1. Introduction
Nowadays, people are looking for recyclable, eco-friendly and multi-purpose materials, which would be applicable in many different fields of industry. A good example of such a material is activated carbon (AC), which is highly valued among scientists and industrial R&D workers [
1,
2,
3]. This is mainly due to its high surface area, electric conductivity, thermal and chemical stability as well as porosity. AC also boasts an adjustable pore size distribution and the possibility of surface functionalization [
4,
5]. These features make it a versatile, multi-purpose material with a broad spectrum of possible applications.
Regarding the applications, AC can be used as an adsorbent, which is shown in a study made by Michałek et al. [
6], describing an AC derived from cherry seeds through pyrolysis, applied for noble metal adsorption. Castro-Cardenas et al. [
7] used a microwave hydrothermal carbonization technique to obtain activated carbon from invasive pleco fish biomass. Their material was further used for fluoride and cadmium ion adsorption from water. Another example of AC application for environmental protection is a biochar made by Sreńscek-Nazzal et al. [
8] derived from banana peels activated with KOH and urea (nitrogen doping), which is capable of capturing CO
2.
Biomass-based ACs can serve as a material for components of energy storage systems as well. Soursos et al. made an electrocatalyst for oxygen reduction reactions (ORR) and oxygen evolution reactions (OER) supported on corncob-based AC, which is an essential component of Zn-Air batteries. The carbon was obtained through pyrolysis in a partially oxidizing atmosphere (20% O
2) and then activated with KOH at 850 °C [
9]. Hydrothermal liquefaction is an AC synthetic method used by Asare et al. [
10] to obtain activated carbon from algae. Their AC was further used as electrode material for supercapacitor construction. An interesting approach can be seen in a study published by Wang et al. [
11], which considers enzyme treatment of walnut green husk to obtain activated carbon, further used for assembling a solid–solid phase-change material (SSCPM). Such a system is an example of a specific manner of solar–thermal energy utilization.
Carbons obtained from biomass can also serve as remarkable supports for catalysts, just like the material obtained by Yue et al. [
12] from reed, which grows in sea or fresh waters. Through the pyrolysis of industrial organic residues, Mateus et al. [
13] developed a series of M/AC-type catalysts for CO
2 methanation by turning cork waste from the wine industry into activated carbon, which was further impregnated with Ni and Ce. For the same purpose the wheat straw-derived Ni/AC improved with CeO
2 [
14] or rice husk-derived Ni/AC doped with Mn can also be used [
15]. We can see a similar approach in the case of other catalysts for CO
2 methanation, such as Ce and Mg-promoted Ni catalysts supported on carbonized cellulose [
16], as well as for Ni/Ce-AC catalyst supported on biochar made of pine sawdust [
17]. Even low-grade coal [
18] can be used as a starting material for AC production. Ni/AC nanomaterials are widely used for other catalytic processes, such as Chen et al.’s FENi electrocatalyst for oxygen evolution, supported on carbon nanotubes [
19] or a similar system made by Wanga et al. [
20] consisting of bimetallic phosphide on carbonaceous matrix, used for the same purpose as the previous one. Furthermore, Ren and Liu developed a catalyst for direct conversion of syngas into methane, which is based on nickel and supported on AC made from wheat straw [
21]. Moreover, a NiFe/biochar catalyst synthesized by Gonzalez-Castano et al. [
22] allows for synthetic production of natural gas ingredients. An interesting approach is shown in a study made by Lourenço et al. [
23], which considers catalytic electrochemical reduction of CO
2 to CO with the use of ZnO/biochar composites. In that case, the biochar was obtained through pyrolysis of chitosan and brewed coffee waste.
Quite unique nickel-based catalytic systems for methanation were developed by Quatorze et al. They used a carbon-ZrO
2 nanocomposite as a support, while the “carbonic” part was assembled with functionalized activated carbon or carbon nanotubes. Such a combination is not only durable and resistant to sintering, but also provides an exceptionally high catalytic performance. Tests of the best variant, the Ni/CNT:ZrO
2 (70:30), boast 85% conversion of CO
2, its methane selectivity reaches 99.5% and the operating temperature is 370 °C. The system maintained stability during a 70 h test without any structural or chemical modifications [
24]. To conclude, AC is a very valuable material that can be used not only in Ni-based catalysts but has a much broader spectrum of applications. It not only has many desirable features but can also be obtained in a cheap and eco-friendly way.
Although AC can be synthesized from various types of biomasses, plant material is the most commonly used type of biomass for its production. There are already commercially available activated carbons made of plant biomass, such as coconut shells [
25], bamboo [
26] or different types of wood [
27,
28]. Scientists would rather choose non-obvious starting materials for AC synthesis, but only a few studies consider golden rod as a plant of choice.
Golden rod is a 1.5–2 m tall weed-like plant with narrow leaves, a 6–8 mm thick, partially woodened stem and eye-catching yellow flowers. It is often spotted in meadows, near agricultural fields, in wastelands or at the sides of railroads. Due to its low ecological and economical value, vast regeneration capabilities, rapid growth [
29] and expansive nature [
30], golden rod is often considered as a pest plant [
31,
32]. These features make it a perfect starting material for obtaining activated carbon.
2. Materials and Methods
Activated carbon preparation: golden rod was collected from the suburban area of Jaworzno (Poland, Silesian Voivodeship), near Przemsza river at the beginning of November 2023. Leaves were separated from stems, which were cut into 2–3 cm long pieces. Both the leaf and stem pieces were dried for 1 month in a dry and warm environment. Then, leaves were crushed by hand (samples 2 and 2b; “b” is for “bis”) or ground in a mincer to a powder (sample 2p; “p” is for “powder”). Pyrolysis was performed in a tube furnace (Czylok, Jastrzębie-Zdrój, Poland) in a nitrogen (99.999% Air Liquide, Kraków, Poland) atmosphere (flow rate 10 mL/min.), with the temperature and time detailed in
Table 1. Carbons 1, 2, 3, 4 and 5 were additionally ground in a mortar to fine powder (samples designated with letters “p” or “b” were not ground after pyrolysis).
Ni/AC catalysts preparation: 18.45 g of Ni(NO3)2·6H2O (WARCHEM, Zakręt, Poland) was dissolved in 1 L of distilled water to get 63 mM solution of Ni. Then, 1.5 g of carbon 1, 2, 2b, 2p and 5 were carried into glass bottles filled with 100 mL of previously made Ni solution, closed with screw tops and treated with ultrasounds for 10 min. In the next step, bottles with mixtures were put into the heated water bath with shaker (ELPIN, Lubawa, Poland) and the temperature was set to 80 °C. Samples were heated and shook for 2 h and then mixtures were filtered through small-pore filter on gravitational funnel. Subsequently, filtrate was collected and stored, while filters with impregnated biochar were put in beakers and dried at 105 °C for 2 days. After drying, impregnated charcoals were calcined in a tube furnace: starting temperature was 20–25 °C, heating rate of 10 °C/min., going up to 500 °C, hold for 3 h with nitrogen flow 10 mL/min. All samples were collected and stored in glass vials and then analyzed to find the connection between their preparation conditions and physicochemical properties.
DSC-TGA analysis: an assessment was performed for thermal stability. It was performed using a SDT Q.600 thermogravimeter (TA Instrument, New Castle, DE, USA). The measurement process occurred in Ar conditions and the gas flow was 100 mL/min.
MP-AES analysis: analysis was conducted to evaluate the effectiveness of Ni adsorption on the carbons’ surfaces. It was performed using an Agilent 4210 MP-AES (Santa Clara, CA, USA) analyzer. The first series was made to choose the carbon with the best adsorbing properties. Before measurement, the device was calibrated with the use of 1, 5, 10, 20 and 40 ppm Ni, as well as some other metals including V, Fe and Mo solutions in 1 M HNO3. Then, 1 mL of each filtrate (1, 2, 2b, 2p and 5) after impregnation (except the “W”, which was not used for impregnation and served as a benchmark) were transferred into vials and diluted according to sequence dilution method with ratios of 1:10 and 1:100 (which gave 15 research samples + benchmark—the “W”). In the second MP-AES study the Ni content was measured directly on impregnated carbon. It was done only for the 2p sample to provide more accurate data. Before solution preparation, 0.0405 g of Ni-impregnated 2p was leached in 50 mL of 1 M HCl in a microwave reactor Magnum II (Ertec, Wrocław, Poland). Heating time was set to 1 h, maximum pressure reached 45 bars and the minimum was 42 bars. Maximum temperature reached 240 °C, while its minimal value was 230 °C. After leaching, the solution was filtered gravitationally to remove the remains of carbon. Samples were prepared with the same method as for the first series of MP-AES studies, but the calibration was done with more standards: 5 ppm Sn in 1 M HCl; 5 ppm Ti, Si in H2O; 1 ppm As in HNO3; 5 ppm W in H2O; 1, 5 and 10 ppm SS IV in HNO3; 1, 5, 10 ppm V, Ni, Fe, Mo in 1 M HNO3.
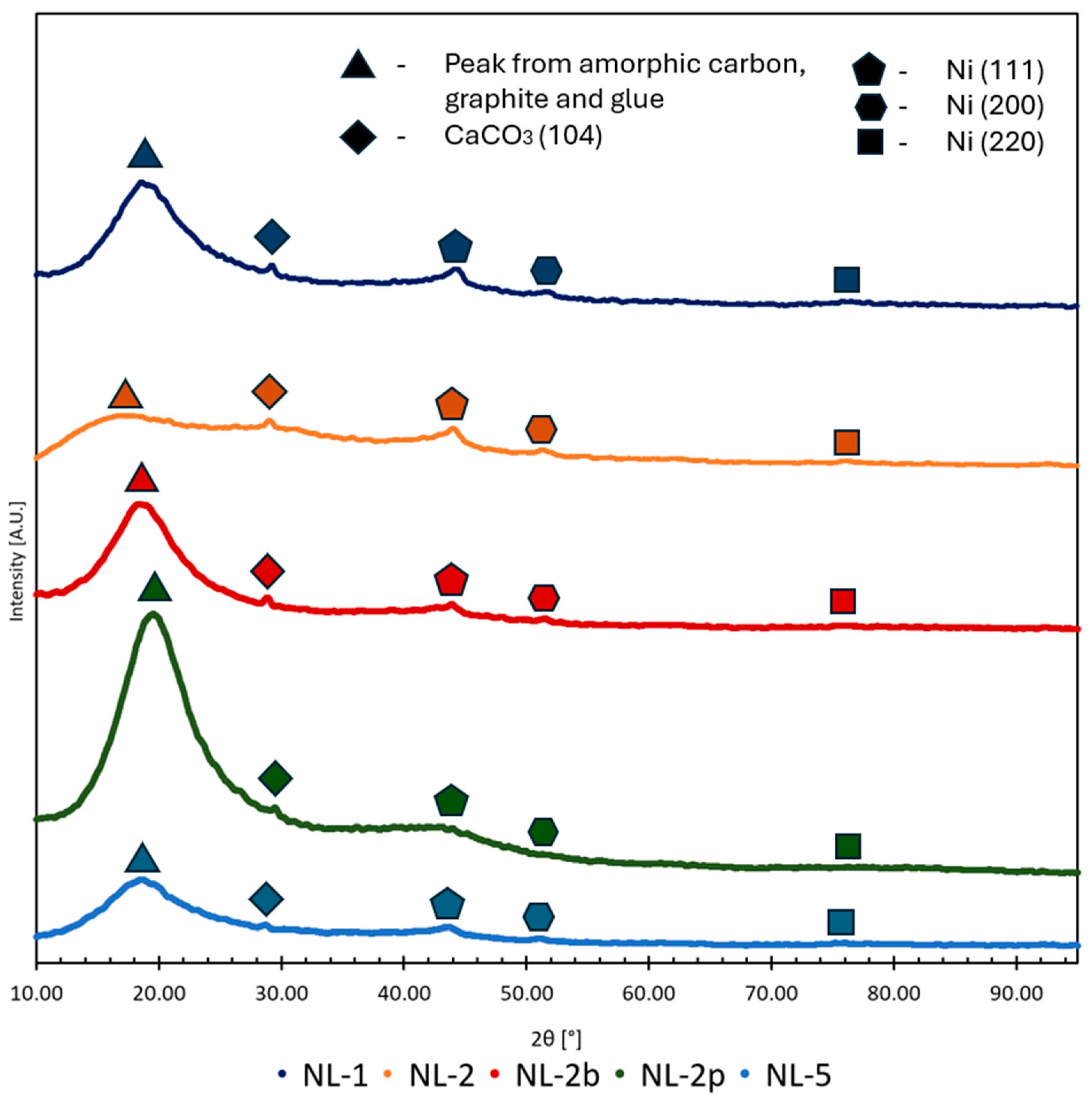
XRD analysis was conducted for verification of our material’s elemental composition. The experiment was performed with the Rigaku MiniFlex II Desktop Powder X-ray Diffractometer (Tokyo, Japan).
BET analysis was performed using a Micromeritics ASAP 2010 device (Norcross, GA, USA). Samples were degassed at 350 °C for 24 h. Measuring procedure started after reaching a 2.2 kPa. The temperature of a cell was maintained at 77.35 K. The break between measurements was 2 min.
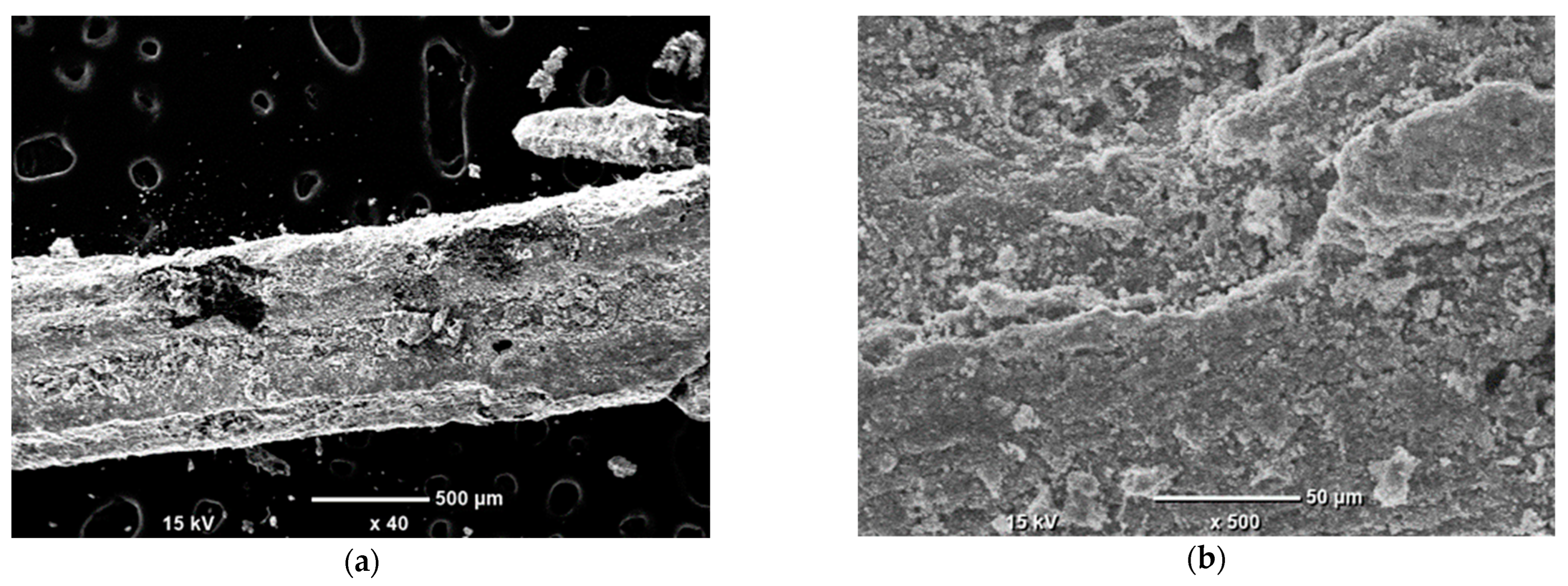
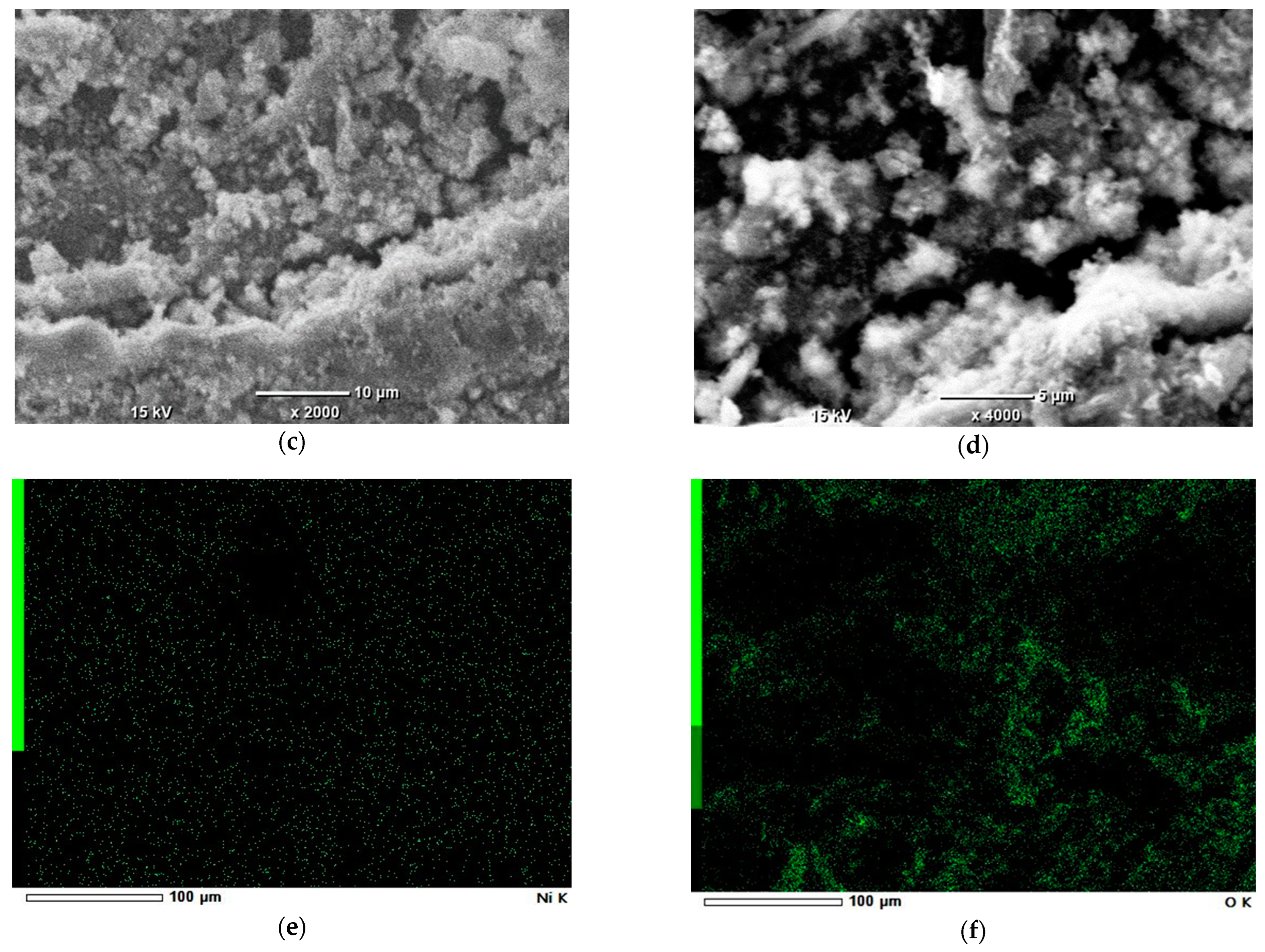
SEM-EDS study was done to observe the surficial structure of carbons and Ni nanoparticle distribution. The observation was conducted using a Scanning Electron Microscope JEOL-6000 Plus (Tokyo, Japan). Observations were made under a high vacuum, at a 10 kV accelerating voltage. The magnification range was from 40 to 4000 times. Observed grain of Ni impregnated on 2p carbon had the approx. size of 1 × 1 × 3 mm.
Ash content was measured to determine the level of mineralization during pyrolysis. Samples were burned in the same tube furnace as for pyrolysis, at the following parameters: 0 min. pre-hold, heating time 12 h, peak temperature 800 °C, hold time 0 min., inert cooling.