A Fundamental Study on the Preparation of Sodium Tungstate from Wolframite via the Smelting Process
Abstract
1. Introduction
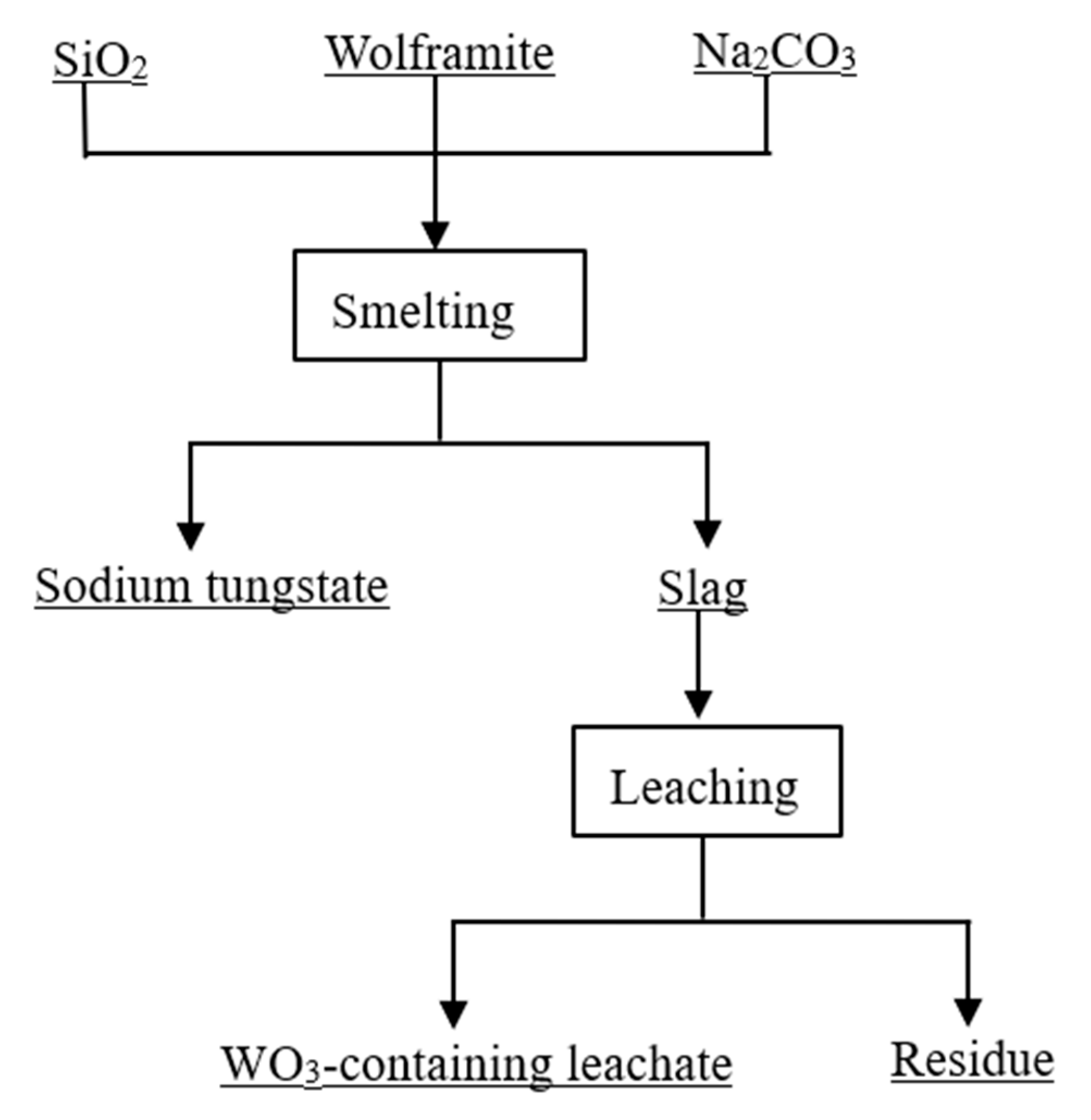
2. Experimental Procedure
3. Results and Discussion
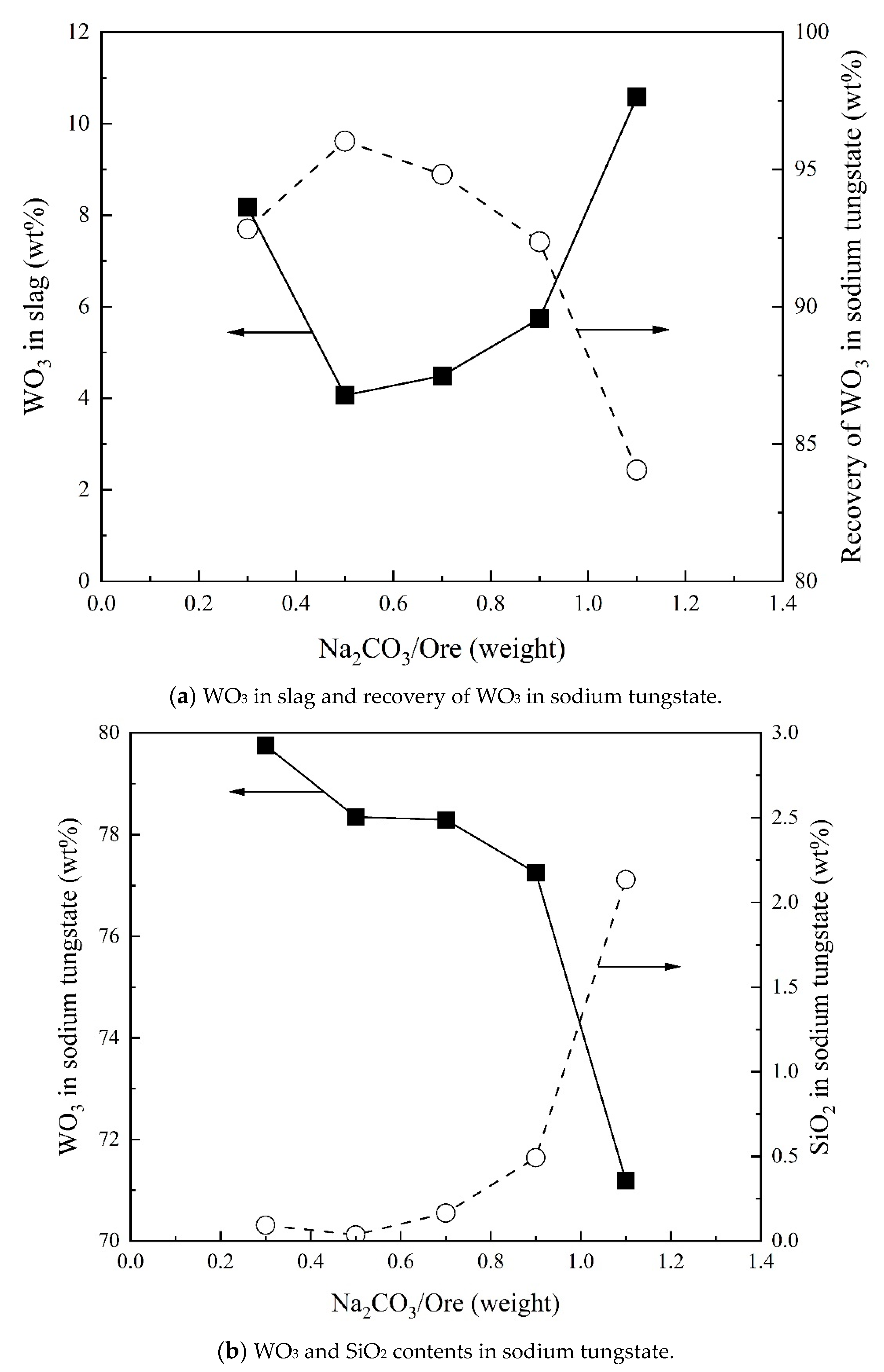
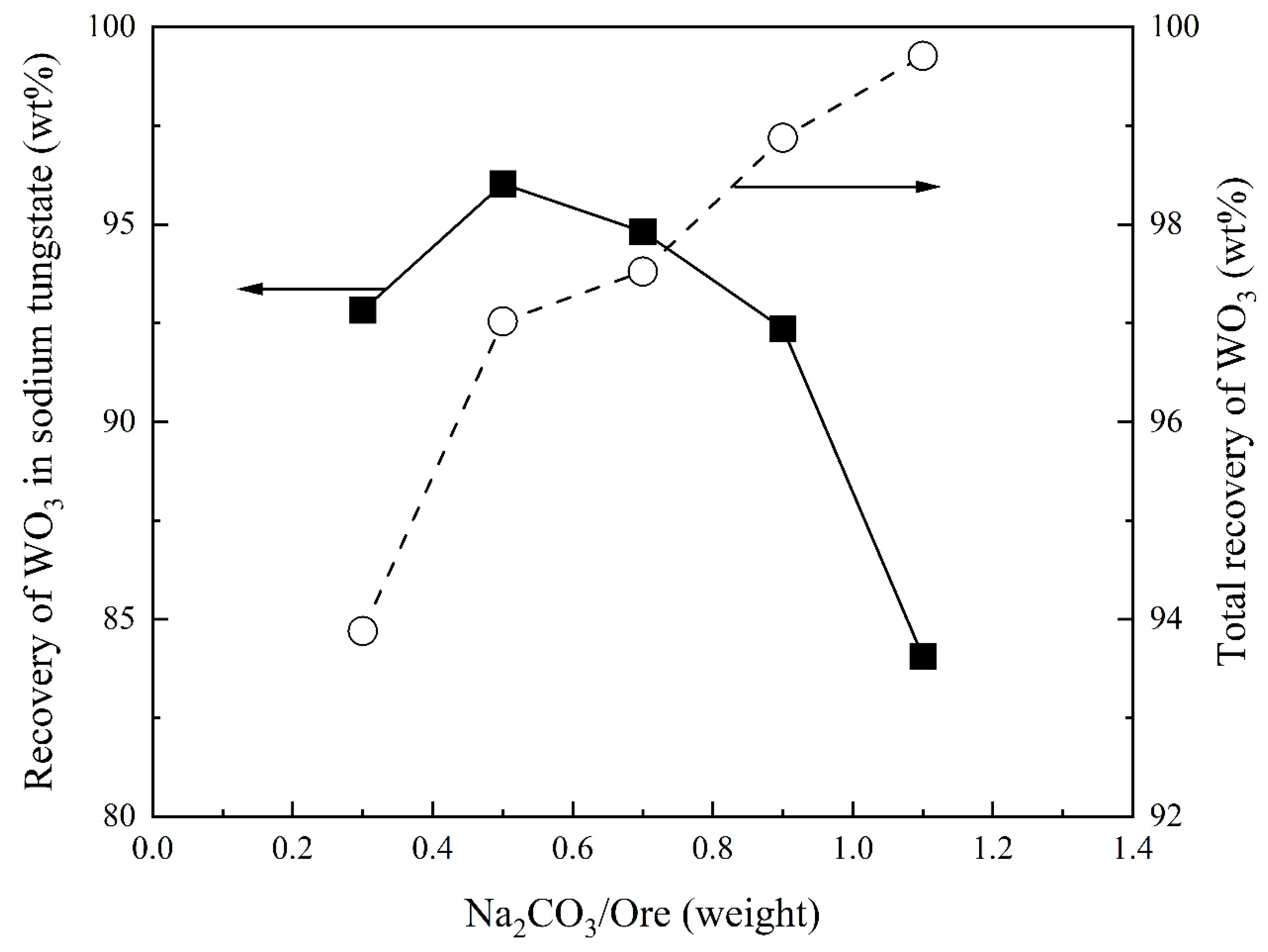
3.1. Effect of Na2CO3/Ore on Recovery of WO3 and Composition of Sodium Tungstate
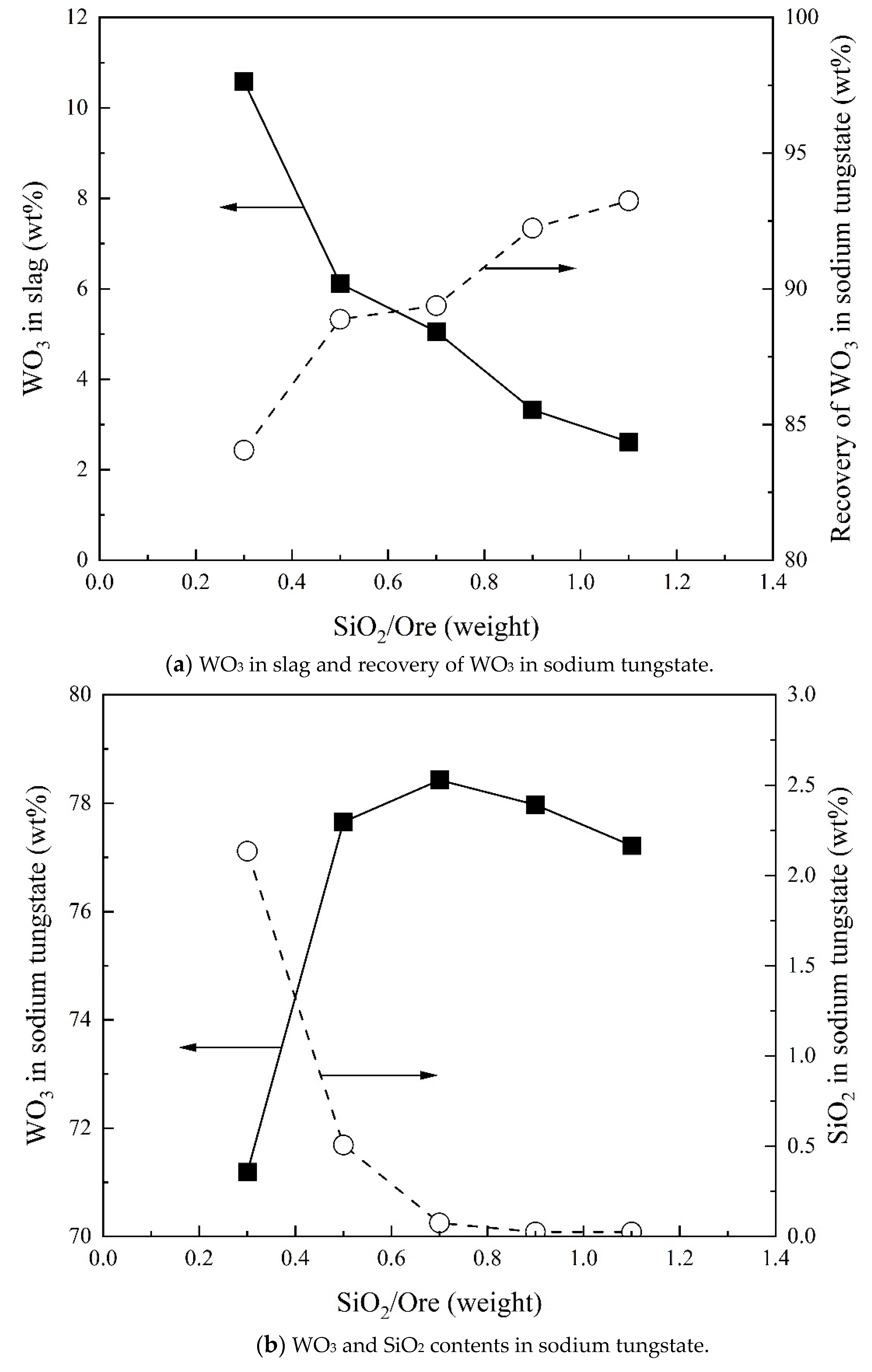
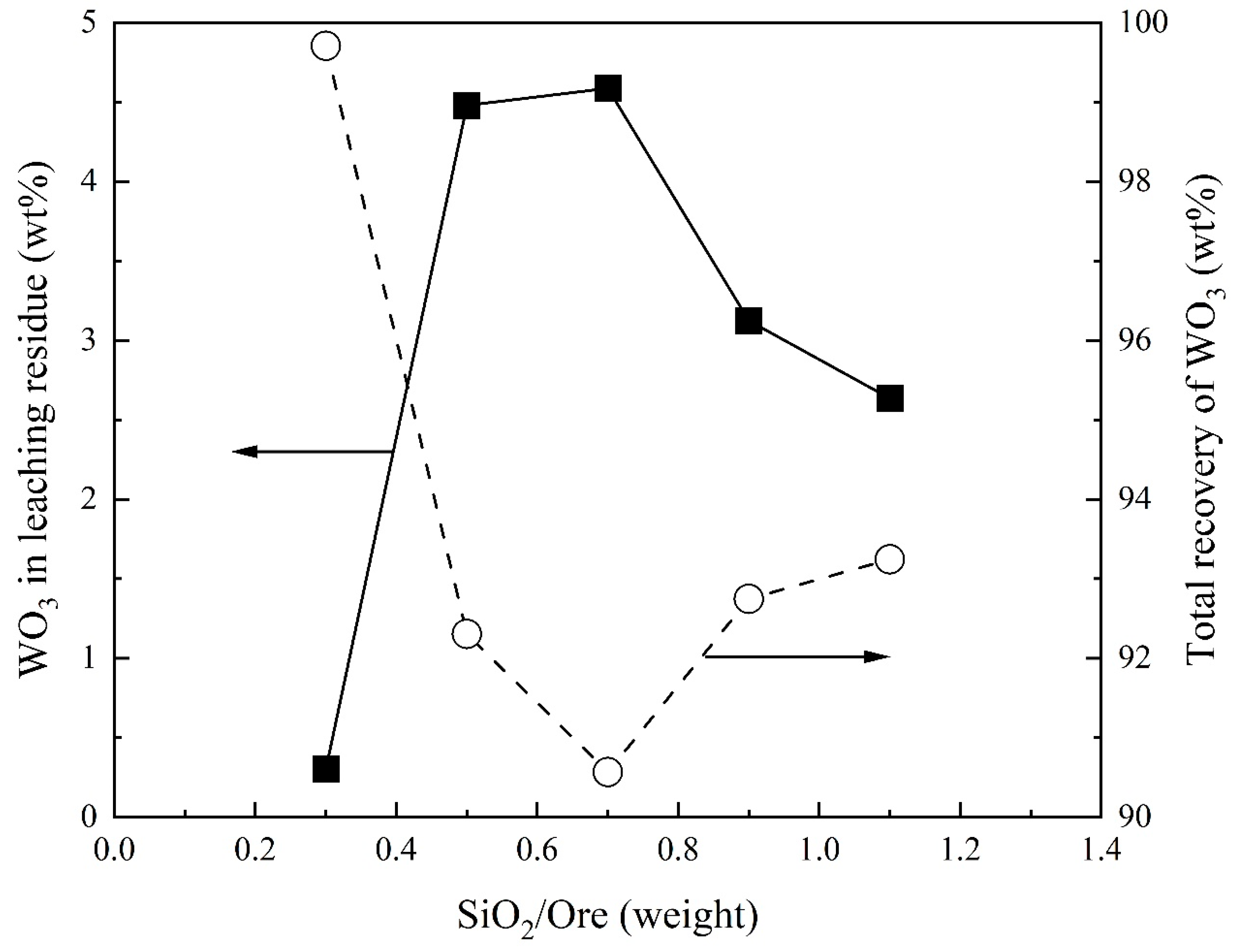
3.2. Effect of SiO2/Ore on Recovery of WO3 and Composition of Sodium Tungstate
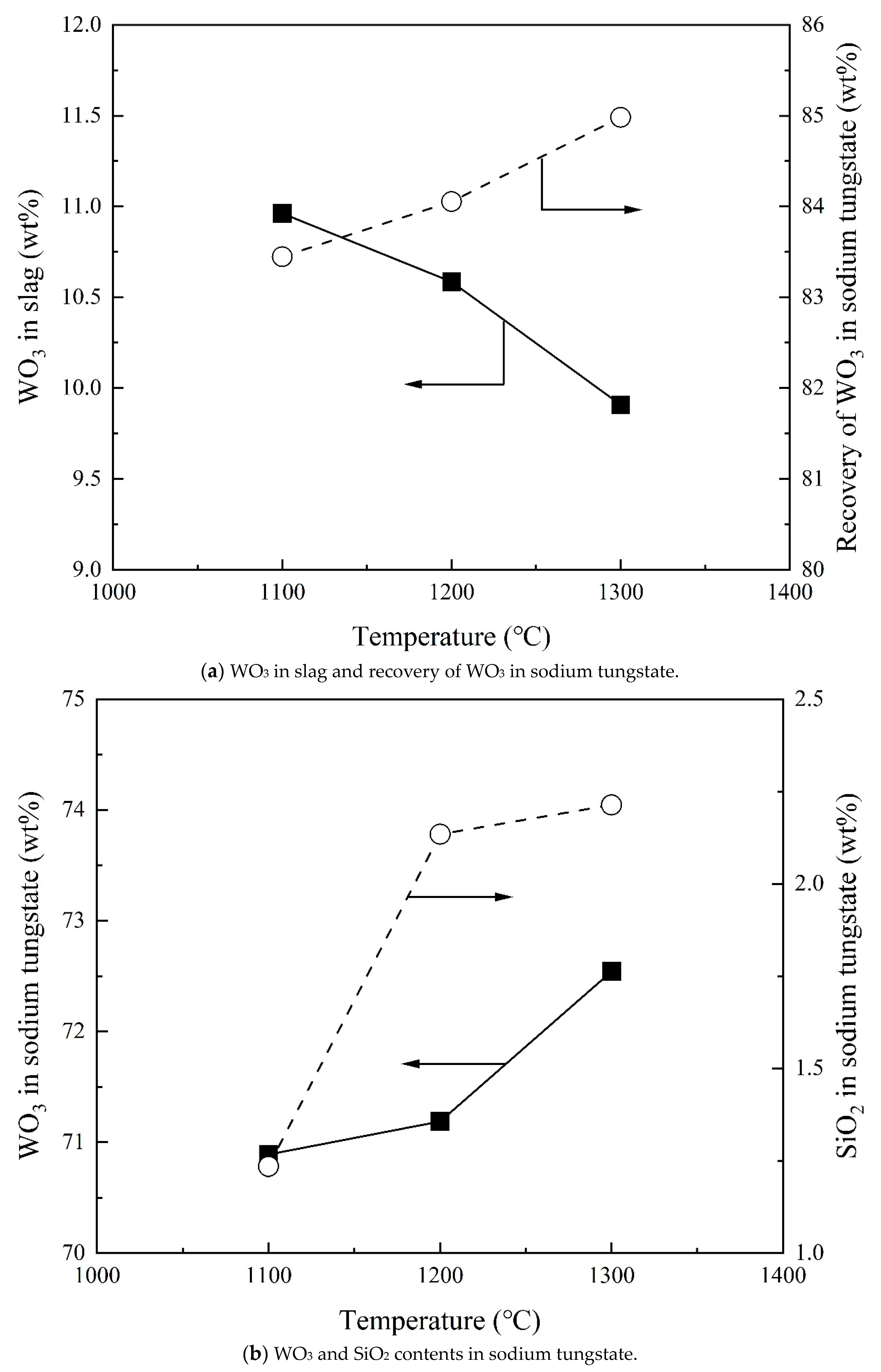
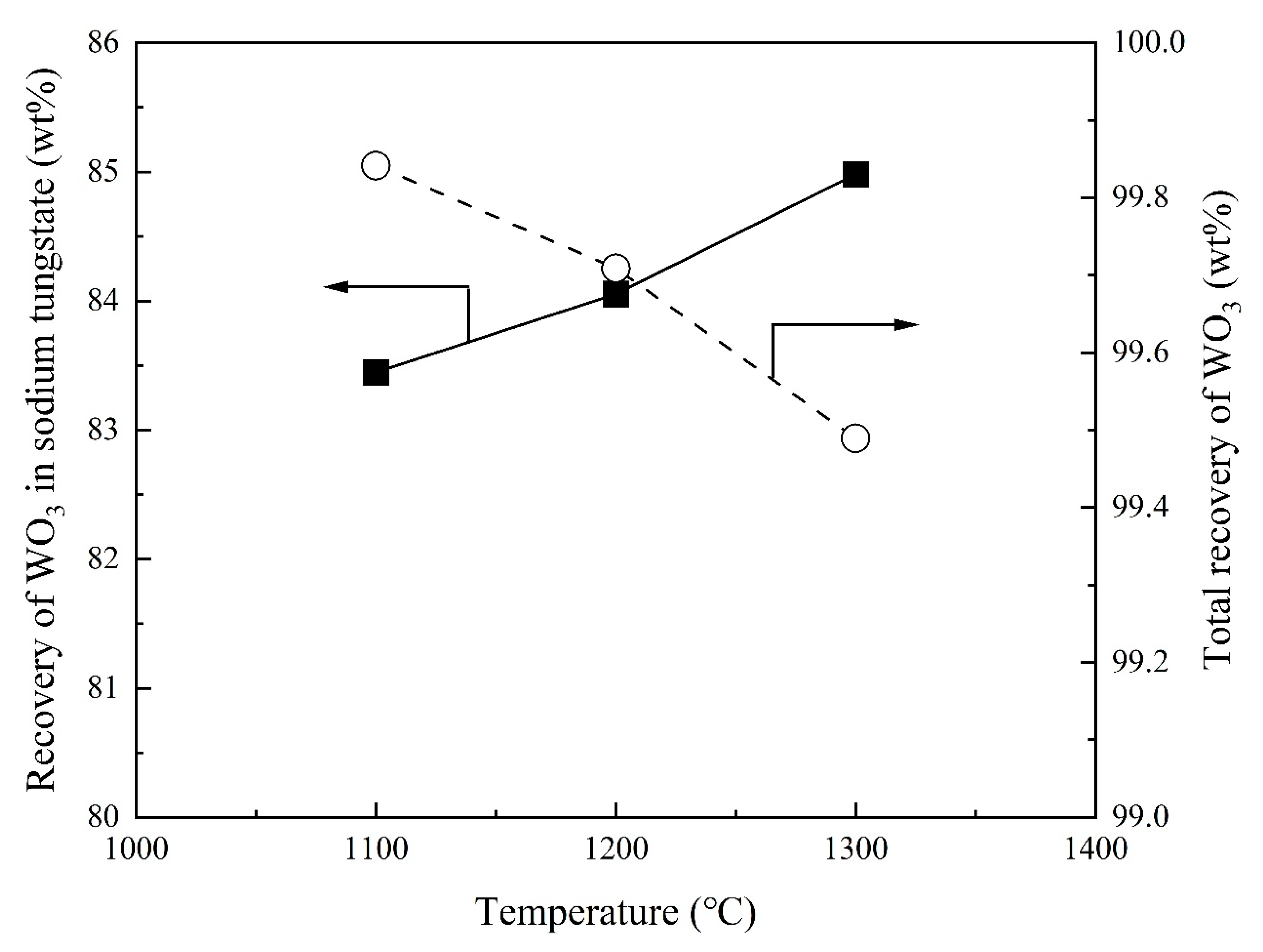
3.3. Effect of Temperature on Recovery of WO3 and Composition of Sodium Tungstate
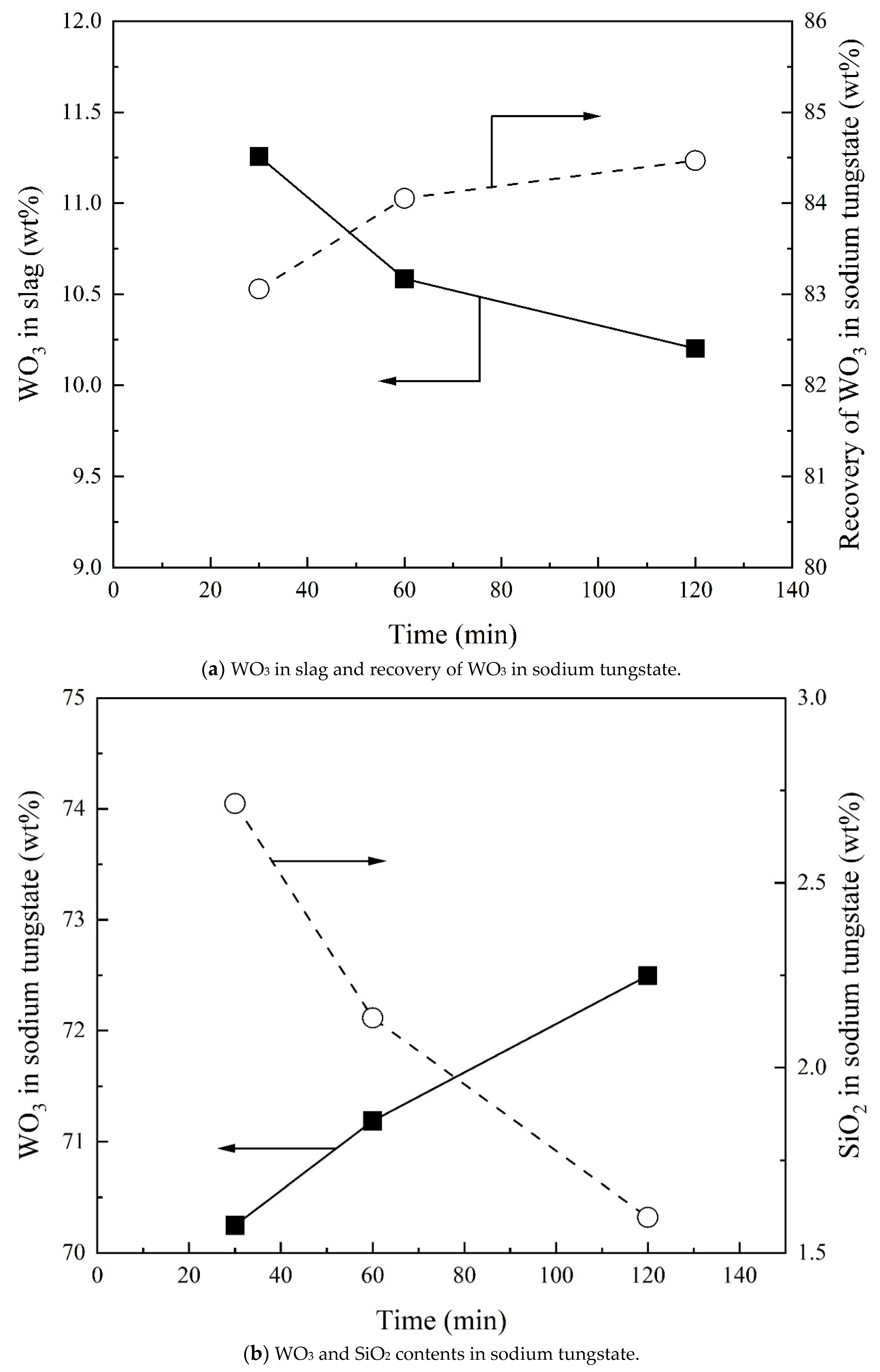
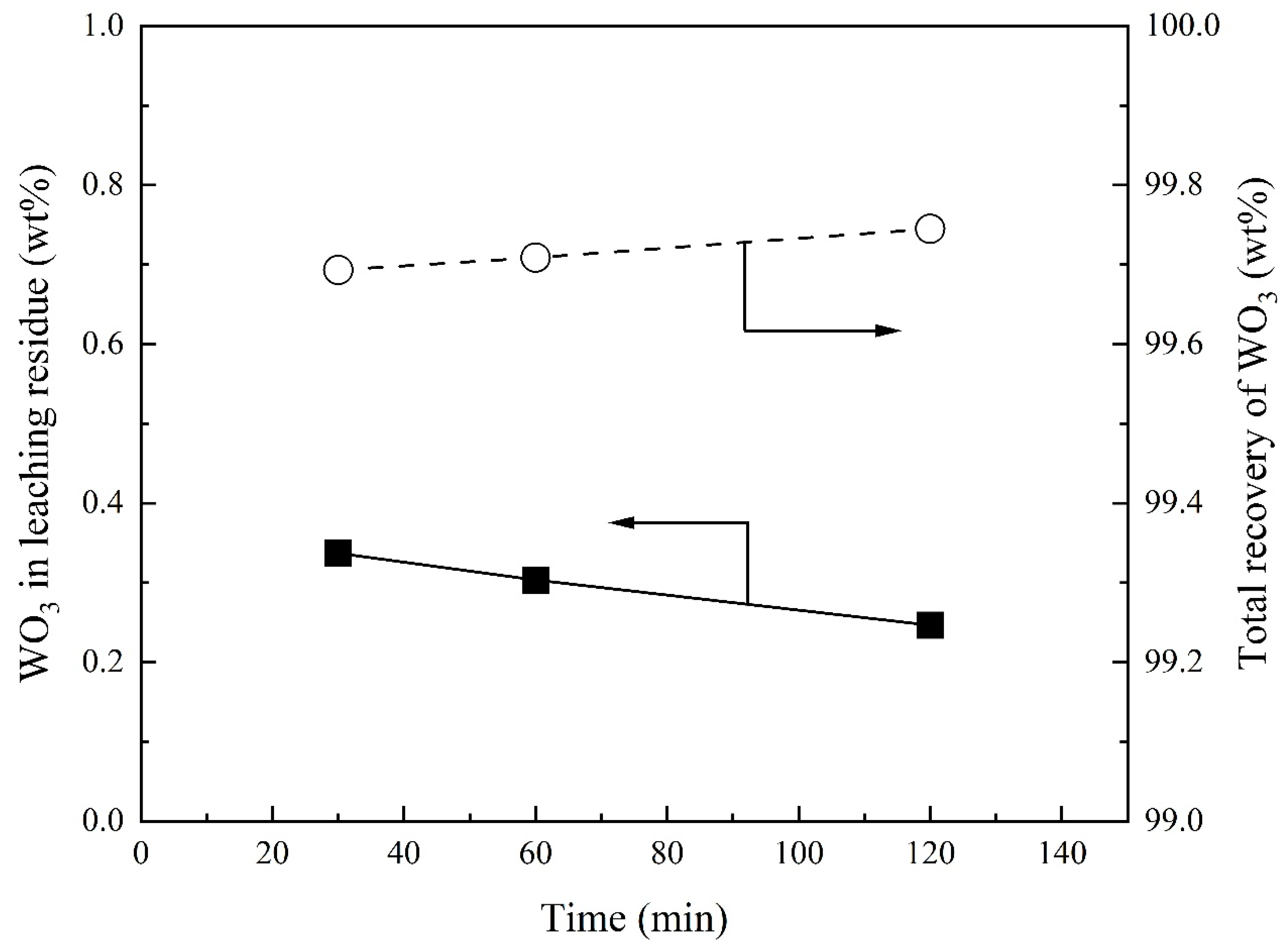
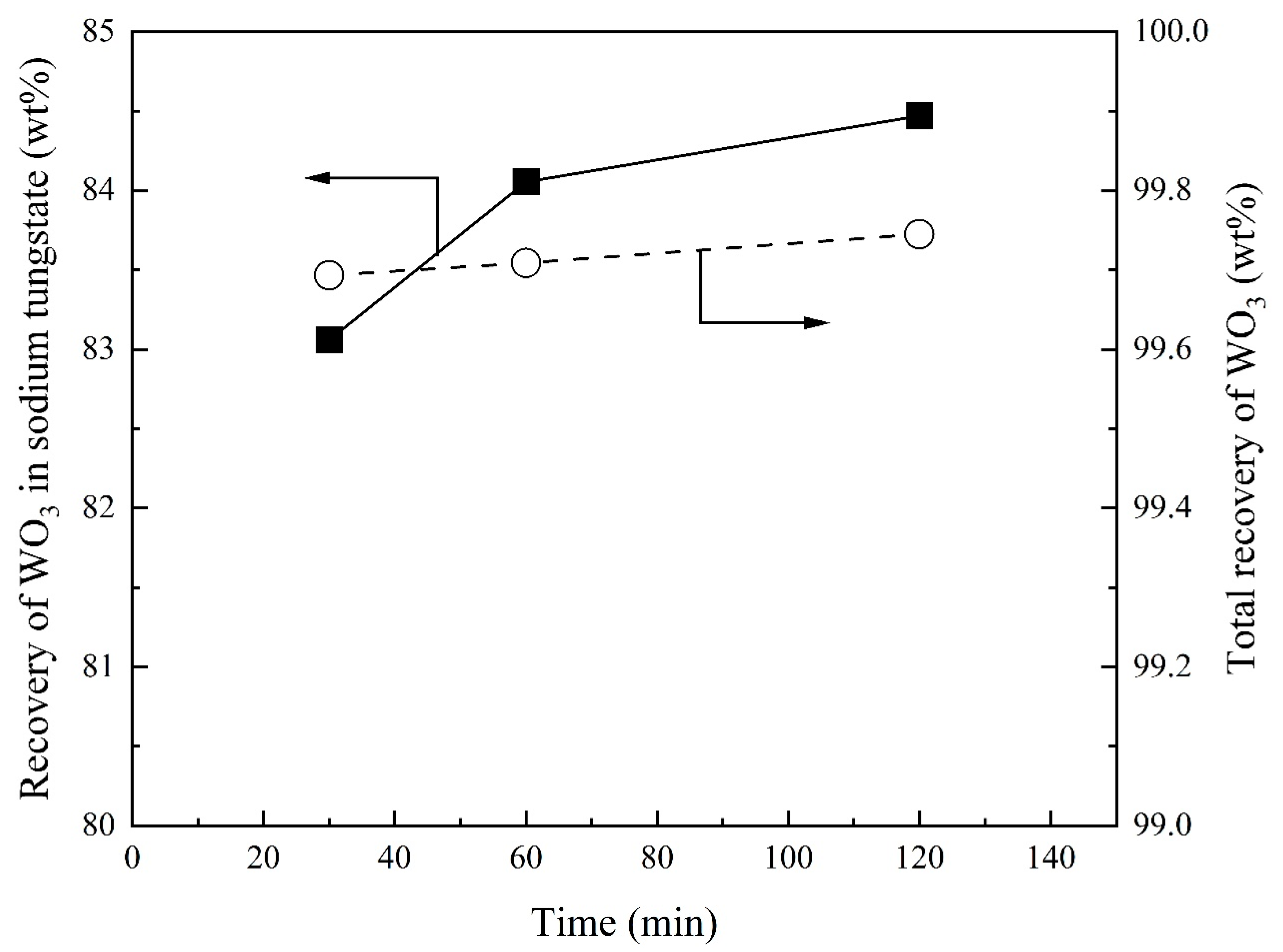
3.4. Effect of Reaction Time on Recovery of WO3 and Composition of Sodium Tungstate
4. Conclusions
- Direct recovery of tungsten can be achieved in the form of Na2WO4 initially increasing and then decreasing with increasing Na2CO3/Ore ratio. The SiO2/Ore ratio increases the direct recovery of tungsten continuously. Temperature and reaction time slightly increase the direct recovery of tungsten. At a Na2CO3/Ore ratio of 0.5 and SiO2/Ore ratio of 0.3, 96% tungsten can be directly recovered as sodium tungstate.
- The WO3 content in sodium tungstate decreases with increasing Na2CO3/Ore ratio and has a maximum with SiO2/Ore ratio. Temperature and reaction time can slightly increase the WO3 content in sodium tungstate. A percentage of 78% WO3 in sodium tungstate (≈99% sodium tungstate) can be obtained at a range of Na2CO3/Ore and SiO2/Ore ratios.
- Total recovery of tungsten increases with increasing Na2CO3/Ore ratio. The SiO2/Ore ratio initially decreases and then increases the total recovery of tungsten. The total recovery of tungsten decreases slightly with increasing temperature and is almost independent of the reaction time above 30 min.
- Up to 11.3 wt% WO3 was reported in the silicate slag. Up to 2.7% SiO2, 0.9 wt% SO3, 2.1 wt% Fe2O3, and 3.6 wt% MnO were reported in the sodium tungstate. These experimental data will support the development of a WO3-containing thermodynamic database.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, Z.; Golev, A.; Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability evaluation of essential critical raw materials: Cobalt, niobium, tungsten and rare earth elements. J. Phys. D Appl. Phys. 2018, 51, 203001. [Google Scholar] [CrossRef]
- Li, X.Y.; Ye, Y.Q.; Zhang, F.L.; Wang, D. Recommended Management Strategies and Resources Status of Chinese Tungsten in the New Period. Mod. Min. 2018, 34, 25–28. [Google Scholar]
- Lassner, E.; Schubert, W. Tungsten-Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds, 1st ed.; Springer Science+Business Media: New York, NY, USA, 1999; pp. 283–374. [Google Scholar]
- Kurlov, A.S.; Gusev, A.I. Tungsten Carbides-Structure, Properties and Application in Hardmetals, 1st ed.; Springer: New York, NY, USA, 2013; pp. 1–3. [Google Scholar]
- Shen, L.; Li, X.; Lindberg, D.; Taskinen, P. Tungsten extractive metallurgy: A review of processes and their challenges for sustainability. Miner. Eng. 2019, 142, 105934. [Google Scholar] [CrossRef]
- Gedgagov, E.I.; Besser, A.D.; Yanakov, V.Y.; Smolyarchuk, V.P. Analyzing the raw materials market and methods for processing tungsten concentrates to obtain competitive products. Theor. Found. Chem. Eng. 2009, 43, 529. [Google Scholar] [CrossRef]
- Premchand. Processing of low grade tungsten ore concentrates by hydrometallurgical route with particular reference to India. Bull. Mater Sci. 1996, 19, 295. [Google Scholar] [CrossRef]
- Gaur, R.S. Modern Hydrometallurgical Production Methods for Tungsten. JOM 2006, 58, 45–49. [Google Scholar] [CrossRef]
- Baimbetov, B.; Moldabayeva, G.; Yeleuliyeva, A.; Jumankulova, S.; Taimassova, A.; Adilzhan, A.; Baisultanov, R.; Yakob, E.; Serikbayev, V. Prospects of Processing Tungsten Ores from the Akchatau Deposit. Processes 2024, 12, 77. [Google Scholar] [CrossRef]
- Su, K.; Ma, X.D.; Zhao, B.J. Harmless Treatment and Valuable Metals Recovery of Tungsten Leaching Residues: A Thermodynamic and Experimental Study. JOM 2021, 73, 1937–1946. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.D.; Su, K.; Liao, C.F.; Zhao, B.J. Fundamental studies for high temperature processing of tungsten leaching residues for alloy formation. Tungsten 2020, 2, 362–370. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.L.; Nie, C.X. Comprehensive Treatments of Tungsten Slags in China: A Critical Review. J. Environ. Manag. 2020, 270, 110927. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.R.; Liu, X.H.; Chen, X.Y.; Li, J.T.; He, L.H.; Zhao, Z.W. Comprehensive utilization and development trend of tungsten smelting slag. Conserv. Util. Miner. Resour 2019, 39, 119. [Google Scholar]
- Dai, Y.Y.; Zhong, H.; Zong, H.Y. Novel process for preparation of mangano-manganic oxide from tungsten residue. Chin. J. Nonferrous Met. 2012, 22, 1242. [Google Scholar]
- Chen, Y.L.; Guo, X.Y.; Wang, Q.M.; Tian, H.Q.; Huang, S.B.; Zhang, J.X. Tungsten and arsenic substance flow analysis of a hydrometallurgical process for tungsten extracting from wolframite. Tungsten 2021, 3, 348–360. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.W.; Cao, C.F.; Liang, Y.; Li, J.T. Behavior of arsenic in process of removing molybdenum by sulfide method. J. Cent. South Univ. 2012, 42, 435–439. [Google Scholar]
- Zhao, Z.W. Tungsten Metallurgy: Fundamentals and Applications, 1st ed.; Tsinghua University Press: Beijing, China, 2013; pp. 230–270. [Google Scholar]
- Gomes, J.M.; Raddatz, A.E.; Carnahan, T.G. Preparation of tungsten carbide by gas sparging tungstate melts. J. Met. 1985, 37, 29–32. [Google Scholar] [CrossRef]
- Malyshev, V.V.; Uskova, N.N.; Gab, A.I. High-temperature selective extraction of Tungsten from Tungsten concentrates in ionic melts. Russ. J. Inorg. Chem. 2002, 47, 1622–1623. [Google Scholar]
- Gostishchev, V.V.; Boiko, V.F. Obtaining Tungsten Powder from the Scheelite Concentrate in ion melts. Theor. Found. Chem. Eng. 2008, 42, 728–730. [Google Scholar] [CrossRef]
- Stemprok, M. Geological significance of immiscibility in fused silicate systems containing tungsten and molybdenum. Int. Geol. Rev. 1975, 17, 1306–1316. [Google Scholar] [CrossRef]
- Zhao, B.J.; Su, K.; Ma, X.D. Experimental Determination of Phase Equilibria in the Na2O-SiO2-WO3 System. Metals 2021, 11, 2014. [Google Scholar] [CrossRef]
- Xu, L.Q.; Zhao, B.J. Extraction of Sodium Tungstate from Tungsten Ore by Pyrometallurgical Smelting. Metals 2023, 13, 312. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermodynamic software and databases 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]



| WO3 | CaO | FeO | MnO | SiO2 | S |
|---|---|---|---|---|---|
| 78.0 | 1.1 | 10.7 | 7.9 | 1.7 | 0.6 |
| Exp No | Ore (g) | Temp (°C) | Time (min) | Na2CO3 (g) | SiO2 (g) |
|---|---|---|---|---|---|
| W1 | 10 | 1200 | 60 | 7 | 3 |
| W2 | 10 | 1200 | 60 | 9 | 3 |
| W3 | 10 | 1200 | 60 | 11 | 3 |
| W4 | 10 | 1200 | 60 | 11 | 5 |
| W5 | 10 | 1200 | 60 | 11 | 7 |
| W6 | 10 | 1200 | 30 | 11 | 3 |
| W7 | 10 | 1200 | 120 | 11 | 3 |
| W8 | 10 | 1100 | 60 | 11 | 3 |
| W9 | 10 | 1300 | 60 | 11 | 3 |
| W10 | 10 | 1200 | 60 | 5 | 3 |
| W11 | 10 | 1200 | 60 | 3 | 3 |
| W12 | 10 | 1200 | 60 | 11 | 9 |
| W13 | 10 | 1200 | 60 | 11 | 11 |
| Exp No | Weight (g) | Composition (wt%) | WO3 Loss in Slag (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | MnO | SO3 | WO3 | Na2O | |||
| W1 | 8.3 | 18.2 | 40.1 | 10.7 | 0.0 | 4.5 | 26.4 | 5.2 |
| W2 | 9.6 | 15.7 | 34.4 | 9.2 | 0.1 | 5.7 | 34.9 | 7.6 |
| W3 | 10.9 | 13.7 | 28.9 | 7.7 | 0.1 | 10.6 | 38.9 | 15.9 |
| W4 | 13.2 | 11.3 | 40.3 | 6.7 | 0.1 | 6.1 | 35.5 | 11.1 |
| W5 | 15.2 | 9.9 | 48.3 | 5.8 | 0.1 | 5.1 | 30.8 | 10.6 |
| W6 | 10.9 | 13.6 | 28.8 | 7.9 | 0.2 | 11.3 | 38.3 | 16.9 |
| W7 | 11.0 | 13.9 | 29.7 | 7.4 | 0.2 | 10.2 | 38.5 | 15.5 |
| W8 | 10.9 | 14.0 | 29.2 | 7.8 | 0.1 | 11.0 | 37.9 | 16.6 |
| W9 | 11.0 | 13.7 | 29.2 | 7.3 | 0.2 | 9.9 | 39.6 | 15.0 |
| W10 | 7.1 | 20.8 | 46.7 | 11.0 | 0.3 | 4.1 | 17.1 | 4.0 |
| W11 | 6.3 | 20.2 | 53.6 | 7.7 | 0.0 | 8.2 | 10.3 | 7.2 |
| W12 | 16.9 | 9.1 | 54.8 | 5.1 | 0.1 | 3.3 | 27.6 | 7.8 |
| W13 | 18.7 | 8.2 | 59.6 | 4.6 | 0.1 | 2.6 | 25.0 | 6.8 |
| Exp No | Weight (g) | Composition (wt%) | Direct Recovery of WO3 (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | MnO | SO3 | WO3 | Na2O | |||
| W1 | 8.8 | 0.0 | 0.2 | 0.0 | 0.9 | 78.3 | 20.6 | 94.8 |
| W2 | 8.6 | 0.0 | 0.5 | 0.0 | 0.9 | 77.3 | 21.4 | 92.4 |
| W3 | 8.5 | 0.2 | 2.1 | 0.3 | 0.8 | 71.2 | 25.4 | 84.1 |
| W4 | 8.3 | 0.0 | 0.5 | 0.0 | 0.8 | 77.7 | 21.0 | 88.9 |
| W5 | 8.2 | 0.0 | 0.1 | 0.0 | 0.9 | 78.4 | 20.6 | 89.4 |
| W6 | 8.6 | 0.3 | 2.7 | 0.4 | 0.8 | 70.2 | 25.6 | 83.1 |
| W7 | 8.4 | 0.1 | 1.6 | 0.4 | 0.8 | 72.5 | 24.6 | 84.5 |
| W8 | 8.5 | 0.1 | 1.2 | 0.1 | 0.7 | 70.9 | 27.0 | 83.4 |
| W9 | 8.5 | 0.2 | 2.2 | 0.6 | 0.8 | 72.5 | 23.6 | 85.0 |
| W10 | 8.9 | 0.4 | 0.0 | 0.8 | 0.7 | 78.3 | 19.7 | 96.0 |
| W11 | 8.4 | 2.1 | 0.1 | 3.6 | 0.4 | 79.8 | 14.0 | 92.8 |
| W12 | 8.6 | 0.0 | 0.0 | 0.0 | 0.5 | 78.0 | 21.4 | 92.2 |
| W13 | 8.7 | 0.0 | 0.0 | 0.0 | 0.6 | 77.2 | 22.1 | 93.2 |
| Exp No | Weight (g) | Composition (wt%) | Total Recovery of WO3 (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | MnO | SO3 | WO3 | Na2O | |||
| W1 | 8.1 | 18.9 | 41.4 | 11.2 | 0.0 | 2.2 | 26.2 | 97.5 |
| W2 | 8.6 | 18.2 | 37.2 | 10.7 | 0.0 | 0.9 | 32.9 | 98.9 |
| W3 | 6.9 | 31.1 | 30.5 | 17.6 | 0.0 | 0.3 | 20.5 | 99.7 |
| W4 | 12.4 | 12.0 | 42.6 | 7.1 | 0.1 | 4.5 | 33.7 | 92.3 |
| W5 | 14.9 | 10.0 | 49.5 | 5.9 | 0.1 | 4.6 | 29.9 | 90.6 |
| W6 | 6.6 | 36.6 | 24.7 | 22.1 | 0.0 | 0.3 | 16.2 | 99.7 |
| W7 | 7.5 | 29.6 | 27.8 | 18.0 | 0.0 | 0.2 | 24.3 | 99.7 |
| W8 | 7.3 | 29.8 | 28.7 | 18.5 | 0.0 | 0.2 | 22.8 | 99.8 |
| W9 | 7.2 | 30.5 | 30.6 | 15.4 | 0.0 | 0.5 | 23.0 | 99.5 |
| W10 | 6.9 | 21.4 | 47.3 | 11.3 | 0.1 | 3.1 | 16.7 | 97.0 |
| W11 | 6.2 | 20.8 | 54.2 | 7.9 | 0.0 | 7.1 | 9.9 | 93.9 |
| W12 | 16.8 | 8.9 | 55.3 | 5.1 | 0.1 | 3.1 | 27.5 | 92.7 |
| W13 | 18.5 | 8.1 | 60.2 | 4.7 | 0.1 | 2.6 | 24.3 | 93.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Zhao, B. A Fundamental Study on the Preparation of Sodium Tungstate from Wolframite via the Smelting Process. Metals 2024, 14, 299. https://doi.org/10.3390/met14030299
Xu L, Zhao B. A Fundamental Study on the Preparation of Sodium Tungstate from Wolframite via the Smelting Process. Metals. 2024; 14(3):299. https://doi.org/10.3390/met14030299
Chicago/Turabian StyleXu, Liqiang, and Baojun Zhao. 2024. "A Fundamental Study on the Preparation of Sodium Tungstate from Wolframite via the Smelting Process" Metals 14, no. 3: 299. https://doi.org/10.3390/met14030299
APA StyleXu, L., & Zhao, B. (2024). A Fundamental Study on the Preparation of Sodium Tungstate from Wolframite via the Smelting Process. Metals, 14(3), 299. https://doi.org/10.3390/met14030299






