Thermal Stability Calculation of Typical Phases in Tungsten Cathodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Calculation Method
2.2. Thermal Stability of Different Cathodes in Different Environments
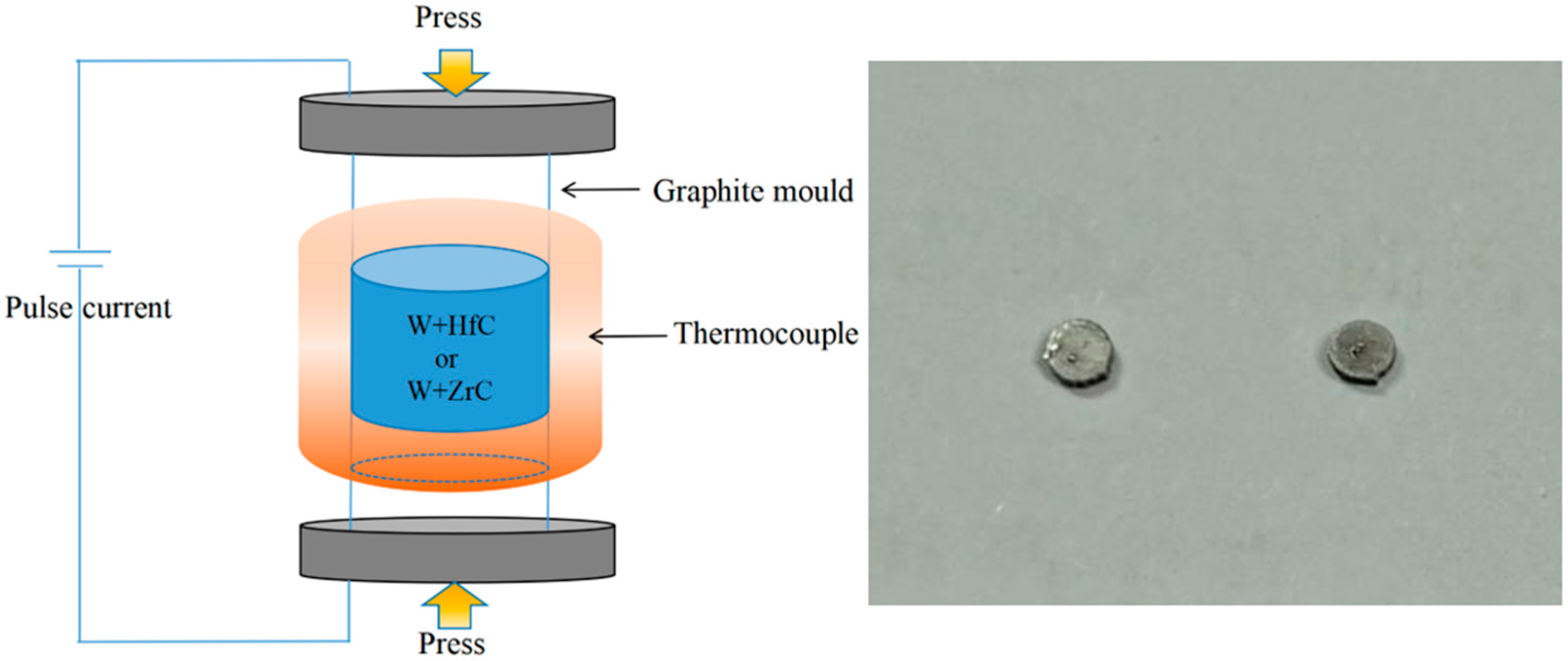
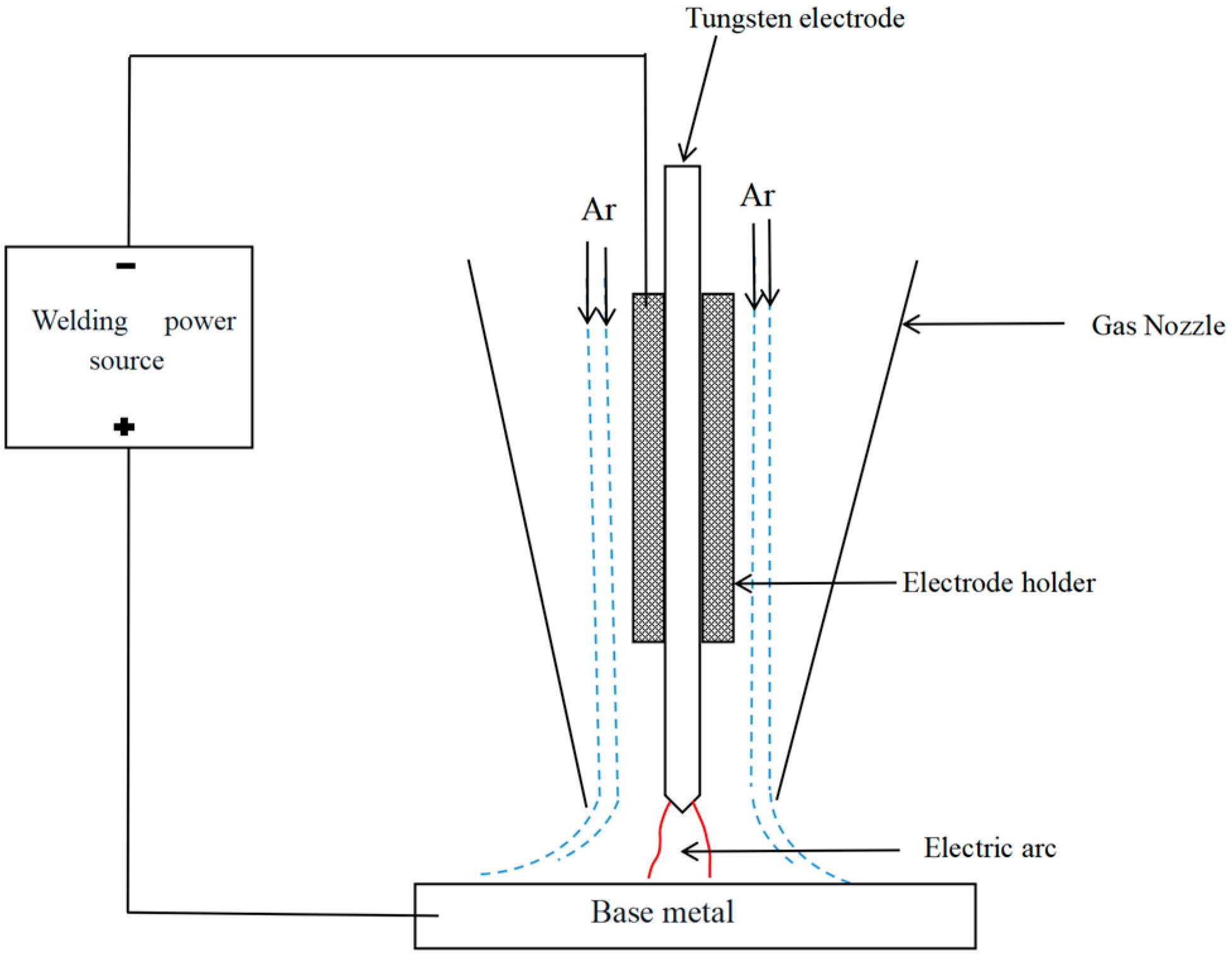
2.3. Experimental Verification
3. Results and Discussion
3.1. Thermodynamic Analysis Under a Protective Atmosphere
3.2. Thermodynamic Analysis Under Vacuum Conditions
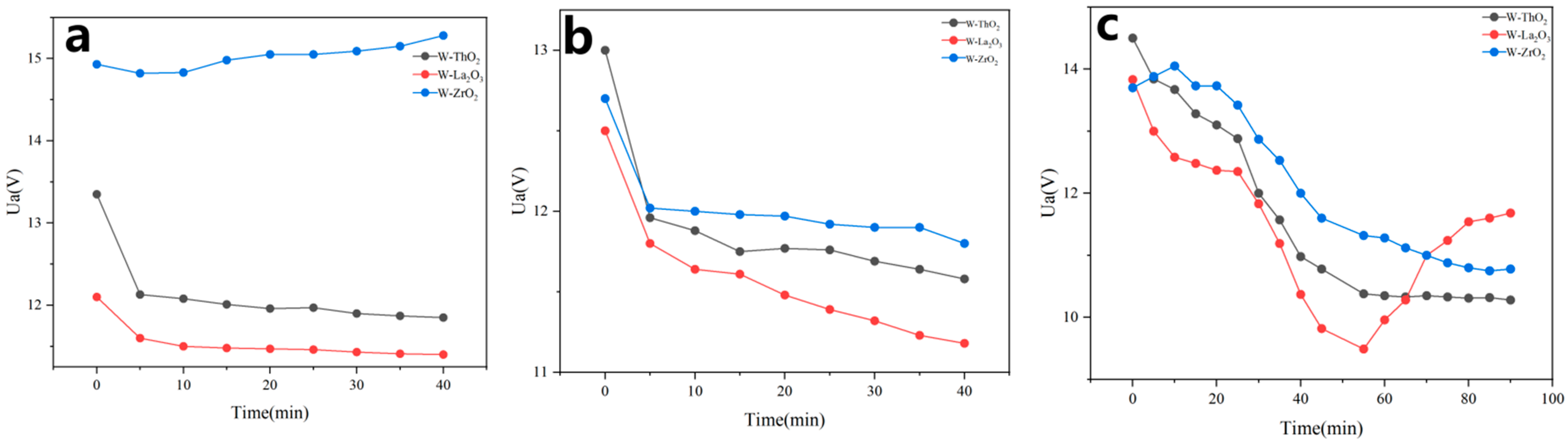
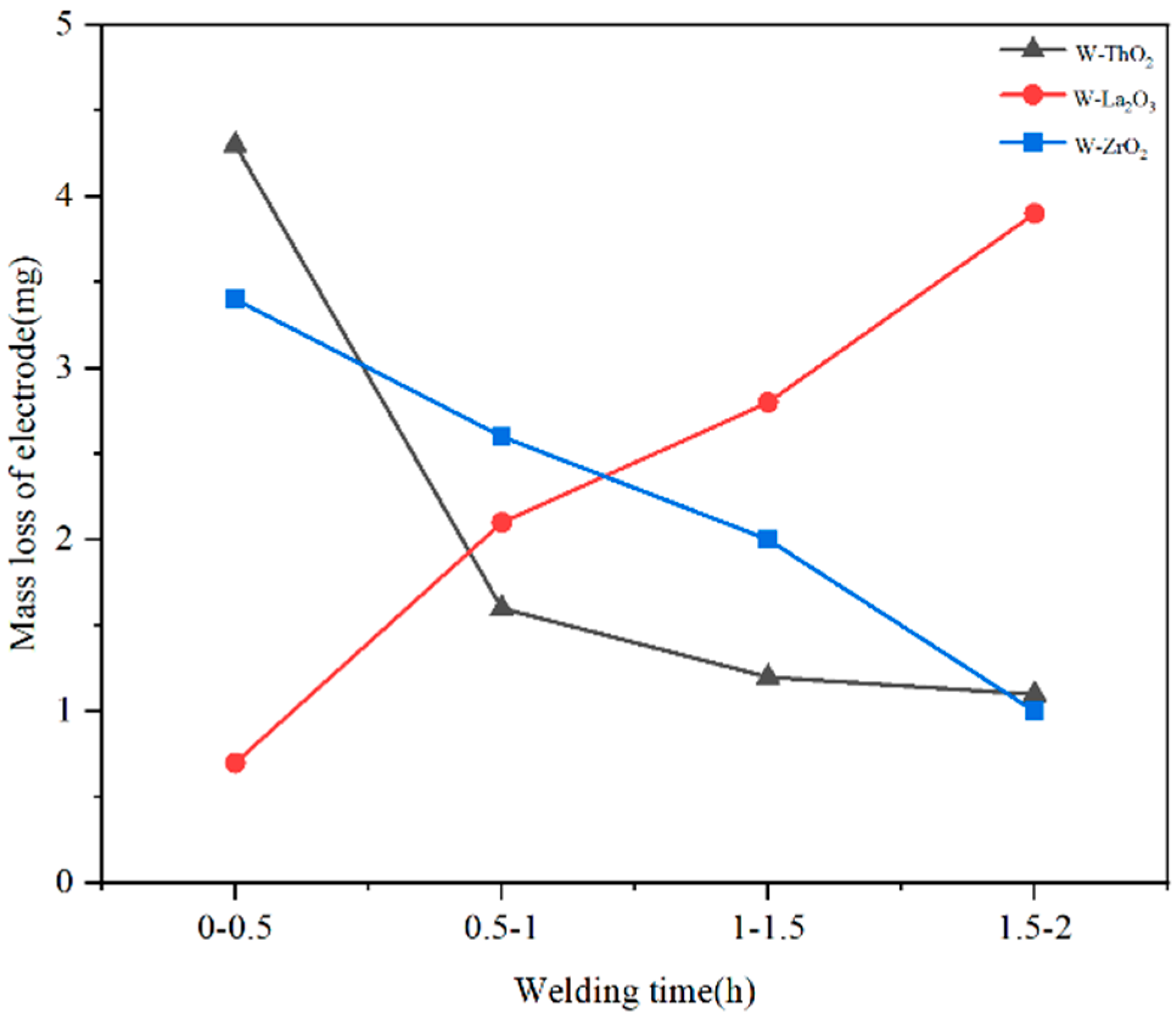
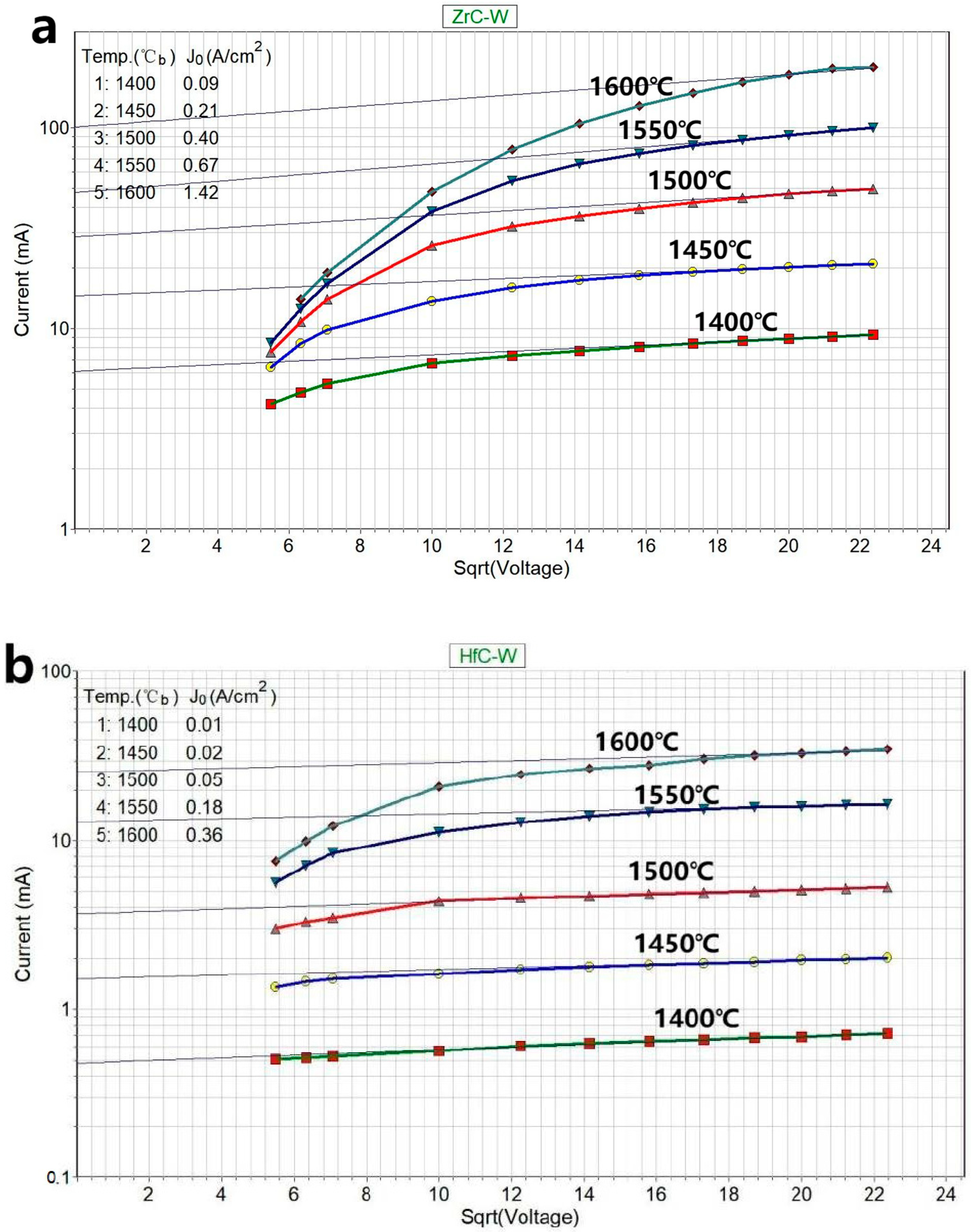
3.3. Testing the Emission Stability of Several Materials
3.3.1. Testing in a Protective Atmosphere
3.3.2. Testing in a Vacuum Environment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kirkwood, D.M.; Gross, S.J.; Balk, T.J.; Beck, M.J.; Booske, J.; Busbaher, D.; Jacobs, R.; Kordesch, M.E.; Mitsdarffer, B.; Morgan, D.; et al. Frontiers in Thermionic Cathode Research. IEEE Trans. Electron Devices 2018, 65, 2061–2071. [Google Scholar] [CrossRef]
- Liu, X.; Vancil, B.K.; Durham, D.B.; Ogletree, D.F.; Barnard, E.S.; Balk, T.J. Observation of Ultrathin Sc-Containing Surface Layer on Life-Tested Scandate Cathodes. IEEE Trans. Electron Devices 2023, 70, 2876–2882. [Google Scholar] [CrossRef]
- Nemchinsky, V. Erosion of thermionic cathodes in welding and plasma arc cutting systems. IEEE Trans. Plasma Sci. 2013, 42, 199–215. [Google Scholar] [CrossRef]
- Ives, R.L.; Falce, L.R.; Miram, G.; Collins, G. Controlled-Porosity Cathodes for High-Current-Density Applications. IEEE Trans. Plasma Sci. 2010, 38, 1345–1353. [Google Scholar] [CrossRef]
- Swartzentruber, P.D. Microstructure and Work Function of Dispenser Cathode Coatings: Effects on Thermionic Emission. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2014. [Google Scholar]
- Liu, X.; Zhou, Q.; Maxwell, T.L.; Vancil, B.K.; Beck, M.J.; Balk, T.J. Scandate cathode surface characterization: Emission testing, elemental analysis and morphological evaluation. Mater. Charact. 2019, 148, 188–200. [Google Scholar] [CrossRef]
- Liu, X. Characterization of Nanostructure, Materials, and Electron Emission Performance of Next-Generation Thermionic Scandate Cathodes. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2019. [Google Scholar]
- Liu, X.; Vancil, B.K.; Beck, M.J.; Balk, T.J. Near-Surface Material Phases and Microstructure of Scandate Cathodes. Materials 2019, 12, 636. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, X.; Maxwell, T.; Vancil, B.; Balk, T.J.; Beck, M.J. BaxScyOz on W (0 0 1), (1 1 0), and (1 1 2) in scandate cathodes: Connecting to experiment via μO and equilibrium crystal shape. Appl. Surf. Sci. 2018, 458, 827–838. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Y.; Dong, L.; Liu, Z.; Liu, P.; Cai, Y.; Liu, H.; Wang, J. Vacuum evaporation synthesizing Sc film layer and elucidation of near-surface Ba-O-Sc electronic configuration for dispenser cathode. Appl. Surf. Sci. 2024, 679, 161241. [Google Scholar] [CrossRef]
- Li, N.; Zhang, K.; Feng, J. Effect of Sr-Doping on the Emission-Active Materials of Scandate Cathodes. IEEE Trans. Electron Devices 2023, 70, 5883–5889. [Google Scholar] [CrossRef]
- Paschen, P. Alternatives to thorium additions to tungsten-based materials. JOM 1996, 48, 45–47. [Google Scholar] [CrossRef]
- Gäfvert, T.; Pagels, J.; Holm, E. Thorium exposure during tungsten inert gas welding with thoriated tungsten electrodes. Radiat. Prot. Dosim. 2003, 103, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-Y.; Yang, Y.-F.; Zhang, X.-K.; Li, S.-L.; Hu, P.; Wang, J.-S. A review on recent progress of thermionic cathode. Tungsten 2020, 2, 289–300. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, Y.; Zhou, Y.; Wang, S.; Meng, J.; Cao, N.; Shi, W. Study on Morphology and Chemical States of Surface Active Layer of Th-W Cathode. Materials 2022, 15, 2726. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Y.; Sun, J.; Liu, H.; Li, Z.; Wang, J. Cathodes in magnetrons: A. review. MetalMat 2024, 1, e14. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, Y.; Chen, S.; Zhang, T.; Lv, Z.; Luo, Z. Focusing cathode tip characteristics in cooling tungsten. Energy 2019, 167, 982–993. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.C.; Cao, J.; Li, Y. Development and application of tungsten electrode materials. Mater. Sci. Forum 2015, 817, 348–354. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Zhou, C.; Wei, Y.; Kong, C.; Zheng, X.; Zhang, X.; Yang, Z.; Zheng, J.; Cong, Y.; et al. Endurance-test and theoretical prediction of a rare earth nanocathode for the applied field magnetoplasmadynamic thruster. Plasma Sci. Technol. 2023, 25, 104005. [Google Scholar] [CrossRef]
- Zhou, S.; Liang, S.; Guo, Y.; Yang, J.; Nie, Z. Study on microstructure and migration mechanism of LaW cathode. Mater. Charact. 2023, 195, 112547. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, Y.; Zhou, Y.; Wang, S.; Meng, J. Electron emission mechanism of scandium-tungsten cathodes. Int. J. Refract. Met. Hard Mater. 2022, 105, 105828. [Google Scholar] [CrossRef]
- Wang, X.; D’avella, S.; Liang, Z.; Zhang, B.; Wu, J.; Zscherpel, U.; Tripicchio, P.; Yu, X. On the effect of the attention mechanism for automatic welding defects detection based on deep learning. Expert Syst. Appl. 2025, 268, 126386. [Google Scholar] [CrossRef]
- Kottke, N.G.; Tajmar, M.; Hey, F.G. Hollow cathode testing of Y2O3, La2O3-doped tungsten and LaB6 emitters with krypton and iodine. Vacuum 2024, 220, 112812. [Google Scholar] [CrossRef]
- Gadzhiev, M.K.; Sargsyan, M.A.; Tereshonok, D.V.; Tyuftyaev, A.S. Investigation of the argon arc binding to the lanthanated tungsten cathode. Europhys. Lett. 2015, 111, 25001. [Google Scholar] [CrossRef]
- Dong, L.; Wang, J.; Liu, W.; Li, C.; Zhang, J.; Yang, Y.; Zhou, F. Fabrication and thermionic emission properties of lanthanum carbide doped tungsten cathodes. Mater. Lett. 2015, 146, 47–50. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Liang, S.; Yang, J.; Nie, Z. Effect of ZrH2 Doping on Electron Emission Performance of Rare Earth Tungsten Electrode. Coatings 2023, 13, 666. [Google Scholar] [CrossRef]
- Yang, J.; Nie, Z.; Wang, Y. Microstructure and emission ability of rare earth oxides doped molybdenum cathodes. Appl. Surf. Sci. 2003, 215, 87–95. [Google Scholar] [CrossRef]
- Luo, L.; Shi, J.; Lin, J.; Zan, X.; Zhu, X.; Xu, Q.; Wu, Y. Microstructure and performance of rare earth element-strengthened plasma-facing tungsten material. Sci. Rep. 2016, 6, 32701. [Google Scholar] [CrossRef] [PubMed]
- Petriceanu, M.; Ioniță, F.G.; Piticescu, R.R.; Nicoară, A.I.; Matei, A.C.; Ioța, M.A.; Tudor, I.A.; Caramarin, Ș.; Ciobota, C.F. Effect of Doping ZrO2 on Structural and Thermal Properties. Inorganics 2024, 12, 290. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Z.; Wang, M.; Deng, H.; Yang, J.; Jiang, Y.; Zhang, T.; Wang, X.; Fang, Q.; Liu, C. The superior thermal stability and tensile properties of hot rolled W-HfC alloys. Int. J. Refract. Met. Hard Mater. 2019, 81, 42–48. [Google Scholar] [CrossRef]
- Gheribi, A.; Audet, C.; Le Digabel, S.; Bélisle, E.; Bale, C.; Pelton, A. Calculating optimal conditions for alloy and process design using thermodynamic and property databases, the FactSage software and the Mesh Adaptive Direct Search algorithm. Calphad 2012, 36, 135–143. [Google Scholar] [CrossRef]
- Yuan, J.; Dai, J.-Q.; Zhao, M.-W.; Zhong, Y.-Y.; Deng, D.-W. First-principles study on thermodynamic stability and electronic structures of the ferroelectric binary HfO2 and ZrO2 (001) polar surfaces. Surf. Interfaces 2025, 56, 105523. [Google Scholar] [CrossRef]
- Mokhena, T.C.; John, M.J.; Mochane, M.J.; Mtibe, A. Zirconia-based nanocomposites. In Handbook of Polymer and Ceramic Nanotechnology; Springer International Publishing: Cham, Switzerland, 2021; pp. 1505–1525. [Google Scholar]
- Li, M.; Zhao, N.; Wang, C.; Zou, Y.; Chen, X.; Mei, Y. Improving thermal stability and electrochemical performance of polymer solid electrolyte membranes with a novel TiO2@ PA-APP composite additive. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135640. [Google Scholar] [CrossRef]
- Cavaliere, P.; Sadeghi, B.; Shabani, A. Spark plasma sintering: Process fundamentals. In Spark Plasma Sintering of Materials: Advances in Processing and Applications; Springer: Cham, Switzerland, 2019; pp. 3–20. [Google Scholar]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Al Aqeeli, N.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark plasma sintering of metals and metal matrix nanocomposites: A review. J. Nanomater. 2012, 2012, 983470. [Google Scholar] [CrossRef]
- Veleva, L.; Schaeublin, R.; Battabyal, M.; Plociski, T.; Baluc, N. Investigation of microstructure and mechanical properties of W–Y and W–Y2O3 materials fabricated by powder metallurgy method. Int. J. Refract. Met. Hard Mater. 2015, 50, 210–216. [Google Scholar] [CrossRef]
- Ushio, M. Arc discharge and electrode phenomena. Pure Appl. Chem. 1988, 60, 809–814. [Google Scholar] [CrossRef][Green Version]
- Sadek, A.A.; Ushio, M.; Matsuda, F. Effect of rare earth metal oxide additions to tungsten electrodes. Metall. Trans. A 1990, 21, 3221–3236. [Google Scholar] [CrossRef]
- Ushio, M.; Sadek, A.A.; Matsuda, F. Comparison of temperature and work functionmeasurements obtained with different GTAelectrodes. Plasma Chem. Plasmaprocess. 1991, 11, 81–101. [Google Scholar] [CrossRef]
- Xie, Z.M.; Liu, R.; Zhang, T.; Fang, Q.F.; Liu, C.S.; Liu, X.; Luo, G.N. Achieving high strength/ductility in bulk W-Zr-Y2O3 alloy plate with hybrid microstructure. Mater. Des. 2016, 107, 144–152. [Google Scholar] [CrossRef]
- Haidar, J. A theoretical model for gas metal arc welding and gas tungsten arc welding. I. J. Appl. Phys. 1998, 84, 3518–3529. [Google Scholar] [CrossRef]
| T/°C | 1200 ~ 1400 | 1400~ 1600 | 1600~ 1800 | 1800~ 2000 | 2000~ 2200 | 2200~ 2400 | 2400~ 2600 | 2600~ 2800 | 2800~ 3000 | 3000~ 3200 | 3200~ 3400 | 3400 + | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Materials | |||||||||||||
| Th-W | ThO2(s) | ThO2(l) | |||||||||||
| La-W | La2O3(s) | La2O3(l) | |||||||||||
| Y-W | Y2O3(s) | Y2O3(l) | |||||||||||
| Ti-W | TiO2(s) | TiO2(l) | |||||||||||
| Zr-W | ZrO2(s) | ZrO2(l) | |||||||||||
| Hf-W | HfO2(s) | HfO2(l) | |||||||||||
| Gd-W | Gd2O3(s) | Gd2O3(l) | |||||||||||
| Lu-W | Lu2O3(s) | Lu2O3(l) | |||||||||||
| Er-W | Er2O3(s) | Er2O3(l) | |||||||||||
| Color | Description | ||||||||||||
| solid oxide | |||||||||||||
| liquid oxide | |||||||||||||
| T/°C | 1200 ~ 1400 | 1400 ~ 1600 | 1600~ 1800 | 1800~ 2000 | 2000~ 2200 | 2200~ 2400 | 2400~ 2600 | 2600~ 2800 | 2800~ 3000 | 3000~ 3200 | 3200~ 3400 | 3400 + | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Materials | ||||||||||||||
| Th-W | ThC2(s) | Th(l) | ||||||||||||
| La-W | LaC2(s) | La(l) | ||||||||||||
| Y-W | YC2(s) | Y(l) | ||||||||||||
| Ti-W | TiC(s) | |||||||||||||
| Zr-W | ZrC(s) | |||||||||||||
| Hf-W | HfC(s) | |||||||||||||
| Color | Description | |||||||||||||
| solid carbide | ||||||||||||||
| liquid element | ||||||||||||||
| T/°C | 1200 ~ 1400 | 1400 ~ 1600 | 1600 ~ 1800 | 1800 ~ 2000 | 2000 ~ 2200 | 2200 ~ 2400 | 2400 ~ 2600 | 2600 ~ 2800 | 2800 ~ 3000 | 3000 ~ 3200 | 3200 ~ 3400 | 3400 + | Carbide : Oxide | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Materials | ||||||||||||||||||
| Th-W | ThC2(s) ThO2(s) | ThO2(s) Th(l) | Th(l) | (a) 1:1 | ||||||||||||||
| La-W | LaC2(s) La2O3(s) | La2O3(s) La(l) | La(l) La2O3(l) | La(l) | La(g) | |||||||||||||
| Y-W | Y2O3(s) YC2(s) | Y2O3(s) Y(l) | Y2O3(l) Y(l) | Y(g) | ||||||||||||||
| Ti-W | TiC(s) TiO2(s) | TiC(s) TiO2(l) | Ti(l) TiO2(l) | Ti(l) | Ti(g) | |||||||||||||
| Zr-W | ZrO2(s) ZrC(s) | ZrC(s) ZrO2(l) | Zr(l) ZrO2(l) | |||||||||||||||
| Hf-W | HfC(s) HfO2(s) | HfC(s) HfO2(l) | ||||||||||||||||
| Th-W | ThC2(s) ThO2(s) | ThO2(s) Th(l) | Th(l) | (b) 1:2 | ||||||||||||||
| La-W | LaC2(s) La2O3(s) | La2O3(s) La(l) | La(l) La2O3(l) | La(l) La(g) | ||||||||||||||
| Y-W | Y2O3(s) YC2(s) | Y2O3(s) Y(l) | Y2O3(l) Y(l) | Y(l) Y(g) | ||||||||||||||
| Ti-W | TiC(s) TiO2(s) | TiC(s) TiO2(l) | Ti(l) TiO2(l) | TiO2(l) Ti(g) | Ti(g) | |||||||||||||
| Zr-W | ZrO2(s) ZrC(s) | ZrC(s) ZrO2(l) | Zr(l) ZrO2(l) | |||||||||||||||
| Hf-W | HfC(s) HfO2(s) | HfC(s) HfO2(l) | ||||||||||||||||
| Th-W | ThC2(s) ThO2(s) | ThO2(s) Th(l) | Th(l) | (c) 2:1 | ||||||||||||||
| La-W | LaC2(s) La2O3(s) | La2O3(s) La(l) | La(l) La2O3(l) | La(l) | La(g) | |||||||||||||
| Y-W | Y2O3(s) YC2(s) | Y2O3(s) Y(l) | Y2O3(l) Y(l) | Y(g) | ||||||||||||||
| Ti-W | TiC(s) TiO2(s) | TiC(s) TiO2(l) | Ti(l) TiC(s) | Ti(g) TiC(s) | ||||||||||||||
| Zr-W | ZrO2(s) ZrC(s) | ZrC(s) ZrO2(l) | Zr(l) ZrC(s) | |||||||||||||||
| Hf-W | HfC(s) HfO2(s) | HfC(s) HfO2(l) | ||||||||||||||||
| Color | Description | |||||||||||||||||
| solid +solid | ||||||||||||||||||
| liquid + solid | ||||||||||||||||||
| liquid + liquid | ||||||||||||||||||
| gaseous + liquid | ||||||||||||||||||
| geseous | ||||||||||||||||||
| liquid | ||||||||||||||||||
| gaseous + solid | ||||||||||||||||||
| T/°C | 1000 ~ 1100 | 1100 ~ 1200 | 1200 ~ 1300 | 1300 ~ 1400 | 1400 ~ 1500 | 1500 ~ 1600 | 1600 ~ 1700 | 1700 ~ 1800 | 1800 ~ 1900 | 1900 ~ 2000 | 2000 + | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Materials | ||||||||||||
| Th-W | ThO2(s) | ThO2(g) | ||||||||||
| La-W | La2O3(s) | La(g) | ||||||||||
| Y-W | Y2O3(s) | Y(g) | ||||||||||
| Ti-W | TiO2(s) | TiO2(g) | Ti(g) | |||||||||
| Zr-W | ZrO2(s) | ZrO2(g) | ||||||||||
| Hf-W | HfO2(s) | |||||||||||
| Gd-W | Gd2O3(s) | Gd(g) | ||||||||||
| Lu-W | Lu2O3(s) | Lu(g) | ||||||||||
| Er-W | Er2O3(s) | Er(g) | ||||||||||
| Color | Description | |||||||||||
| solid oxide | ||||||||||||
| gaseous element | ||||||||||||
| gaseous oxide | ||||||||||||
| T/°C | 1000 ~ 1100 | 1100 ~ 1200 | 1200 ~ 1300 | 1300 ~ 1400 | 1400 ~ 1500 | 1500 ~ 1600 | 1600 ~ 1700 | 1700 ~ 1800 | 1800 ~ 1900 | 1900 ~ 2000 | 2000 + | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Materials | ||||||||||||
| Th-W | ThC2(s) | Th(g) | ||||||||||
| La-W | LaC2(s) | La(g) | ||||||||||
| Y-W | YC2(s) | Y(g) | ||||||||||
| Ti-W | TiC(s) | Ti(g) | ||||||||||
| Zr-W | ZrC(s) | Zr(g) | ||||||||||
| Hf-W | HfC(s) | |||||||||||
| Color | Description | |||||||||||
| solid carbide | ||||||||||||
| gaseous element | ||||||||||||
| T/°C | 1000 ~ 1100 | 1100 ~ 1200 | 1200 ~ 1300 | 1300 ~ 1400 | 1400 ~ 1500 | 1500 ~ 1600 | 1600 ~ 1700 | 1700 ~ 1800 | 1800 ~ 1900 | 1900 ~ 2000 | 2000 + | Carbide : Oxide | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Materials | ||||||||||||||||||
| Th-W | ThC2(s) ThO2(s) | ThC2(s)Th(s) | ThC2(s)Th(g) | Th(g) | (a) 1:1 | |||||||||||||
| La-W | LaC2(s)La2O3(s) | La(g) | ||||||||||||||||
| Y-W | Y2O3(s) YC2(s) | Y(g) | ||||||||||||||||
| Ti-W | TiC(s)TiO2(s) | TiO2(s) Ti(g) | Ti(g) | |||||||||||||||
| Zr-W | ZrC(s) ZrO2(s) | ZrO2(s)Zr(s) | ZrO2(s)Zr(g) | Zr(g) | ||||||||||||||
| Hf-W | HfC(s) HfO2(s) | Hf(g) HfO2(s) | ||||||||||||||||
| Th-W | ThC2(s) ThO2(s) | ThO2(s)Th(s) | ThO2(s)Th(g) | Th(g) | (b) 1:2 | |||||||||||||
| La-W | LaC2(s)La2O3(s) | La(g) La2O3(s) | La(g) | |||||||||||||||
| Y-W | Y2O3(s) YC2(s) | Y(g) Y2O3(s) | Y(g) | |||||||||||||||
| Ti-W | TiC(s)TiO2(s) | TiO2(s) Ti(g) | Ti(g) | |||||||||||||||
| Zr-W | ZrC(s) ZrO2(s) | ZrO2(s)Zr(s) | ZrO2(s)Zr(g) | Zr(g) | ||||||||||||||
| Hf-W | HfC(s) HfO2(s) | Hf(g) HfO2(s) | ||||||||||||||||
| Th-W | ThC2(s) ThO2(s) | ThC2(s)Th(s) | ThC2(s) Th(g) | Th(g) | (c) 1:3 | |||||||||||||
| La-W | LaC2(s)La2O3(s) | La(g) | ||||||||||||||||
| Y-W | Y2O3(s) YC2(s) | Y(g) | ||||||||||||||||
| Ti-W | TiC(s)TiO2(s) | TiC(s) Ti(g) | Ti(g) | |||||||||||||||
| Zr-W | ZrC(s) ZrO2(s) | ZrC(s)Zr(s) | ZrC(s)Zr(g) | Zr(g) | ||||||||||||||
| Hf-W | HfC(s) HfO2(s) | HfC(s) Hf(g) | Hf(g) | |||||||||||||||
| Color | Description | |||||||||||||||||
| solid +solid | ||||||||||||||||||
| solid+solid element | ||||||||||||||||||
| solid+gaseous element | ||||||||||||||||||
| gaseous element | ||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, J.; Zhang, P.; Nie, Z. Thermal Stability Calculation of Typical Phases in Tungsten Cathodes. Metals 2025, 15, 254. https://doi.org/10.3390/met15030254
Wang J, Yang J, Zhang P, Nie Z. Thermal Stability Calculation of Typical Phases in Tungsten Cathodes. Metals. 2025; 15(3):254. https://doi.org/10.3390/met15030254
Chicago/Turabian StyleWang, Jiaxuan, Jiancan Yang, Po Zhang, and Zuoren Nie. 2025. "Thermal Stability Calculation of Typical Phases in Tungsten Cathodes" Metals 15, no. 3: 254. https://doi.org/10.3390/met15030254
APA StyleWang, J., Yang, J., Zhang, P., & Nie, Z. (2025). Thermal Stability Calculation of Typical Phases in Tungsten Cathodes. Metals, 15(3), 254. https://doi.org/10.3390/met15030254





