Corrosion Behavior of Advanced High-Strength Steels (AHSS) in Chloride Solutions for Automotive Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructural Characterization
2.3. Electrochemical Technique
3. Results and Discussion
3.1. Microstructure
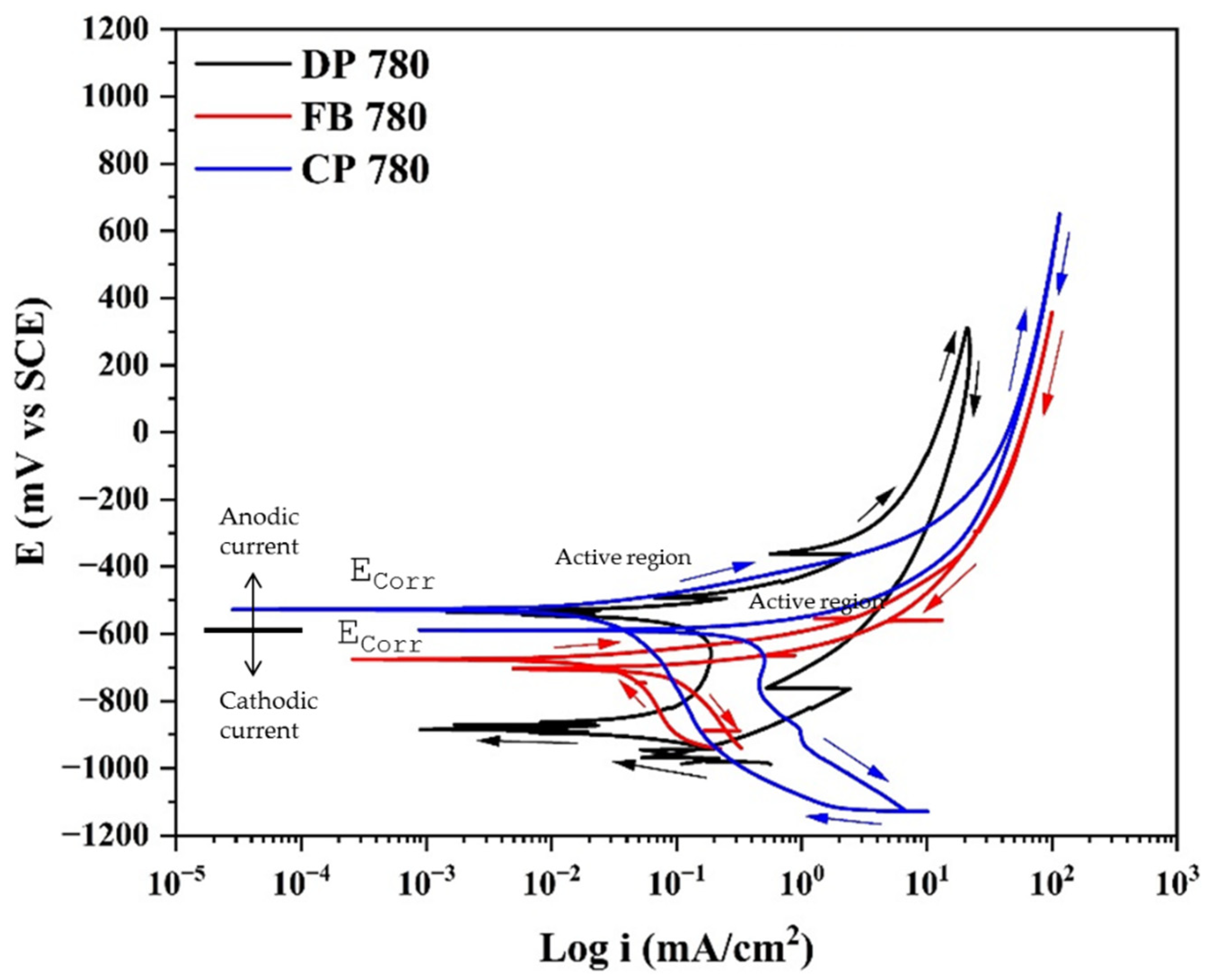
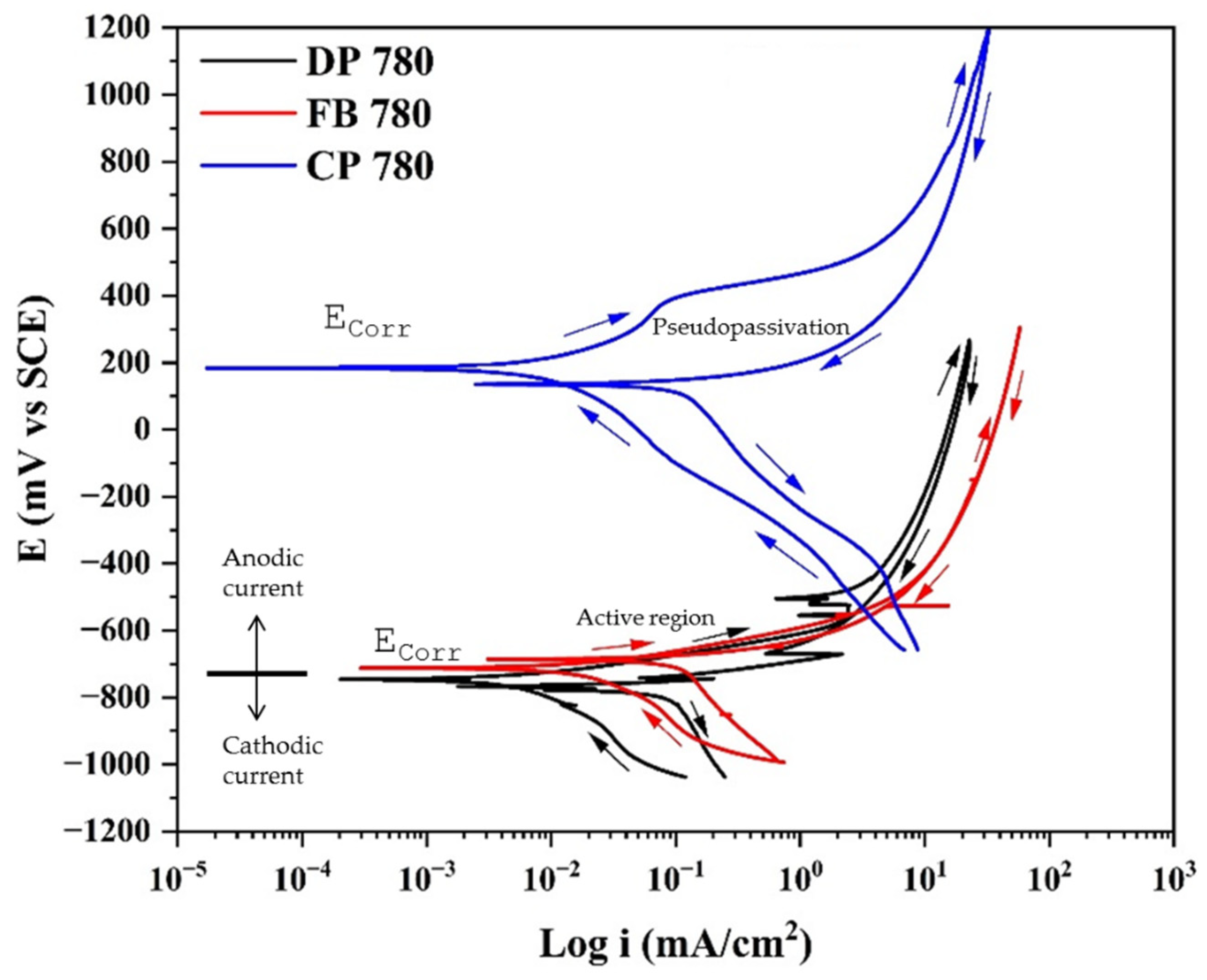
3.2. Cyclic Potentiodynamic Polarization
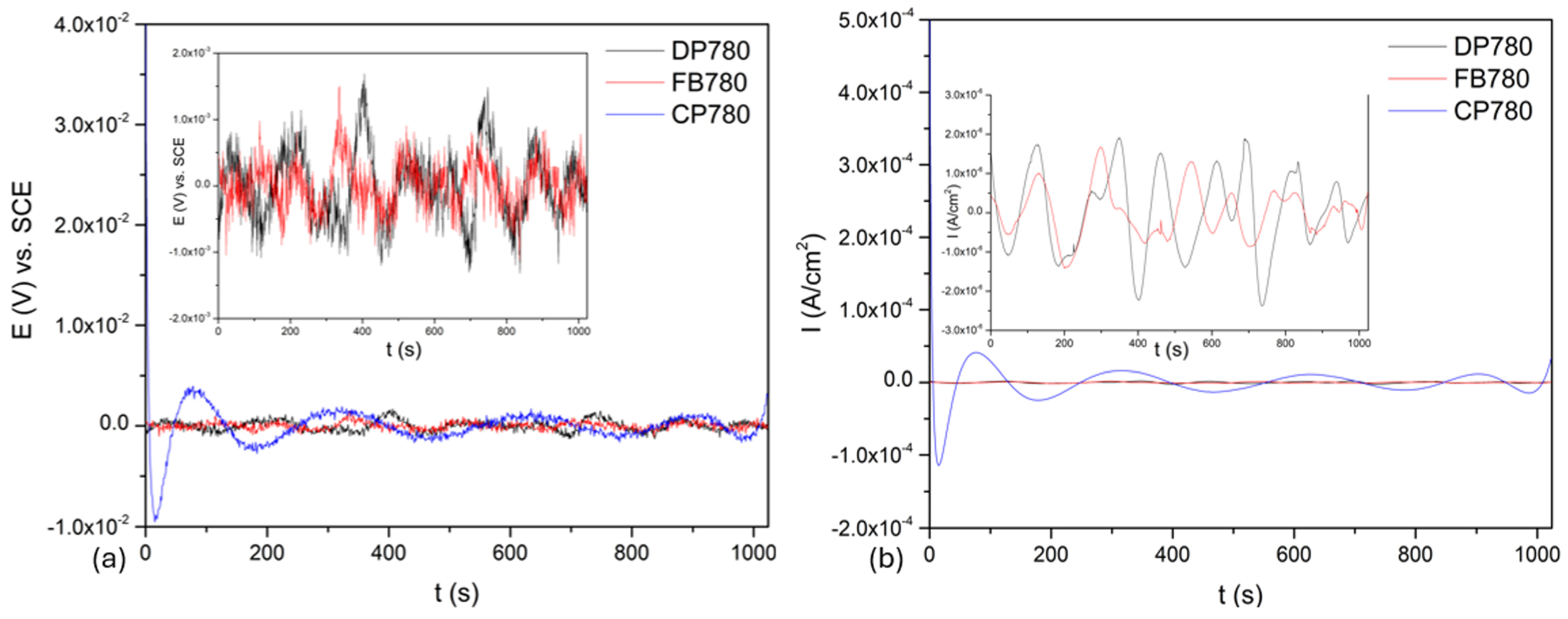
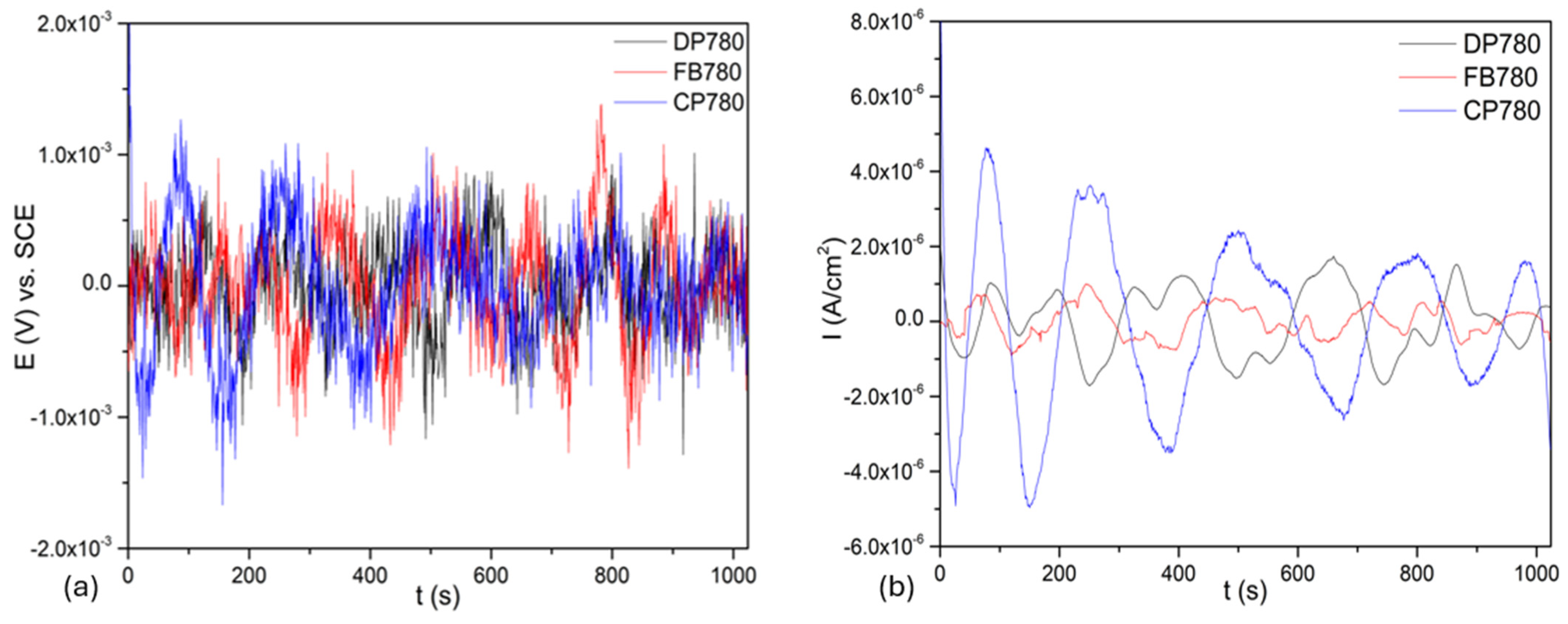
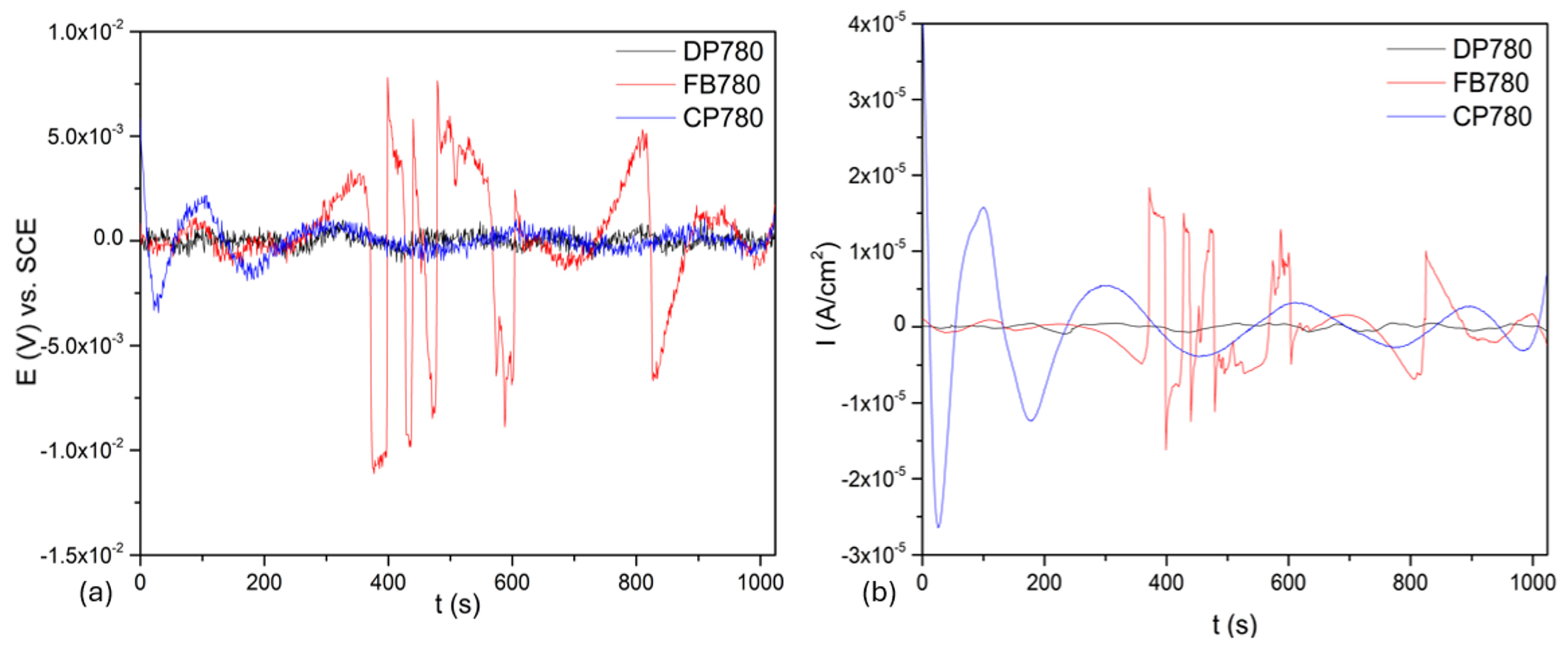
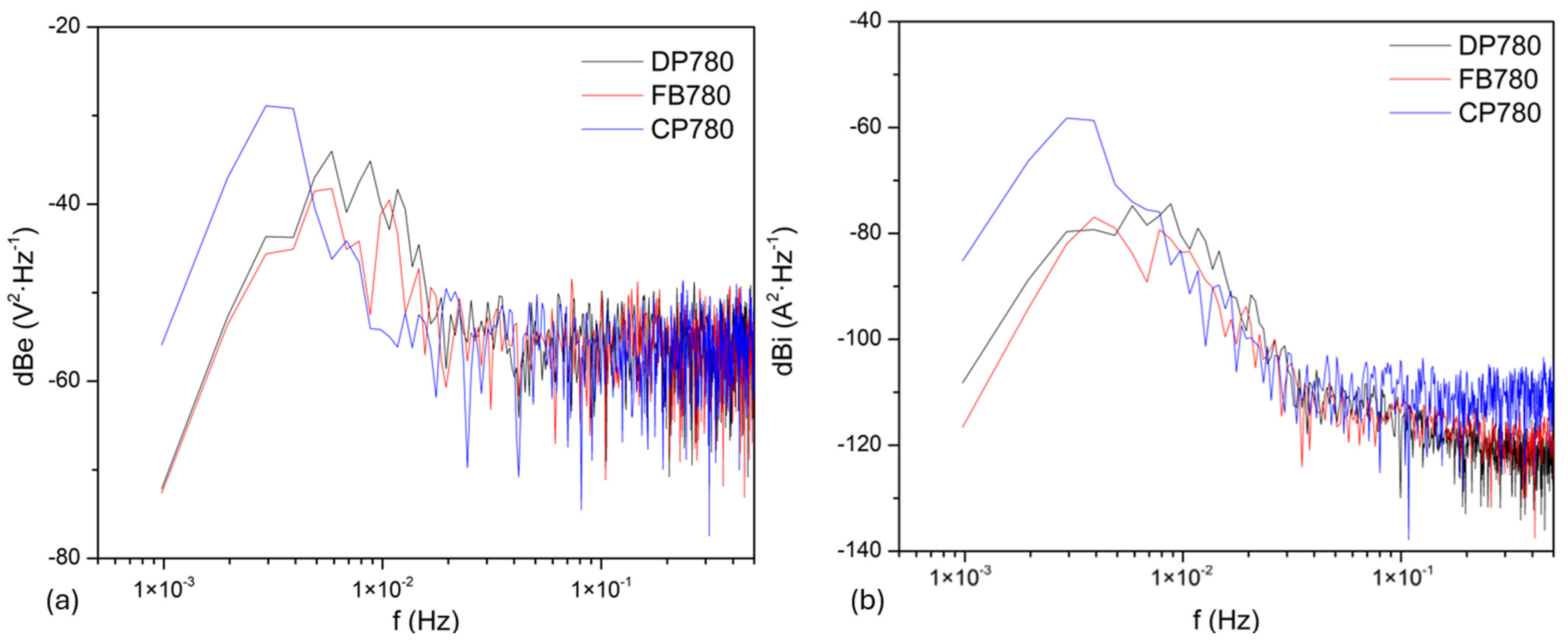
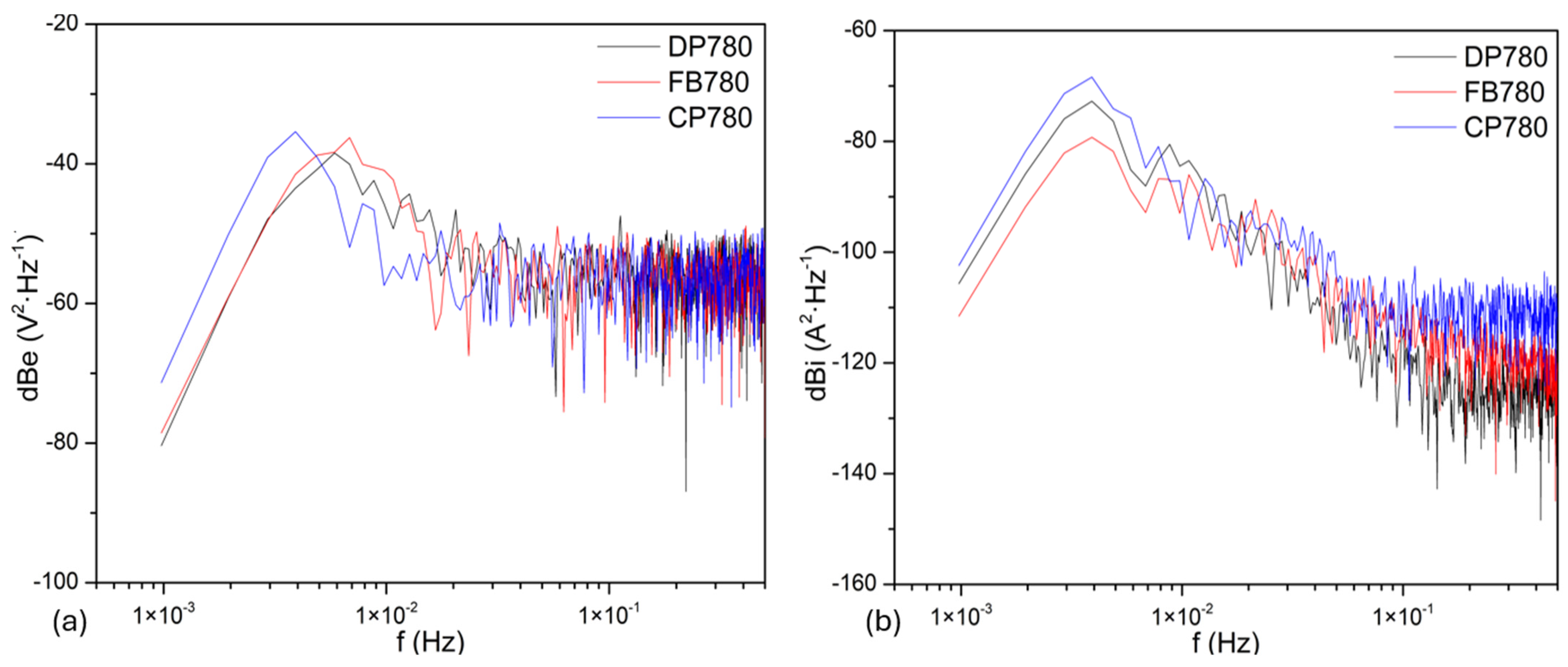
3.3. Electrochemical Noise
3.3.1. Time-Domain Analysis
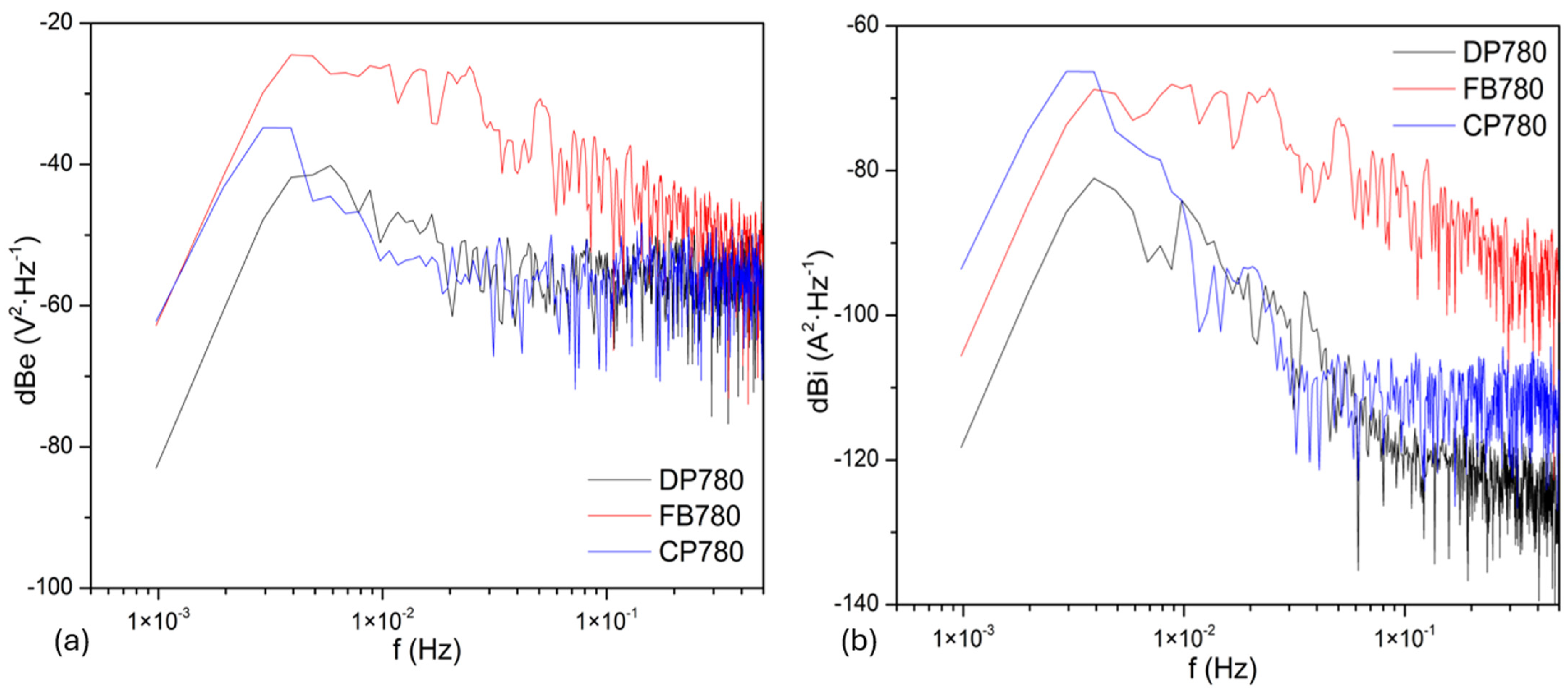
3.3.2. Frequency Domain Analysis
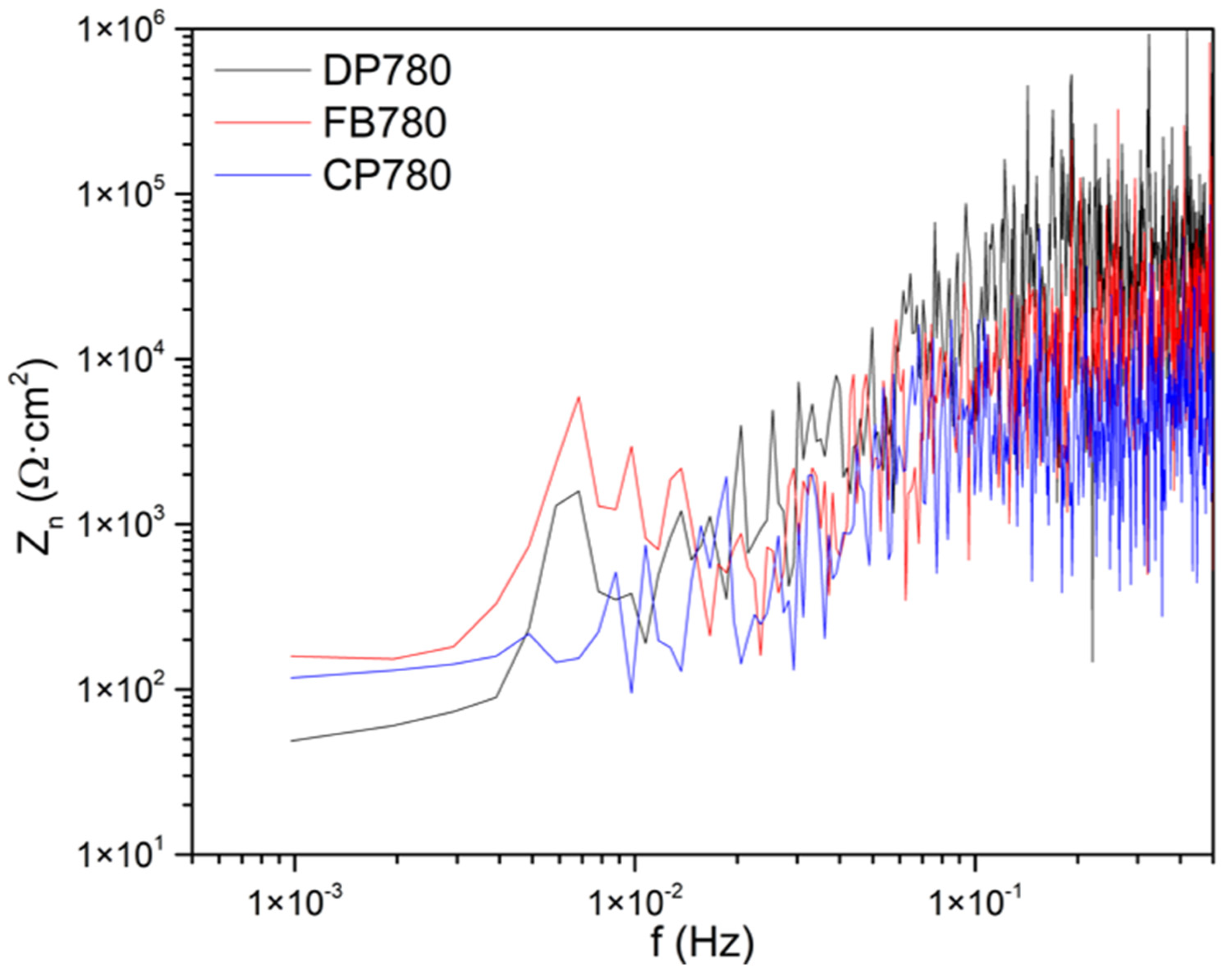
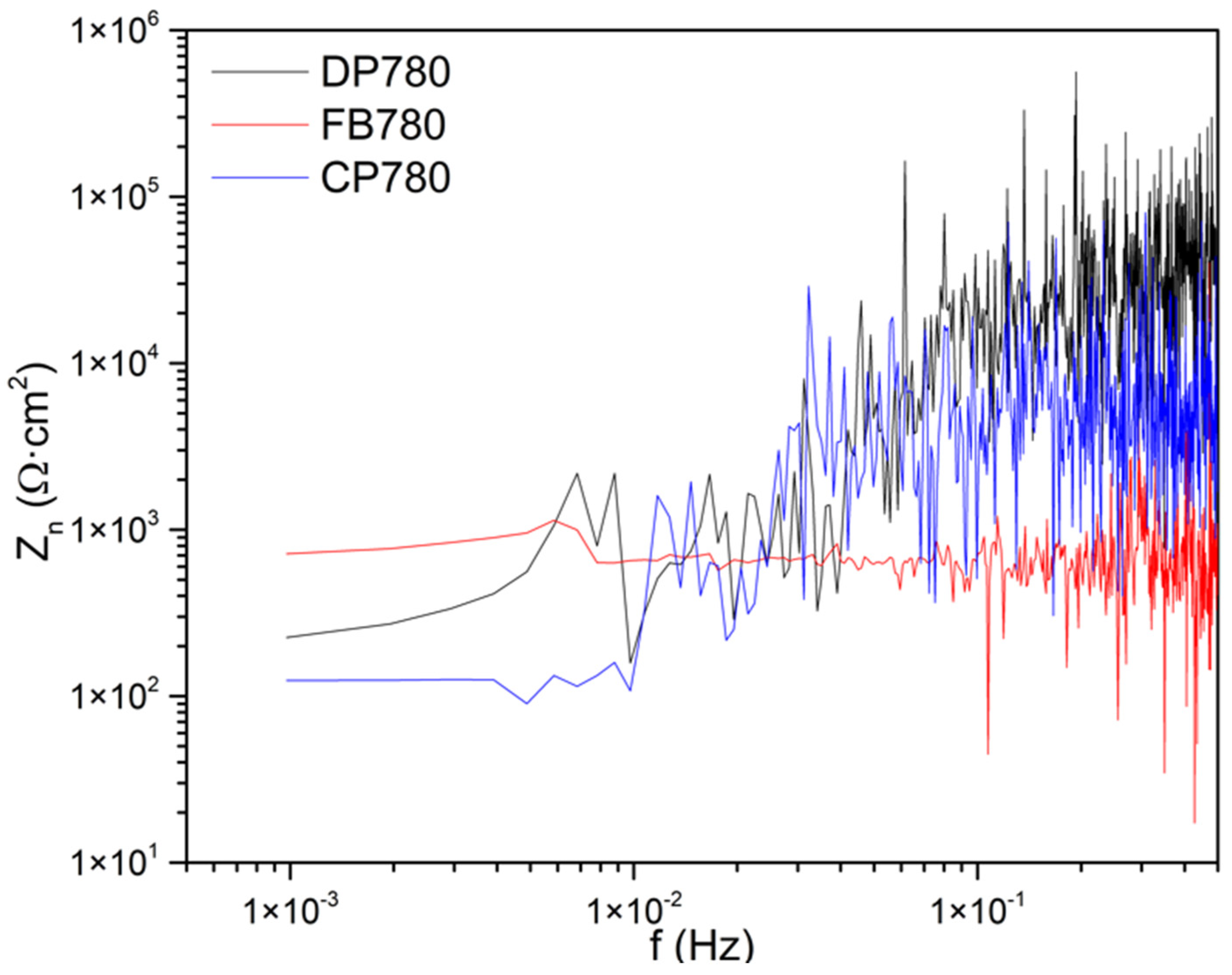
Noise Impedance
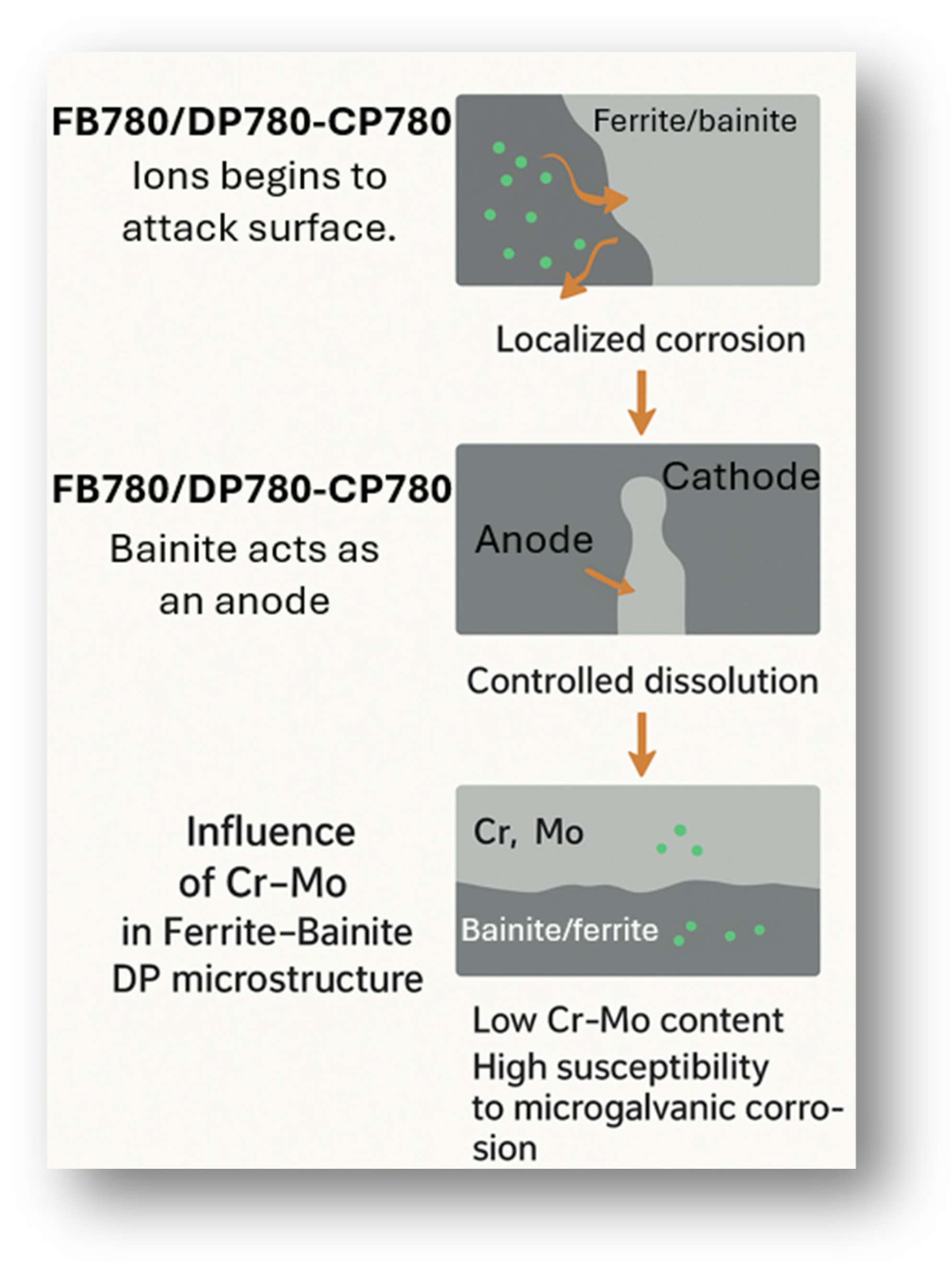
4. Discussion
5. Conclusions
- AHSS steels in their DP780, CP780, and FB780 grades presented galvanic pairs accompanied by a surface autocorrosion process. This process is generated by the diffusion of ions on the surface, resulting in uniform corrosion that degrades specific areas such as ferrite, inclusions, or some defects in the microstructure. With localized corrosion, chloride attack is encouraged, causing a uniform dissolution of the ferritic phase and resulting in pitting that is uniformly distributed and dissolves the material on the surface. The presence of inclusions influences the beginning of localized attacks.
- In CPP, the anodic breach reaction is associated with oxygen reduction, controlled by mass transfer.
- Both techniques demonstrated that localized corrosion is the predominant system in the AHSS.
- Results indicated that CPP revealed localized corrosion as the type of corrosion present in the system. This is due to the two phases that exist in the system.
- The results obtained by CPP determined that the Ecorr of CP 780 is higher (noble values), indicating a higher energy required to begin the corrosion process.
- The difference between the results of CPP and EN is due to the mechanism of corrosion. CPP helps determine the corrosion kinetics when a corrosion uniform process dominates; however, as corrosion of AHHS is dominated by a nucleation pitting corrosion process, CPP does not apply to this analysis due to the Tafel law, which applies to uniform corrosion processes.
- Zn showed that FB780 presented higher corrosion resistance when characterized by this method. That behavior is observable in all media and can be attributed to the presence of Mo in the alloy. On the other hand, CP780 exhibited lower resistance in various solutions, including Rn and Zn.
- Samples presented mixed corrosion, with a predominance of uniform corrosion, which occurs when a pitting process is diffused. In this case, the diffusion provoked the distribution of pitting in the susceptible phases.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuziak, R.; Kawalla, R.; Waengler, S. Advanced high strength steels for automotive industry. Arch. Civ. Mech. Eng. 2008, 8, 103–117. [Google Scholar] [CrossRef]
- Keeler, S.; Kimchi, M. Advanced High-Strength Steels Application Guidelines Version 5.0; WorldAutoSteel: Brussels, Belgium, 2014. [Google Scholar]
- Tamarelli, C.M. AHSS 101: The Evolving Use of Advanced High-Strength Steel for Automotive Applications; Steel Market Development Institute: Southfield, MI, USA, 2011; pp. 1–42. [Google Scholar]
- Montoya-Rangel, M.; Garza-Montes, O.N.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical noise measurements of advanced high-strength steels in different solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Auto/Steel Partnership. “A Special Edition of AHSS Case Studies.” Advanced High-Strength Steel Applications: Design and Stamping Process Guidelines. 2010. Available online: https://www.scribd.com/document/77898471/AHSS-Applications-Guidelines-Final (accessed on 1 October 2025).
- Keleştemur, O.; Yildiz, S. Effect of various dual-phase heat treatments on the corrosion behavior of reinforcing steel used in the reinforced concrete structures. Constr. Build. Mater. 2009, 23, 78–84. [Google Scholar] [CrossRef]
- Galán, J.; Samek, L.; Verleysen, P.; Verbeken, K.; Houbaert, Y. Advanced high strength steels for automotive industry. Rev. Metal. 2012, 48, 118–131. [Google Scholar] [CrossRef]
- Maffei, B.; Salvatore, W.; Valentini, R. Dual-phase steel rebars for high-ductile r.c. structures, Part 1: Microstructural and mechanical characterization of steel rebars. Eng. Struct. 2007, 29, 3325–3332. [Google Scholar] [CrossRef]
- Khan, A.S.; Baig, M.; Choi, S.H.; Yang, H.S.; Sun, X. Quasi-static and dynamic responses of advanced high strength steels: Experiments and modeling. Int. J. Plast. 2012, 30–31, 1–17. [Google Scholar] [CrossRef]
- Lesch, C.; Kwiaton, N.; Klose, F.B. Advanced High Strength Steels (AHSS) for Automotive Applications−Tailored Properties by Smart Microstructural Adjustments. Steel Res. Int. 2017, 88, 1700210. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.B.; Ray, K.K. Influence of bainite/martensite-content on the tensile properties of low carbon dual-phase steels. Mater. Sci. Eng. A 2008, 474, 270–282. [Google Scholar] [CrossRef]
- Qu, S.; Zhang, Y.; Pang, X.; Gao, K. Influence of temperature field on the microstructure of low carbon microalloyed ferrite-bainite dual-phase steel during heat treatment. Mater. Sci. Eng. A 2012, 536, 136–142. [Google Scholar] [CrossRef]
- Abedini, O.; Behroozi, M.; Marashi, P.; Ranjbarnodeh, E.; Pouranvari, M. Intercritical heat treatment temperature dependence of mechanical properties and corrosion resistance of dual phase steel. Mater. Res. 2019, 22, e20170969. [Google Scholar] [CrossRef]
- Mintz, B. Hot dip galvanising of transformation induced plasticity and other intercritically annealed steels. Int. Mater. Rev. 2001, 46, 169–197. [Google Scholar] [CrossRef]
- Uzun, H.; Önal, E. Mechanical Properties and Corrosion Behaviors in 3.5% NaCl Solution of Grade-A and Dual-Phase Steels Welded by FCAW. In Proceedings of the European Conference in Technology and Society—EuroTecS-2013, Sarajevo, Bosnia and Herzegovina, 27–28 June 2013. [Google Scholar]
- Schmitta, J.H.; Iungb, T. New developments of advanced high-strength steels for automotive applications. Comptes Rendus. Physique 2018, 19, 641–656. [Google Scholar] [CrossRef]
- Montoya-Rangel, M.; Garza-Montes-de-Oca, N.; Gaona-Tiburcio, C.; Almeraya-Calderón, F. Corrosion mechanism of advanced high strength dual-phase steels by electrochemical noise analysis in chloride solutions. Mater. Today Commun. 2023, 35, 105663. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, X.; Li, J. Corrosion Resistance of the Welded Joint of Submarine Pipeline Steel with Ferrite Plus Bainite Dual-Phase Microstructure. Steel Res. Int. 2015, 86, 1260–1270. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, P.H.; Guan, Y.; Chen, Q.F.; Pu, S.K. The corrosion resistance of ultra-low carbon bainitic steel. Corros. Sci. 2009, 51, 954–961. [Google Scholar] [CrossRef]
- Xia, D.; Song, S.; Wang, J.; Shi, J.; Bi, H.; Gao, Z. Determination of corrosion types from electrochemical noise by phase space reconstruction theory. Electrochem. Commun. 2012, 15, 88–92. [Google Scholar] [CrossRef]
- Monticelli, C. Evaluation of Corrosion Inhibitors by Electrochemical Noise Analysis. J. Electrochem. Soc. 1992, 139, 706. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Jáquez-Muñoz, J.M.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olgui-Coca, J.; Lopez-Leon, L.D.; Estupiñán-López, F.; Lira-Martínez, A.; Gaona Tiburcio, C. Corrosion Resistance of Titanium Alloys Anodized in Alkaline Solutions. Metals 2023, 13, 1510. [Google Scholar] [CrossRef]
- Suresh, G.U.; Mudali, U.K. Electrochemical Noise Analysis of Pitting Corrosion of Type 304L Stainless Steel. Corrosion 2014, 70, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Homborg, A.M.; Cottis, R.A.; Mol, J.M.C. An integrated approach in the time, frequency and time-frequency domain for the identification of corrosion using electrochemical noise. Electrochim. Acta. 2016, 222, 627–640. [Google Scholar] [CrossRef]
- Mansfeld, F.; Sun, Z. Technical Note: Localization Index Obtained from Electrochemical Noise Analysis. Corrosion 1999, 55, 915–918. [Google Scholar] [CrossRef]
- Mehdipour, M.; Naderi, R.; Markhali, B.P. Electrochemical study of effect of the concentration of azole derivatives on corrosion behavior of stainless steel in H2SO4. Prog. Org. Coatings 2014, 77, 1761–1767. [Google Scholar] [CrossRef]
- Ikpeseni, S.C.; Ameh, E.S. Effect of Temperature and Microstructure on the Corrosion Behaviour of a low Carbon Dual Phase Steel. Am J Eng. Res. 2017, 6, 1–7. [Google Scholar]
- Si, Y. Investigation of Galvanic Corrosion Behavior of Dual Phase Steel. ECS Trans. 2016, 72, 13. [Google Scholar] [CrossRef]
- Fushimi, K.; Yanagisawa, K.; Nakanishi, T.; Hasegawa, Y.; Kawano, T.; Kimura, M. Microelectrochemistry of dual-phase steel corroding in 0.1 M sulfuric acid. Electrochim. Acta 2013, 114, 83–87. [Google Scholar] [CrossRef]
- Nagiub, A.M. Electrochemical Noise Analysis for Different Green Corrosion Inhibitors for Copper Exposed to Chloride Media. Corros. Sci. 2017, 35, 201–210. [Google Scholar] [CrossRef]
- Gerengi, H.; Sen, N.; Uygur, I.; Kaya, E. Corrosion behavior of dual phase 600 and 800 steels in 3.5 wt.% NaCl environment. J. Adhes. Sci. Technol. 2020, 34, 903–915. [Google Scholar] [CrossRef]
- Park, I.J.; Kim, S.T.; Lee, I.S.; Park, Y.S.; Moon, M.B. A study on corrosion behavior of DP-type and TRIP-type cold rolled steel sheet. Mater. Trans. 2009, 50, 1440–1447. [Google Scholar] [CrossRef]
- Park, I.J.; Lee, S.M.; Kang, M.; Lee, S.; Lee, Y.K. Pitting corrosion behavior in advanced high strength steels. J. Alloys Compd. 2015, 619, 205–210. [Google Scholar] [CrossRef]
- ASTM E3-95; Standard Practice for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2007.
- Gaona-Tiburcio, C.; Almeraya-Calderón, F.; Chacon-Nava, J.; Matutes-Aquino, A.M.; Martinez-Villafañe, A. Electrochemical response of permanent magnets in different solutions. J. Alloys Compd. 2004, 369, 78–80. [Google Scholar] [CrossRef]
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM International: West Conshohocken, PA, USA, 1999.
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- ASTM G199-09; Standard Guide for Electrochemical Noise Measurement. ASTM International: West Conshohocken, PA, USA, 2014.
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Méndez-Ramírez, C.T.; Baltazar-Zamora, M.Á.; Estupinán-López, F.; Bautista-Margulis, R.G.; Cuevas-Rodríguez, J.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. Corrosion of Titanium Alloys Anodized Using Electrochemical Techniques. Metals 2023, 13, 476. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Méndez-Ramírez, C.T.; Martínez-Ramos, C.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Estupinan-Lopez, F.; Landa-Ruiz, L.; Nieves-Mendoza, D.; Almeraya-Calderon, F. Electrochemical Noise Analysis: An Approach to the Effectivity of Each Method in Different Materials. Materials 2024, 17, 4013. [Google Scholar] [CrossRef]
- Almeraya-Calderon, F.; Villegas-Tovar, M.; Maldonado-Bandala, E.; Lara-Banda, M.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Nieves-Mendoza, D.; Lopez-Leon, L.D.; Jaquez-Muñoz, J.M.; Estupiñán-López, F.; et al. Use of Electrochemical Noise for the Study of Corrosion by Passivated CUSTOM 450 and AM 350 Stainless Steels. Metals 2024, 14, 341. [Google Scholar] [CrossRef]
- Thewlis, G. Classification and quantification of microstructures in steels. J. Mater. Sci. Technol. 2004, 20, 143–160. [Google Scholar] [CrossRef]
- Kang, Y.; Han, Q.; Zhao, X.; Cai, M. Influence of nanoparticle reinforcements on the strengthening mechanisms of an ultrafine-grained dual phase steel containing titanium. Mater. Des. 2013, 44, 331–339. [Google Scholar] [CrossRef]
- Martínez-Villafañe, A.; Almeraya-Calderón, F.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.; Porcayo-Calderón, J. High-Temperature Degradation and Protection of Ferritic and Austenitic Steels in Steam Generators. J. Mater. Eng. Perform. 1998, 7, 108–113. [Google Scholar] [CrossRef]
- Lara Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Cabral, M.J.A.; Estupinan, F.; Baltazar-Zamora, M.A.; Almeraya-Calderon, F. Corrosion Behaviour of 304 Austenitic, 15-5PH and 17-4PH Passive Stainless Steels in acid solutions. Int. J. Electrochem. Sci. 2018, 13, 10314–10324. [Google Scholar] [CrossRef]
- Kwon, O.; Lee, K.; Kim, G.; Chin, K.-G. New Trends in Advanced High Strength Steel Developments For Automotive Application. Mat. Sci. Forum. 2010, 638–642, 136–141. [Google Scholar] [CrossRef]
- Aydin, K.; Essadiqi, E.; Yue, S. Development of 3rd generation AHSS with medium Mn content alloying compositions. Mater. Sci. Eng. A 2013, 564, 501–508. [Google Scholar] [CrossRef]
- Saeidi, N.; Ekrami, A. Comparison of mechanical properties of martensite/ferrite and bainite/ferrite dual phase 4340 steels. Mater. Sci. Eng. A 2009, 523, 125–129. [Google Scholar] [CrossRef]
- Kelly, R.G.; Inman, M.E.; Hudson, J.L. Analysis of electrochemical noise for type 410 stainless steel in chloride solutions. In Electrochemical Noise Measurement for Corrosion Applications; ASTM International: West Conshohocken, PA, USA, 1996. [Google Scholar]
- Treseder, R.S. NACE Corrosion Engineers Reference Book, 2nd ed.; NACE International: Houston, TX, USA, 1991. [Google Scholar]
- Ramirez-Arteaga, A.M.; Gonzalez-Rodriguez, J.G.; Campillo, B.; Gaona-Tiburcio, C.; Dominguez-Patiño, G.; Leduc Lezama, L.; Chacon-Nava, J.G.; Neri-Flores, M.A.; Martinez-Villafañe, A. An electrochemical study of the corrosion behavior of a dual phase steel in 0.5 m H2SO4. Int. J. Electrochem. Sci. 2010, 5, 1786–1798. [Google Scholar] [CrossRef]
- Tafel, J. Über die Polarisation bei kathodischer Wasserstoffentwicklung. Z. Phys. Chem. 1905, 50, 641–712. [Google Scholar] [CrossRef]
- Butler, J.A.V. Studies in heterogeneous equilibria. Part II—The kinetic interpretation on the Nernst theory of electromotive force. Trans. Faraday Soc. 1924, 19, 729–733. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Lara-Banda, M.; Almeraya-Calderón, F.; Jáquez-Muñoz, J.M.; Nieves-Mendoza, D.; Baltazar-Zamora, M.A.; Olguín-Coca, J.; Estupiñan-Lopez, F.; Cabral Miramontes, J.; Santiago-Hurtado, G.; Gaona-Tiburcio, C. A Study of the Corrosion Behavior of AHSS Complex-Phase CP 780 Employing an Electrochemical Noise Technique Analyzed by Different Methods. Metals 2025, 15, 59. [Google Scholar] [CrossRef]
- Martínez-Aparicio, B.; Martínez-Bastidas, D.; Gaona-Tiburcio, C. Ulises Martin, José Cabral-Miramontes & Facundo Almeraya-Calderón Localized corrosion of 15–5 PH and 17–4 PH stainless steel in NaCl solution. J. Solid. State Electrochem. 2023, 27, 2993–3001. [Google Scholar] [CrossRef]
- Martínez-Ramos, C.; Olguin-Coca, J.; Lopez-Leon, L.D.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Castañeda-Robles, I.; Jaquez-Muñoz, J.M.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; et al. Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals 2023, 13, 1850. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Montoya-R, M.; Garza Montes de Oca, N.; Castorena, G.H.J.; Estupiñan, L.F.; Cabral, M.J.; Maldonado, B.E.; Gaona-Tiburcio, C. Corrosion behavior of multilayer coatings deposited by PVD on Inconel 718 in Chloride and Sulphuric Acid solutions. Int. J. Electrochem. Sci. 2019, 14, 9596–9609. [Google Scholar] [CrossRef]
- Gómez, J.A.; Antonissen, J.; Palacio, C.A.; Grave, E. De Effects of Si as alloying element on corrosion resistance of weathering steel. Corros. Sci. 2012, 59, 198–203. [Google Scholar] [CrossRef]
- Cottis, R.; Turgoose, S. Electrochemical Impedance and Noise; NACE International: Shanghai, China, 1999; p. 153. [Google Scholar]
- Mansfeld, F.; Oldham, H. A modification of the Stern—Geary linear polarization equation. Corros. Sci. 1971, 11, 787–796. [Google Scholar] [CrossRef]
- Bogaerts, J.F.; Chen, W.F. The physical meaning of noise resistance. Corros. Sci. 1995, 37, 1839–1842. [Google Scholar] [CrossRef]
- Homborg, A.M.; van Westing, E.P.M.; Tinga, T.; Zhang, X.; Oonincx, P.J.; Ferrari, G.M.; de Wit, J.H.W.; Mol, J.M.C. Novel Time–Frequency Characterization of Electrochemical Noise Data in Corrosion Studies Using Hilbert Spectra. Corros. Sci. 2013, 66, 97–110. [Google Scholar] [CrossRef]
- Botona Pedemonte, F.J.; Aballe Villero, A.; Marcos Bárcena, M. Ruido Electroquímico. Métodos de Análisis; Septem Ediciones: Barcelona, Spain, 2002; ISBN 84-95687-33-X. [Google Scholar]
- Gaona-Tiburcio, C.; Aguilar, L.M.R.; Zambrano-Robledo, P.; Estupiñán-López, F.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Castillo-González, E.; Almeraya-Calderón, F. Electrochemical Noise Analysis of Nickel Based Superalloys in Acid Solutions. Int. J. Electrochem. Sci. 2014, 9, 523–533. [Google Scholar] [CrossRef]
- Park, C.J.; Kwon, H.S. Electrochemical noise analysis of localized corrosion of duplex stainless steel aged at 475 °C. Mater. Chem. Phys. 2005, 91, 355–360. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Cottis, R.A. Shot noise and statistical parameters for the estimation of corrosion mechanisms. Corros. Sci. 2005, 47, 3280–3299. [Google Scholar] [CrossRef]
- Lara, M.; Gaona, C.; Zambrano, P.; Delgado, M.; Cabral, J.; Nieves, D.; Maldonado, E.; Estupiñan, F.; Chacón, J.; Almeraya, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using. Materials 2020, 12, 2836. [Google Scholar] [CrossRef]
- Reid, S.A.; Eden, D.A. Assessment of Corrosion. US9264824B1, 24 July 2001. [Google Scholar]
- Cottis, R. Interpretation of Electrochemical Noise Data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]
- Eden, D.A. Electrochemical Noise—The First Two Octaves. In Proceedings of the NACE International Corrosion/98, San Diego, CA, USA, 22–27 March 1998; pp. 1–31. [Google Scholar]
- Calabrese, L.; Galeano, M.; Proverbio, E. Identifying Corrosion Forms on Synthetic Electrochemical Noise Signals by the Hilbert–Huang Transform Method. Corros. Eng. Sci. Technol. 2018, 53, 492–501. [Google Scholar] [CrossRef]
- Bertocci, U.; Huet, F.; Nogueira, R.P.; Rousseau, P. Drift Removal Procedures in the Analysis of Electrochemical Noise. Corrosion 2002, 58, 337–347. [Google Scholar] [CrossRef]
- Homborg, A.M.; Tinga, T.; Van Westing, E.P.M.; Zhang, X.; Ferrari, G.M.; De Wit, J.H.W.; Mol, J.M.C. A Critical Appraisal of the Interpretation of Electrochemical Noise for Corrosion Studies. Corrosion 2014, 70, 971–987. [Google Scholar] [CrossRef]
- Enestam, S.; Bankiewicz, D.; Tuiremo, J.; Mäkelä, K.; Hupa, M. Are NaCl and KCl equally corrosive on superheater materials of steam boilers? Fuel 2013, 104, 294–306. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Shibaeva, T.V.; Laurinavichyute, V.K.; Tsirlina, G.A.; Arsenkin, A.M.; Grigorovich, K.V. The effect of microstructure and non-metallic inclusions on corrosion behavior of low carbon steel in chloride containing solutions. Corros. Sci. 2014, 80, 299–308. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Zhang, Z.; Cao, F.H.; Zhang, J.Q. Dimensional analysis applied to pitting corrosion measurements. Electrochim. Acta 2008, 53, 2688–2698. [Google Scholar] [CrossRef]
- Bertucci, U.; Gabrielli, C.; Huet, F.; Keddam, M.; Rousseau, P. Noise Resistance Applied to Corrosion Measurements: II. Experimental Tests. J. Electroche. Soc. 1997, 144, 37. [Google Scholar] [CrossRef]
- Xia, D.-H.; Song, S.-Z.; Behnamian, Y. Detection of corrosion degradation using electrochemical noise (EN): Review of signal processing methods for identifying corrosion forms. Corros. Eng. Sci. Technol. 2016, 51, 527–544. [Google Scholar] [CrossRef]
- Almeraya, C.F.; Estupiñán, F.; Zambrano, R.P.; Martínez-Villafañe, A.; Borunda, T.A.; Colás, R.; Gaona-Tiburcio, C. Análisis de los transitorios de ruido electroquímico para aceros inoxidables 316 Y – DUPLEX 2205 en NaCl Y FeCl (·) Electrochemical noise transient analysis for 316 and Duplex 2205 stainless steels in NaCl and FeCl. Rev. Metal. 2012, 48, 147–156. [Google Scholar] [CrossRef]
- Galvan-Martinez, R.; Orozco-Cruz, R.; Torres-Sanchez, R.; Martinez, E.A. Corrosion study of the X52 steel immersed in seawater with a corrosion inhibitor using a rotating cylinder electrode. Mater. Corros. 2010, 61, 872–876. [Google Scholar] [CrossRef]
- Sakairi, M.; Sasaki, R.; Kaneko, A.; Seki, Y.; Nagasawa, D. Evaluation of metal cation effects on galvanic corrosion behavior of the A5052 aluminum alloy in low chloride ion containing solution by electrochemical noise impedance. Electrochim. Acta 2014, 131, 123. [Google Scholar] [CrossRef]
- Marcus, P. (Ed.) Corrosion Mechanisms in Theory and Practice, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Cottis, R.A.; Homborg, A.M.; Mol, J.M.C. The Relationship between Spectral and Wavelet Techniques for Noise Analysis. Electrochim. Acta 2016, 202, 277–287. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Vinaya; Madhusudhan, R.; Sah, R.; Manjini, S. Mechanical and Electrochemical Behavior of Dual-Phase Steels Having Varying Ferrite Martensite Volume Fractions. J. Mater. Eng. Perform. 2019, 28, 3600–3613. [Google Scholar] [CrossRef]
- ASTM F2129-08; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant. ASTM International: West Conshohocken, PA, USA, 2001. [CrossRef]
- Villegas-Tovar, J.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Baltazar-Zamora, M.A.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderón, F. Electrochemical Corrosion Behavior of Passivated Precipitation Hardening Stainless Steels for Aerospace Applications. Metals 2023, 13, 835. [Google Scholar] [CrossRef]
- Silverman, D.C. Practical Corrosion Prediction Using Electrochemical Techniques. In Uhlig’s Corrosion Handbook, 3rd ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 1129–1166. [Google Scholar]
- Kumar, A.M.; Toor, I.U.H. Localized Corrosion Evaluation of Newly Developed Stainless-Steel Alloys in Chloride Medium through Dynamic and Localized Micro Electrochemical Techniques. J. Mater. Res. Technol. 2023, 26, 5668–5682. [Google Scholar] [CrossRef]
- Li, T.; Chien, S.C.; Ren, Z.; Windl, W.; Ernst, F.; Frankel, G.S. Understanding the Efficacy of Concentrated Interstitial Carbon in Enhancing the Pitting Corrosion Resistance of Stainless Steel. Acta Mater. 2021, 221, 117433. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Georges, M.; Frankel, G.S. Understanding the Potentiodynamic Critical Pitting Temperature. Corros. Sci. 2024, 235, 112216. [Google Scholar] [CrossRef]
- Frankel, G.S. Fundamentals of Corrosion Kinetics. In Active Protective Coatings; Springer Series in Materials Science; Springer: Dordrecht, The Netherlands, 2016; Volume 233, pp. 17–32. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.J. Impact of Interfacial Phase Evolution at the Steel-Coating Interface on Hydrogen Diffusion and Corrosion Degradation in Hot-Dip Galvanized AHSS. Surf. Coatings Technol. 2025, 513, 132511. [Google Scholar] [CrossRef]
- Reyes, S.; Rodriguez, L.; Parra, G.; Lemus, M.; Microstructural, V.; Enrique Salas Reyes, A.; Ángel Lara Rodriguez, G.; Rafael González Parra, J.; Hugo Mercado Lemus, V. Microstructural Characterization and Corrosion Behavior of Similar and Dissimilar Welded Advanced High-Strength Steels (AHSS) by Rotary Friction Welding. Materials 2024, 17, 918. [Google Scholar] [CrossRef] [PubMed]
- Haisch, T.; Mittemeijer, E.J.; Schultze, J.W. On the influence of microstructure and carbide content of steels on the electrochemical dissolution process in aqueous NaCl electrolytes. Mater. Corros. 2002, 53, 740–755. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Zhao, X.; Dong, C.; Kang, S. Effects of Nucleation Site and Morphology of Carbide-Free Bainite on Microstructures and Properties of Bainite/Martensite Multiphase Steels. Mater. Sci. Eng. A 2019, 744, 86–93. [Google Scholar] [CrossRef]
- Qu, S.; Pang, X.; Wang, Y.; Gao, K. Corrosion Behavior of Each Phase in Low Carbon Microalloyed Ferrite–Bainite Dual-Phase Steel: Experiments and Modeling. Corros. Sci. 2013, 75, 67–77. [Google Scholar] [CrossRef]
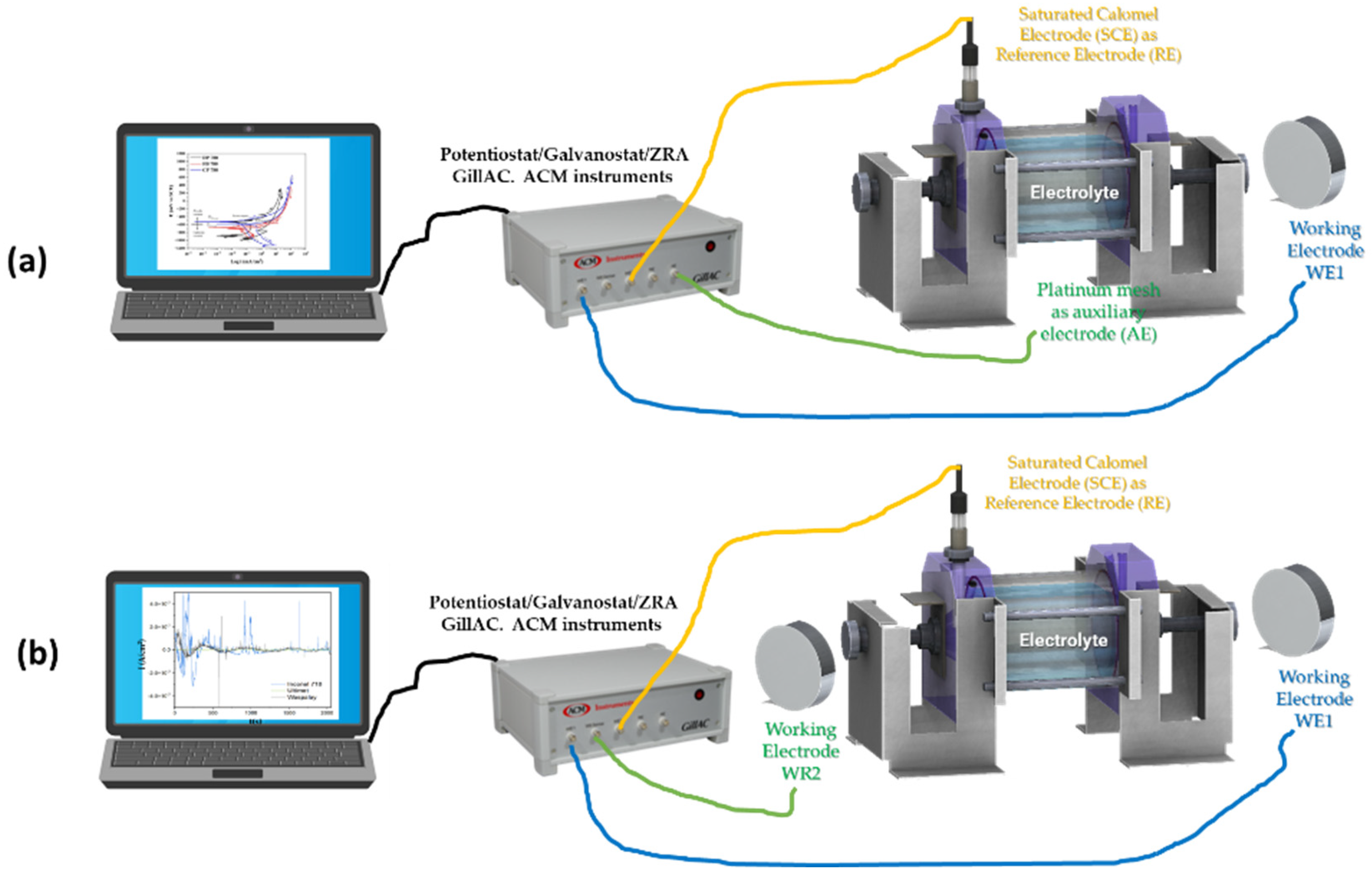



| Element | Dual-Phase DP780 | Ferritic–Bainitic FB780 | Complex-Phase CP780 |
|---|---|---|---|
| Fe | Balance | Balance | Balance |
| C | 0.10 | 0.09 | 0.09 |
| Mn | 2.61 | 1.73 | 1.669 |
| Cr | 0.420 | 0.640 | 0.771 |
| Mo | - | 0.006 | - |
| Si | 0.510 | 0.300 | 0.511 |
| Ti | 0.080 | 0.021 | 0.007 |
| Nb | - | - | 0.045 |
| AHSS Steels | Solution | Ecorr (mV vs. SCE) | Rp (Ω·cm2) | Epit (mV) | icorr (mA/cm2) | Hysteresis |
|---|---|---|---|---|---|---|
| DP 780 | NaCl | −883 | 38 | 297 | 1.69 × 10−2 | Positive |
| FB 780 | −678 | 9.62 | 335 | 1.98 × 10−2 | Positive | |
| CP 780 | −525 | 1133.1 | 638 | 2.20 × 10−3 | Positive | |
| DP 780 | CaCl2 | −670 | 24 | 275 | 2.18 × 10−2 | Positive |
| FB 780 | −710 | 28.7 | 296 | 1.30 × 10−2 | Positive | |
| CP 780 | 205 | 1754.6 | 1173 | 1.63 × 10−3 | Positive | |
| DP 780 | MgCl2 | −748 | 41 | 255 | 3.76 × 10−3 | Positive |
| FB 780 | −709 | 16.5 | 303 | 1.502 × 10−2 | Positive | |
| CP 780 | 184 | 3595.3 | 1178 | 1.62 × 10−3 | Positive |
| Corrosion Type | LI |
|---|---|
| Localized | 1.0–0.1 |
| Mixt | 0.1–0.01 |
| Uniform | 0.01–0.001 |
| Corrosion Type | Potential | Current | ||
|---|---|---|---|---|
| Skewness | Kurtosis | Skewness | Kurtosis | |
| Uniform | <±1 | <3 | <±1 | <3 |
| Pitting | <−2 | >>3 | >±2 | >>3 |
| Transgranular (SCC) | 4 | 20 | −4 | 20 |
| Intergranular (SCC 1) | −6.6 | 18 to 114 | 1.5 to 3.2 | 6.4 to 15.6 |
| Intergranular (SCC 2) | −2 to −6 | 5 to 45 | 3 to 6 | 10 to 60 |
| Alloy | LI | Kurtosis | Skewness | Rn (Ω·cm2) |
|---|---|---|---|---|
| NaCl | ||||
| DP780 | 0.016 | 2.3 | −0.17 | 531 |
| FB780 | 0.037 | 2.9 | 0.3 | 584 |
| CP780 | 0.081 | 147 | 8 | 81 |
| CaCl2 | ||||
| DP780 | 0.022 | 1.98 | −0.06 | 384 |
| FB780 | 0.008 | 2.09 | 0.07 | 1026 |
| CP780 | 0.007 | 2.9 | 0.01 | 209 |
| MgCl2 | ||||
| DP780 | 0.01 | 2.4 | −0.53 | 972 |
| FB780 | 0.17 | 5.9 | 1.17 | 700 |
| CP780 | 0.016 | 10 | 0.26 | 124 |
| β Intervals | Corrosion Type | |||||
|---|---|---|---|---|---|---|
| Uniform | Pitting | Passive | ||||
| Minimum | Maximum | Minimum | Maximum | Minimum | Maximum | |
| dB(V)·Decade−1 | 0 | −7 | −20 | −25 | −15 | −25 |
| dB(A)·Decade−1 | 0 | −7 | −7 | −14 | −1 | 1 |
| Alloy | ψ0 | Zn0 |
|---|---|---|
| NaCl | ||
| DP780 | −108 | 253 |
| FB780 | −116 | 845 |
| CP780 | −85 | 88 |
| CaCl2 | ||
| DP780 | −105 | 48 |
| FB780 | −111 | 158 |
| CP780 | −102 | 117 |
| MgCl2 | ||
| DP780 | −118 | 225 |
| FB780 | −105 | 716 |
| CP780 | −93 | 124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeraya-Calderón, F.; Montoya-Rangel, M.; Nieves-Mendoza, D.; Jáquez-Muñoz, J.M.; Diaz-Olivares, A.; Lara-Banda, M.; Maldonado-Bandala, E.; Estupinan-Lopez, F.; Cabral-Miramontes, J.; Olguin-Coca, J.; et al. Corrosion Behavior of Advanced High-Strength Steels (AHSS) in Chloride Solutions for Automotive Applications. Metals 2025, 15, 1116. https://doi.org/10.3390/met15101116
Almeraya-Calderón F, Montoya-Rangel M, Nieves-Mendoza D, Jáquez-Muñoz JM, Diaz-Olivares A, Lara-Banda M, Maldonado-Bandala E, Estupinan-Lopez F, Cabral-Miramontes J, Olguin-Coca J, et al. Corrosion Behavior of Advanced High-Strength Steels (AHSS) in Chloride Solutions for Automotive Applications. Metals. 2025; 15(10):1116. https://doi.org/10.3390/met15101116
Chicago/Turabian StyleAlmeraya-Calderón, Facundo, Marvin Montoya-Rangel, Demetrio Nieves-Mendoza, Jesus Manuel Jáquez-Muñoz, Abel Diaz-Olivares, Maria Lara-Banda, Erick Maldonado-Bandala, Francisco Estupinan-Lopez, Jose Cabral-Miramontes, Javier Olguin-Coca, and et al. 2025. "Corrosion Behavior of Advanced High-Strength Steels (AHSS) in Chloride Solutions for Automotive Applications" Metals 15, no. 10: 1116. https://doi.org/10.3390/met15101116
APA StyleAlmeraya-Calderón, F., Montoya-Rangel, M., Nieves-Mendoza, D., Jáquez-Muñoz, J. M., Diaz-Olivares, A., Lara-Banda, M., Maldonado-Bandala, E., Estupinan-Lopez, F., Cabral-Miramontes, J., Olguin-Coca, J., & Gaona-Tiburcio, C. (2025). Corrosion Behavior of Advanced High-Strength Steels (AHSS) in Chloride Solutions for Automotive Applications. Metals, 15(10), 1116. https://doi.org/10.3390/met15101116








