Influence of the Applied External Magnetic Field on the Deposition of Ni–Cu Alloys
Abstract
1. Introduction
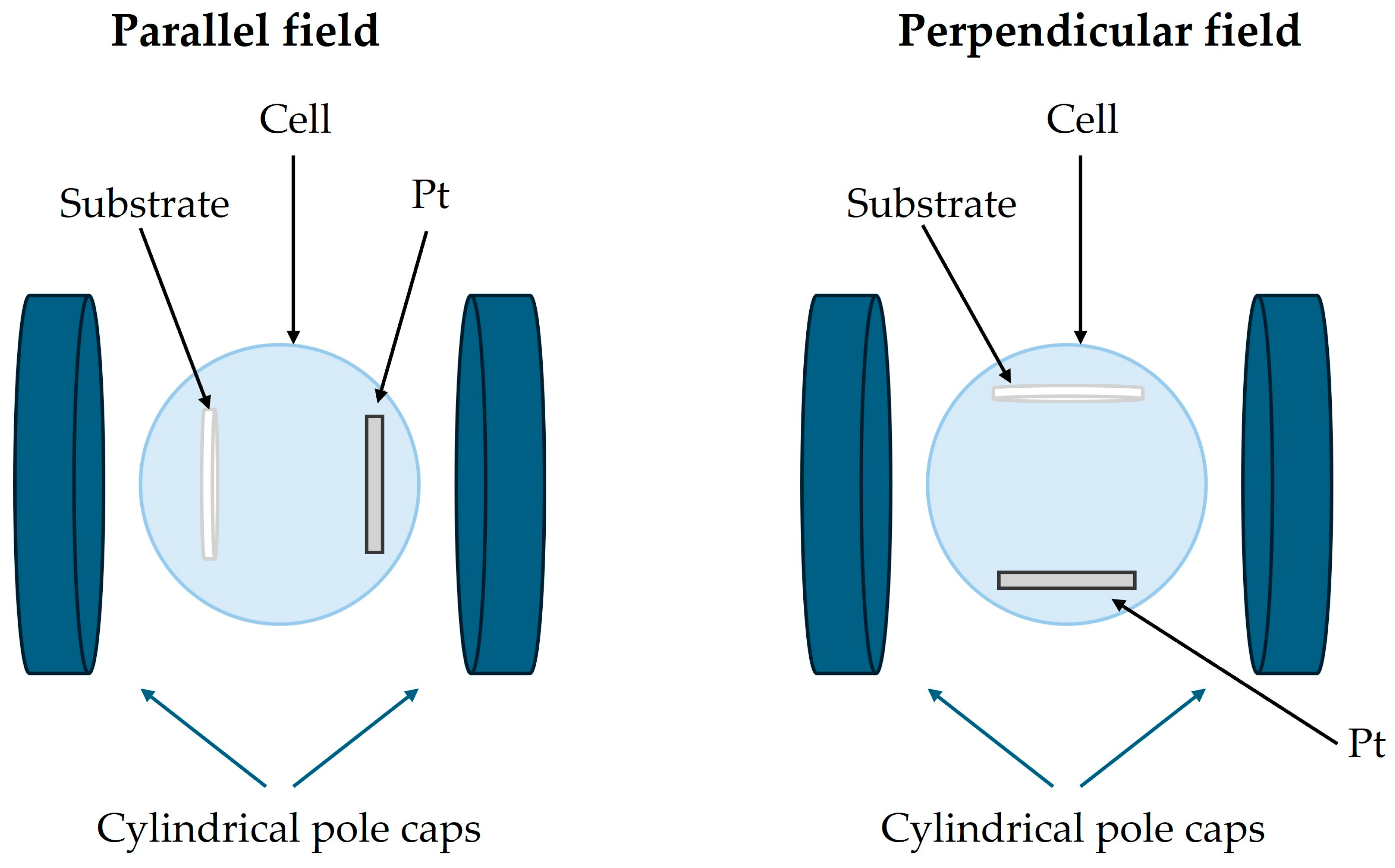
2. Materials and Methods
3. Results
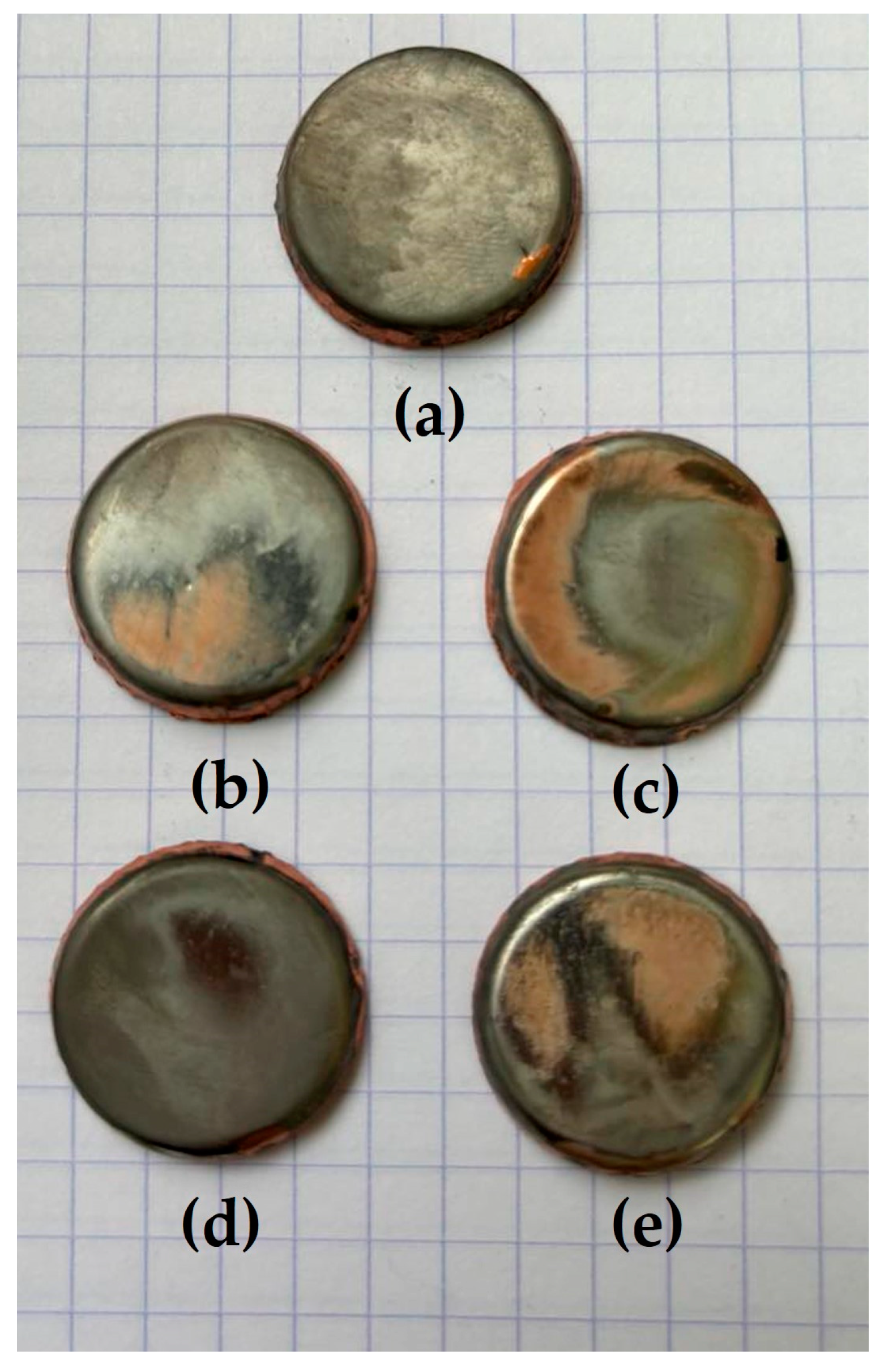
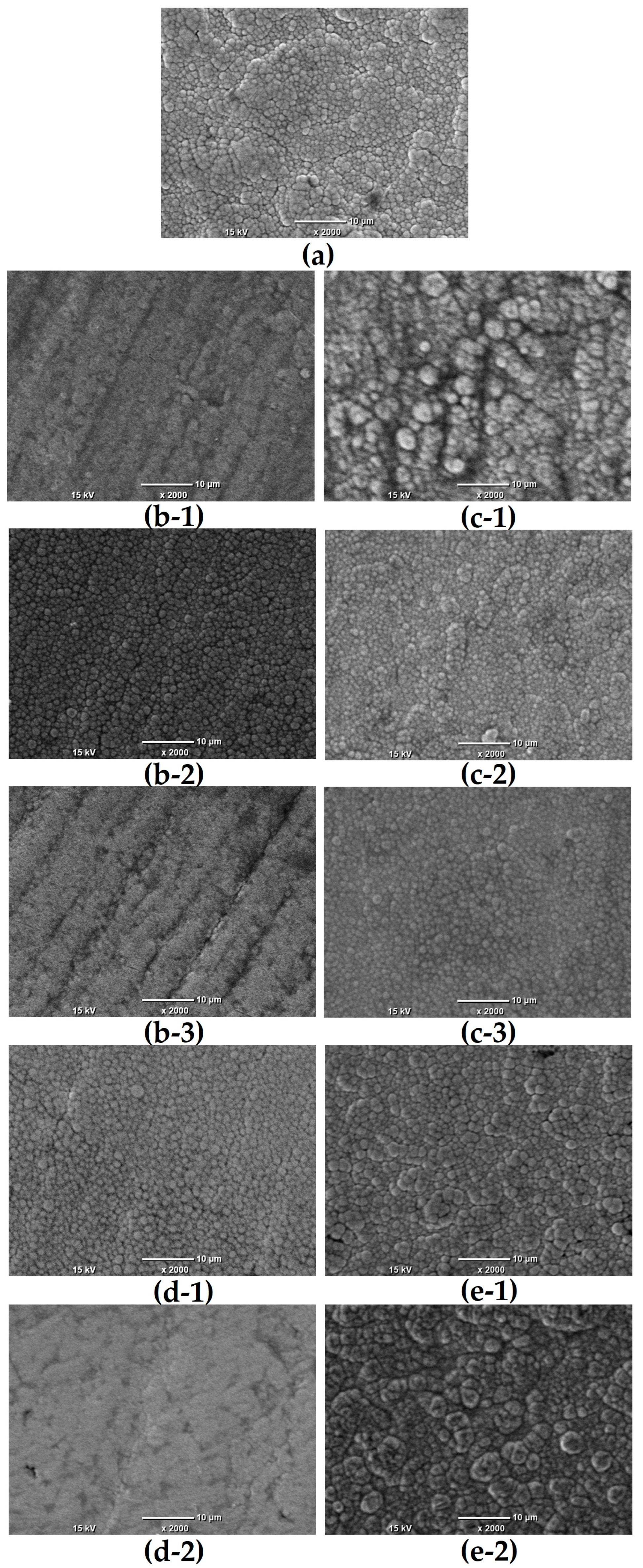
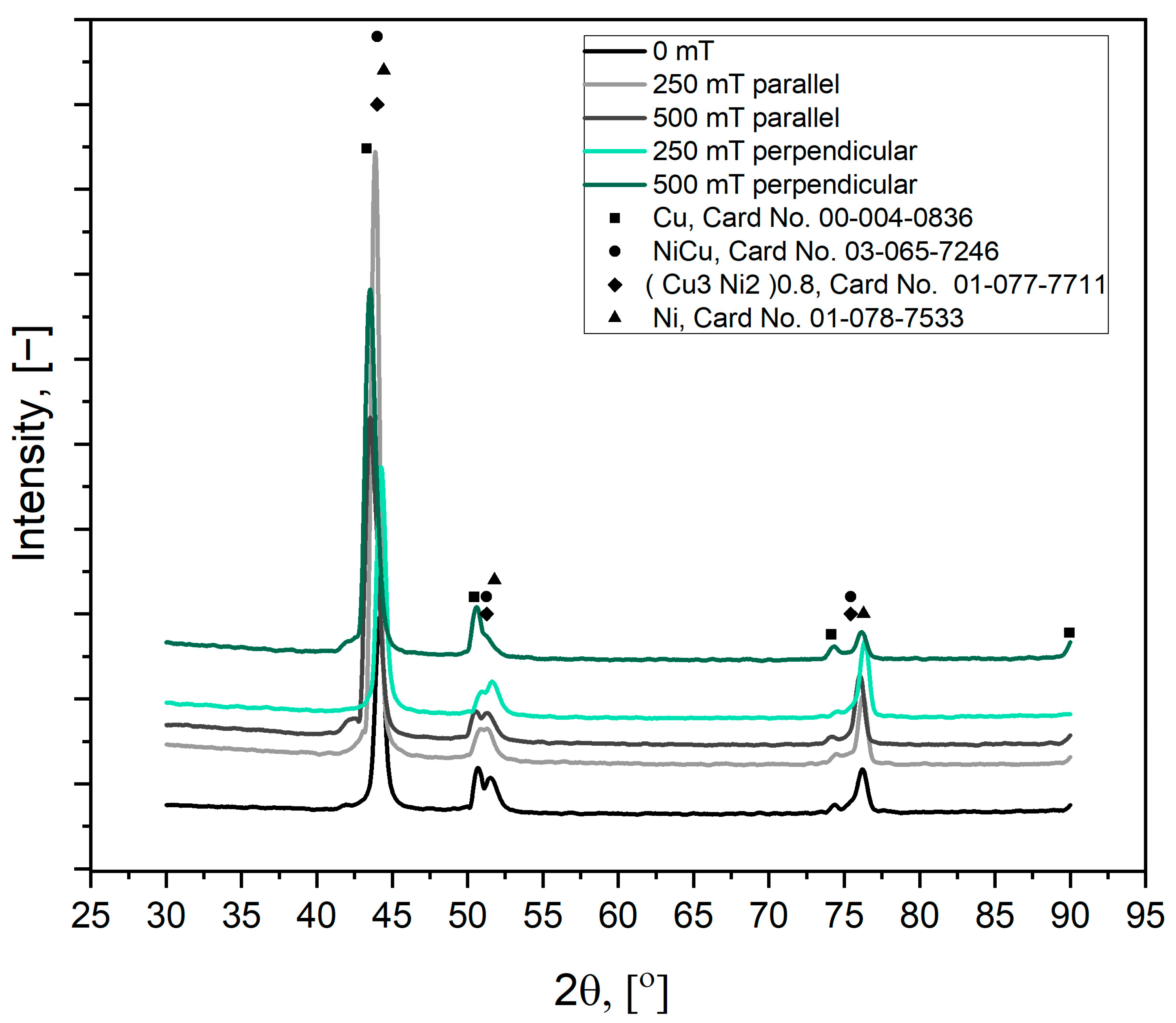
3.1. Morphology and Composition
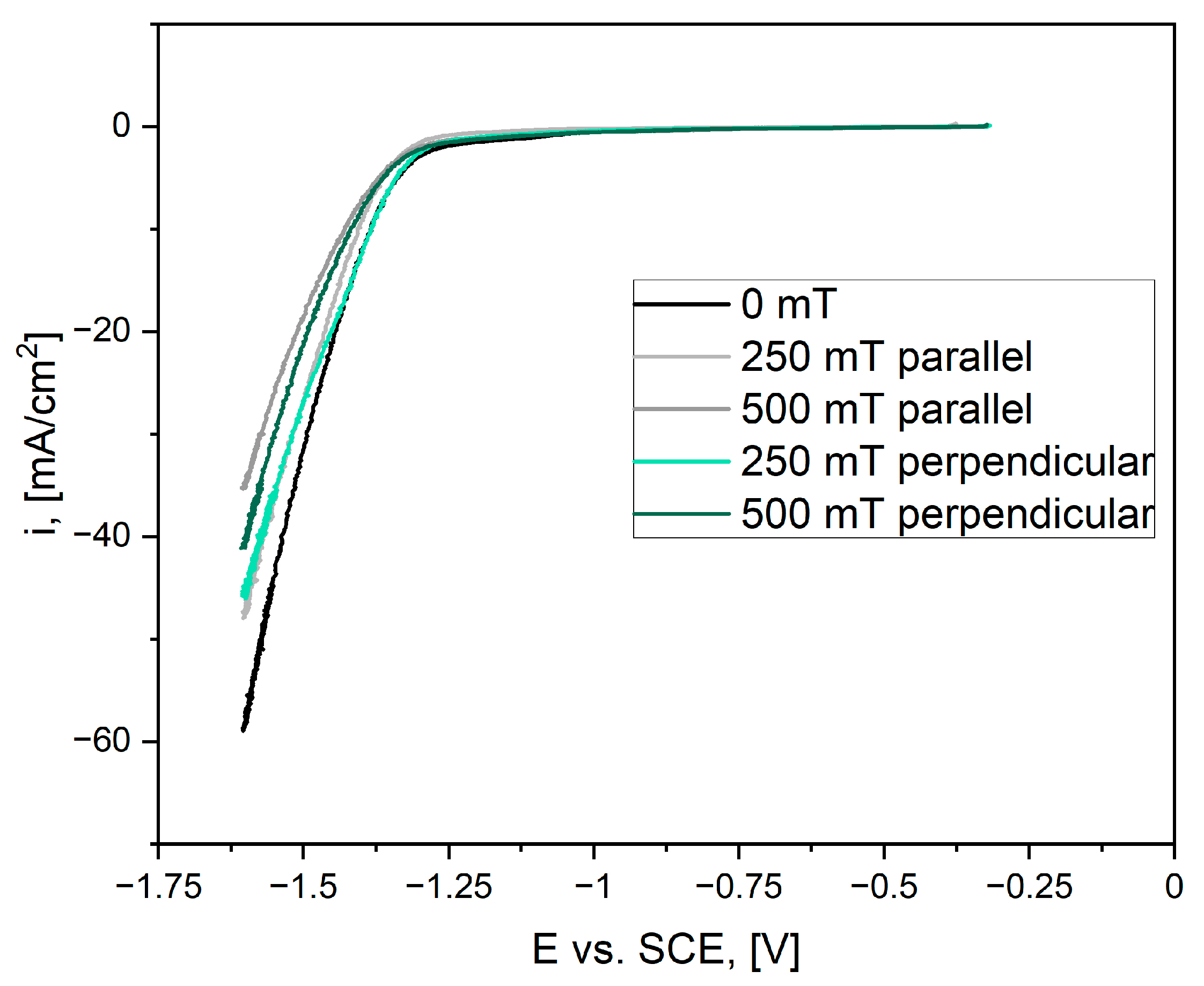
3.2. Catalytic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarac, U.; Öksüzoğlu, R.M.; Baykul, M.C. Deposition potential dependence of composition, microstructure, and surface morphology of electrodeposited Ni–Cu alloy films. J. Mater. Sci. Mater. Electron. 2012, 23, 2110–2116. [Google Scholar] [CrossRef]
- Chassaing, E.; Quang, K.V.; Wiart, R. Mechanism of copper-nickel alloy electrodeposition. J. Appl. Electrochem. 1987, 17, 1267–1280. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Yang, H.; Dai, J.; Zhu, R.; Gong, J.; Peng, L.; Ding, W. Electrodeposition mechanism and characterization of Ni–Cu alloy coatings from a eutectic-based ionic liquid. Appl. Surf. Sci. 2014, 288, 530–536. [Google Scholar] [CrossRef]
- Staroń, S.; Ledwig, P.; Dubiel, B. Electrodeposited Ni-Cu Coatings with Hierarchical Surface Morphology. Metall. Mater. Trans. A 2022, 53, 2071–2085. [Google Scholar] [CrossRef]
- Goranova, D.; Avdeev, G.; Rashkov, R. Electrodeposition and characterization of Ni-Cu alloys. Surf. Coat. Technol. 2014, 240, 204–210. [Google Scholar] [CrossRef]
- Ngamlerdpokin, K.; Tantavichet, N. Electrodeposition of nickel-copper alloys to use as a cathode for hydrogen evolution in an alkaline media. Int. J. Hydrog. Energy 2014, 39, 2505–2515. [Google Scholar] [CrossRef]
- Brito, M.M.; Artisiani, R.A.; Carlos, I.A. The electrodeposition of Ni-Cu and Ni-Cu-P from aspartate-based baths. J. Alloys Compd. 2022, 890, 161761. [Google Scholar] [CrossRef]
- Kockar, H.; Bayirli, M.; Alper, M. A new example of the diffusion-limited aggregation: Ni–Cu film patterns. Appl. Surf. Sci. 2010, 256, 2995–2999. [Google Scholar] [CrossRef]
- Alper, M.; Baykul, M.C.; Péter, L.; Tóth, J.; Bakonyi, İ. Preparation and Characterisation of Electrodeposited Ni–Cu/Cu Multilayers. J. Appl. Electrochem. 2004, 34, 841–848. [Google Scholar] [CrossRef]
- Myung, N.V.; Nobe, K. Electrodeposition of Ni/Cu multilayers. Trade J. 2000, 87, 125–134. [Google Scholar]
- Basori; Soegijono, B.; Susetyo, F.B. Magnetic field exposure on electroplating process of ferromagnetic nickel ion on copper substrate. J. Phys. Conf. Ser. 2022, 2377, 012002. [Google Scholar] [CrossRef]
- Bund, A.; Ispas, A.; Mutschke, G. Magnetic field effects on electrochemical metal depositions. Sci. Technol. Adv. Mater. 2008, 9, 024208. [Google Scholar] [CrossRef]
- Kołczyk-Siedlecka, K.; Kutyła, D.; Skibińska, K.; Jędraczka, A.; Żabiński, P. Catalytic Properties of Electroless Nickel-Based Coatings Modified by the Magnetic Field. Arch. Met. Mater 2024, 69, 17–24. [Google Scholar]
- Kołczyk-Siedlecka, K.; Skibińska, K.; Kutyła, D.; Kwiecińska, A.; Kowalik, R.; Żabiński, P. Influence of magnetic field on electroless metallization of 3D prints by copper and nickel. Arch. Metall. Mater. 2019, 64, 17–22. [Google Scholar] [CrossRef]
- Kovalyov, S.V.; Girin, O.B.; Debiemme-Chouvy, C.; Mishchenko, V.I. Copper electrodeposition under a weak magnetic field: Effect on the texturing and properties of the deposits. J. Appl. Electrochem. 2021, 51, 235–243. [Google Scholar] [CrossRef]
- Dobosz, I.; Kutyła, D.; Kac, M.; Włoch, G.; Żabiński, P. The influence of homogenous external magnetic field on morphology and magnetic properties of CoRu nanowire arrays. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 262, 114795. [Google Scholar] [CrossRef]
- Hang, T.; Hu, A.; Li, M.; Mao, D. Structural control of a cobalt nanocone array grown by directional electrodeposition. CrystEngComm 2010, 12, 2799–2802. [Google Scholar] [CrossRef]
- Huang, M.; Weber, N.; Mutschke, G. A Simulation Framework for Electrochemical Processes with Electrolyte Flow. J. Electrochem. Soc. 2023, 170, 073502. [Google Scholar] [CrossRef]
- Huang, M.; Uhlemann, M.; Eckert, K.; Mutschke, G. Pulse Reverse Plating of Copper Micro-Structures in Magnetic Gradient Fields. Magnetochemistry 2022, 8, 66. [Google Scholar] [CrossRef]
- Liang, P.; Li, Q.; Chen, L.; Tang, Z.; Li, Z.; Wang, Y.; Tang, Y.; Han, C.; Lan, Z.; Zhi, C.; et al. The magnetohydrodynamic effect enables a dendrite-free Zn anode in alkaline electrolytes. J. Mater. Chem. A 2022, 10, 11971–11979. [Google Scholar] [CrossRef]
- Bau, H.H. Applications of Magneto Electrochemistry and Magnetohydrodynamics in Microfluidics. Magnetochemistry 2022, 8, 140. [Google Scholar] [CrossRef]
- Mitra, K.; Adalder, A.; Mandal, S.; Ghorai, U.K. Enhancing Electrochemical Reactivity with Magnetic Fields: Unraveling the Role of Magneto-Electrochemistry. Small Methods 2024, e2301132. [Google Scholar] [CrossRef]
- Luo, S.; Elouarzaki, K.; Xu, Z.J. Electrochemistry in Magnetic Fields. Angew. Chemie Int. Ed. 2022, 61, e202203564. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.; Guo, M.; Hong, A.; Yu, T.; Luo, X.; Yuan, C.; Lei, W.; Wang, S. Magnetic Enhancement for Hydrogen Evolution Reaction on Ferromagnetic MoS 2 Catalyst. Nano Lett. 2020, 20, 2923–2930. [Google Scholar] [CrossRef]
- Sambalova, O.; Billeter, E.; Yildirim, O.; Sterzi, A.; Bleiner, D.; Borgschulte, A. Magnetic field enhancement of electrochemical hydrogen evolution reaction probed by magneto-optics. Int. J. Hydrogen Energy 2021, 46, 3346–3353. [Google Scholar] [CrossRef]
- Salinas, G.; Lozon, C.; Kuhn, A. Unconventional applications of the magnetohydrodynamic effect in electrochemical systems. Curr. Opin. Electrochem. 2023, 38, 101220. [Google Scholar] [CrossRef]
- Deng, Y.; Lai, W.; Xu, B. A Mini Review on Doped Nickel-Based Electrocatalysts for Hydrogen Evolution Reaction. Energies 2020, 13, 4651. [Google Scholar] [CrossRef]
- Angeles-Olvera, Z.; Crespo-Yapur, A.; Rodríguez, O.; Cholula-Díaz, J.; Martínez, L.; Videa, M. Nickel-Based Electrocatalysts for Water Electrolysis. Energies 2022, 15, 1609. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, Y.; Cai, W.; Xia, T.; Jiang, W.-J.; Ding, W.; Cao, J.-P. Boosting hydrogen evolution activity and durability of Pd–Ni–P nanocatalyst via crystalline degree and surface chemical state modulations. Int. J. Hydrogen Energy 2019, 44, 31053–31061. [Google Scholar] [CrossRef]
- Mech, K.; Wróbel, M.; Wojnicki, M.; Mech-Piskorz, J.; Żabiński, P.; Kowalik, R. Electrodeposition of NiPd alloy from aqueous chloride electrolytes. Appl. Surf. Sci. 2016, 388, 809–816. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, X.; Zuo, Y.; Ju, P.; Tang, Y. Electrodeposited Pd-Ni-Mo film as a cathode material for hydrogen evolution reaction. Electrochim. Acta 2015, 174, 1041–1049. [Google Scholar] [CrossRef]
- Fukumoto, M.; Takahashi, H.; Kutyła, D.; Wojnicki, M.; Żabiński, P. Morphological investigation and electrochemical performance evaluation of novel porous Ni–Pt produced by Al-deposition/dissolution in molten salts for hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 49, 754–765. [Google Scholar] [CrossRef]
- Fiameni, S.; Herraiz-Cardona, I.; Musiani, M.; Pérez-Herranz, V.; Vázquez-Gómez, L.; Verlato, E. The HER in alkaline media on Pt-modified three-dimensional Ni cathodes. Int. J. Hydrogen Energy 2012, 37, 10507–10516. [Google Scholar] [CrossRef]
- Eiler, K.; Krawiec, H.; Kozina, I.; Sort, J.; Pellicer, E. Electrochemical characterisation of multifunctional electrocatalytic mesoporous Ni-Pt thin films in alkaline and acidic media. Electrochim. Acta 2020, 359, 136952. [Google Scholar] [CrossRef]
- Kutyła, D.; Kołczyk-Siedlecka, K.; Kwiecińska, A.; Skibińska, K.; Kowalik, R.; Żabiński, P. Preparation and characterization of electrodeposited Ni-Ru alloys: Morphological and catalytic study. J. Solid State Electrochem. 2019, 23, 3089–3097. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Fo, Y.; Zhang, B.; Molochas, C.; Gao, J.; Li, W.; Cui, X.; Zhou, X.; Jiang, L.; et al. Engineering of unique Ni-Ru nano-twins for highly active and robust bifunctional hydrogen oxidation and hydrogen evolution electrocatalysis. Chem. Eng. J. 2023, 454, 139959. [Google Scholar] [CrossRef]
- Mao, J.; He, C.-T.; Pei, J.; Liu, Y.; Li, J.; Chen, W.; He, D.; Wang, D.; Li, Y. Isolated Ni Atoms Dispersed on Ru Nanosheets: High-Performance Electrocatalysts toward Hydrogen Oxidation Reaction. Nano Lett. 2020, 20, 3442–3448. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jin, P.; Liu, B.; Zhao, L.; Cai, J.; Wei, Z.; Zuo, S.; Zhang, J.; Feng, L. Ultrafine carbon encapsulated NiRu alloys as bifunctional electrocatalysts for boosting overall water splitting: Morphological and electronic modulation through minor Ru alloying. J. Mater. Chem. A 2020, 8, 9049–9057. [Google Scholar] [CrossRef]
- Kutyła, D.; Salcı, A.; Kwiecińska, A.; Kołczyk-Siedlecka, K.; Kowalik, R.; Żabiński, P.; Solmaz, R. Catalytic activity of electrodeposited ternary Co–Ni–Rh thin films for water splitting process. Int. J. Hydrogen Energy 2020, 45, 34805–34817. [Google Scholar] [CrossRef]
- Skibińska, K.; Kutyła, D.; Yang, X.; Krause, L.; Marzec, M.M.; Żabiński, P. Rhodium-decorated nanoconical nickel electrode synthesis and characterization as an electrochemical active cathodic material for hydrogen production. Appl. Surf. Sci. 2022, 592, 153326. [Google Scholar] [CrossRef]
- Nguyen, N.-A.; Choi, H.-S. Effect of Ni/Rh ratios on characteristics of NixRhy nanosponges towards high-performance hydrogen evolution reaction. Data Br. 2019, 24, 103941. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-A.; Nguyen, V.-T.; Shin, S.; Choi, H.-S. NiRh nanosponges with highly efficient electrocatalytic performance for hydrogen evolution reaction. J. Alloys Compd. 2019, 789, 163–173. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Chang, Y.; Lu, Y.; Zhao, S.; Xu, D.; Dai, Z.; Han, M.; Bao, J. Component-Controlled Synthesis of Necklace-Like Hollow Ni X Ru y Nanoalloys as Electrocatalysts for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 17326–17336. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.J.; Franceschini, E.A.; Lacconi, G.I. Ni and Ni x Co y Alloys Electrodeposited on Stainless Steel AISI 316L for Hydrogen Evolution Reaction. Electrocatalysis 2018, 9, 459–470. [Google Scholar] [CrossRef]
- González-Buch, C.; Herraiz-Cardona, I.; Ortega, E.; García-Antón, J.; Pérez-Herranz, V. Synthesis and characterization of macroporous Ni, Co and Ni–Co electrocatalytic deposits for hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2013, 38, 10157–10169. [Google Scholar] [CrossRef]
- Lupi, C.; Dell’Era, A.; Pasquali, M. Nickel–cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int. J. Hydrogen Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- Vernickaite, E.; Tsyntsaru, N.; Sobczak, K.; Cesiulis, H. Electrodeposited tungsten-rich Ni-W, Co-W and Fe-W cathodes for efficient hydrogen evolution in alkaline medium. Electrochim. Acta 2019, 318, 597–606. [Google Scholar] [CrossRef]
- Żabiński, P.; Mech, K.; Kowalik, R. Electrocatalytically active Co–W and Co–W–C alloys electrodeposited in a magnetic field. Electrochim. Acta 2013, 104, 542–548. [Google Scholar] [CrossRef]
- Laszczyńska, A. Electrodeposited alloy electrodes in the Co–W–Mo system as highly efficient catalysts for hydrogen production from alkaline water electrolysis. Mater. Chem. Phys. 2024, 314, 128876. [Google Scholar] [CrossRef]
- Laszczyńska, A.; Szczygieł, I. Electrocatalytic activity for the hydrogen evolution of the electrodeposited Co–Ni–Mo, Co–Ni and Co–Mo alloy coatings. Int. J. Hydrogen Energy 2020, 45, 508–520. [Google Scholar] [CrossRef]
- Goranova, D.; Lefterova, E.; Rashkov, R. Electrocatalytic activity of Ni-Mo-Cu and Ni-Co-Cu alloys for hydrogen evolution reaction in alkaline medium. Int. J. Hydrogen Energy 2017, 42, 28777–28785. [Google Scholar] [CrossRef]
- Negem, M.; Nady, H. Electroplated Ni-Cu nanocrystalline alloys and their electrocatalytic activity for hydrogen generation using alkaline solutions. Int. J. Hydrogen Energy 2017, 42, 28386–28396. [Google Scholar] [CrossRef]
- Cui, X.; Xiao, P.; Wang, J.; Zhou, M.; Guo, W.; Yang, Y.; He, Y.; Wang, Z.; Yang, Y.; Zhang, Y.; et al. Highly Branched Metal Alloy Networks with Superior Activities for the Methanol Oxidation Reaction. Angew. Chemie Int. Ed. 2017, 56, 4488–4493. [Google Scholar] [CrossRef]
- Koza, J.A.; Uhlemann, M.; Gebert, A.; Schultz, L. The effect of magnetic fields on the electrodeposition of CoFe alloys. Electrochim. Acta 2008, 53, 5344–5353. [Google Scholar] [CrossRef]
- Huang, M.; Skibinska, K.; Zabinski, P.; Wojnicki, M.; Włoch, G.; Eckert, K.; Mutschke, G. On the prospects of magnetic-field-assisted electrodeposition of nano-structured ferromagnetic layers. Electrochim. Acta 2022, 420, 140422. [Google Scholar] [CrossRef]
- Rode, S.; Henninot, C.; Matlosz, M. Complexation Chemistry in Nickel and Copper-Nickel Alloy Plating from Citrate Baths. J. Electrochem. Soc. 2005, 152, C248. [Google Scholar] [CrossRef]
- Goranova, D.; Rashkov, R.; Avdeev, G.; Tonchev, V. Electrodeposition of Ni–Cu alloys at high current densities: Details of the elements distribution. J. Mater. Sci. 2016, 51, 8663–8673. [Google Scholar] [CrossRef]
- Watanabe, T. Control of Macrostructure in Plated Films and Fabrication of Three-Dimensional Microstructure. In Nano Plating—Microstructure Formation Theory of Plated Films and a Database of Plated Films; Elsevier: Amsterdam, The Netherlands, 2004; pp. 195–205. [Google Scholar]
- Landolt, D. Electrochemical and materials science aspects of alloy deposition. Electrochim. Acta 1994, 39, 1075–1090. [Google Scholar] [CrossRef]
- Krause, L.; Skibińska, K.; Rox, H.; Baumann, R.; Marzec, M.M.; Yang, X.; Mutschke, G.; Żabiński, P.; Lasagni, A.F.; Eckert, K. Hydrogen Bubble Size Distribution on Nanostructured Ni Surfaces: Electrochemically Active Surface Area Versus Wettability. ACS Appl. Mater. Interfaces 2023, 15, 18290–18299. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, S.; Kutyła, D.; Zabinski, P. The Influence of the Magnetic Field on Ni Thin Film Preparation by Electrodeposition Method and Its Electrocatalytic Activity towards Hydrogen Evolution Reaction. Coatings 2023, 13, 1816. [Google Scholar] [CrossRef]
- Solmaz, R.; Döner, A.; Kardaş, G. The stability of hydrogen evolution activity and corrosion behavior of NiCu coatings with long-term electrolysis in alkaline solution. Int. J. Hydrog. Energy 2009, 34, 2089–2094. [Google Scholar] [CrossRef]
- Lee, J.M.; Bae, K.M.; Jung, K.K.; Jeong, J.H.; Ko, J.S. Creation of microstructured surfaces using Cu–Ni composite electrodeposition and their application to superhydrophobic surfaces. Appl. Surf. Sci. 2014, 289, 14–20. [Google Scholar] [CrossRef]
- Eugénio, S.; Silva, T.M.; Carmezim, M.J.; Duarte, R.G.; Montemor, M.F. Electrodeposition and characterization of nickel–copper metallic foams for application as electrodes for supercapacitors. J. Appl. Electrochem. 2014, 44, 455–465. [Google Scholar] [CrossRef]
- Mao, Y.-H.; Chen, C.-Y.; Fu, J.-X.; Lai, T.-Y.; Lu, F.-H.; Tsai, Y.-C. Electrodeposition of nickel-copper on titanium nitride for methanol electrooxidation. Surf. Coat. Technol. 2018, 350, 949–953. [Google Scholar] [CrossRef]


| Sample | Point | Chemical Composition, [% at.] | ||
|---|---|---|---|---|
| Cu | Ni | O | ||
| 0 mT | - | 47.7 | 49.6 | 2.7 |
| 250 mT parallel | 1 | 80.1 | 16.7 | 3.2 |
| 2 | 35.5 | 61.3 | 3.2 | |
| 3 | 62.7 | 35.1 | 2.2 | |
| 500 mT parallel | 1 | 82.2 | 3.6 | 14.2 |
| 2 | 34.1 | 61.3 | 4.6 | |
| 3 | 29.0 | 65.8 | 5.2 | |
| 250 mT perpendicular | 1 | 50.1 | 46.8 | 3.1 |
| 2 | 66.1 | 31.8 | 2.1 | |
| 500 mT perpendicular | 1 | 36.1 | 59.9 | 4.0 |
| 2 | 77.0 | 8.2 | 14.8 | |
| Sample | Chemical Composition [% Mass] | |
|---|---|---|
| Cu | Ni | |
| 0 mT | 54.15 | 45.85 |
| 250 mT parallel | 70.69 | 29.31 |
| 500 mT parallel | 77.38 | 22.62 |
| 250 mT perpendicular | 54.75 | 45.25 |
| 500 mT perpendicular | 76.69 | 23.31 |
| Sample | Crystallite Size [nm] |
|---|---|
| 0 mT | 12 |
| 250 mT parallel | 13 |
| 500 mT parallel | 13 |
| 250 mT perpendicular | 12 |
| 500 mT perpendicular | 15 |
| Sample | Contact Angle [°] |
|---|---|
| 0 mT | 71 ± 4 |
| 250 mT parallel | 73 ± 8 |
| 500 mT parallel | 71 ± 8 |
| 250 mT perpendicular | 68 ± 7 |
| 500 mT perpendicular | 65 ± 7 |
| Sample | EONSET [V] |
|---|---|
| 0 mT | −1.38 |
| 250 mT parallel | −1.35 |
| 500 mT parallel | −1.37 |
| 250 mT perpendicular | −1.33 |
| 500 mT perpendicular | −1.38 |
| Sample | Tafel Slope [mV/dec] |
|---|---|
| 0 mT | 172 |
| 250 mT parallel | 143 |
| 500 mT parallel | 181 |
| 250 mT perpendicular | 138 |
| 500 mT perpendicular | 173 |
| Sample | Time [s] |
|---|---|
| 0 mT | 192 |
| 250 mT parallel | 188 |
| 500 mT parallel | 192 |
| 250 mT perpendicular | 190 |
| 500 mT perpendicular | 192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skibińska, K.; Elsharkawy, S.; Kołczyk-Siedlecka, K.; Kutyła, D.; Żabiński, P. Influence of the Applied External Magnetic Field on the Deposition of Ni–Cu Alloys. Metals 2024, 14, 281. https://doi.org/10.3390/met14030281
Skibińska K, Elsharkawy S, Kołczyk-Siedlecka K, Kutyła D, Żabiński P. Influence of the Applied External Magnetic Field on the Deposition of Ni–Cu Alloys. Metals. 2024; 14(3):281. https://doi.org/10.3390/met14030281
Chicago/Turabian StyleSkibińska, Katarzyna, Safya Elsharkawy, Karolina Kołczyk-Siedlecka, Dawid Kutyła, and Piotr Żabiński. 2024. "Influence of the Applied External Magnetic Field on the Deposition of Ni–Cu Alloys" Metals 14, no. 3: 281. https://doi.org/10.3390/met14030281
APA StyleSkibińska, K., Elsharkawy, S., Kołczyk-Siedlecka, K., Kutyła, D., & Żabiński, P. (2024). Influence of the Applied External Magnetic Field on the Deposition of Ni–Cu Alloys. Metals, 14(3), 281. https://doi.org/10.3390/met14030281









