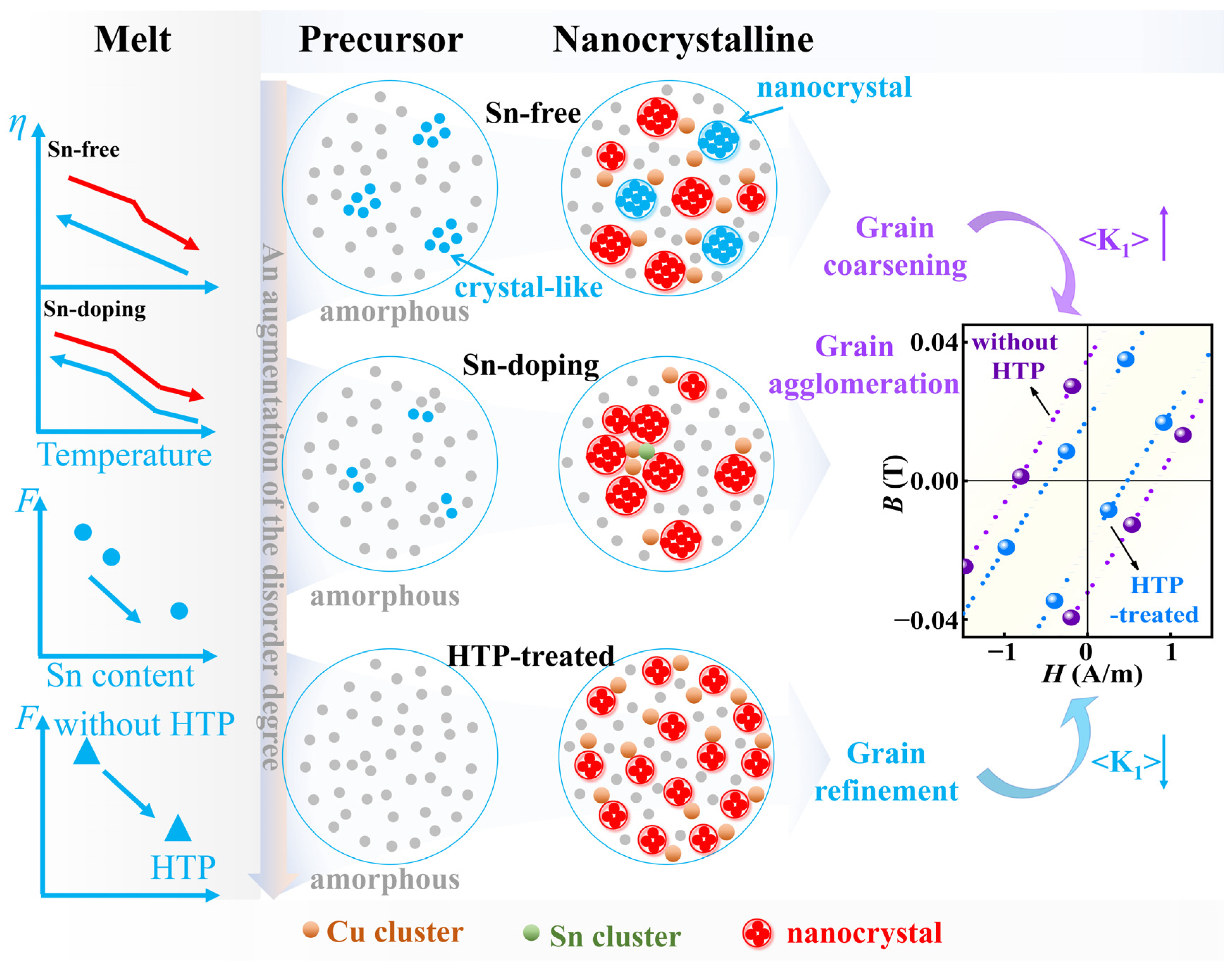
To clearly elucidate the effect of Sn content and HTP on the
F of melts, AFA of precursor, and crystallization behavior, a schematic showing the correlation among viscosity, precursor structure, nanocrystalline structure, and SMPs is illustrated in
Figure 8. With the addition of Sn, the viscosity of the master alloy melt decreases gradually at the same temperature, along with a reduction in the
F value of the melt (
Table 1). This indicates the Sn-doping melt shows a relatively weak relative change of
M during cooling, compared to the Sn-free melt. The lower
F in the Sn-doping melt makes it less prone to forming a crystal-like structure during cooling, resulting in improved AFA of precursors. In the Sn-free precursor, a portion of crystal-like structures tend to form easily. During the subsequent heat treatment, these crystal-like structures grow into distinct nanocrystals. In addition, the formation of Cu cluster nucleation sites facilitates the nucleation of new nanocrystals around them. The growth competition with crystal-like structures and new nanocrystals results in relatively uniformly distributed nanocrystals under optimal nanocrystallization. For the Sn-doping alloy, obvious grain aggregation has been observed in the nanocrystallized structure. This may be attributed, on the one hand, to the large reduction of crystal-like structures, which reduces the inhibitory effect on the uneven nucleation distribution of new nanocrystals [
53]. On the other hand, the increase in the size of Cu clusters, along with the formation of Sn clusters around them, facilitates easier nucleation and growth of nanocrystals, resulting in an agglomerated grain structure. According to the Herzer model, the average magnetocrystalline anisotropy, <
K1>, is proportional to the average size of nanocrystals (
D) [
52]. Therefore, the addition of Sn causes grain agglomeration, which increases the <
K1>, leading to the increase in
Hc of nanocrystalline ribbons. Due to the addition of 0.3 at% Sn, Sn0.3 has a lower viscosity than Sn0 and Sn0.1. The
M of Sn0.3 melt (
MHT = 2.62,
MLT = 1.78) is higher than that of Sn0 (
MHT = 2.00,
MLT = 1.31) and Sn0.1 (
MHT = 2.14,
MLT = 1.43) in HT region and LT region, which indicates that the melt is relatively unstable in both temperature regions. However, the ratio of high and low-temperature
M, that is
F value, can measure the change degree of melt stability and the trend of crystalline phase formation during cooling. The smaller the
F value is, the weaker the trend is, and the better the AFA of the alloy is. Sn0.3 (
F = 1.48) has a lower
F value than Sn0 (
F = 1.53) and Sn0.1 (
F = 1.50), so its precursor structure shows a higher degree of disorder. In the process of heat treatment, because the heat of mixing between Sn and Cu is positive (7 kJ/mol), the addition of 0.3 at% Sn promotes the clustering of Cu clusters and increases the size of Cu clusters before nanocrystallization, which increases the Cu cluster size of 1.5 nm compared with Sn0 and Sn0.1. Therefore, in the process of nanocrystallization of Sn0.3, with the clustering of Sn and the increase in the size of Cu clusters, the grains tend to precipitate near the nucleation position, resulting in the aggregation of grains with the same orientation. This phenomenon of grain aggregation makes the magnetocrystalline anisotropy (
K1) of grains within the ferromagnetic exchange length difficult to average, which makes the <
K1> relatively large. Generally speaking, the
Hc is directly proportional to the <
K1>, and the
μ is inversely proportional to the <
K1>, so the increase in <
K1> in Sn0.3 deteriorates its SMPs.
HTP treatment reduces the
F of Sn-free and Sn-doping master alloys (
Table 1), reducing the number of heterogeneous nucleation sites in the alloy, so that the precursor structure shows a high degree of disorder. The alloy melt may release some energy when it forms short-order clusters during cooling [
33]. We use high-temperature DSC to compare the thermodynamic behavior of the master alloys before and after HTP treatment (
Figure S5, Supporting Information). The DSC result shows that the amount of released energy (Δ
H) values of the HTP-treated sample is increased from 2.8–4.0 J/g to 5.1–5.8 J/g, compared to the samples without HTP, indicating that HTP-treated samples are more inclined to form short-order clusters, resulting in the improvement of the degree of disorder of the precursors. Consequently, a nanocrystalline-amorphous dual-phase structure with uniform and fine nanocrystals distributed in the amorphous matrix is achieved after optimal heat treatment. This HTP treatment helps to refine the average size of nanocrystals, leading to the reduction in the <
K1>, thereby reducing the
Hc of nanocrystalline ribbons.