Steel slag is one of the main industrial solid wastes produced in the steel-making process. Approximately 100–150 kg of steel slag is produced per ton of crude steel. In 2022, China’s crude steel output reached 1.013 billion tons, producing about 120 million tons of steel slag. The cumulative stockpile exceeds 1 billion tons [
1]. However, as the overall utilization rate is relatively low, the problem of the efficient treatment of steel slag needs to be solved urgently, and there are broad prospects for its utilization. Its mineral composition includes iron phase, dicalcium silicate (C
2S), three calcium silicate (C
3S), olivine, and others [
2]. Slag also contains free calcium oxide (f-CaO). Volume expansion occurs during the hydration process, causing the product to crack, which seriously affects the safe use of tailing. Iron and steel enterprises mostly use cold slag storage treatment. Prolonged natural aging can reduce the f-CaO content, but there exists the problem of an excessively long aging time. The storage process requires large amounts of land, and the leaching of heavy metal ions can pollute underground water sources, thus causing harm to human health [
3].
On the other hand, at present, the greenhouse effect caused by CO
2 emissions is becoming more and more serious. The emission reduction and capture of CO
2 have become hot spots of global research [
4], and CO
2 emissions have affected environmental, social, economic, and other aspects. The iron and steel industry is one of the largest carbon-emitting industries, presenting an urgent need for CO
2 capture and storage solutions [
5].
More and more researchers are focusing on combining steel slag utilization and CO
2 capture, thereby promoting the green development of the comprehensive utilization of solid waste in the steel industry. Carbonation allows the permanent storage of the greenhouse gas CO
2 in the solid form of carbonate [
6]. Carbonation, as an advanced technology that uses solid waste to save resources and energy, is an effective method to treat steel slag [
7]. In recent years, the study of the carbonation of steel slag has been increasing and is mainly divided into direct carbonation and indirect carbonation [
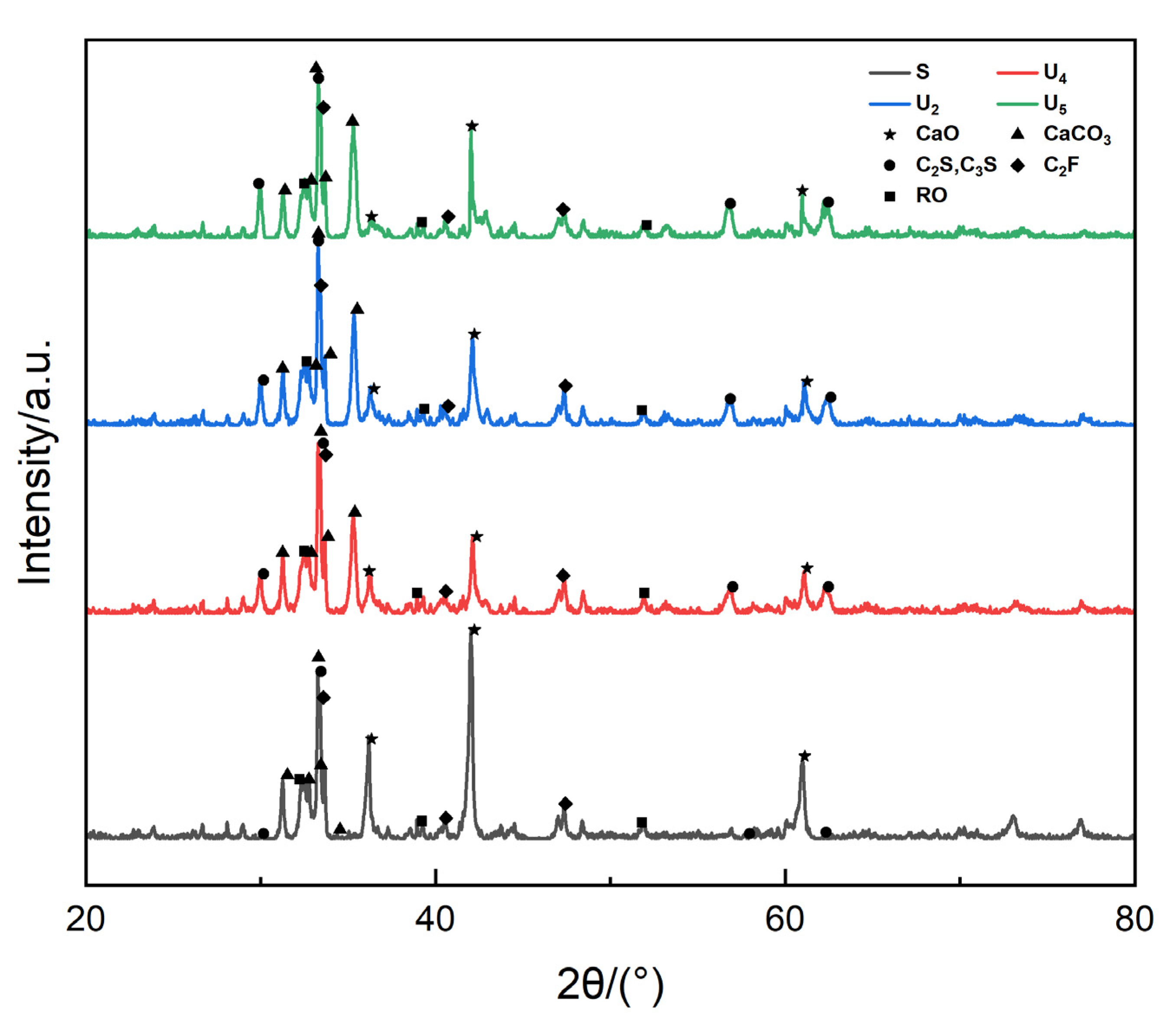
8]. However, indirect carbonation technology is too complex and is therefore not conducive to large-scale promotion and application. Therefore, in the field of direct carbonation technology, Chang et al. [
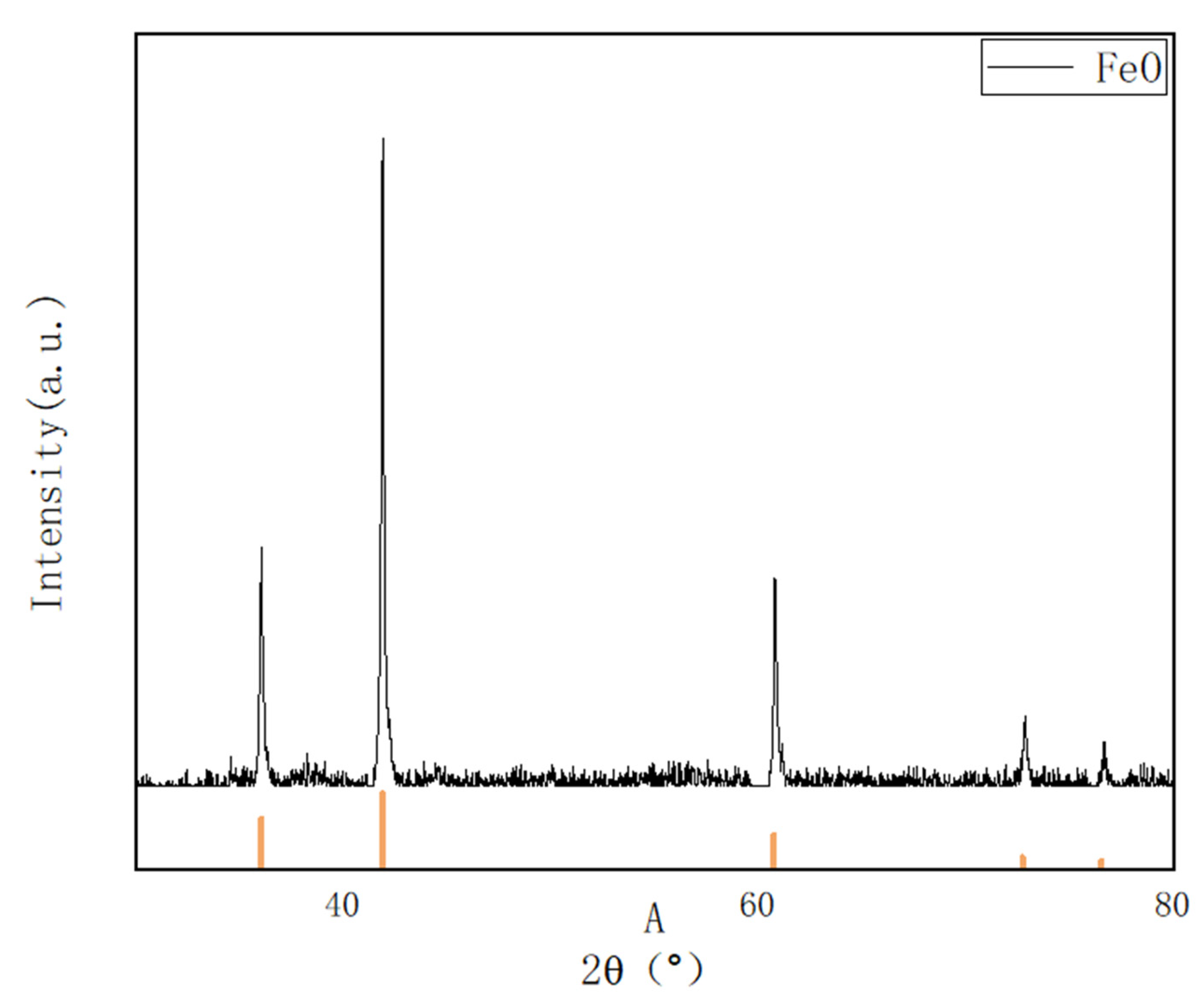
9] and Bo et al. [
10] used XRD for the phase analysis of steel slag before and after carbonization and found that the intensity of C
2S and C
3S diffraction peaks before and after the carbonization of steel slag decreased, the diffraction peaks of Ca(OH)
2 and f-CaO essentially disappeared, and there were obvious CaCO
3 and SiO
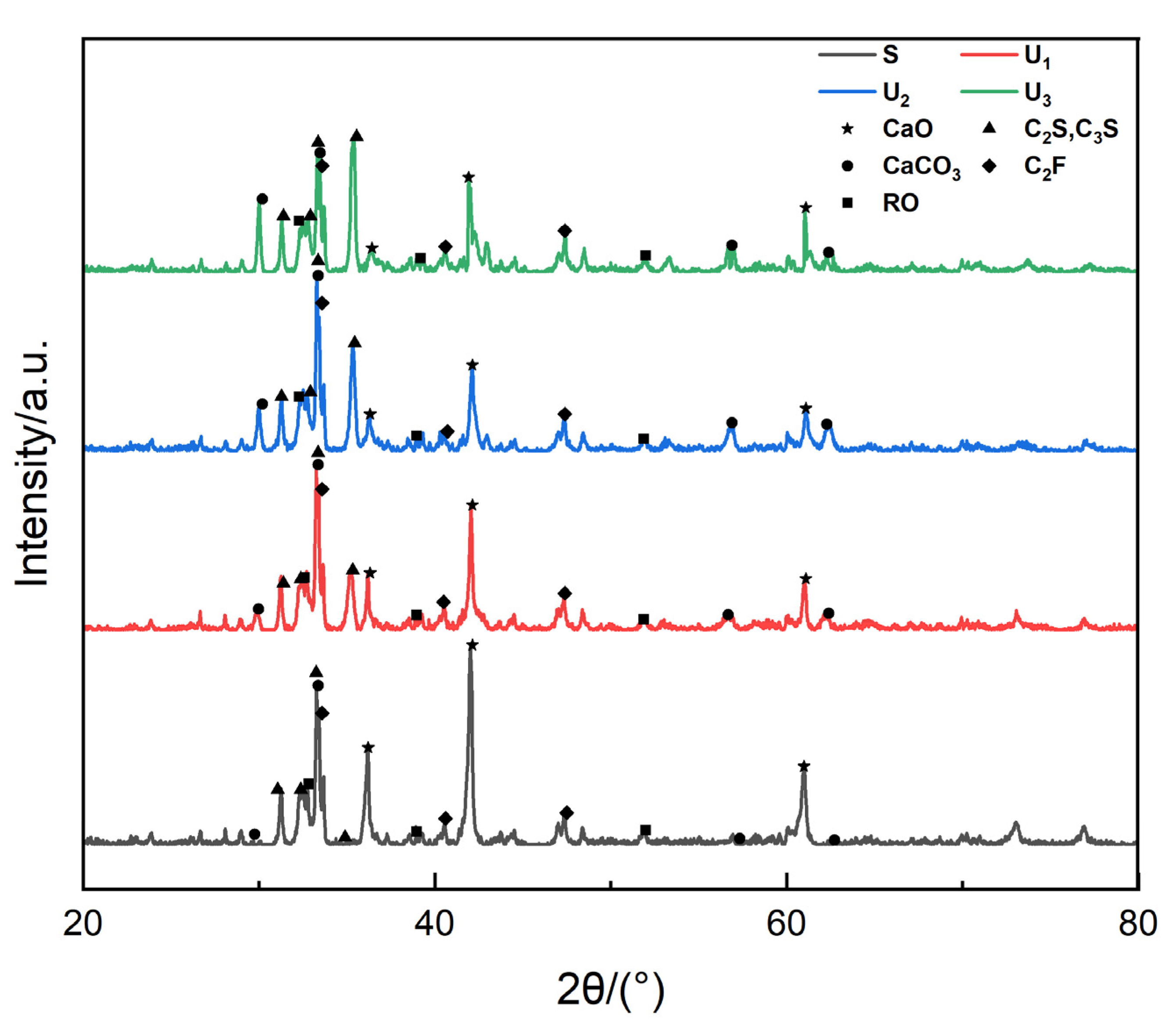
2 diffraction peaks. Their study indicated the feasibility of conducting carbonation. By using XRD analysis, Liang [
11] found that a divalent metal oxide solid solution (RO) phase and calcium ferrite (C
2F) basically had no effect on the carbonation reaction, and the proper addition of H
2O was beneficial to carbonation; Fang [
12] studied the carbonization efficiency of steel slag minerals via QXRD and found that portlandite absorbed the greatest amount of CO
2 and the carbon sequestration of γ-C
2S, β-C
2S, akermanite, merwinite, and cuspidine gradually decreased. Chang [
13] used water to bring CO
2 gas into a rotating container with steel slag, using the rotor to stir the steel slag, and achieved the CO
2 capture and fixation of steel slag in water. The results showed that the maximum conversion of slag was 93.5% carbon transformation with T = 65 °C, a rotation speed of 750 r/min, and t = 30 min. Stolaroff [
14] proposed a feasible technology of slag carbonation that involves spraying circulating water on a bed of ground slag, a solution containing Ca(OH)
2, to collect CO
2 from the air. However, the carbonation efficiency is low, the water consumption is large, the fixed cost is high, and the technology still needs to be optimized. Dong et al. [
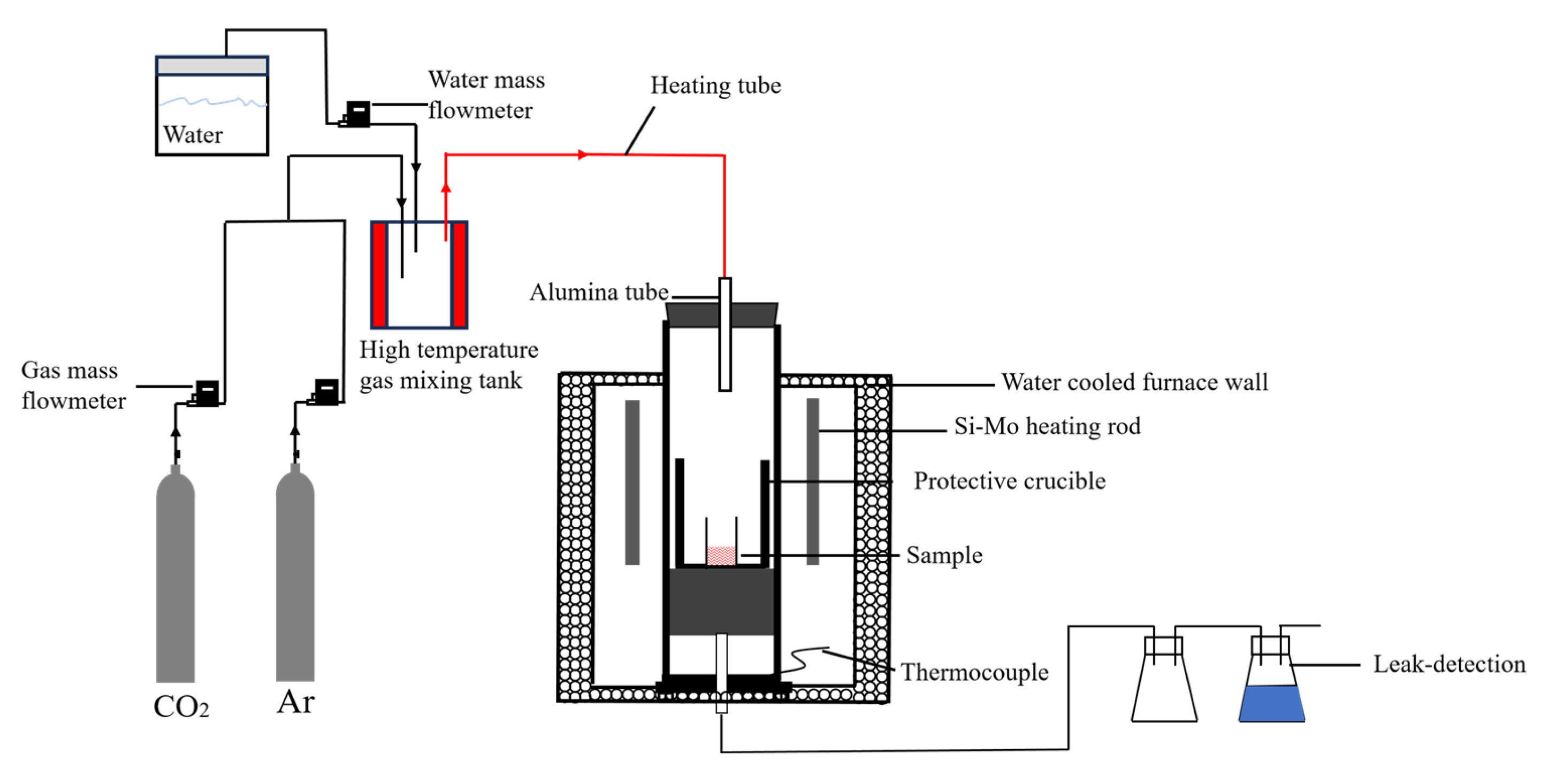
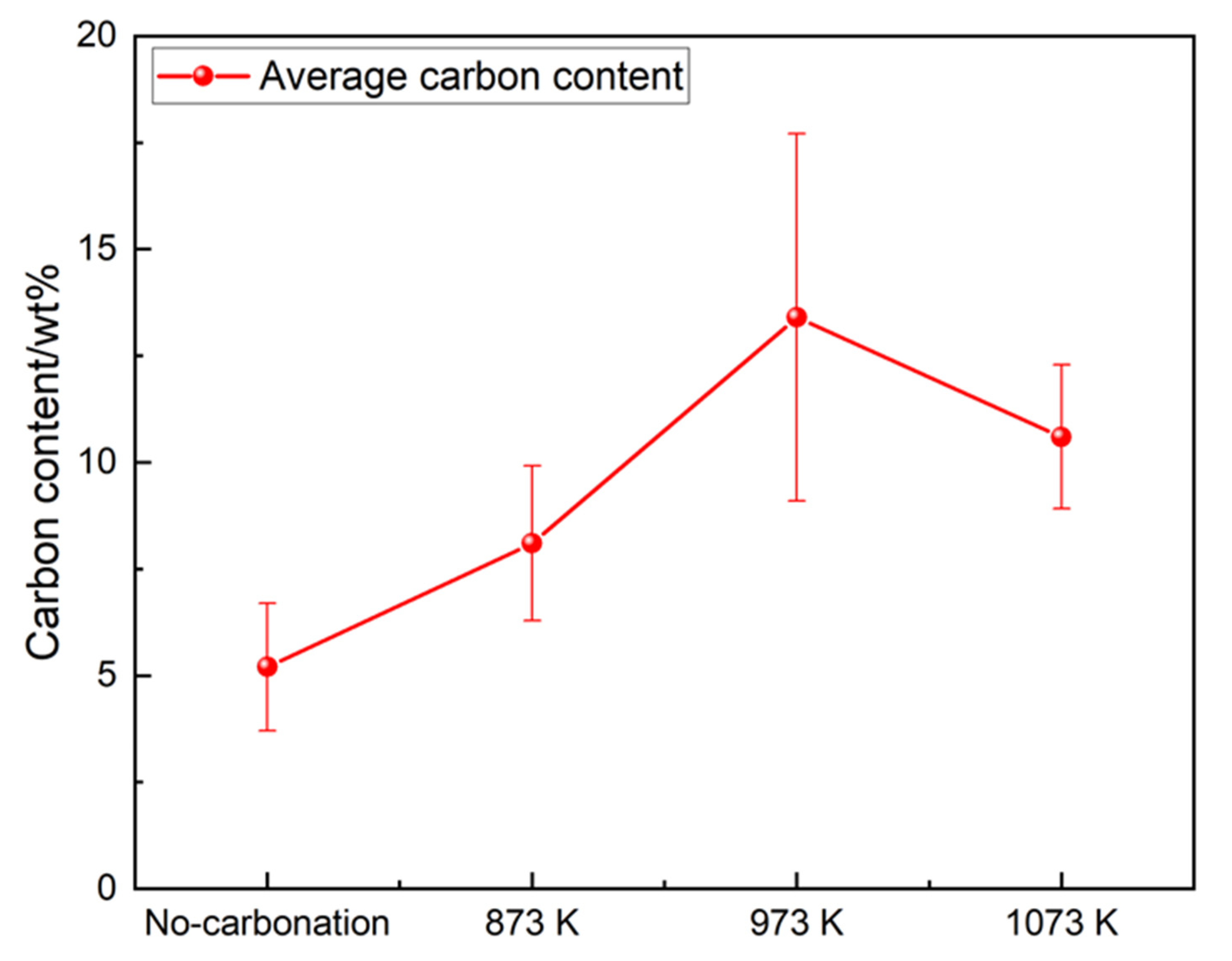
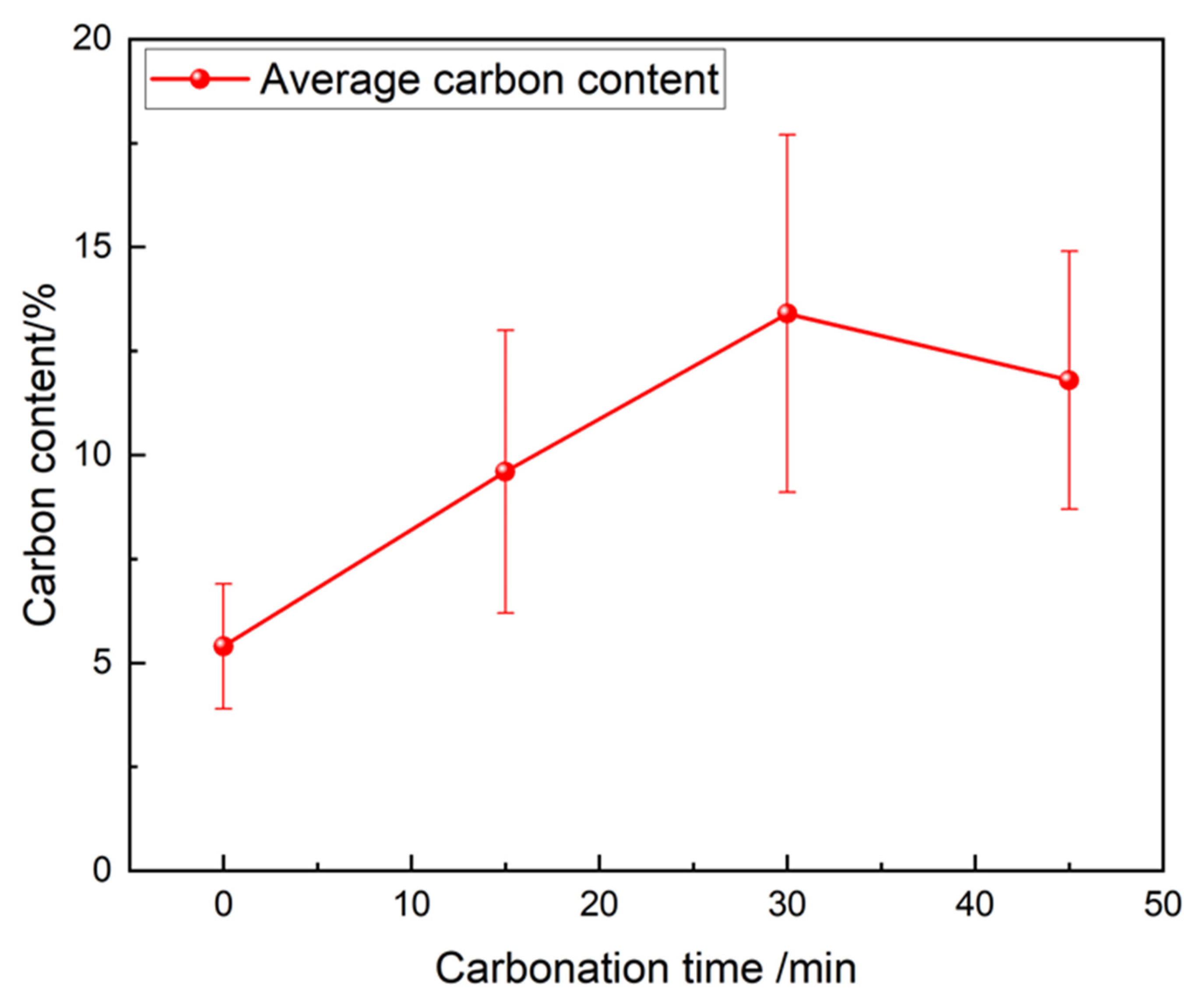
15] studied the carbonation reaction of steel slag at a temperature of 350–850 °C, a reaction time of 0.5–3 h, and a particle size of 0.074–2 mm. The results showed that the optimal reaction conditions were 700 °C, reaction time 0.5-1 h, particle size 0.18 mm, and the digestion efficiency of f-CaO can reach 90%. Peng et al. [
16] conducted a study on the carbonation of hot steel slag, and the results showed that by utilizing CO
2 gas as a modifier for hot steel slag, the phase composition of the steel slag improved greatly, and the amount of f-CaO reduced to less than 1%. The above relevant research indicates that CO
2 gas injection in the disposal process of hot steel slag can significantly reduce the content of f-CaO during the carbonation process. However, the reaction conditions of direct carbonation are still not clear; in particular, the optimum temperatures and reaction times are quite different under
pCO2 > 0.6 in CO
2-H
2O mixed atmosphere. The direct carbonation behavior of slag with CO
2 containing water vapor still need more detailed research.