Rare Earth Metal Ion-Associates in Ln3+—CO32−—H2O System
Abstract
1. Introduction
- 1.
- By Mass
- Light (La–Nd);
- Medium (Sm–Gd);
- Heavy (Dy–Lu, Y).
- 2.
- By Importance, to eliminate the disbalance between global production and consumption of individual REEs
- Critical (Nd, Eu, Tb, Dy, Er, Y);
- Non-critical (La, Pr, Sm, Gd, Lu);
- Surplus (Ce, Ho, Tm, Yb).
2. Materials and Methods
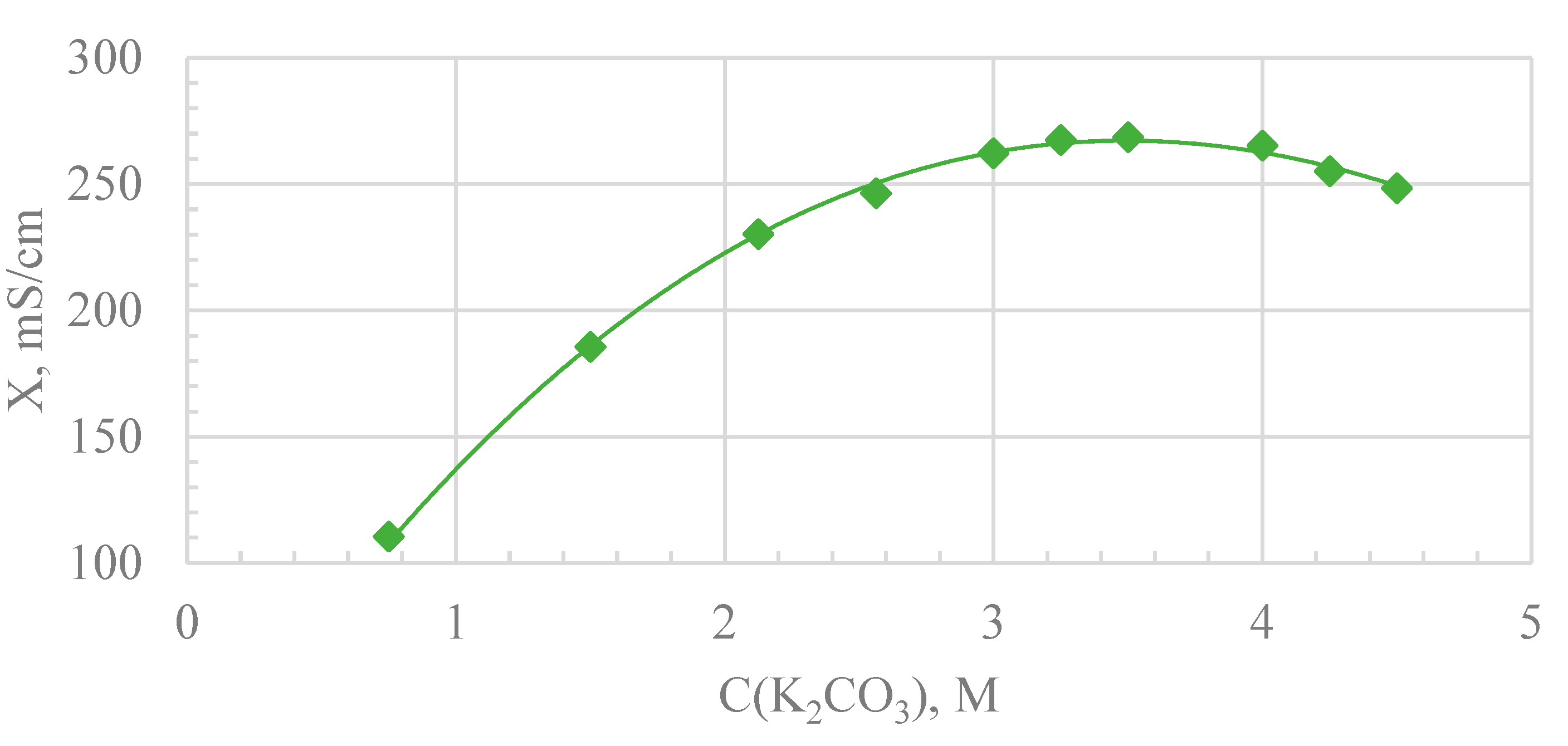
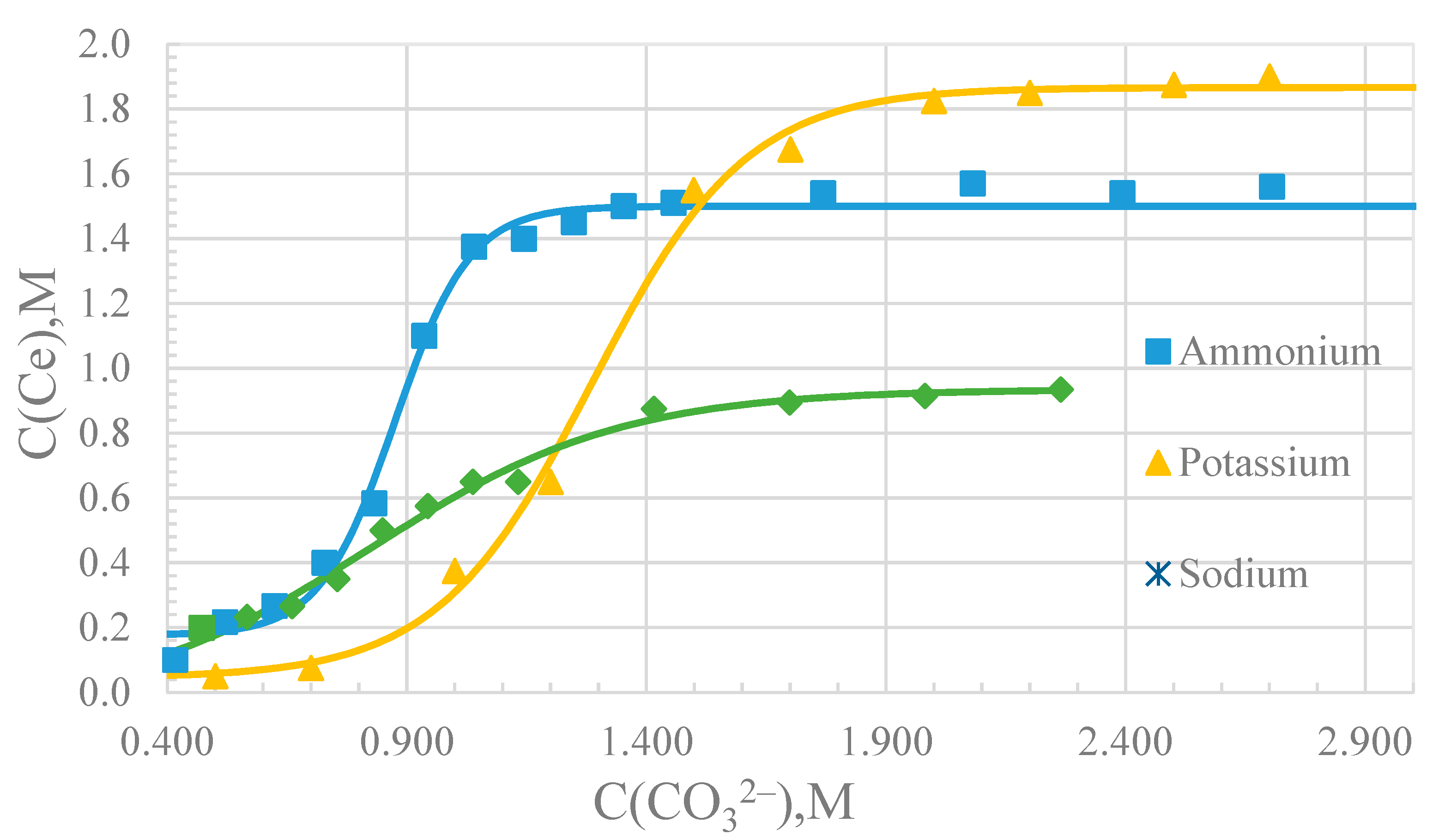
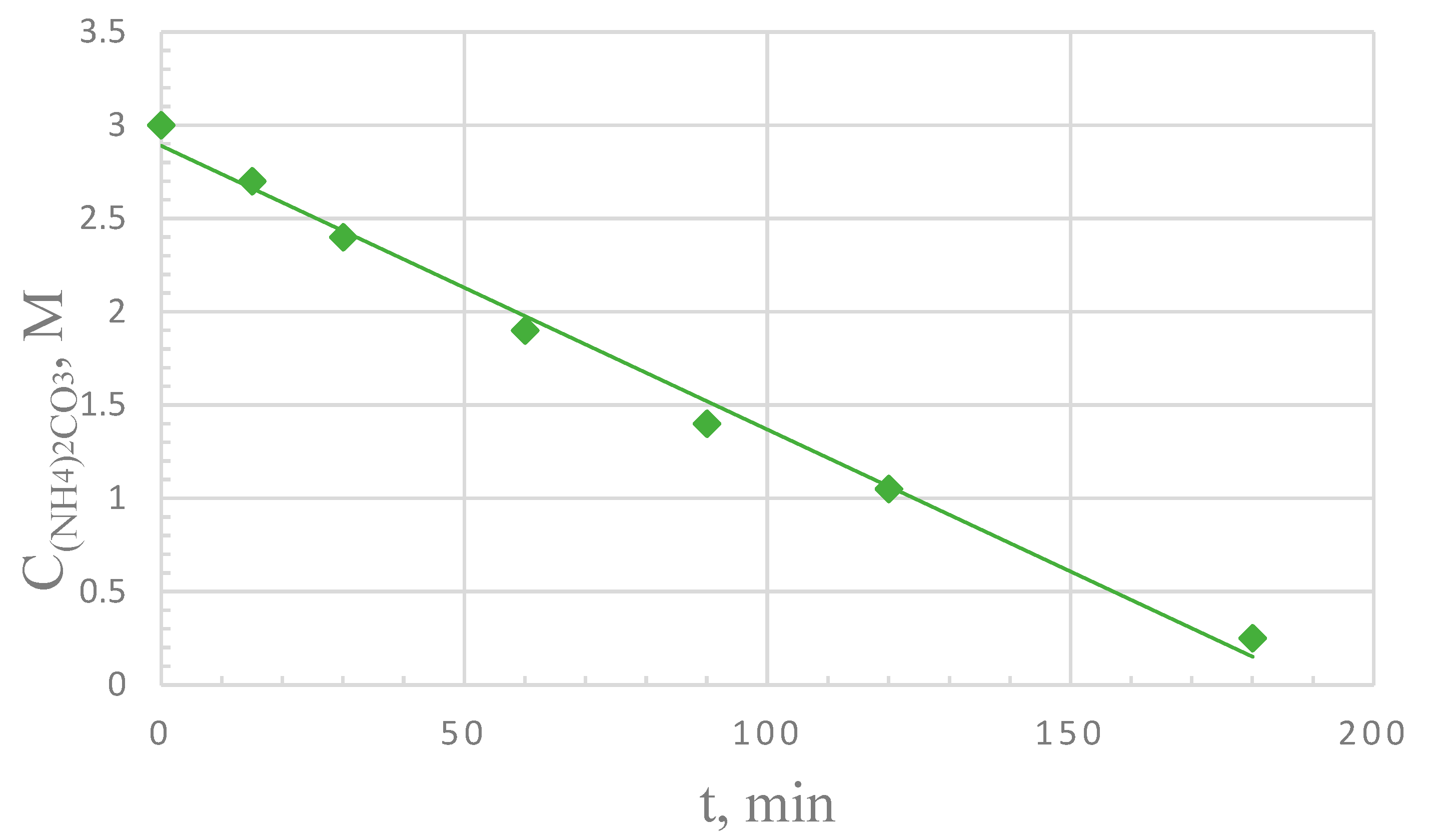
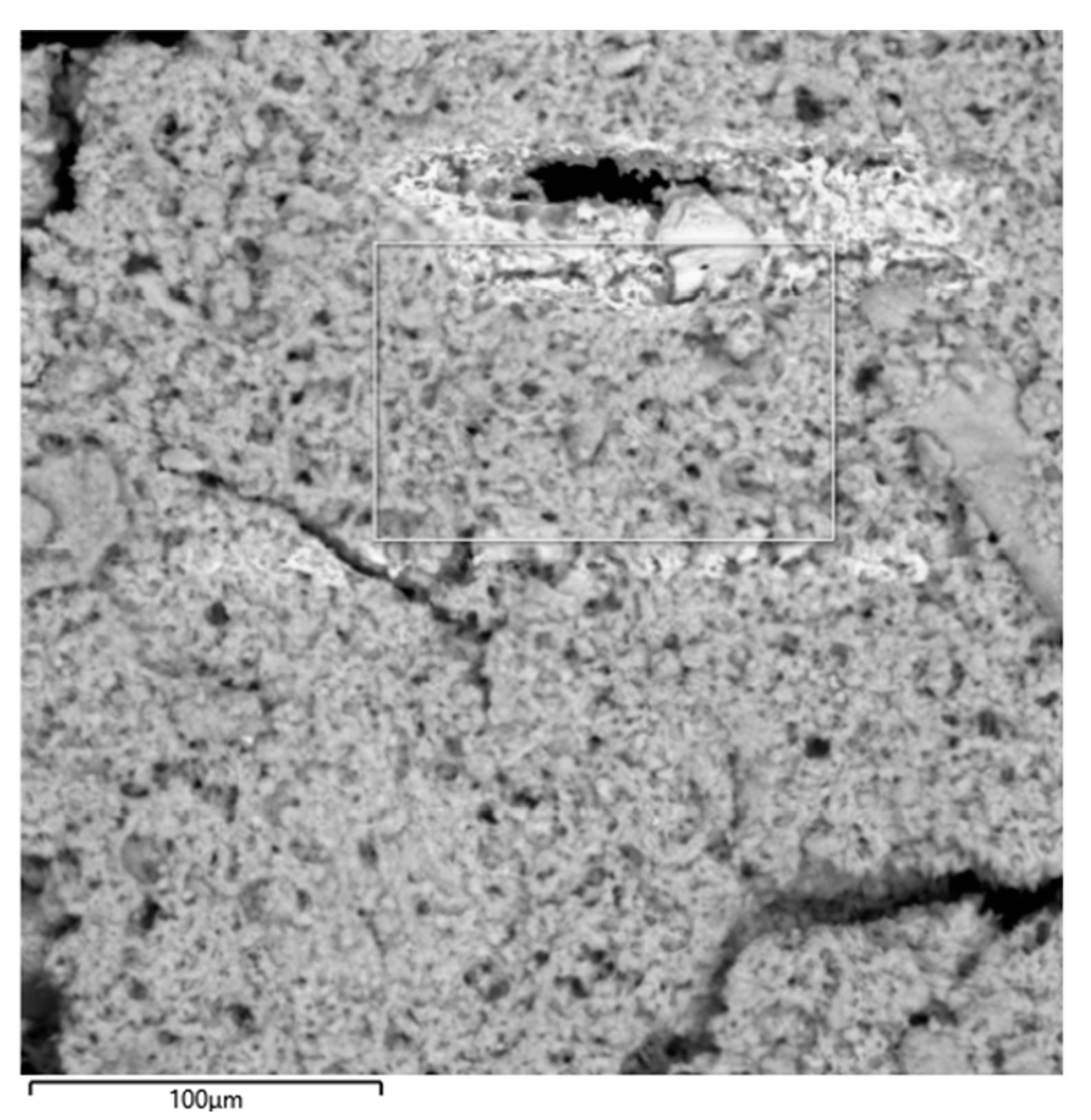
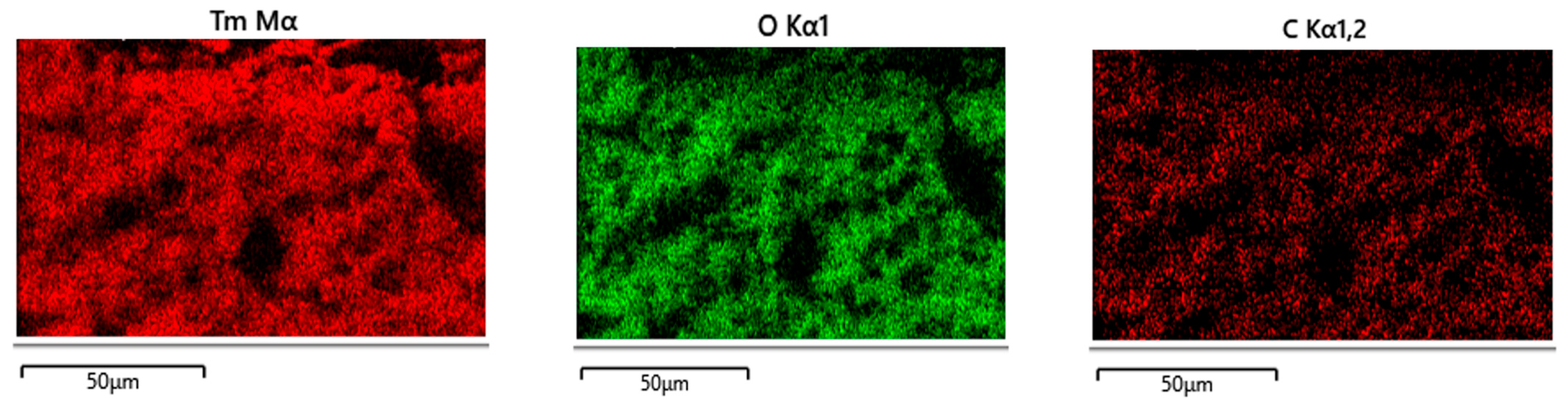
3. Results
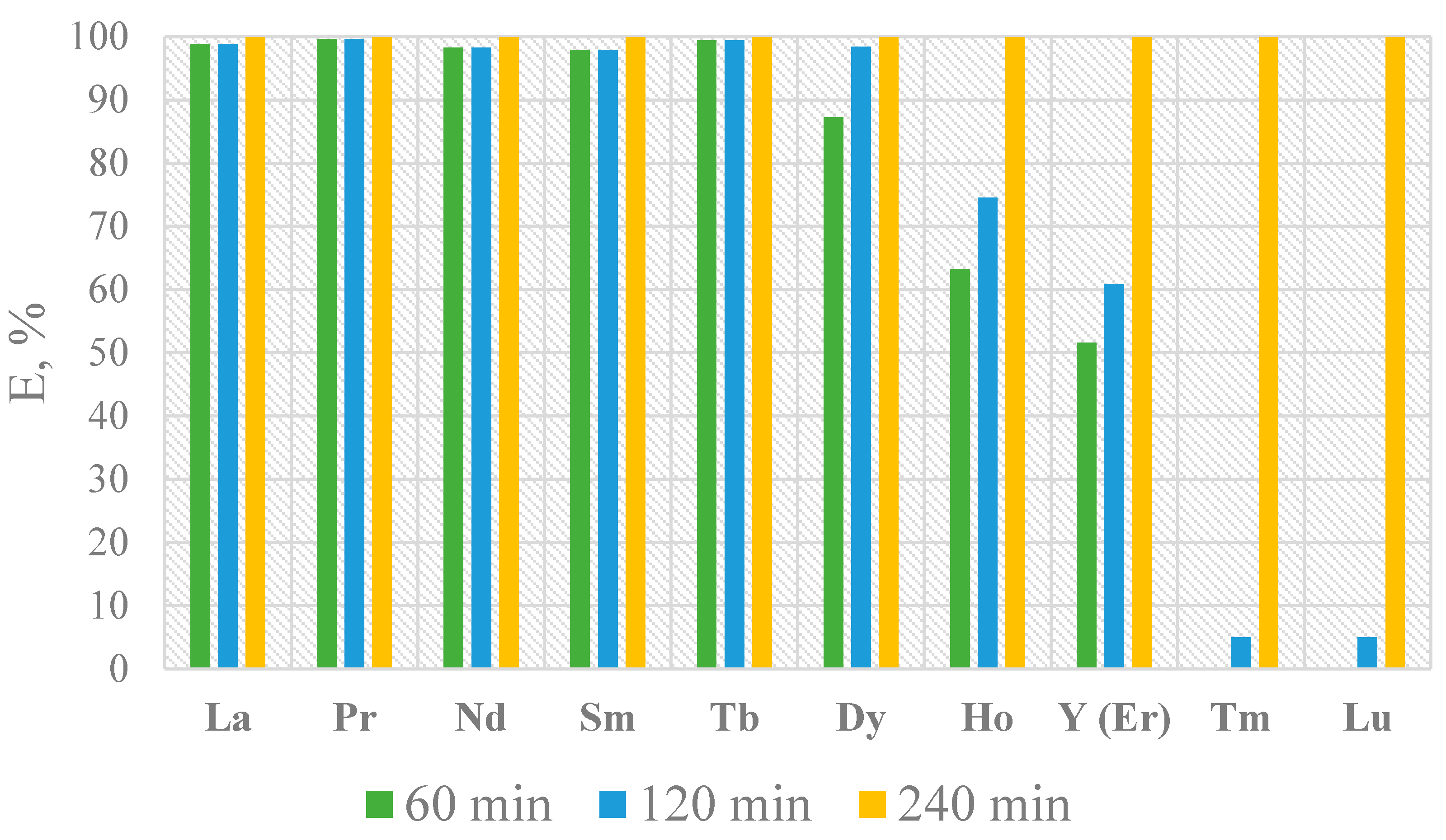
- Yield of Ln2(CO3)3 after 60 min: La—98.8 %; Nd—98.3 %; Pr—99.6 %; Sm—97.9 %; Tb—99.4 %; Dy—87.2 %; Ho—63.3 %; Y (Er)—51.6 %; Tm—0 %; Lu—0 %.
- Yield of Ln2(CO3)3 after 120 min: La—98.8 %; Nd—98.3 %; Pr—99.6 %; Sm—97.9 %; Tb—99.4 %; Dy—98.4 %; Ho—74.5 %; Y (Er)—60.9 %; Tm—5 %; Lu—5 %.
- Yield of Ln2(CO3)3 at 240 min reached approximately 100 %, including Tm and Lu.
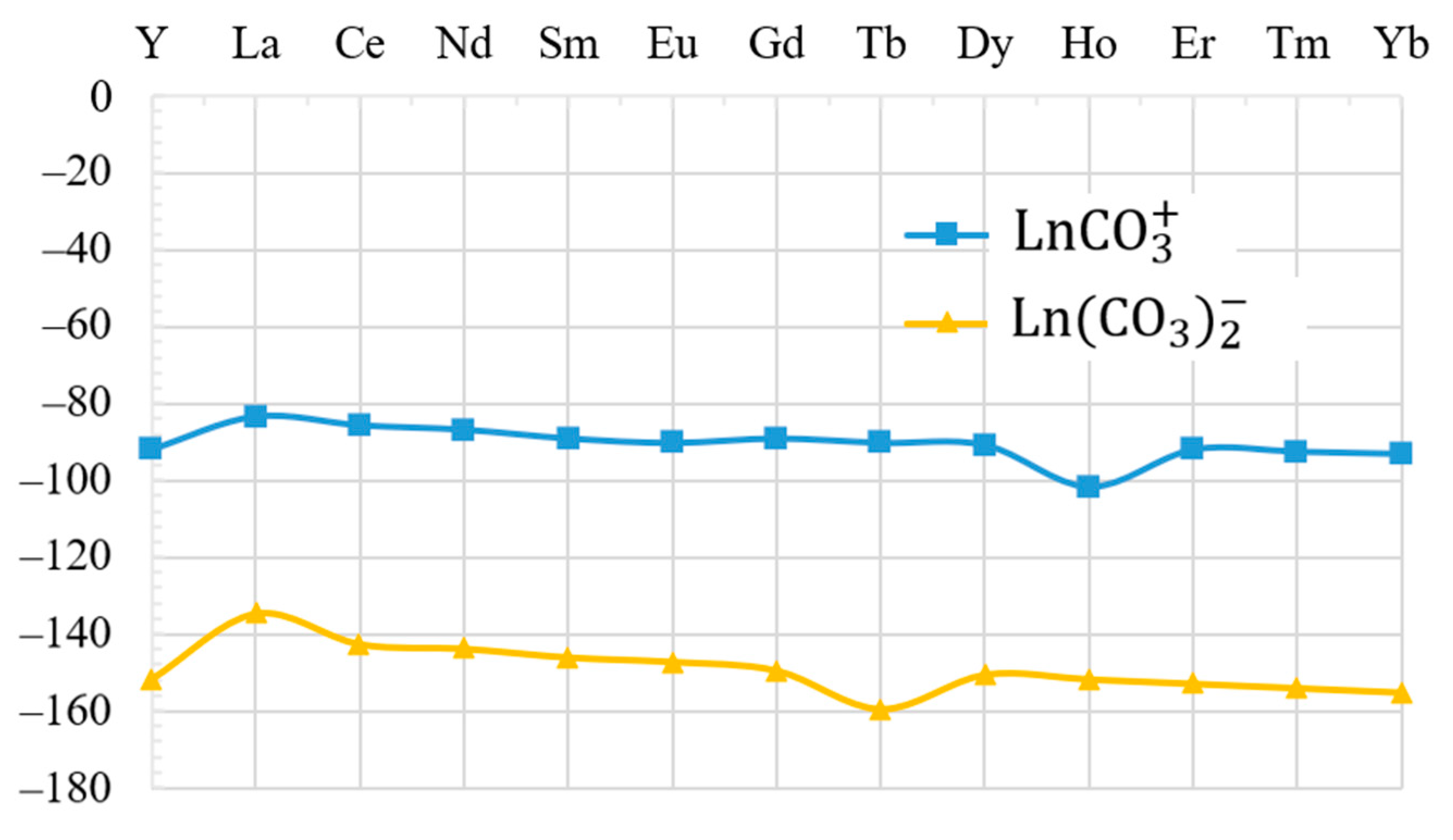
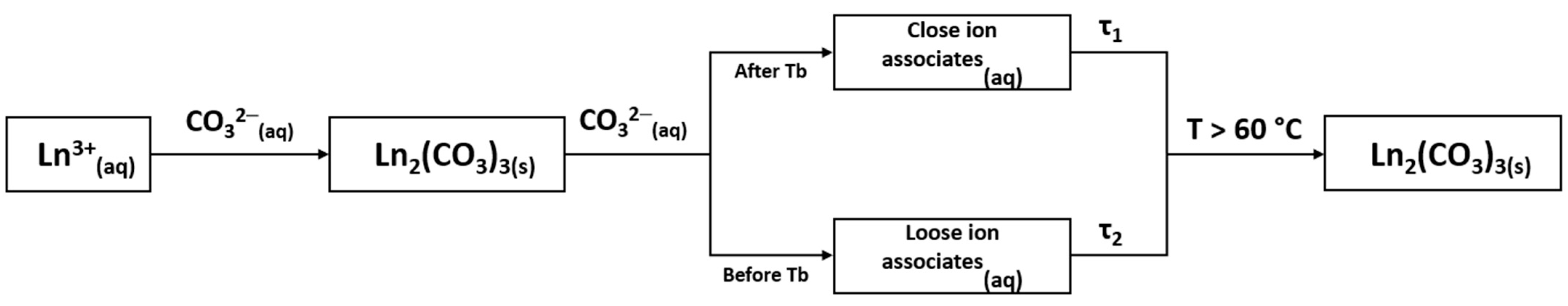
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cherepovitsyn, A.; Solovyova, V.; Dmitrieva, D. New challenges for the sustainable development of the rare-earth metals sector in Russia: Transforming industrial policies. Resour. Policy 2023, 81, 103347. [Google Scholar] [CrossRef]
- Cuadros-Muñoz, J.-R.; Jimber-del-Río, J.-A.; Sorhegui-Ortega, R.; Zea-De la Torre, M.; Vergara-Romero, A. Contribution of Rare Earth Elements Is Key to the Economy of the Future. Land 2024, 13, 1220. [Google Scholar] [CrossRef]
- Pathapati, S.V.S.H.; Free, M.L.; Sarswat, P.K. A Comparative Study on Recent Developments for Individual Rare Earth Elements Separation. Processes 2023, 11, 2070. [Google Scholar] [CrossRef]
- Leiva, C.; Arroyo-Torralvo, F.; Luna-Galiano, Y.; Villegas, R.; Vilches, L.F.; Fernández Pereira, C. Valorization of Bayer Red Mud in a Circular Economy Process: Valuable Metals Recovery and Further Brick Manufacture. Processes 2022, 10, 2367. [Google Scholar] [CrossRef]
- Saidakhmetov, P.; Piyanzina, I.; Faskhutdinova, A.; Nedopekin, O.; Adyrbekova, G.; Baiman, G.; Suyerkulova, Z.; Romanova, I. Ab Initio Magnetic Properties Simulation of Nanoparticles Based on Rare Earth Trifluorides REF3 (RE = Tb, Dy, Ho). Crystals 2023, 13, 1487. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.Á. Biohydrometallurgy for Rare Earth Elements Recovery from Industrial Wastes. Molecules 2021, 26, 6200. [Google Scholar] [CrossRef]
- Pirzada, M.D.S. Alternative Resources of Rare Earth Elements in Pakistan. Mater. Proc. 2024, 17, 26. [Google Scholar] [CrossRef]
- Amirshahi, S.; Jorjani, E. Preliminary Flowsheet Development for Mixed Rare Earth Elements Production from Apatite Leaching Aqueous Solution Using Biosorption and Precipitation. Minerals 2023, 13, 909. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Wade, B.P.; Gilbert, S.E.; Alford, R. Mineralogy and Distribution of REE in Oxidised Ores of the Mount Weld Laterite Deposit, Western Australia. Minerals 2023, 13, 656. [Google Scholar] [CrossRef]
- Mitrofanova, G.V.; Chernousenko, E.V.; Kompanchenko, A.A.; Kalugin, A.I. Specific action of collector from phosphoric acid alkyl esters class in flotation of apatite-nepheline ores. J. Min. Inst. 2024, 268, 637–645. [Google Scholar]
- Skublov, S.G.; Levashova, E.V.; Mamykina, M.E.; Gusev, N.I.; Gusev, A.I. The polyphase Belokurikhinsky granite massif, Gorny Altai: Isotope-geochemical study of zircon. J. Min. Inst. 2024, 268, 552–575. [Google Scholar]
- Pashkevich, M.A.; Danilov, A.S. Ecological security and sustainability. J. Min. Inst. 2023, 260, 153–154. [Google Scholar]
- Ma, F.; Chen, L.; Lin, Z.; Liu, Z.; Zhang, W.; Guo, R. Microstructure and Key Properties of Phosphogypsum-Red Mud-Slag Composite Cementitious Materials. Materials 2022, 15, 6096. [Google Scholar] [CrossRef] [PubMed]
- Prischepa, O.M.; Kireev, S.B.; Nefedov, Y.V.; Martynov, A.V.; Lutsky, D.S.; Krykova, T.N.; Sinitsa, N.; Xu, R. Theoretical and methodological approaches to identifying deep accumulations of oil and gas in oil and gas basins of the Russian Federation. Front. Earth Sci. 2023, 11, 1192051. [Google Scholar] [CrossRef]
- Mukaba, J.-L.; Eze, C.P.; Pereao, O.; Petrik, L.F. Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals 2021, 11, 1051. [Google Scholar] [CrossRef]
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Elbagory, M.; Shaker, E.M.; El-Nahrawy, S.; Omara, A.E.-D.; Khalifa, T.H. The Concurrent Application of Phosphogypsum and Modified Biochar as Soil Amendments Influence Sandy Soil Quality and Wheat Productivity. Plants 2024, 13, 1492. [Google Scholar] [CrossRef]
- Guan, Q.; Sui, Y.; Liu, C.; Wang, Y.; Zeng, C.; Yu, W.; Gao, Z.; Zang, Z.; Chi, R.-a. Characterization and Leaching Kinetics of Rare Earth Elements from Phosphogypsum in Hydrochloric Acid. Minerals 2022, 12, 703. [Google Scholar] [CrossRef]
- Ziying, C.; Zhan, L.; Jia, C.; Parashuram, K.; Fawzi, B.; Hongdeng, Q. Recent advances in selective separation technologies of rare earth elements: A review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar]
- Cheremisina, O.V.; Gorbacheva, A.A.; Balandinsky, D.A.; Luo, Y.; Ponomareva, M.A. Synergistic effect of a mixture of ethoxyphosphoric esters and sodium oleate in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133314. [Google Scholar] [CrossRef]
- Pyagai, I.; Zubkova, O.; Babykin, R.; Toropchina, M.; Fediuk, R. Influence of Impurities on the Process of Obtaining Calcium Carbonate during the Processing of Phosphogypsum. Materials 2022, 15, 4335. [Google Scholar] [CrossRef] [PubMed]
- Lutskiy, D.S.; Lukyantseva, E.S.; Mikheeva, V.Y.; Grigorieva, L.V. Investigation of the extraction of samarium and gadolinium from leaching solutions of phosphorus-containing raw materials using solid extractants. Arab J. Basic Appl. Sci. 2023, 30, 68–73. [Google Scholar] [CrossRef]
- Millero, F.J.; Schreiber, D.R. Use of the ion pairing model to estimate activity coefficients of the ionic components of natural waters. Am. J. Sci. 1982, 282, 1508–1540. [Google Scholar] [CrossRef]
- Byrne, R.H. Inorganic speciation of dissolved elements in seawater: The influence of pH on concentration ratios. Geochem. Trans. 2003, 3, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, B.P.; Sulyanova, E.A. Lanthanide Contraction in LnF3 (Ln = Ce-Lu) and Its Chemical and Structural Consequences: Part 2: Specialized Empirical System of R3+ (R = Y, La, and 14 Ln) and F1− Ionic Radii for RF3 Series. Int. J. Mol. Sci. 2023, 24, 17080. [Google Scholar] [CrossRef]
- Sukhanova, K.G. Trace elements in the silicate minerals of the Borodino Meteorite (H5). J. Min. Inst. 2024, 265, 16–33. [Google Scholar]
- Lan, Q.; Yang, Y.; Xie, Z.; Guo, H.; Liu, D.; Zhang, X. Molecular Dynamics Calculation of the Coordination Behavior of Yb (III) in Sodium Carbonate Solution. Processes 2023, 11, 2624. [Google Scholar] [CrossRef]
- Surkova, A.; Bogomolov, A.; Paderina, A.; Khistiaeva, V.; Boichenko, E.; Grachova, E.; Kirsanov, D. Milk Analysis using a New Optical Multisensor System Based on Lanthanide (III) Complexes. Eng. Proc. 2023, 48, 28. [Google Scholar] [CrossRef]
- Cantrell, K.; Byrne, R. Rare earth element complexation by carbonate and oxalate ions. Geochim. Cosmochim. Acta 1987, 51, 597–605. [Google Scholar] [CrossRef]
- Lobacheva, O.L. Ion Flotation of Ytterbium Water-Salt Systems—An Innovative Aspect of the Modern Industry. Water 2021, 13, 3493. [Google Scholar] [CrossRef]
- Firsching, F.; Mohammadzadei, J. Solubility products of the rare-earth carbonates. J. Chem. Eng. Data 1986, 31, 40–42. [Google Scholar] [CrossRef]
- Ohta, A. Experimental and theoretical studies of REE partitioning between Fe hydroxide and Mn oxide and seawater. Chikyukagaku (Geochem.) 2006, 40, 13–30, (In Japanese with English abstract). [Google Scholar]
- Olender, R.; Nitzan, A. Lattice theory of solvation and dissociation in macromolecular fluids. II. Quasichemical approximation. J. Chem. Phys. 1994, 101, 2338–2349. [Google Scholar] [CrossRef][Green Version]
- Finney, A.R.; Lectez, S.; Freeman, C.L.; Harding, J.H.; Stackhouse, S. Ion Association in Lanthanide Chloride Solutions. Chem. A Eur. J. 2019, 25, 8725–8740. [Google Scholar] [CrossRef] [PubMed]
- Smolyakov, B.; Veselova, G. Limiting Equivalent Conductivities of Ions in Water Between 25 and 200 C. Elektrokhim 1975, 11, 700–704. [Google Scholar]
- Ismail, I.M.; Masuko, Y.; Tomiyasu, H.; Fujii, Y. Conductivity of some rare earth chlorides in sub and supercritical aqueous solutions. J. Supercrit. Fluids 2003, 25, 69–79. [Google Scholar] [CrossRef]
- Kellermeier, M.; Raiteri, P.; Berg, J.K.; Kempter, A.; Gale, J.D.; Gebauer, D. Entropy drives calcium carbonate ion association. ChemPhysChem 2016, 17, 3535–3541. [Google Scholar] [CrossRef]
- Oleynik, I.L. Increasing the Processing Degree of Phosphate Raw Materials with the Associated Extraction of the Rare Earth Metals. The Dissertation Abstract for the Degree of Candidate of Technical Sciences. 2021. Available online: https://spmi.ru/sites/default/files/imci_images/sciens/dissertacii/2021/oleynik_avtoreferat.pdf (accessed on 17 February 2025).
- Pawlowicz, R. Calculating the conductivity of natural waters. Limnol. Oceanogr. Methods 2008, 6, 489–501. [Google Scholar] [CrossRef]
- Fang, Y. Ions Interacting with Macromolecules NMR Studies in Solution. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2017; p. 71. [Google Scholar]
- Litvinova, T.E.; Oleinic, I.S.; Lutskiy, D.S. Thermodynamic justification of the extraction of rare-earth metals during the carbonate conversion of secondary phosphate raw materials. ARPN J. Eng. Appl. Sci. 2020, 15, 2919–2924. [Google Scholar]


| REM | Ohta and Kawabe | Liu and Burne | REM | Ohta and Kawabe | Liu and Burne | ||||
|---|---|---|---|---|---|---|---|---|---|
| lgK1 | lgK2 | lgK1 | lgK2 | lgK1 | lgK2 | lgK1 | lgK2 | ||
| La | 8.33 | 12.52 | 6.52 | 11.58 | Dy | 8.95 | 14.38 | 7.33 | 13.18 |
| Ce | 8.58 | 13.06 | 6.86 | 12.05 | Ho | 8.96 | 14.48 | 7.32 | 13.27 |
| Pr | 8.73 | 13.43 | 7.03 | 12.37 | Er | 9.02 | 14.62 | 7.38 | 13.39 |
| Nd | 8.75 | 13.59 | 7.08 | 12.46 | Tm | 9.10 | 14.79 | 7.45 | 13.54 |
| Sm | 8.90 | 13.95 | 7.25 | 12.82 | Yb | 9.15 | 14.94 | 7.58 | 13.56 |
| Eu | 8.86 | 14.02 | 7.26 | 12.91 | Lu | 9.13 | 14.96 | 7.53 | 13.64 |
| Gd | 8.78 | 13.95 | 7.17 | 12.76 | Y | 8.88 | 14.29 | 7.25 | 12.90 |
| Tb | 8.88 | 14.22 | 7.23 | 13.05 | Sc | - | 17.41 | - | - |
| Input | Output | ||||
|---|---|---|---|---|---|
| № | Item | Mass, g | № | Item | Mass, g |
| 1 | Ammonium carbonate solution: | 219.40 | 1 | Productive solution: | 199.00 |
| Ammonium carbonate | 57.60 | Ammonium carbonate | 34.10 | ||
| Water | 161.80 | Ammonium nitrate | 0.96 | ||
| 2 | Terbium nitrate (III) solution: | 11.18 | Water | 163.94 | |
| Terbium nitrate (III) | 1.38 | 2 | Water | 11.06 | |
| Water | 9.8 | Water (evaporation) | 6.55 | ||
| Total: | 230.58 | Water from Equation (5) | 4.51 | ||
| 3 | Ammonia | 8.51 | |||
| 4 | Carbon dioxide | 11.02 | |||
| 5 | Terbium carbonate (III) | 0.99 | |||
| Total: | 230.58 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litvinova, T.; Gerasev, S.; Sergeev, V.; Lidanovskiy, E. Rare Earth Metal Ion-Associates in Ln3+—CO32−—H2O System. Metals 2025, 15, 239. https://doi.org/10.3390/met15030239
Litvinova T, Gerasev S, Sergeev V, Lidanovskiy E. Rare Earth Metal Ion-Associates in Ln3+—CO32−—H2O System. Metals. 2025; 15(3):239. https://doi.org/10.3390/met15030239
Chicago/Turabian StyleLitvinova, Tatiana, Stepan Gerasev, Vasiliy Sergeev, and Egor Lidanovskiy. 2025. "Rare Earth Metal Ion-Associates in Ln3+—CO32−—H2O System" Metals 15, no. 3: 239. https://doi.org/10.3390/met15030239
APA StyleLitvinova, T., Gerasev, S., Sergeev, V., & Lidanovskiy, E. (2025). Rare Earth Metal Ion-Associates in Ln3+—CO32−—H2O System. Metals, 15(3), 239. https://doi.org/10.3390/met15030239







