The Microstructural Observation in Precipitations of Peak-Aged Al–Zn–Mg Alloys with Various Zn/Mg Ratios
Abstract
1. Introduction
2. Materials and Methods
3. Results
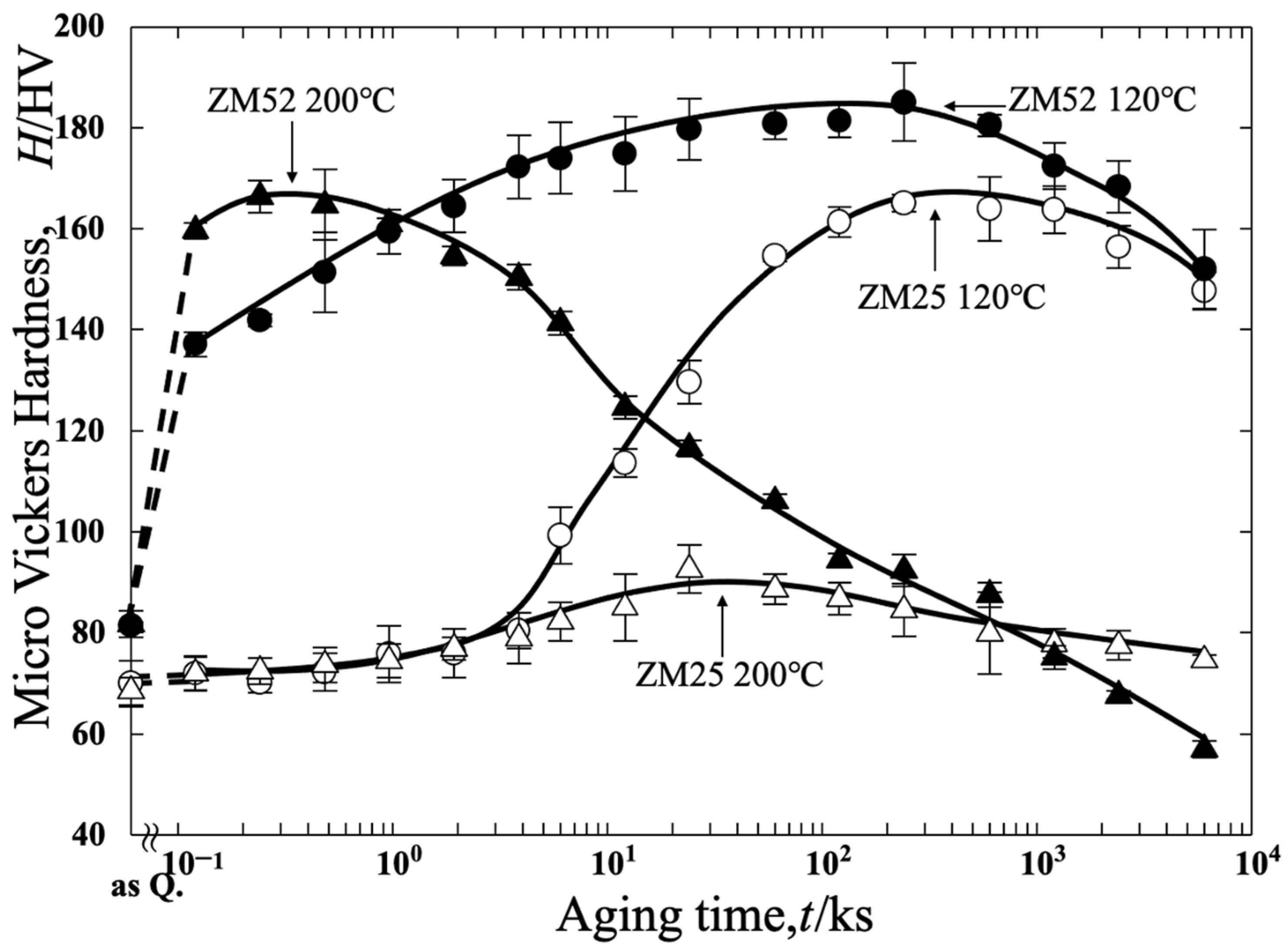
3.1. Vickers Microhardness
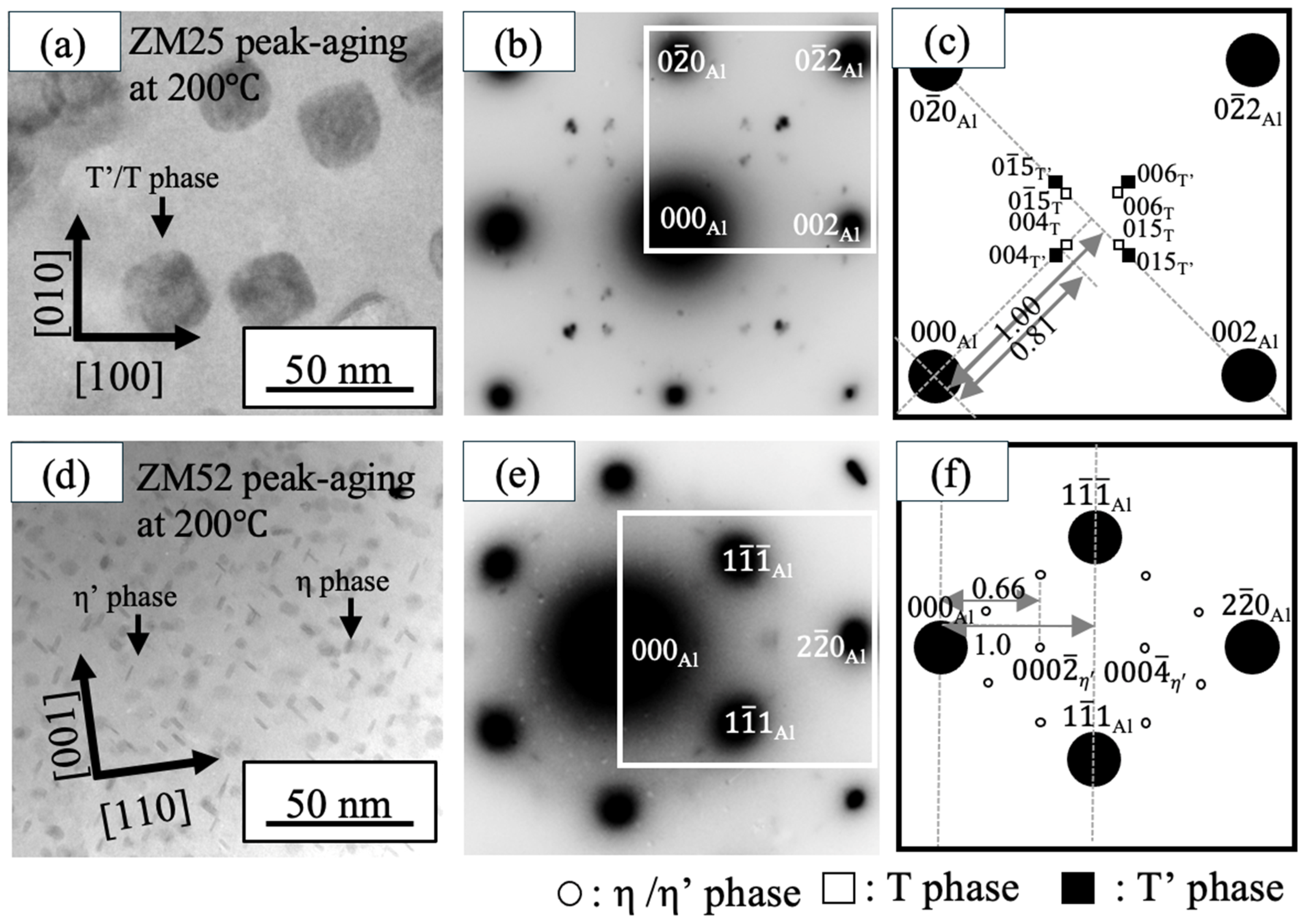
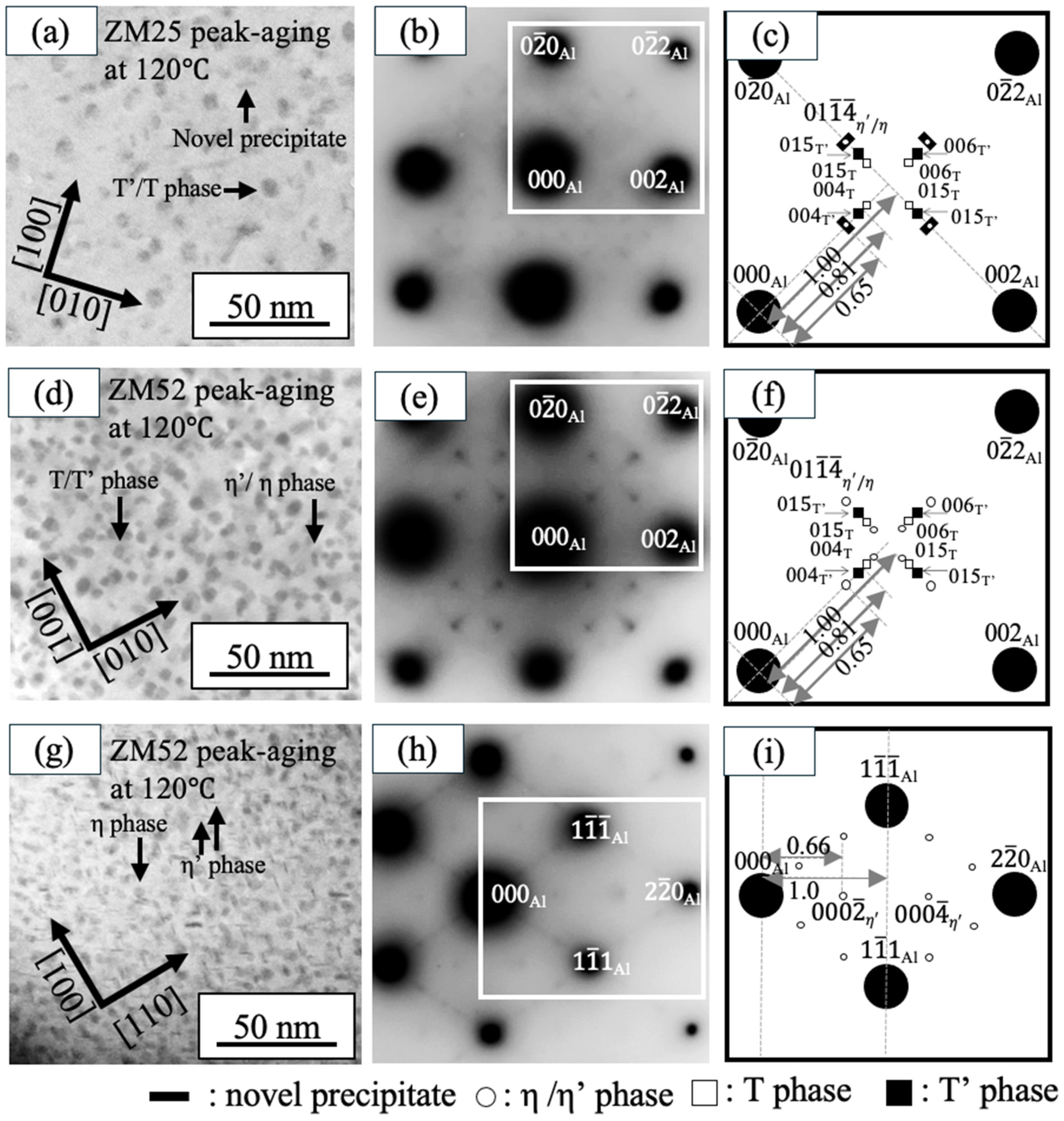
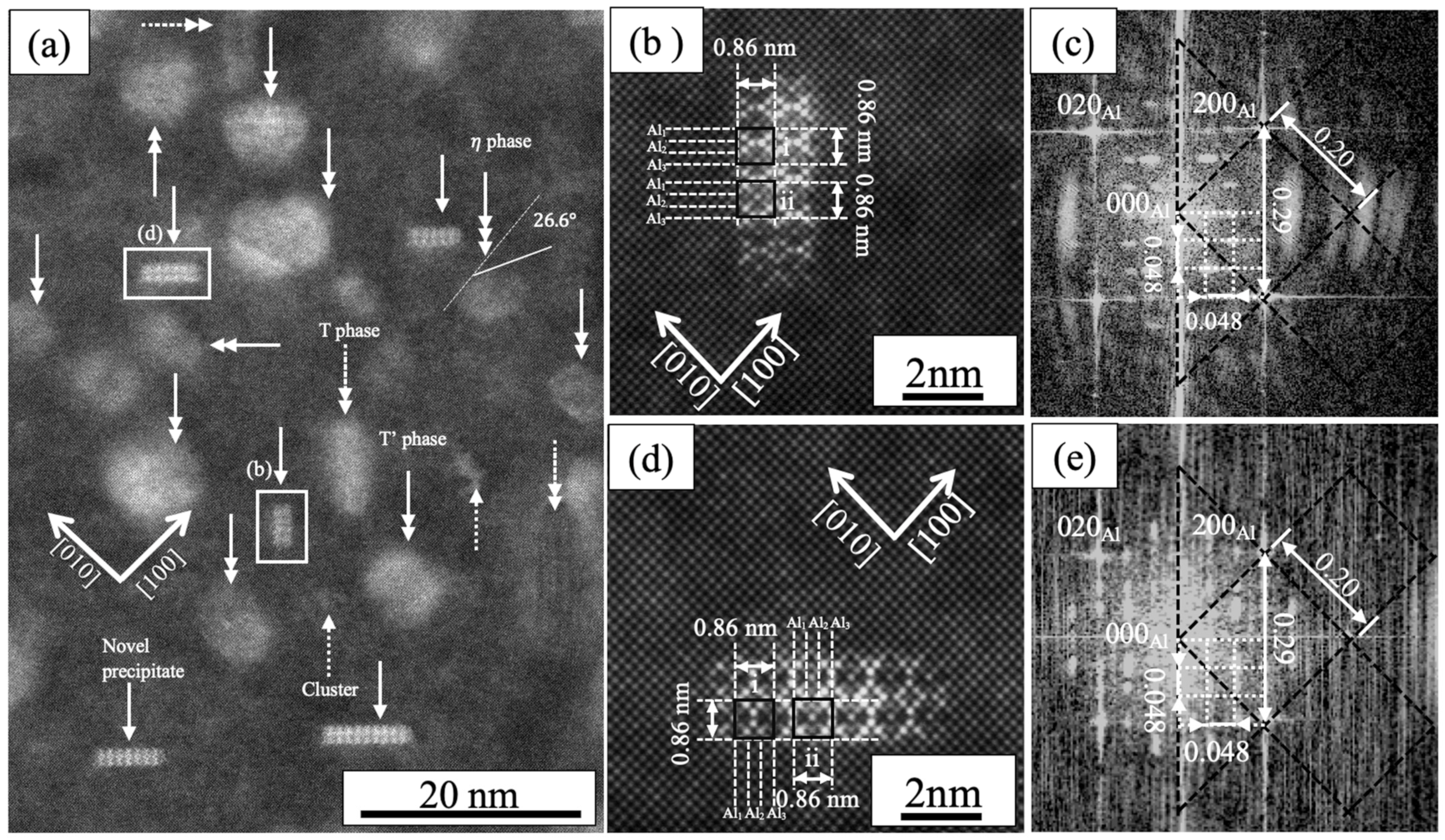
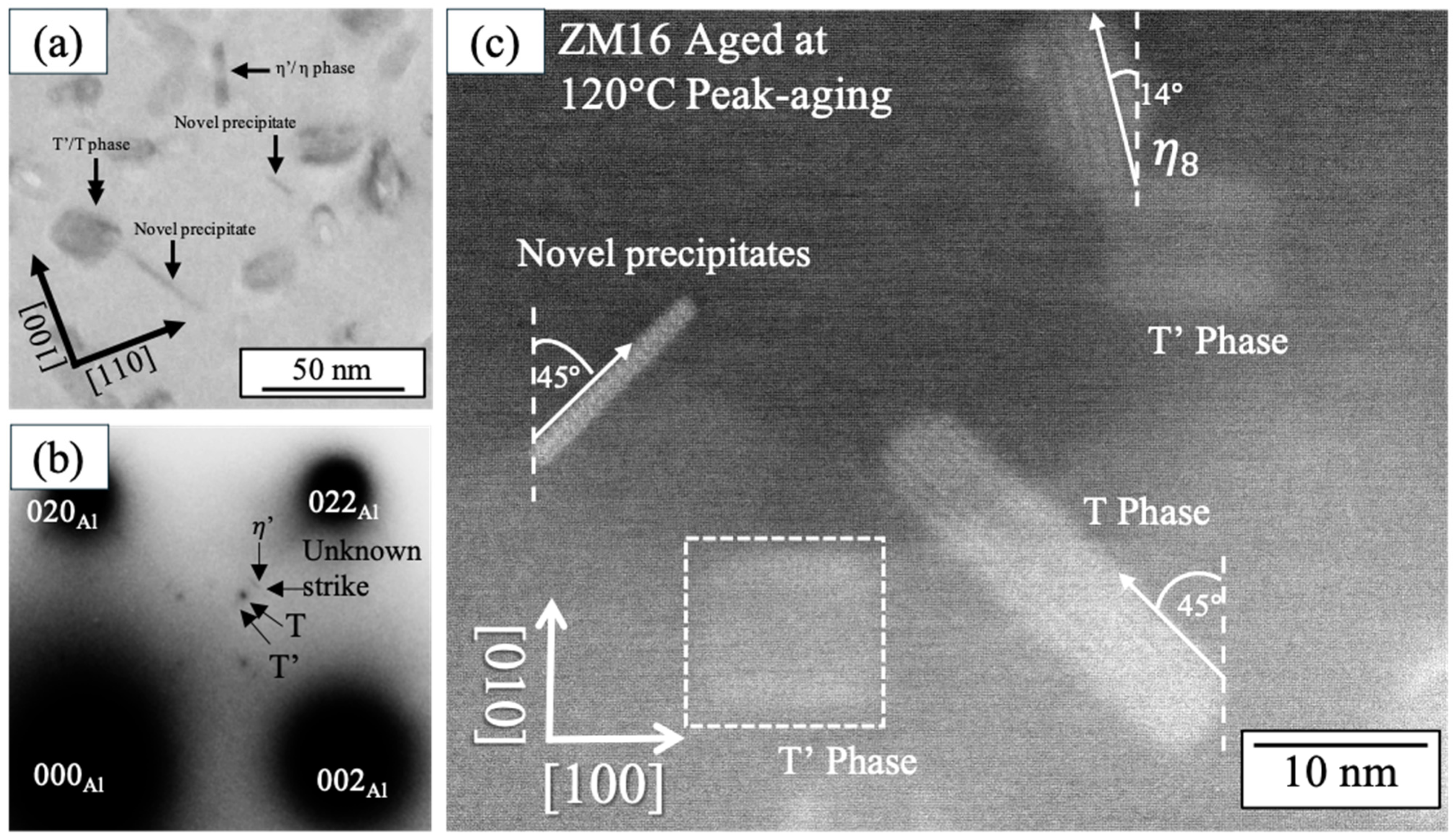
3.2. TEM Observation
4. Discussion
5. Conclusions
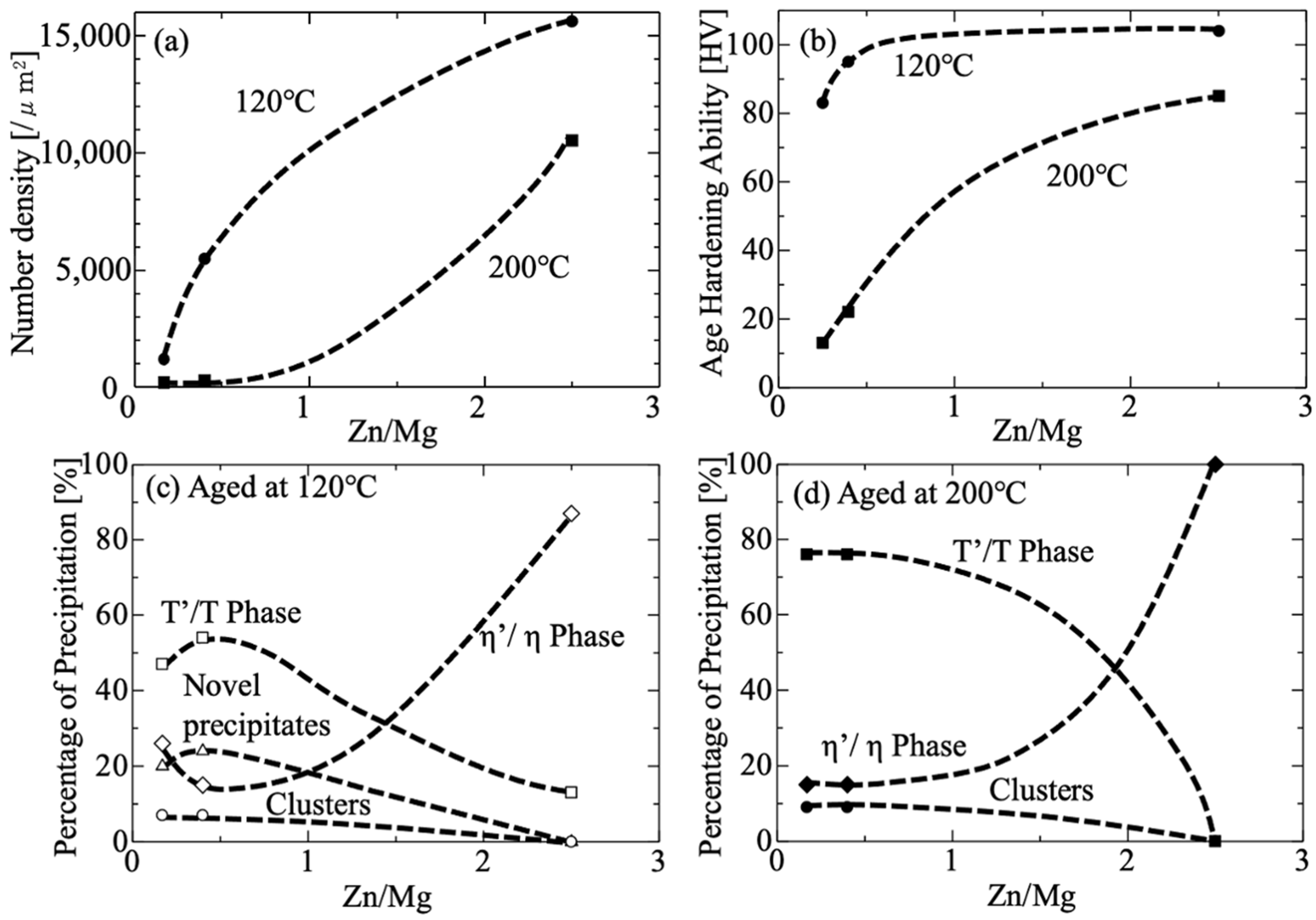
- According to the Vickers Hardness results, the ZM52 alloy exhibits higher as-quenched hardness than ZM25. While both alloys show comparable age-hardening responses at 120 °C, the difference becomes more obvious at 200 °C. ZM52 achieves significantly greater hardening, whereas ZM25 exhibits only a slight increase followed by gradual softening. ZM52 also shows faster cluster formation and reaches peak hardness earlier, particularly at 200 °C.
- Based on the peak-aging conditions observed for each alloy, TEM analyses revealed that increasing the aging temperature leads to a lower number density but larger precipitate size, resulting in reduced hardness. Conversely, at lower aging temperatures, precipitates become finer and more densely distributed, which enhances hardness. However, the effect of aging temperature varied depending on the Zn/Mg ratio. In the ZM25 alloy, the precipitate areal density at 200 °C was approximately five times lower than that at 120 °C, whereas in ZM52 it decreased by only about 1.5 times. The average precipitate size in ZM25 increased nearly sixfold when the aging temperature was raised from 120 °C to 200 °C, while ZM52 showed only a slight change in precipitate size between the two temperatures.
- Alloys with Zn/Mg < 1.0 (ZM25) aged at both 120 °C and 200 °C primarily contain T′/T phases as the dominant strengthening precipitates, followed by η′/η phases. The variation in their number density influences the resulting hardness. In contrast, the high-Zn/Mg alloy (ZM52, Zn/Mg > 2.0) predominantly forms η′/η phases as the main strengthening precipitates at both temperatures.
- Unique precipitates aligning along [110] Al and [10] Al were identified in high-Mg alloys (ZM25) aged at 120 °C. Their presence and relatively high number density suggest that these “novel precipitates” may contribute to the overall strengthening behavior in low-Zn/Mg alloys.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stemper, L.; Tunes, M.A.; Oberhauser, P.; Uggowitzer, P.J.; Pogatscher, S. Age-hardening response of AlMgZn alloys with Cu and Ag additions. Acta Mater. 2020, 195, 541–554. [Google Scholar] [CrossRef]
- Jensrud, O. High-strength aluminium alloys extrusions—A review of the thermo-mechanical process in high performance profile manufacturing. Key Eng. Mater. 2012, 491, 11–18. [Google Scholar] [CrossRef]
- Thronsen, E.; Shah, S.; Hatzoglou, C.; Marioara, C.D.; Wenner, S.; Andersen, S.J.; Holmedal, B.; Holmestad, R. The evolution of precipitates in an Al–Zn–Mg alloy. J. Mater. Res. Technol. 2023, 23, 5666–5680. [Google Scholar] [CrossRef]
- Matsuda, K.; Yasumoto, T.; Bendo, A.; Tsuchiya, T.; Lee, S.; Nishimura, K.; Nunomura, N.; Marioara, C.D.; Levik, A.; Holmestad, R.; et al. Effect of copper addition on precipitation behavior near grain boundary in Al–Zn–Mg alloy. Mater. Trans. 2019, 60, 1688–1696. [Google Scholar] [CrossRef]
- Bigot, A.; Auger, P.; Chambreland, S.; Blavette, D.; Reeves, A. Atomic scale imaging and analysis of T′ precipitates in Al–Mg–Zn alloys. Microsc. Microanal. Microstruct. 1997, 8, 103–113. [Google Scholar] [CrossRef]
- Lei, C.; Ebrahimi, M.; Jiang, H.; Ding, W. Microstructure and mechanical properties of squeeze-cast Al–5.0Mg–3.0Zn–1.0Cu alloys in solution-treated and aged conditions. Trans. Nonferr. Met. Soc. China 2020, 30, 2326–2338. [Google Scholar]
- Stemper, L.; Tunes, M.A.; Dumitraschkewitz, P.; Mendez-Martin, F.; Tosone, R.; Marchand, D.; Curtin, W.A.; Uggowitzer, P.J.; Pogatscher, S. Giant hardening response in AlMgZn(Cu) alloys. Acta Mater. 2021, 206, 116617. [Google Scholar] [CrossRef]
- Matsuda, K.; Kawai, A.; Watanabe, K.; Lee, S.; Marioara, C.D.; Wenner, S.; Nishimura, K.; Matsuzaki, T.; Nunomura, N.; Sato, T.; et al. Extra electron diffraction spots caused by fine precipitates formed at the early stage of aging in Al-Mg-X (X = Si, Ge, Zn)-Cu alloys. Mater. Trans. 2017, 58, 167–175. [Google Scholar] [CrossRef]
- Polmear, I.J. Light Alloys: From Traditional Alloys to Nanocrystals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2006. [Google Scholar]
- Goswami, R.; Lynch, S.; Holroyd, N.H.; Knight, S.P.; Holtz, R.L. Evolution of grain boundary precipitates in Al 7075 upon aging and correlation with stress corrosion cracking behavior. Metall. Mater. Trans. A 2013, 44, 1268–1278. [Google Scholar] [CrossRef]
- Hsiao, T.J.; Chiu, P.H.; Tai, C.L.; Tsao, T.C.; Tseng, C.Y.; Lin, Y.X.; Chen, H.R.; Chung, T.F.; Chen, C.Y.; Wang, S.H.; et al. Effect of Cu Additions on the Evolution of Eta-prime Precipitates in Aged AA 7075 Al–Zn–Mg–Cu Alloys. Metals 2022, 12, 2120. [Google Scholar] [CrossRef]
- Kilic, S.; Kacar, I.; Sahin, M.; Ozturk, F.; Erdem, O. Effects of aging temperature, time, and pre-strain on mechanical properties of AA7075. Mater. Res. 2019, 22, e20190006. [Google Scholar] [CrossRef]
- Mazilkin, A.A.; Baretzky, B.; Enders, S.; Kogtenkova, O.A.; Straumal, B.B.; Rabkin, E.; Valiev, R. Hardness of nanostructured Al–Zn, Al–Mg and Al–Zn–Mg alloys obtained by high-pressure torsion. Defect Diffus. Forum 2006, 249, 155–160. [Google Scholar] [CrossRef]
- Somekawa, H.; Osawa, Y.; Mukai, T. Effect of solid-solution strengthening on fracture toughness in extruded Mg–Zn alloys. Scr. Mater. 2006, 55, 593–596. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, X.; Gao, K.; Wen, S.; Rong, L.; Huang, H.; Wei, W.; Nie, Z. Effect of Zn/Mg ratio on microstructure and mechanical properties of Al–Zn–Mg alloys. Mater. Lett. 2022, 312, 131676. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.; Tang, S.; Zhu, Q.; Song, H.; Cao, L. Co-precipitation of T′ and η′ phase in Al–Zn–Mg–Cu alloys. Mater. Charact. 2020, 169, 110610. [Google Scholar] [CrossRef]
- Lee, S.; Watanabe, K.; Matsuda, K.; Nishimura, K.; Numomura, N.; Toda, H.; Hirayama, K.; Shimizu, K.; Gao, H.; Yamaguchi, M.; et al. Precipitation structure and mechanical properties on peak-aged Al–Zn–Mg alloys including different Zn/Mg ratios. J. Jpn. Inst. Light Met. 2017, 67, 162–167. [Google Scholar] [CrossRef]
- Ahmed, A.; Matsuda, K.; Lee, S.; Tsuchiya, T.; Nishimura, K.; Nunomura, N.; Toda, H.; Hirayama, K.; Shimizu, K.; Yamaguchi, M.; et al. Microstructure observation of T-phase in Al–Zn–Mg alloy with low Zn/Mg ratio. J. Alloys Compd. 2025, 1010, 177781. [Google Scholar] [CrossRef]
- Bendo, A.; Matsuda, K.; Lervik, A.; Tsuru, T.; Nishimura, K.; Nunomura, N.; Holmestad, R.; Marioara, C.D.; Shimizu, K.; Toda, H.; et al. An unreported precipitate orientation relationship in Al–Zn–Mg based alloys. Mater. Charact. 2019, 158, 109958. [Google Scholar] [CrossRef]
- Maloney, S.K.; Hono, K.; Polmear, I.J.; Ringer, S.P. The effects of a trace addition of silver upon elevated temperature ageing of an Al–Zn–Mg alloy. Micron 2001, 32, 741–747. [Google Scholar] [CrossRef]
- Chen, J.; Zhen, L.; Yang, S.; Shao, W.; Dai, S. Investigation of precipitation behavior and related hardening in AA7055 aluminum alloy. Mater. Sci. Eng. A 2009, 500, 34–42. [Google Scholar] [CrossRef]
- Bergman, G.; Waugh, J.L.T.; Pauling, L. The crystal structure of the metallic phase Mg3232(Al, Zn)4949. Acta Crystallogr. 1957, 10, 254–259. [Google Scholar] [CrossRef]
- Thackery, P.A. The nature and morphology of precipitate in Al–Zn–Mg alloys. J. Inst. Met. 1968, 96, 228–235. [Google Scholar]
- Zhang, M.; Li, C.; Zhang, Y.; Liu, S.; Jiang, J.; Tang, J.; Ye, L.; Zhang, X. Effect of hot deformation on microstructure and quenching-induced precipitation behavior of Al–Zn–Mg–Cu alloy. Mater. Charact. 2021, 172, 110861. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.; Tang, S.; Wang, Y.; Zhao, K.; Cao, L. The effect of pre-ageing/stretching on the ageing-hardening behavior of Al–Zn–Mg–Cu alloys correlated with Zn/Mg ratio. Mater. Sci. Eng. A 2022, 830, 142331. [Google Scholar] [CrossRef]
- Jiang, K.; Lan, Y.; Pan, Q.; Deng, Y. Effect of the Zn/Mg ratio on microstructures, mechanical properties, and corrosion performances of Al–Zn–Mg alloys. Materials 2020, 13, 3299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Z.; Pang, S.; Li, X.; Dong, C.; Liaw, P.K. Coherent precipitation and strengthening in compositionally complex alloys: A review. Entropy 2018, 20, 878. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Whitmore, L.; Woo, S.; Aramburu, A.U.; Letzig, D.; Yi, S. Excellent age hardenability with the controllable microstructure of AXW100 magnesium sheet alloy. Sci. Rep. 2020, 10, 22413. [Google Scholar] [CrossRef]
- Li, X.Z.; Hansen, V.; Gjønnes, J.; Wallenberg, L.R. HREM study and structure modeling of the η′ phase, the hardening precipitates in commercial Al–Zn–Mg alloys. Acta Mater. 1999, 47, 2651–2659. [Google Scholar] [CrossRef]
- Deschamps, A.; Hutchinson, C.R. Precipitation kinetics in metallic alloys: Experiments and modeling. Acta Mater. 2021, 220, 117338. [Google Scholar] [CrossRef]
- Fu, Z.; Zhao, X.; Jiao, M.; Ren, X.; Liu, H.; Liu, H. Study on the aging precipitation behavior and kinetics of Al–10.0Zn–3.0Mg–2.8Cu alloy by pre-deformation treatment. Materials 2024, 17, 3729. [Google Scholar] [CrossRef]
- Ishii, H.; Takagi, R.; Takata, N.; Suzuki, A.; Kobashi, M. Influence of added fourth elements on precipitation in heat-resistant Al–Mg–Zn ternary alloys. Mater. Trans. 2022, 63, 513–521. [Google Scholar] [CrossRef]
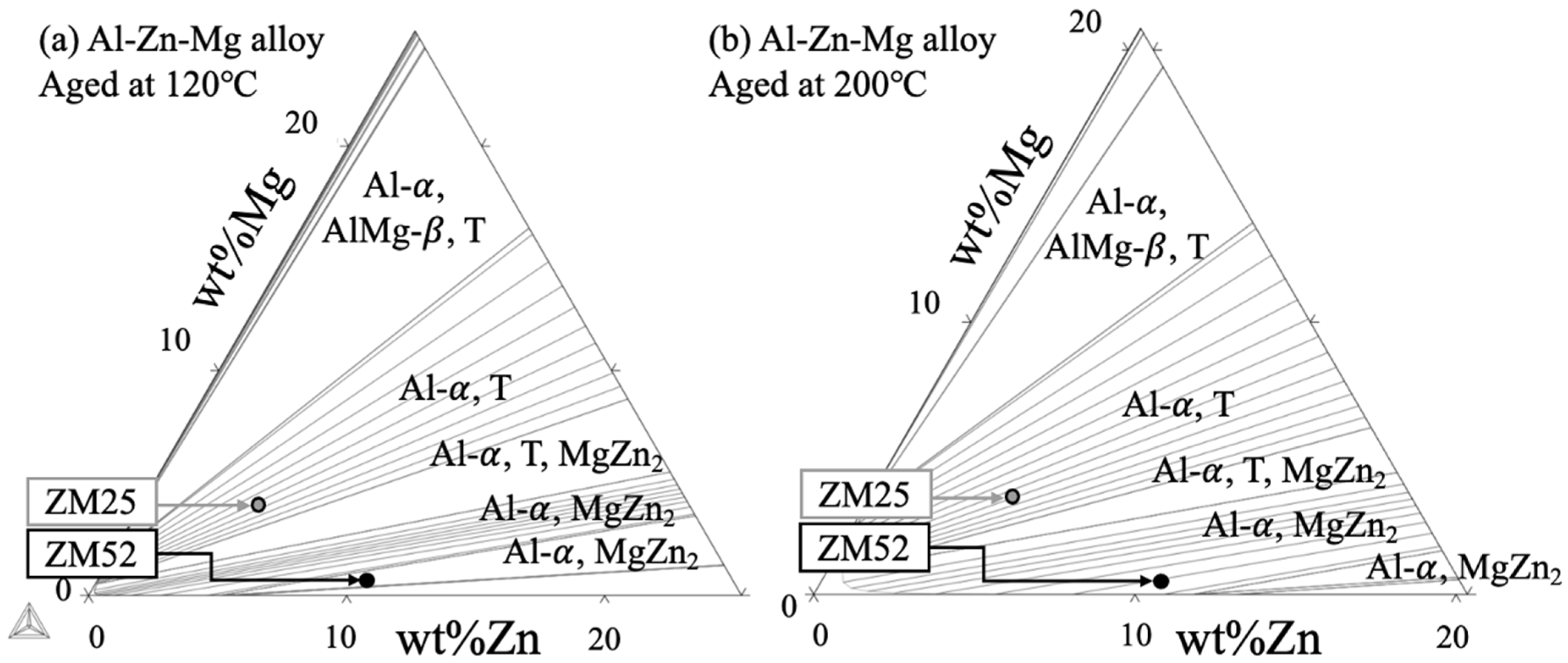
| Alloy | mol% | wt% | Al | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Zn | Mg | Zn/Mg | Zn + Mg | Zn | Mg | Zn/Mg | Zn + Mg | ||
| ZM16 | 1.5 | 5.8 | 0.26 | 7.3 | 3.5 | 5.1 | 0.68 | 8.6 | Bal. |
| ZM25 | 2.1 | 5.2 | 0.40 | 7.3 | 4.9 | 4.6 | 1.1 | 9.5 | Bal. |
| ZM52 | 4.8 | 1.9 | 2.5 | 6.7 | 11 | 1.6 | 6.8 | 8.4 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanphiboon, W.; Lee, S.; Tsuchiya, T.; Ahmed, A.; Ikeno, S.; Yoshida, T.; Matsuda, K. The Microstructural Observation in Precipitations of Peak-Aged Al–Zn–Mg Alloys with Various Zn/Mg Ratios. Metals 2025, 15, 1260. https://doi.org/10.3390/met15111260
Sanphiboon W, Lee S, Tsuchiya T, Ahmed A, Ikeno S, Yoshida T, Matsuda K. The Microstructural Observation in Precipitations of Peak-Aged Al–Zn–Mg Alloys with Various Zn/Mg Ratios. Metals. 2025; 15(11):1260. https://doi.org/10.3390/met15111260
Chicago/Turabian StyleSanphiboon, Wanlalak, Seungwon Lee, Taiki Tsuchiya, Abrar Ahmed, Susumu Ikeno, Tomoo Yoshida, and Kenji Matsuda. 2025. "The Microstructural Observation in Precipitations of Peak-Aged Al–Zn–Mg Alloys with Various Zn/Mg Ratios" Metals 15, no. 11: 1260. https://doi.org/10.3390/met15111260
APA StyleSanphiboon, W., Lee, S., Tsuchiya, T., Ahmed, A., Ikeno, S., Yoshida, T., & Matsuda, K. (2025). The Microstructural Observation in Precipitations of Peak-Aged Al–Zn–Mg Alloys with Various Zn/Mg Ratios. Metals, 15(11), 1260. https://doi.org/10.3390/met15111260






