Low-Carbon Steel Formed by DRECE Method with Hot-Dip Zinc Galvanizing and Potentiodynamic Polarization Tests to Study Its Corrosion Behavior
Abstract
1. Introduction
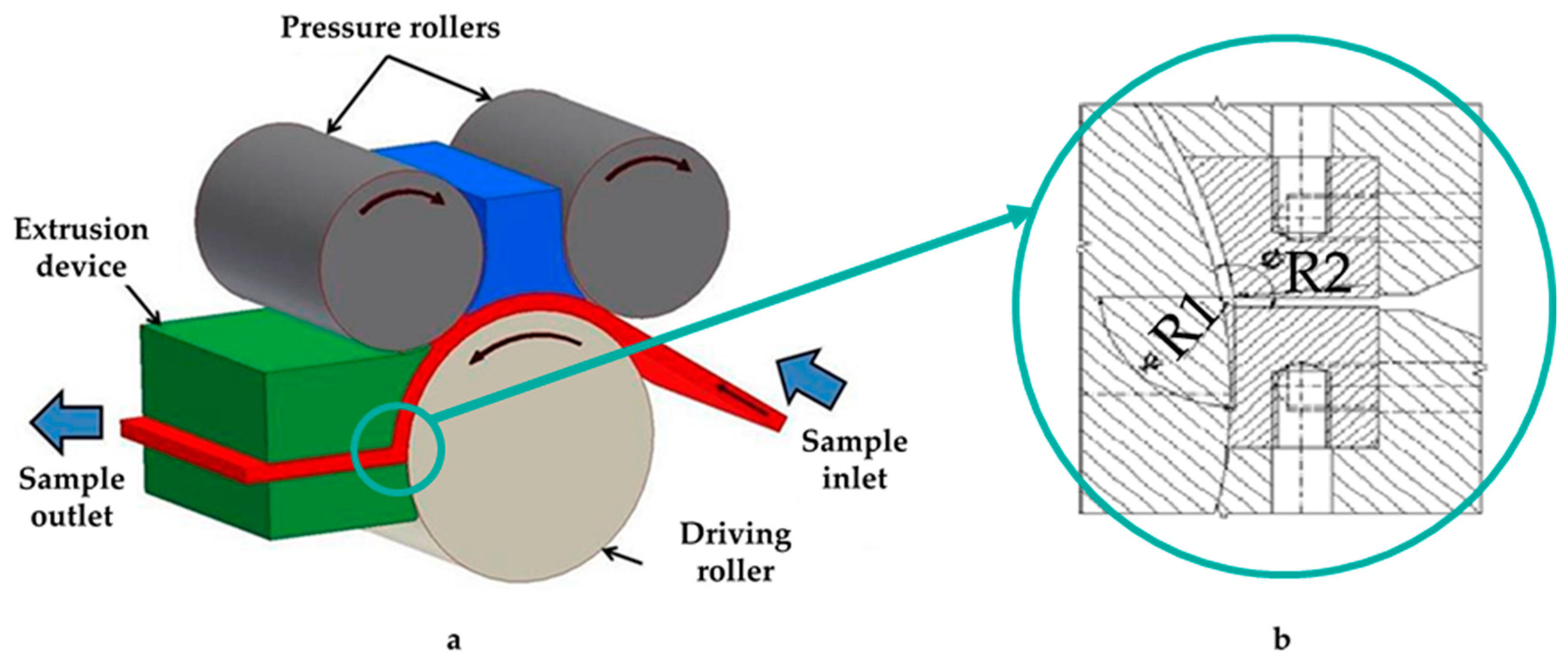
2. Materials and Methods
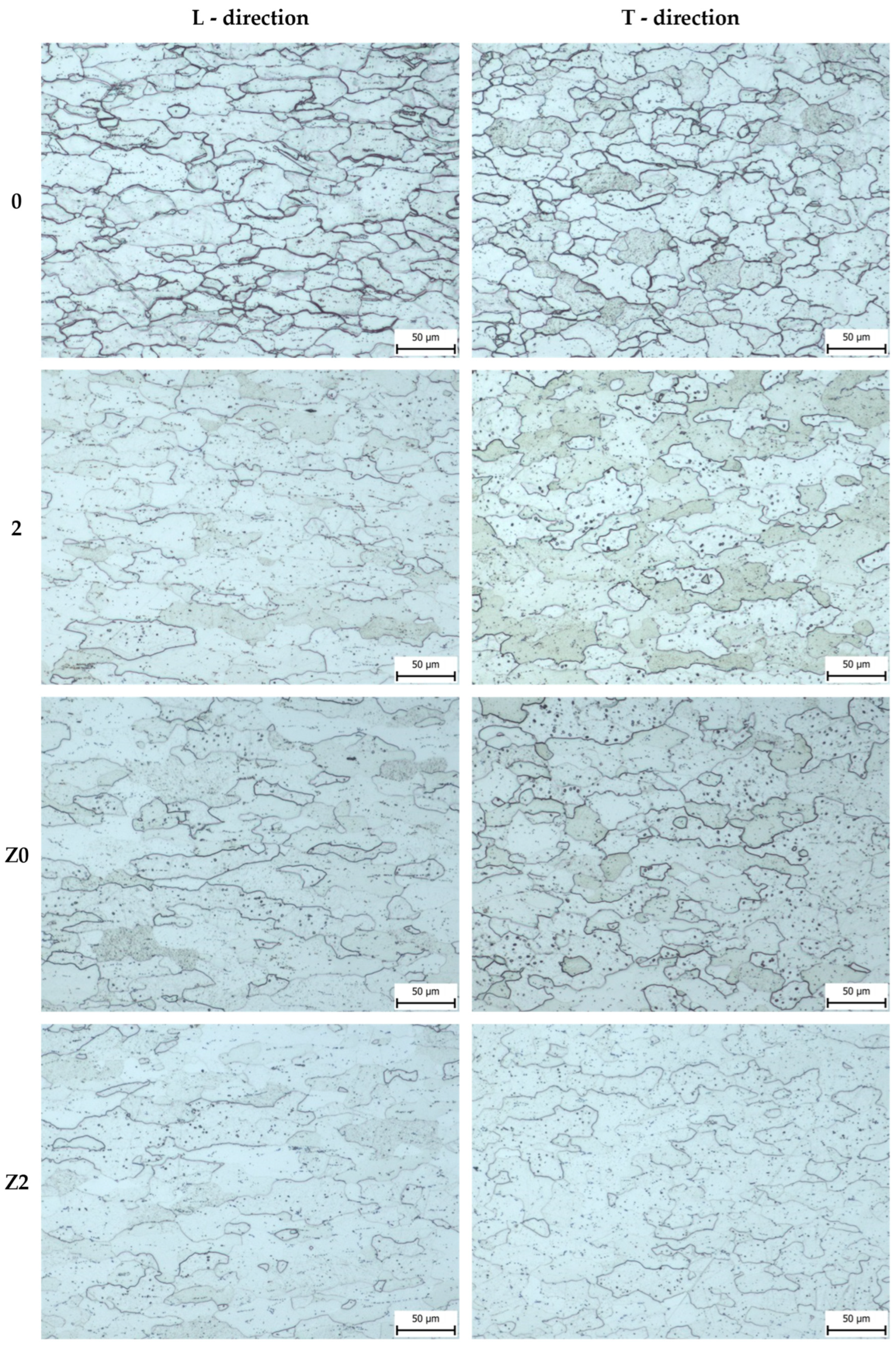
3. Results and Discussion
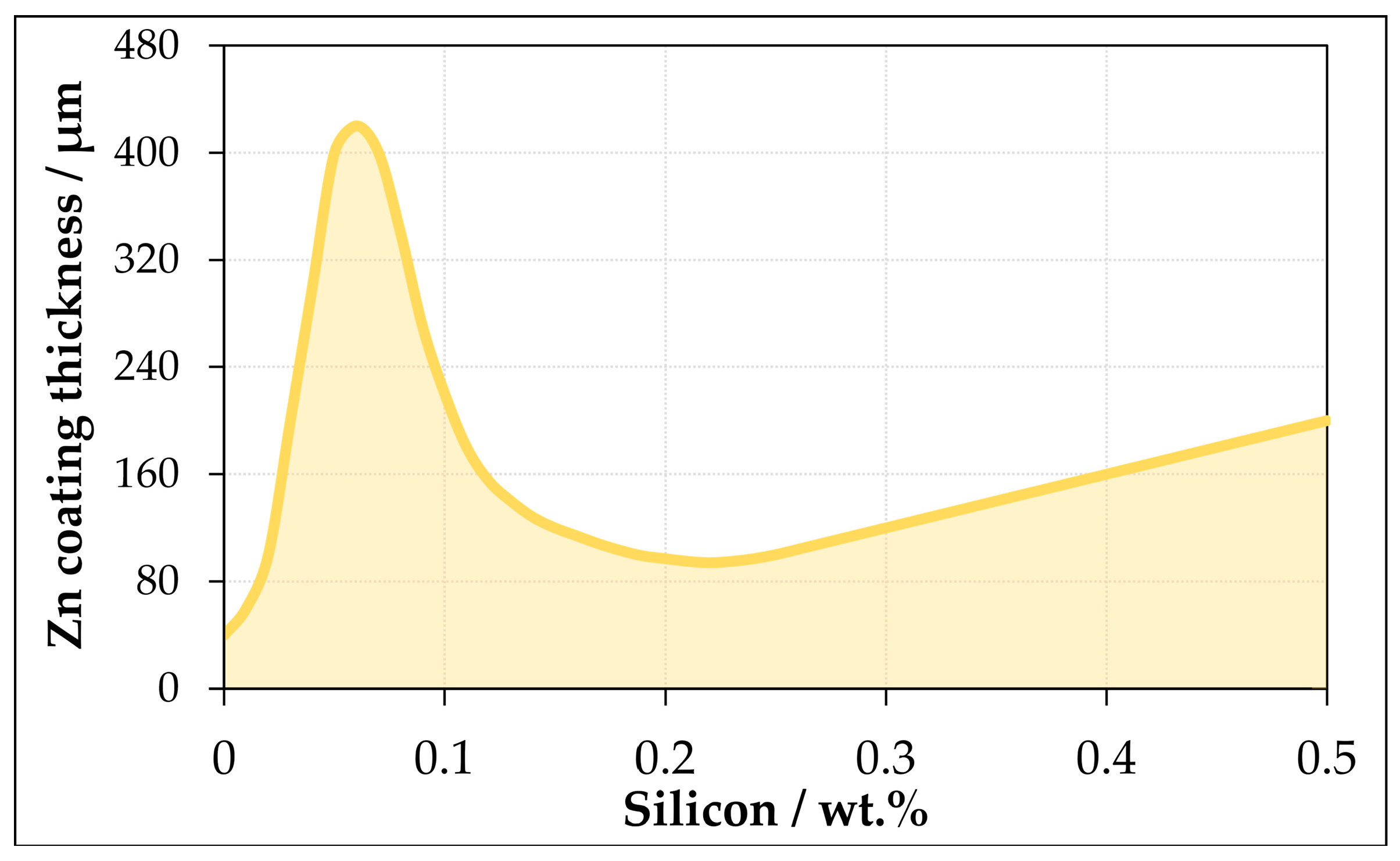
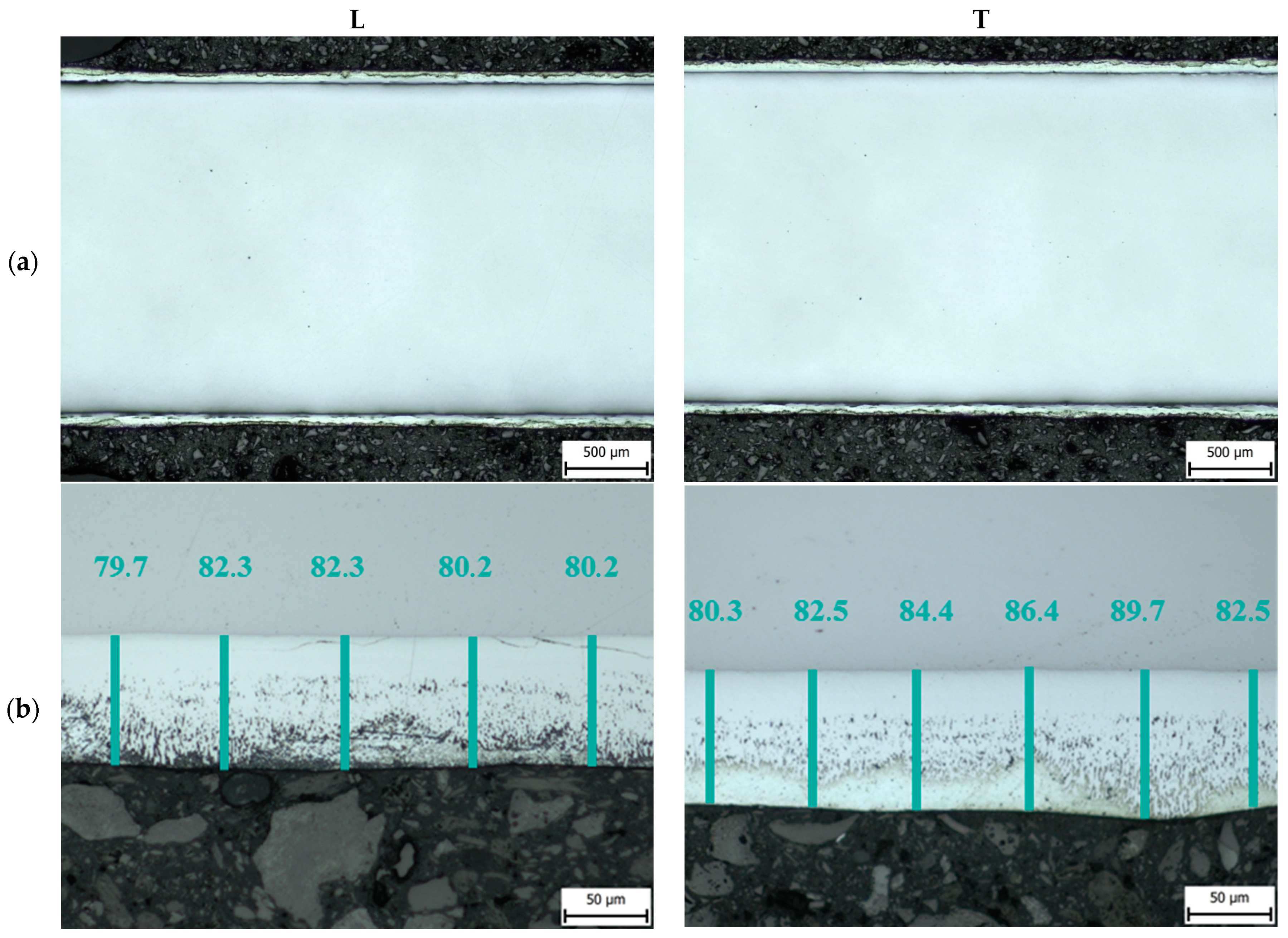
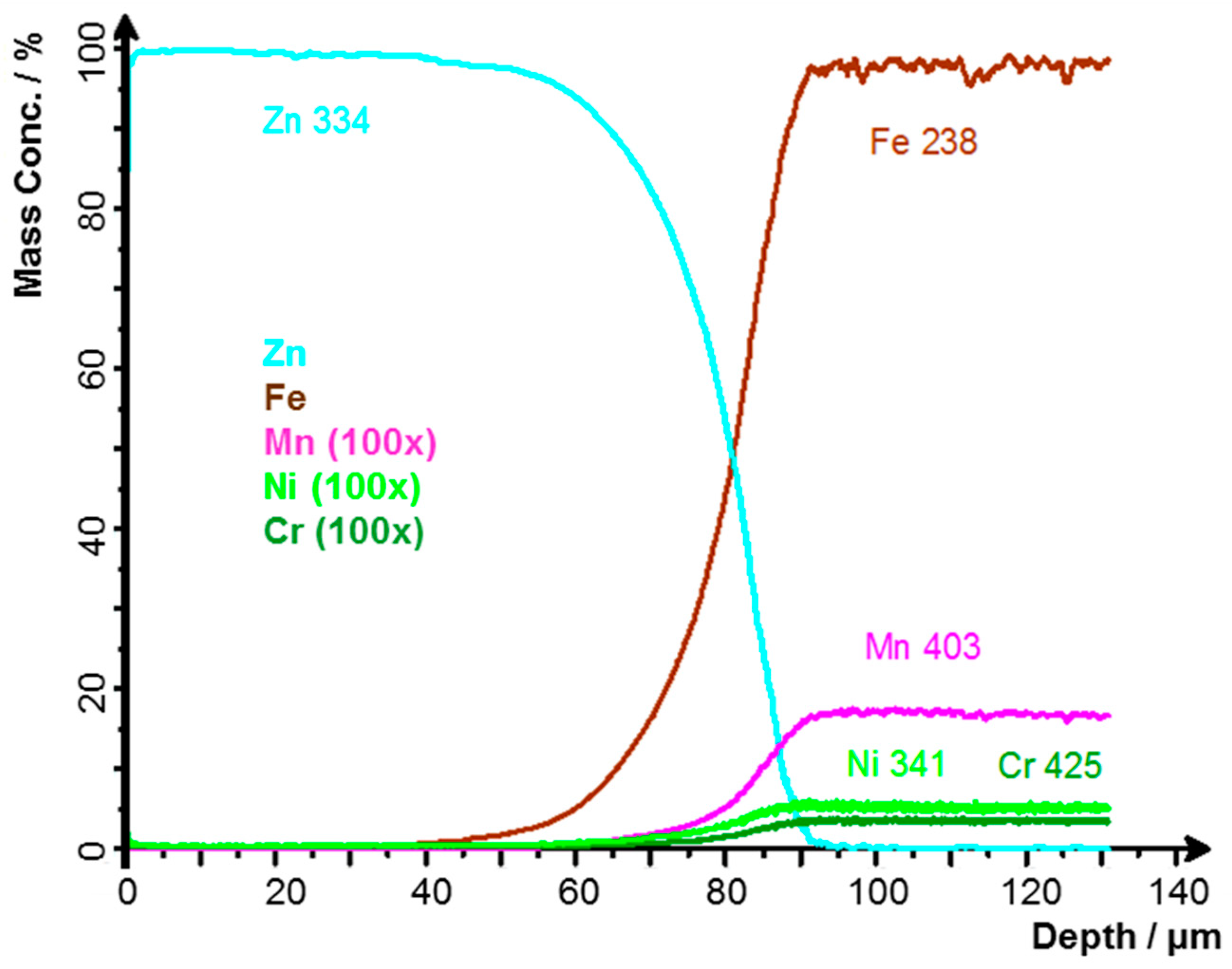
3.1. Thickness of the Zinc Layer
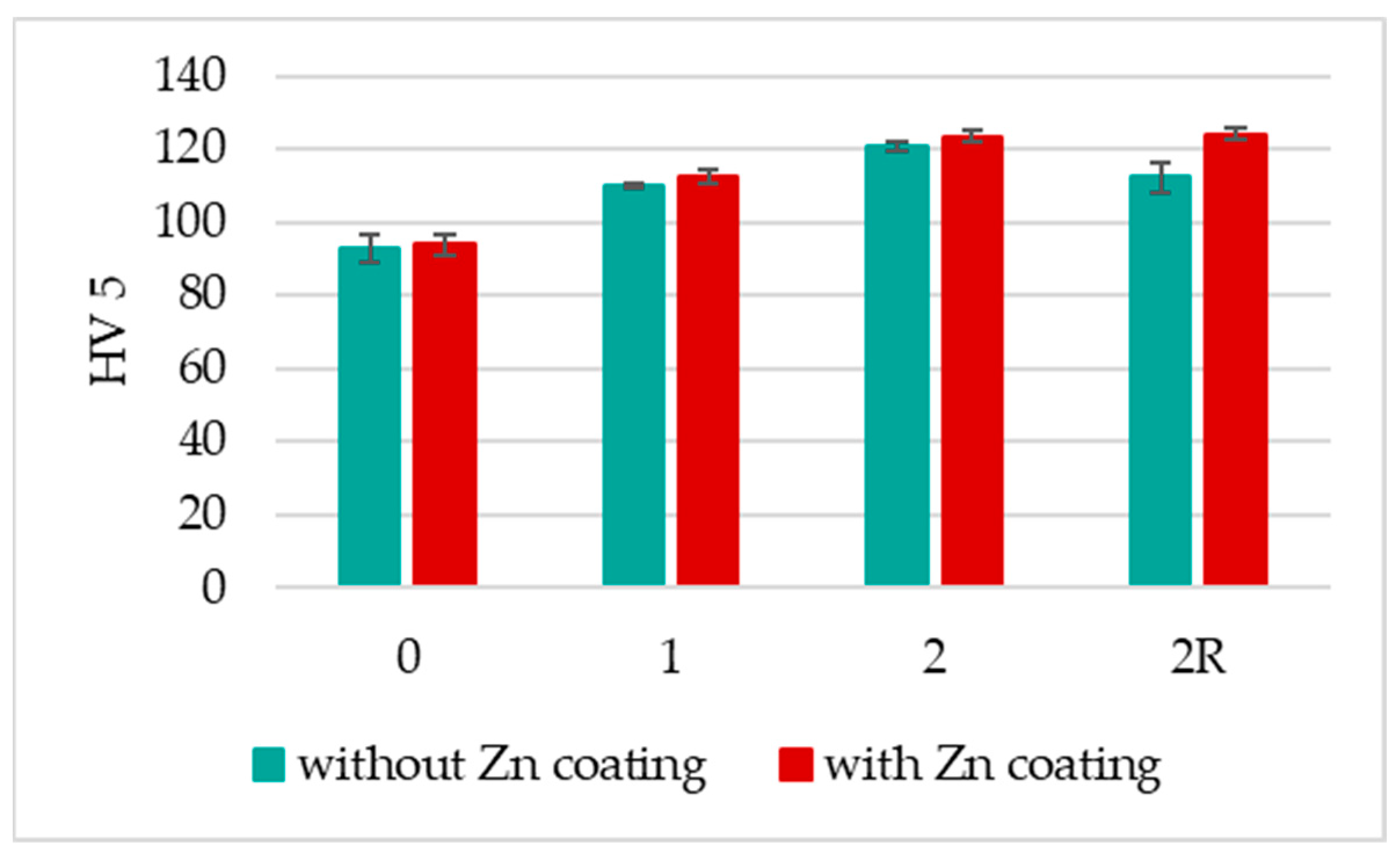
3.2. HV 5 Hardness
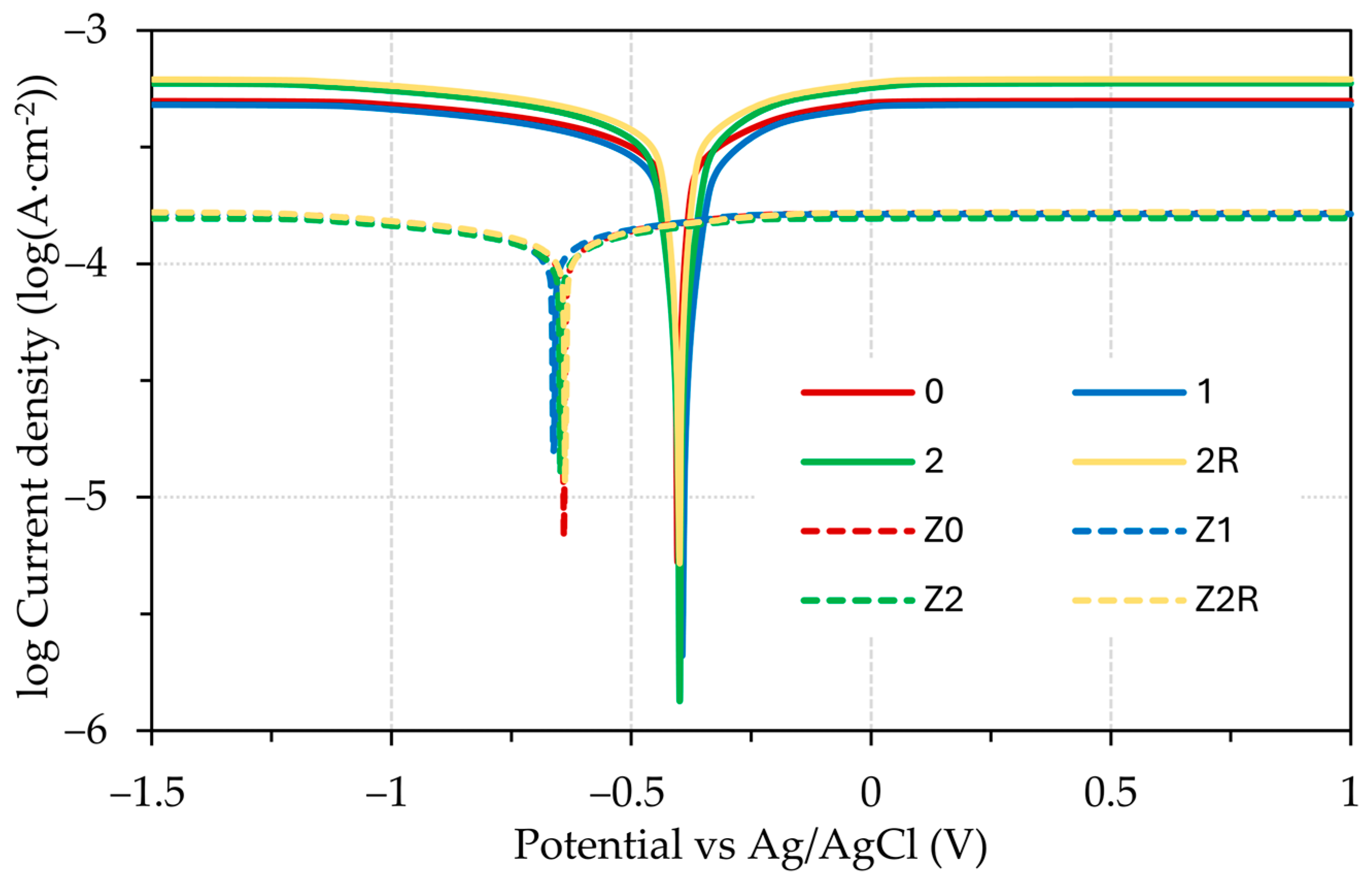
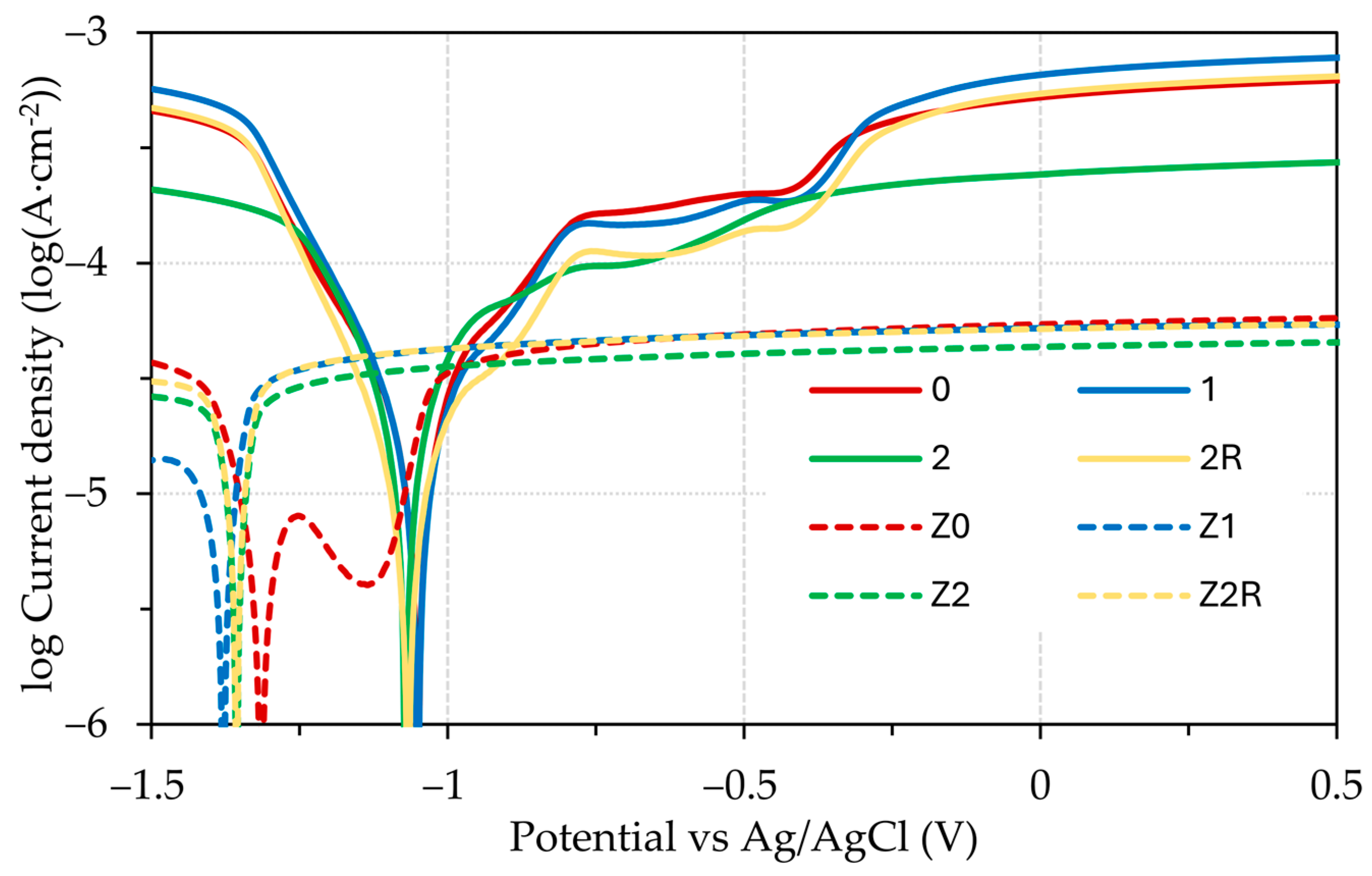
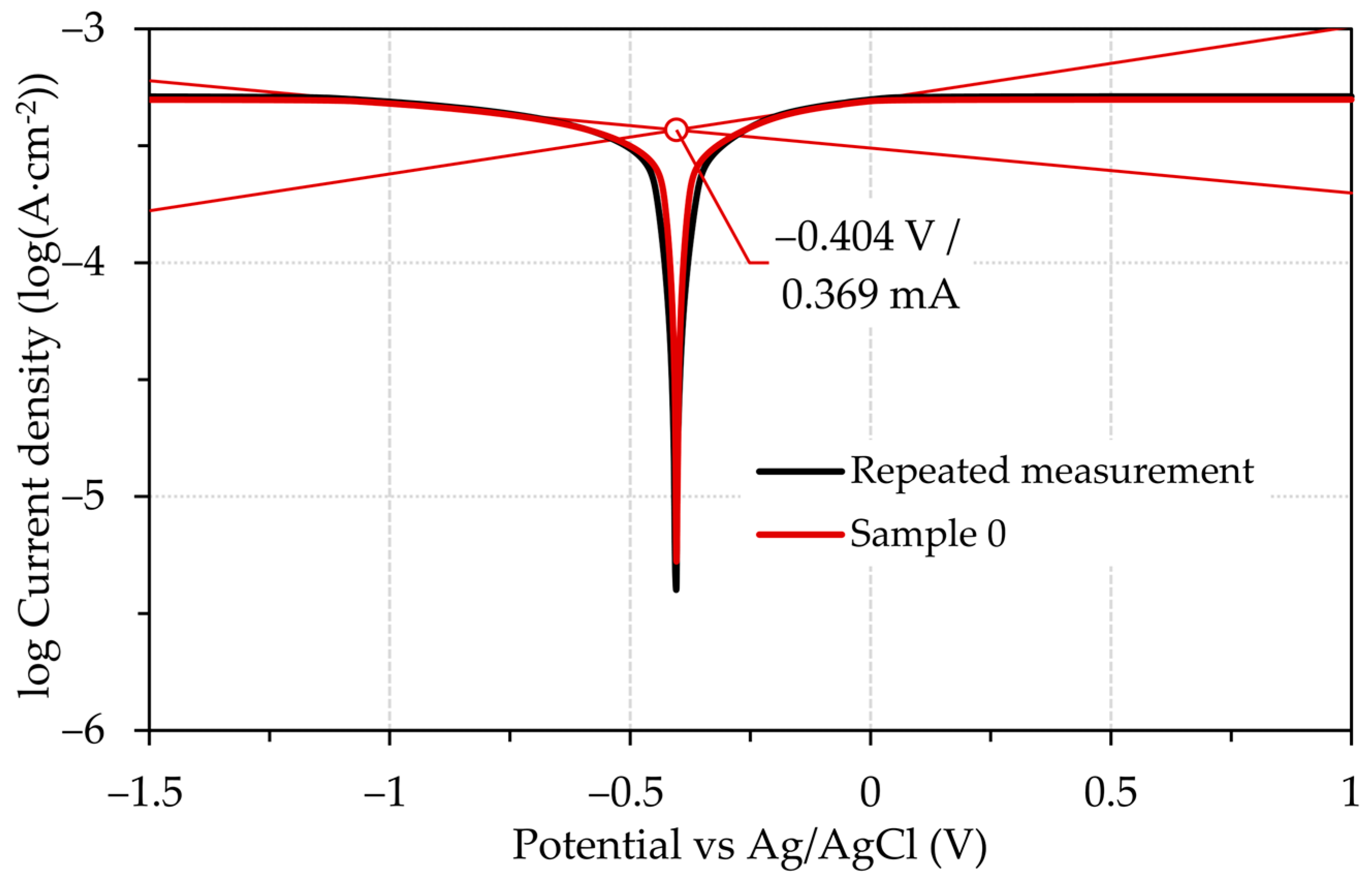
3.3. Electrochemical Testing
4. Conclusions
- The DRECE method showcases its potential to enhance the hardness of the non-alloy structural steel under examination;
- It is established that applying the DRECE method does not diminish the thickness of the zinc coating of the steel specimens;
- Tafel extrapolation confirmed that the DRECE method has no effect on the corrosion resistance of the tested steel specimens;
- According to Tafel extrapolation, the impact of the DRECE method on corrosion resistance is negligible. Conversely, the hot-dip galvanizing of steel significantly enhances its corrosion resistance, which was demonstrated by a 3–4 fold reduction in the corrosion rate and a drop in the corrosion potential value;
- The influence of the electrolyte used is minimal in the case of the 0.1 M HCl and H2SO4 solution. However, when a 5% NaCl solution is employed, the corrosion rate experienced a significant decrease.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toth, L.S.; Gu, C. Ultrafine-Grain Metals by Severe Plastic Deformation. Mater. Charact. 2014, 92, 1–14. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk Nanostructured Materials from Severe Plastic Deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Zrnik, J.; Dobatkin, S.V.; Mamuzič, I. Processing of Metals by Severe Plastic Deformation (SPD)—Structure and Mechanical Properties Respond. Metalurgija 2008, 47, 211–216. [Google Scholar]
- Rusz, S.; Čízek, L.; Michenka, V.; Dutkiewicz, J.; Salajka, M.; Hilšer, O.; Tylšar, S.; Kedroň, J.; Klos, M. New Type of Device for Achievement of Grain Refinement in Metal Strip. Arch. Mater. Sci. Eng. 2014, 69, 38–44. [Google Scholar] [CrossRef]
- Rusz, S.; Klyszewski, A.; Salajka, M.; Hilser, O.; Cizek, L.; Klos, M. Possibilities of Application Methods DRECE in Forming of Non-Ferrous Metals. Arch. Metall. Mater. 2015, 60, 3011–3016. [Google Scholar] [CrossRef]
- Rusz, S.; Hilser, O.; Ochodek, V.; Cada, R.; Svec, J.; Szkandera, P. Influence of SPD Process on Low-Carbon Steel Mechanical Properties. MM Sci. J. 2019, 2019, 2910–2914. [Google Scholar] [CrossRef]
- Rusz, S.; Kraus, M.; Hilšer, O.; Švec, J.; Kedroň, J.; Čížek, L.; Donič, T.; Tański, T.; Krejčí, L. Increasing the Quality of DC01 Steel by Drece Method. In Proceedings of the METAL 2017-26th International Conference on Metallurgy and Materials, Conference Proceedings, Brno, Czech Republic, 24–26 May 2017. [Google Scholar]
- Švec, J.; Rusz, S.; Hilšer, O.; Ochodek, V.; Krejčí, L. Mechanical Properties of Steel DC01 Formed by Drece Method. Trans. VŠB-Tech. Univ. Ostrav. Mech. Ser. 2017, 63, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, M.B.; Kowalczyk, K.; Tkocz, M.; Bulzak, T.; Bednarczyk, I.; Rusz, S. Dual Rolls Equal Channel Extrusion as Unconventional SPD Process of the Ultralow-Carbon Steel: Finite Element Simulation, Experimental Investigations and Microstructural Analysis. Arch. Civil. Mech. Eng. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Jabłońska, M.; Rusz, S.; Bednarczyk, I. Influence of the Drece Process of Severe Plastic Deformation on the Mechanical Properties of the Ultra-Low Carbon Interstitial Free Steel. Arch. Metall. Mater. 2018, 63, 2095–2100. [Google Scholar] [CrossRef]
- Marder, A.R. Metallurgy of Zinc-Coated Steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Kania, H. Structure and Corrosion Resistance of Coatings Obtained by the Batch Double Hot Dip Method in Eutectoid ZnAl Bath with the Addition of Mg and Si. Coatings 2022, 12, 1207. [Google Scholar] [CrossRef]
- Bhat, R.S.; Balakrishna, M.K.; Parthasarathy, P.; Hegde, A.C. Structural Properties of Zn-Fe Alloy Coatings and Their Corrosion Resistance. Coatings 2023, 13, 772. [Google Scholar] [CrossRef]
- Kania, H.; Mendala, J.; Kozuba, J.; Saternus, M. Development of Bath Chemical Composition for Batch Hot-Dip Galvanizing—A Review. Materials 2020, 13, 4168. [Google Scholar] [CrossRef]
- Bellini, C.; Di Cocco, V.; Iacoviello, F.; Mocanu, L.P. Impact of Copper, Tin and Titanium Addition on Bending-Induced Damage of Intermetallic Phases in Hot Dip Galvanizing. Metals 2022, 12, 2035. [Google Scholar] [CrossRef]
- Kreislova, K.; Knotkova, D. The Results of 45 Years of Atmospheric Corrosion Study in the Czech Republic. Materials 2017, 10, 394. [Google Scholar] [CrossRef]
- EN ISO 1461; Hot Dip Galvanized Coatings on Fabricated Iron and Steel Articles Specifications and Test Methods. ISO: Geneva, Switzerland, 2022.
- ISO 14713-2; Zinc Coatings—Guidelines and Recommendations for the Protection against Corrosion of Iron and Steel in Structures—Part 2: Hot Dip Galvanizing. ISO: Geneva, Switzerland, 2019.
- Sánchez, C.; Bustos, O.; Artigas, A.; Bruna, H. Silicon Effect and Microstructural Evolution of Hot Dip Galvanized Coating of Structural Steels. Metals 2023, 13, 1892. [Google Scholar] [CrossRef]
- Vontorová, J.; Mohyla, P. Use of GDOES Method for Evaluation of the Quality and Thickness of Hot Dip Galvanised Coating. Trans. IMF 2018, 96, 313–318. [Google Scholar] [CrossRef]
- Kuklík, V.; Kudláček, J. Hot-Dip Galvanizing of Steel Structures; Elsevier, Butterworth-Heinemann: Amsterdam, The Netherlands, 2016; ISBN 9780081007532. [Google Scholar]
- Xu, W.; Wang, Y.; Yang, H. Analysis of Measurement Accuracy of Zn–Fe Alloy Coatings under Two Take-off Angles. Vacuum 2022, 205, 111456. [Google Scholar] [CrossRef]
- Vontorová, J.; Mohyla, P.; Kreislová, K. Quality of Zinc Coating Formed on Structural Steel by Hot-Dip Galvanizing after Surface Contamination. Coatings 2024, 14, 493. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 4th ed.; Pearson Education Limited: Harlow, UK, 2012; ISBN 978-0-273-74275-3. [Google Scholar]
- Gellings, P.J.; Gierman, G.; Koster, D.; Kuit, J. Synthesis and Characterization of Homogeneous Intermetallic Fe-Zn Compounds. Int. J. Mater. Res. 1980, 71, 70–75. [Google Scholar] [CrossRef]
- Belin, R.; Tillard, M.; Monconduit, L. Redetermination of the Iron-Zinc Phase FeZn13. Acta Crystallogr. C 2000, 56, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.L.; Kashioka, D.; Inomoto, M.; Inui, H.; Takebayashi, H.; Yamaguchi, S. Compression Deformability of Γ and ζ Fe–Zn Intermetallics to Mitigate Detachment of Brittle Intermetallic Coating of Galvannealed Steels. Scr. Mater. 2013, 69, 307–310. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Inomoto, M.; Adachi, H.; Takebayashi, H.; Inui, H. Micropillar Compression Deformation of Single Crystals of the Intermetallic Compound ς-FeZn13. Acta Mater. 2014, 65, 229–239. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Tanaka, K.; Yasuhara, A.; Inui, H. Structure Refinement of the Δ1p Phase in the Fe-Zn System by Single-Crystal X-Ray Diffraction Combined with Scanning Transmission Electron Microscopy. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 275–282. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Yasuhara, A.; Inui, H. Order–Disorder Structure of the Δ1k Phase in the Fe–Zn System Determined by Scanning Transmission Electron Microscopy. Acta Mater. 2014, 81, 345–357. [Google Scholar] [CrossRef]
- Hu, X.; Watanabe, T. Relationship between the Crystallographic Structure of Electrodeposited Fe-Zn Alloy Film and Its Thermal Equilibrium Diagram. Mater. Trans. 2001, 42, 1969–1976. [Google Scholar] [CrossRef]
- Yu, J.; Liu, J.; Zhou, W.; Zhang, J.; Wu, J. Cross-Sectional TEM Observation of Iron–Zinc Intermetallic Γ and Γ1 Phases in Commercial Galvannealed IF Steel Sheets. Mater. Des. 2007, 28, 249–253. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, X. Effects of Steel Coatings Microstructure on Weldability in Resistance Spot Welding of Galvannealed Steel Sheets. Adv. Mater. Res. 2010, 139–141, 610–613. [Google Scholar] [CrossRef]
- Weiss, Z. Emission Yields and the Standard Model in Glow Discharge Optical Emission Spectroscopy: Links to the Underlying Physics and Analytical Interpretation of the Experimental Data. Spectrochim. Acta Part. B At. Spectrosc. 2006, 61, 121–133. [Google Scholar] [CrossRef]
- Priamushko, T.S.; Mikhaylov, A.A.; Babikhina, M.N.; Kudiiarov, V.N.; Laptev, R.S. Glow Discharge Optical Emission Spectrometer Calibration Using Hydrogenated Zr-2.5Nb Alloy Standard Samples. Metals 2018, 8, 372. [Google Scholar] [CrossRef]
- Veverka, J.; Vilémová, M.; Chlup, Z.; Hadraba, H.; Lukáč, F.; Csáki, Š.; Matějíček, J.; Vontorová, J.; Chráska, T. Evolution of Carbon and Oxygen Concentration in Tungsten Prepared by Field Assisted Sintering and Its Effect on Ductility. Int. J. Refract. Met. Hard Mater. 2021, 97, 105499. [Google Scholar] [CrossRef]
- Drápala, J.; Brožová, S.; Szurman, I.; Konečná, K.; Kostiuková, G.; Vontorová, J.; Jonšta, P.; Sobotková, K. Influence of Selected Rare Earth Metals on Structural Characteristics of 42CrMo4 Steel. Metalurgija 2016, 55, 757–760. [Google Scholar]
- ISO 643:2024; Steels—Micrographic Determination of the Apparent Grain Size. ISO: Geneva, Switzerland, 2024.
- ISO 6507-1:2023; Metallic Materials—Vickers Hardness Test, Part 1: Test Method. ISO: Geneva, Switzerland, 2023; p. 35.
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 54., 976–989. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of Scan Rate on the Potentiodynamic Polarization Curve Obtained to Determine the Tafel Slopes and Corrosion Current Density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- Duarte, T.; Meyer, Y.A.; Osório, W.R. The Holes of Zn Phosphate and Hot Dip Galvanizing on Electrochemical Behaviors of Multi-Coatings on Steel Substrates. Metals 2022, 12, 863. [Google Scholar] [CrossRef]
- Vontorová, J.; Váňová, P. Determination of Carburized Layer Thickness by GDOES Method. AIMS Mater. Sci. 2018, 5, 34–43. [Google Scholar] [CrossRef]




| C* | Mn | Si | P | S* | Cr | Ni | Mo | Cu |
| wt% | ||||||||
| 0.0406 | 0.170 | 0.007 | 0.013 | 0.0140 | 0.028 | 0.037 | 0.002 | 0.061 |
| Ti | Co | B | Pb | V | W | Al | Nb | |
| wt% | ||||||||
| <0.001 | 0.006 | 0.0004 | <0.001 | 0.001 | <0.001 | 0.034 | <0.001 | |
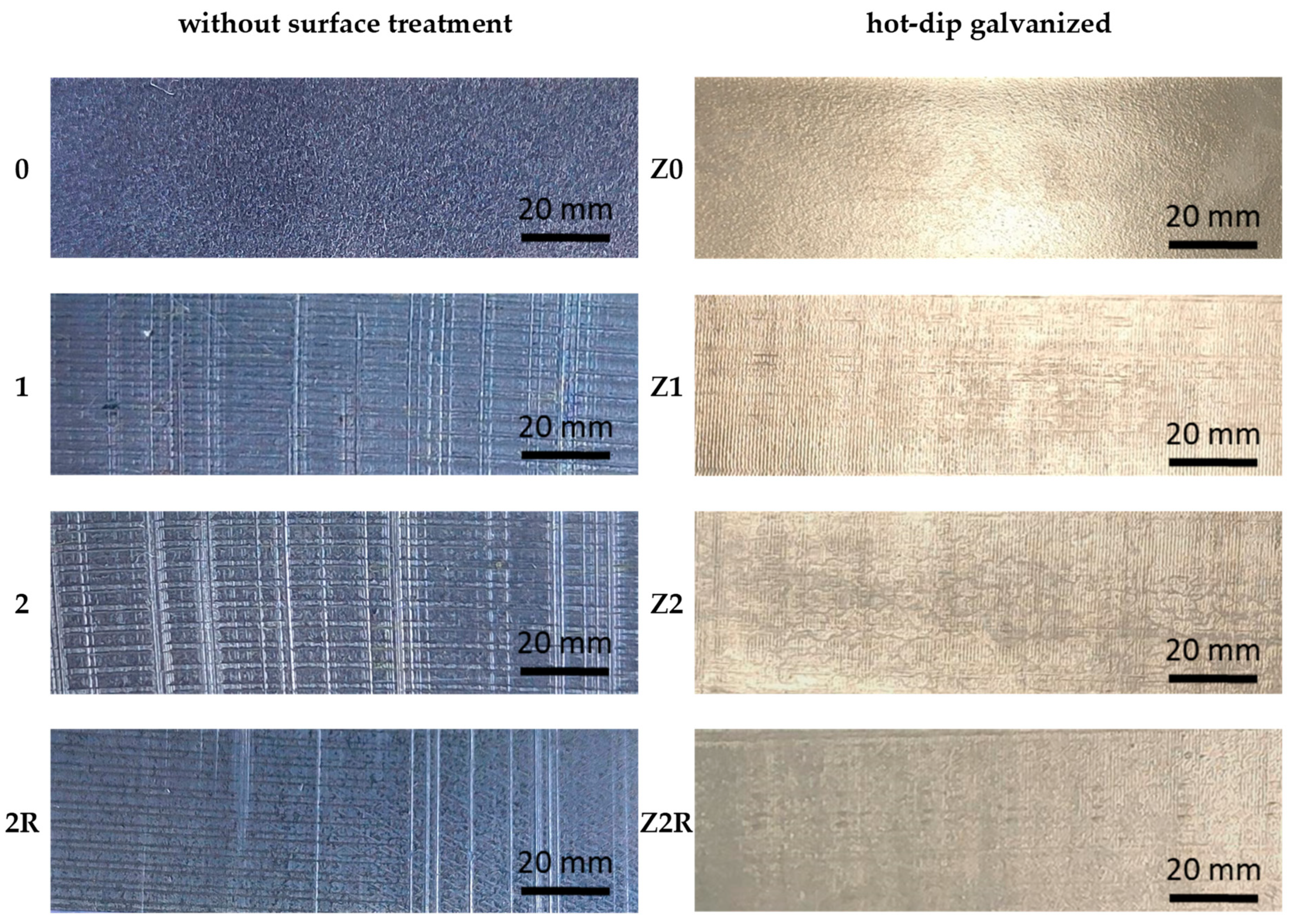
| Without Surface Treatment | Hot-Dip Galvanized | ||
|---|---|---|---|
| 0 | DC03 | Z0 | DC03 |
| 1 | DC03, 1× DRECE | Z1 | DC03, 1× DRECE |
| 2 | DC03, 2× DRECE | Z2 | DC03, 2× DRECE |
| 2R | DC03, 2× DRECE with rotation | Z2R | DC03, 2× DRECE with rotation |
| Sample Label | Thickness of Zn Coating/µm | |
|---|---|---|
| L | T | |
| Z0 | 80.9 ± 3.1 | 84.3 ± 3.5 |
| Z1 | 78.9 ± 3.9 | 83.6 ± 5.2 |
| Z2 | 72.5 ± 3.8 | 71.2 ± 5.3 |
| Z2R | 70.7 ± 3.5 | 75.4 ± 3.8 |
| 0 | 1 | 2 | 2R | Z0 | Z1 | Z2 | Z2R | |
|---|---|---|---|---|---|---|---|---|
| 0.1 M HCl | ||||||||
| Ecorr (V) | −0.404 ± 0.0471 | −0.394 ± 0.0305 | −0.404 ± 0.0316 | −0.404 ± 0.0522 | −0.641 ± 0.0312 | −0.663 ± 0.0311 | −0.649 ± 0.0304 | −0.642 ± 0.0369 |
| icorr (mA·cm−2) | 0.369 ± 0.0300 | 0.350 ± 0.0315 | 0.422 ± 0.0311 | 0.447 ± 0.0314 | 0.127 ± 0.0374 | 0.125 ± 0.0309 | 0.122 ± 0.0301 | 0.128 ± 0.0319 |
| βa (V·decade−1) | 0.315 | 0.333 | 0.326 | 0.315 | 0.291 | 0.310 | 0.277 | 0.259 |
| βc (V·decade−1) | −0.192 | −0.197 | −0.196 | −0.190 | −0.225 | −0.248 | −0.219 | −0.215 |
| Corrosion rate (mm·year−1) | 4.376 | 4.143 | 4.999 | 5.300 | 1.504 | 1.478 | 1.449 | 1.515 |
| Polarization resistance (Ω) | 2318 | 2348 | 1972 | 1922 | 6638 | 6240 | 7154 | 7153 |
| 0.1 M H2SO4 | ||||||||
| Ecorr (V) | −0.409 ± 0.0570 | −0.405 ± 0.0500 | −0.411 ± 0.0420 | −0.409 ± 0.0549 | −0.610 ± 0.0403 | −0.614 ± 0.0523 | −0.675 ± 0.0439 | −0.672 ± 0.0452 |
| icorr (mA·cm−2) | 0.403 ± 0.0451 | 0.374 ± 0.0410 | 0.394 ± 0.0437 | 0.441 ± 0.0400 | 0.117 ± 0.0461 | 0.121 ± 0.0427 | 0.132 ± 0.0517 | 0.130 ± 0.0423 |
| βa (V·decade−1) | 0.316 | 0.303 | 0.321 | 0.308 | 0.368 | 0.384 | 0.296 | 0.302 |
| βc (V·decade−1) | −0.199 | −0.188 | −0.193 | −0.187 | −0.323 | −0.317 | −0.248 | −0.244 |
| Corrosion rate (mm·year−1) | 4.774 | 4.437 | 4.673 | 5.225 | 1.384 | 1.428 | 1.559 | 1.539 |
| Polarization resistance (Ω) | 2093 | 2360 | 2141 | 1991 | 5378 | 5141 | 6063 | 6132 |
| 5% NaCl | ||||||||
| Ecorr (V) | −1.052 ± 0.0327 | −1.049 ± 0.0310 | −1.070 ± 0.0596 | −1.062 ± 0.0321 | −1.304 ± 0.0524 | −1.364 ± 0.0375 | −1.366 ± 0.0300 | −1.364 ± 0.0356 |
| icorr (mA·cm−2) | 0.018 ± 0.0331 | 0.017 ± 0.0302 | 0.019 ± 0.0422 | 0.011 ± 0.0343 | 0.004 ± 0.0380 | 0.004 ± 0.0302 | 0.004 ± 0.0304 | 0.004 ± 0.0409 |
| βa (V·decade−1) | 3.764 | 3.674 | 4.119 | 4.363 | 7.535 | 31.218 | 14.500 | 11.622 |
| βc (V·decade−1) | −4.237 | −4.862 | −4.894 | −5.549 | −8.728 | −6.952 | −24.901 | −22.506 |
| Corrosion rate (mm·year−1) | 0.211 | 0.201 | 0.230 | 0.125 | 0.048 | 0.049 | 0.046 | 0.049 |
| Polarization resistance (Ω) | 3044 | 3004 | 2488 | 4170 | 6602 | 2737 | 2850 | 3085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vontorová, J.; Novák, V.; Váňová, P. Low-Carbon Steel Formed by DRECE Method with Hot-Dip Zinc Galvanizing and Potentiodynamic Polarization Tests to Study Its Corrosion Behavior. Metals 2024, 14, 993. https://doi.org/10.3390/met14090993
Vontorová J, Novák V, Váňová P. Low-Carbon Steel Formed by DRECE Method with Hot-Dip Zinc Galvanizing and Potentiodynamic Polarization Tests to Study Its Corrosion Behavior. Metals. 2024; 14(9):993. https://doi.org/10.3390/met14090993
Chicago/Turabian StyleVontorová, Jiřina, Vlastimil Novák, and Petra Váňová. 2024. "Low-Carbon Steel Formed by DRECE Method with Hot-Dip Zinc Galvanizing and Potentiodynamic Polarization Tests to Study Its Corrosion Behavior" Metals 14, no. 9: 993. https://doi.org/10.3390/met14090993
APA StyleVontorová, J., Novák, V., & Váňová, P. (2024). Low-Carbon Steel Formed by DRECE Method with Hot-Dip Zinc Galvanizing and Potentiodynamic Polarization Tests to Study Its Corrosion Behavior. Metals, 14(9), 993. https://doi.org/10.3390/met14090993







