Thermal Evolution of Expanded Phases Formed by PIII Nitriding in Super Duplex Steel Investigated by In Situ Synchrotron Radiation
Abstract
1. Introduction
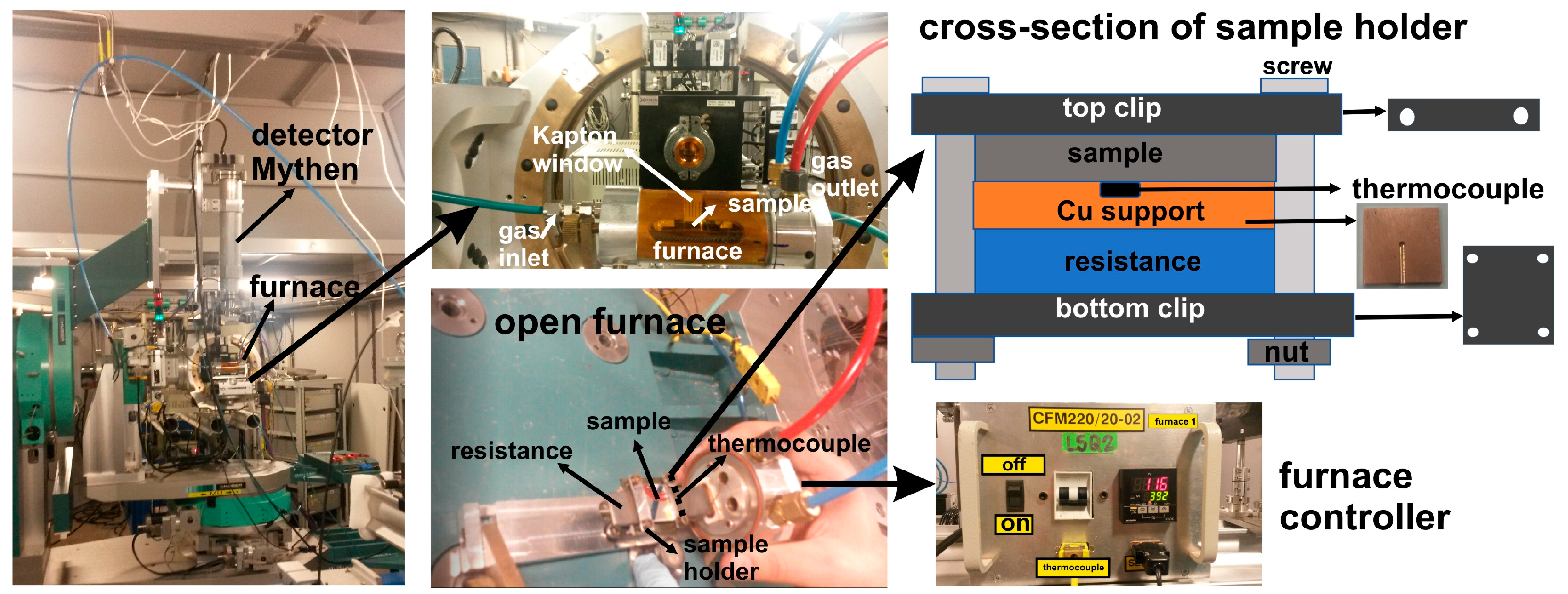
2. Materials and Methods
3. Results and Discussions
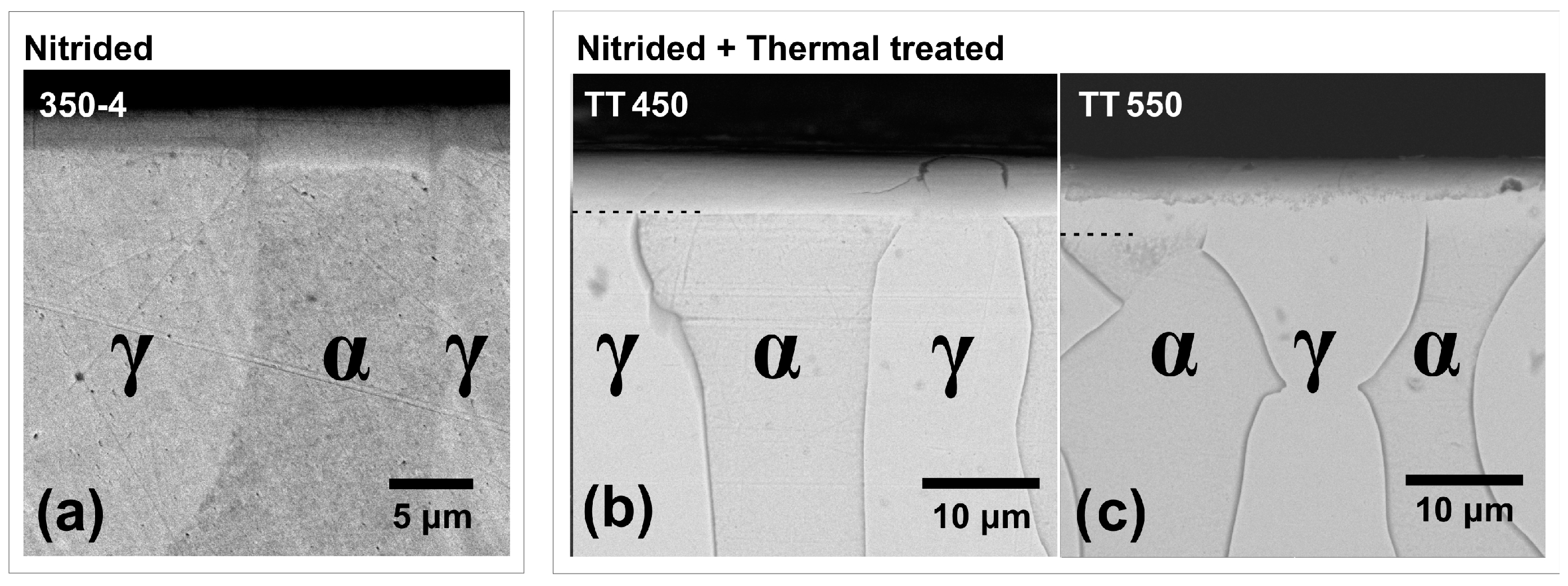
3.1. Microstructure
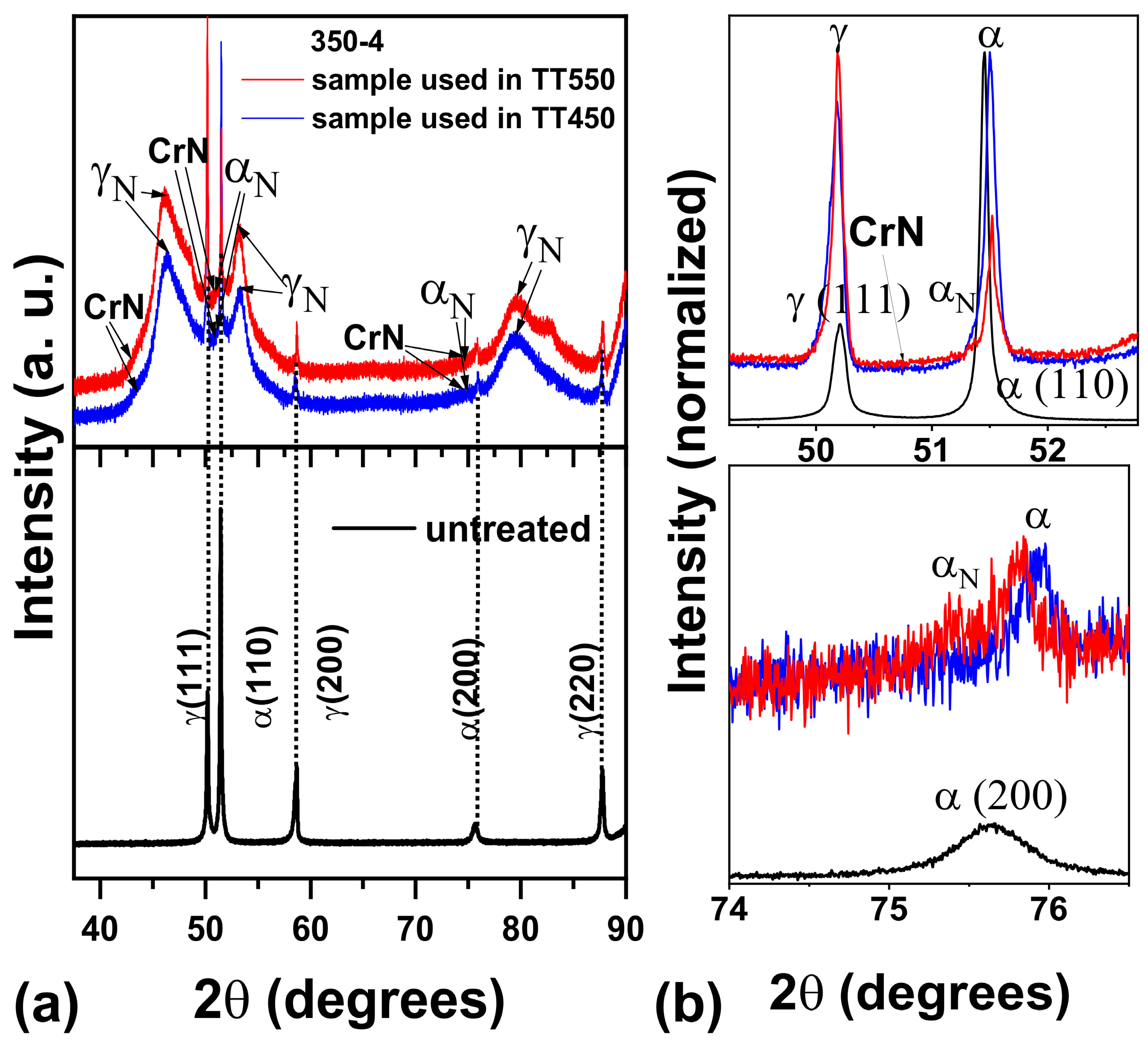
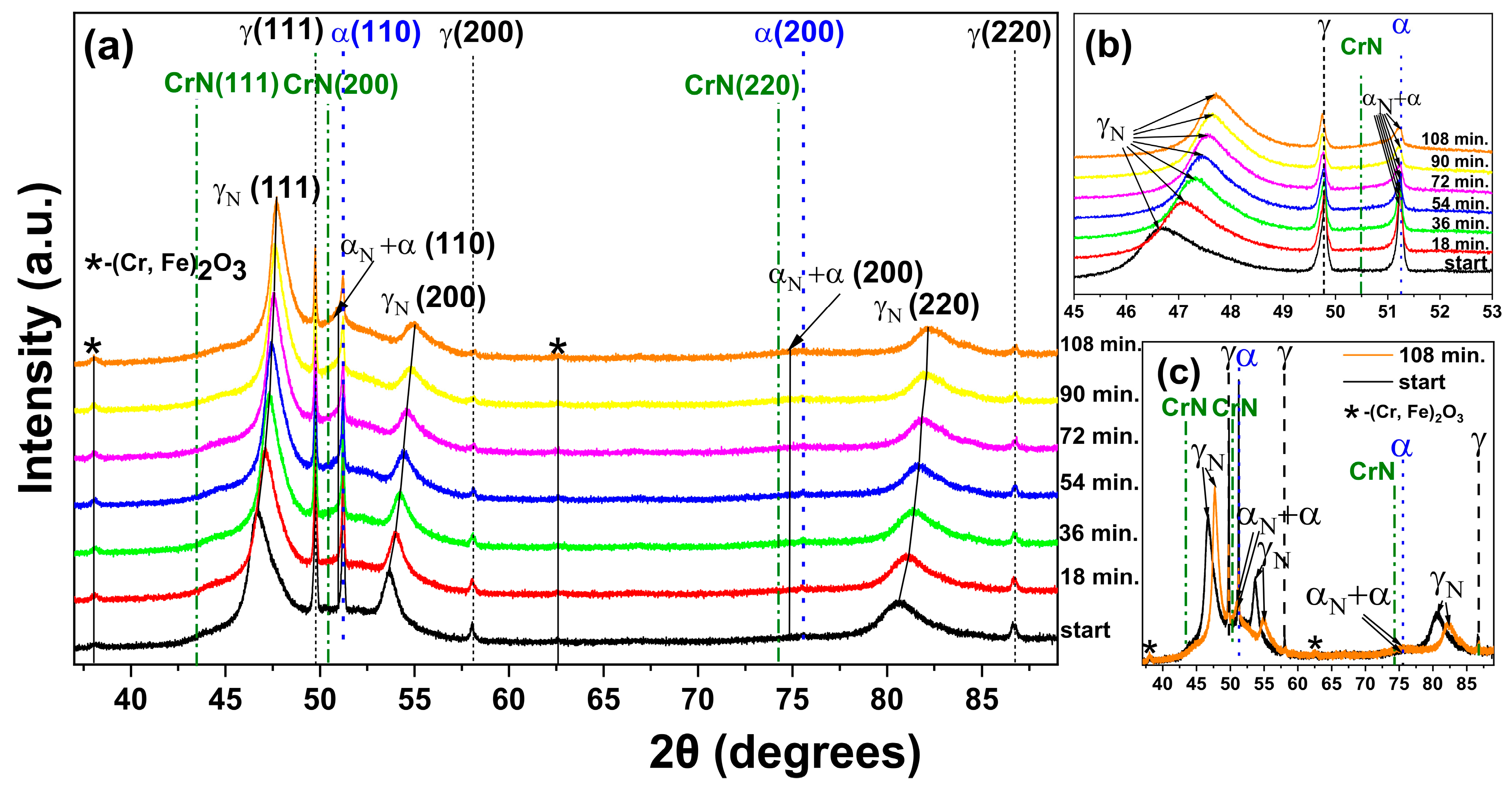
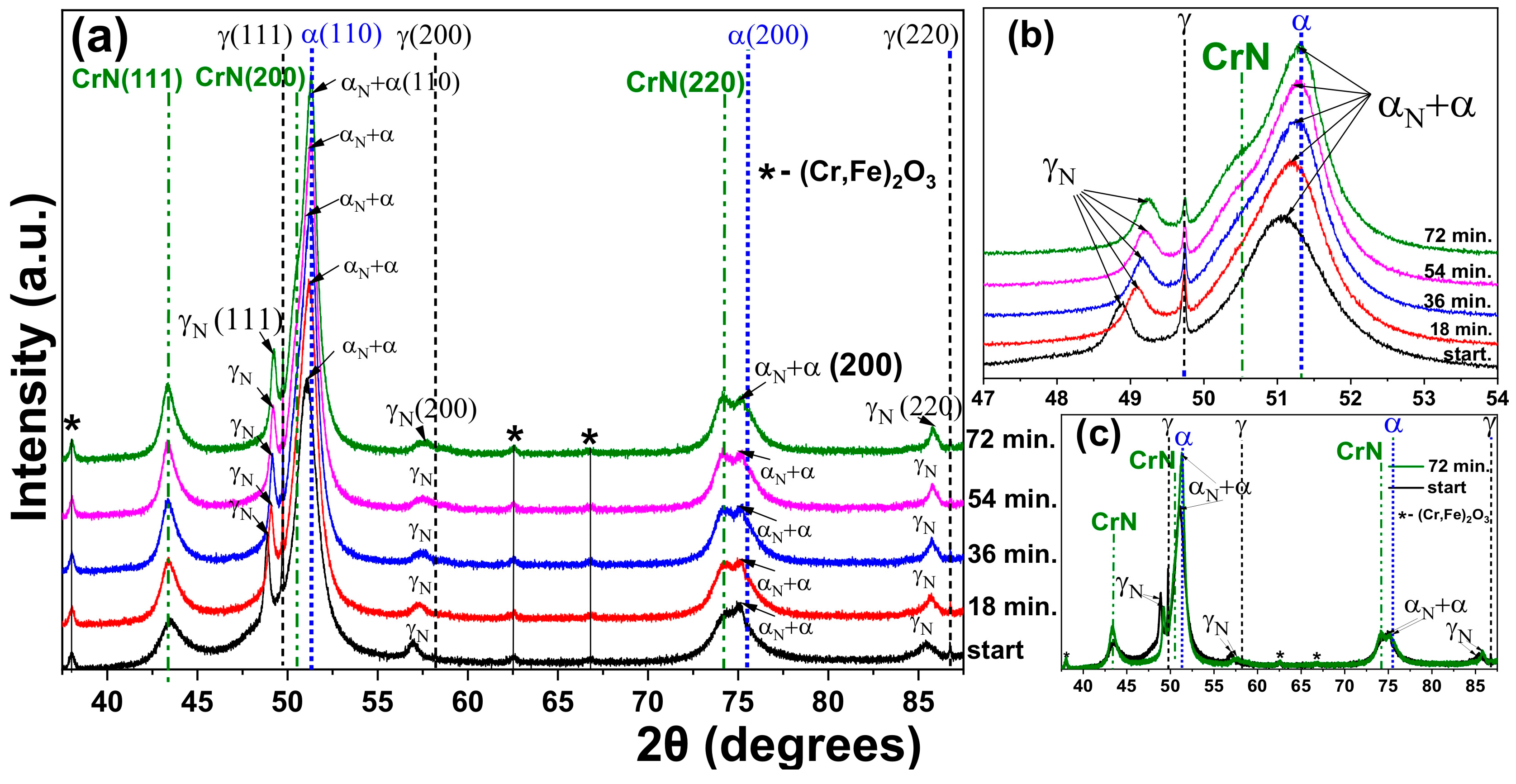
3.2. XRD of the Nitrided Surfaces
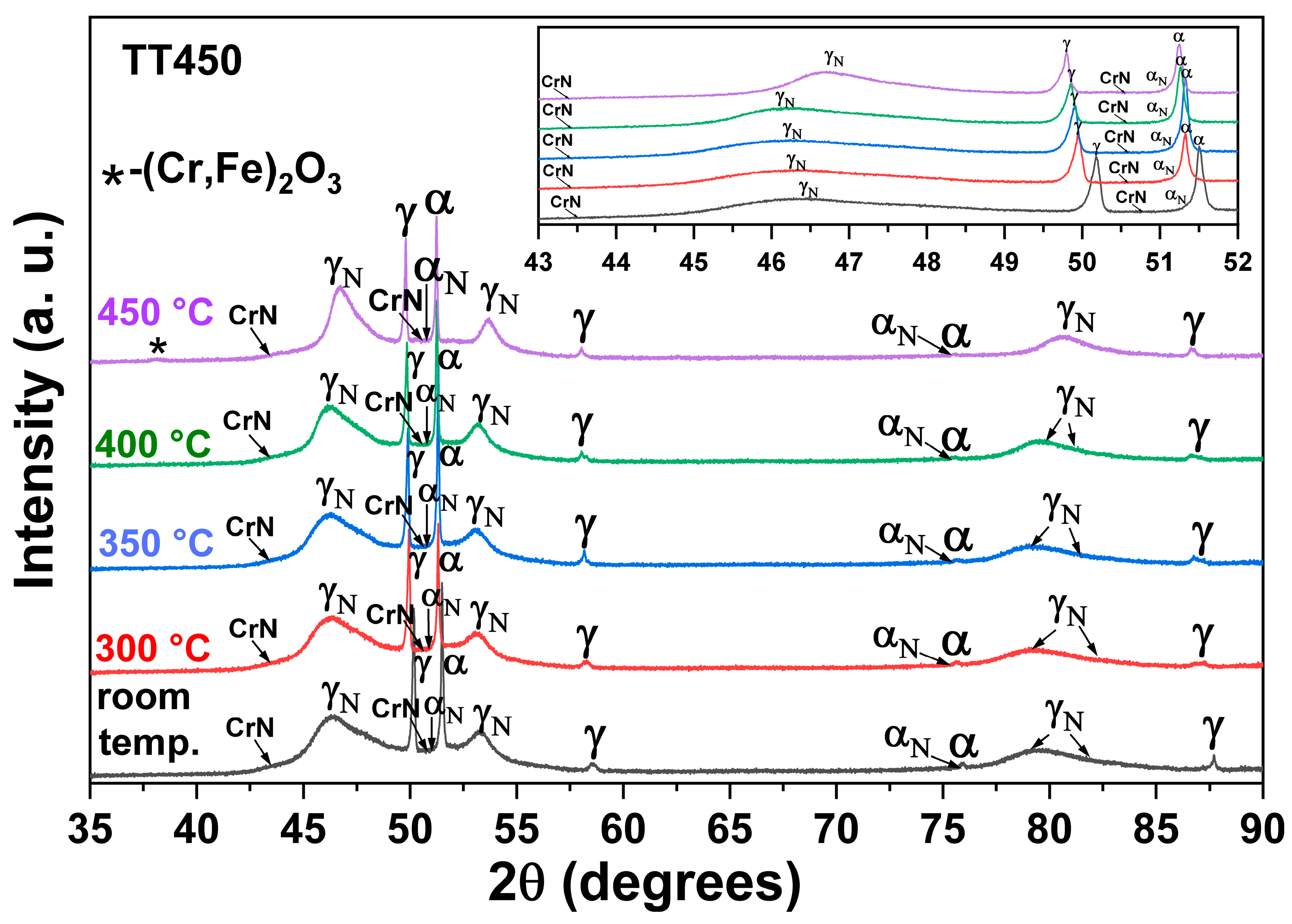
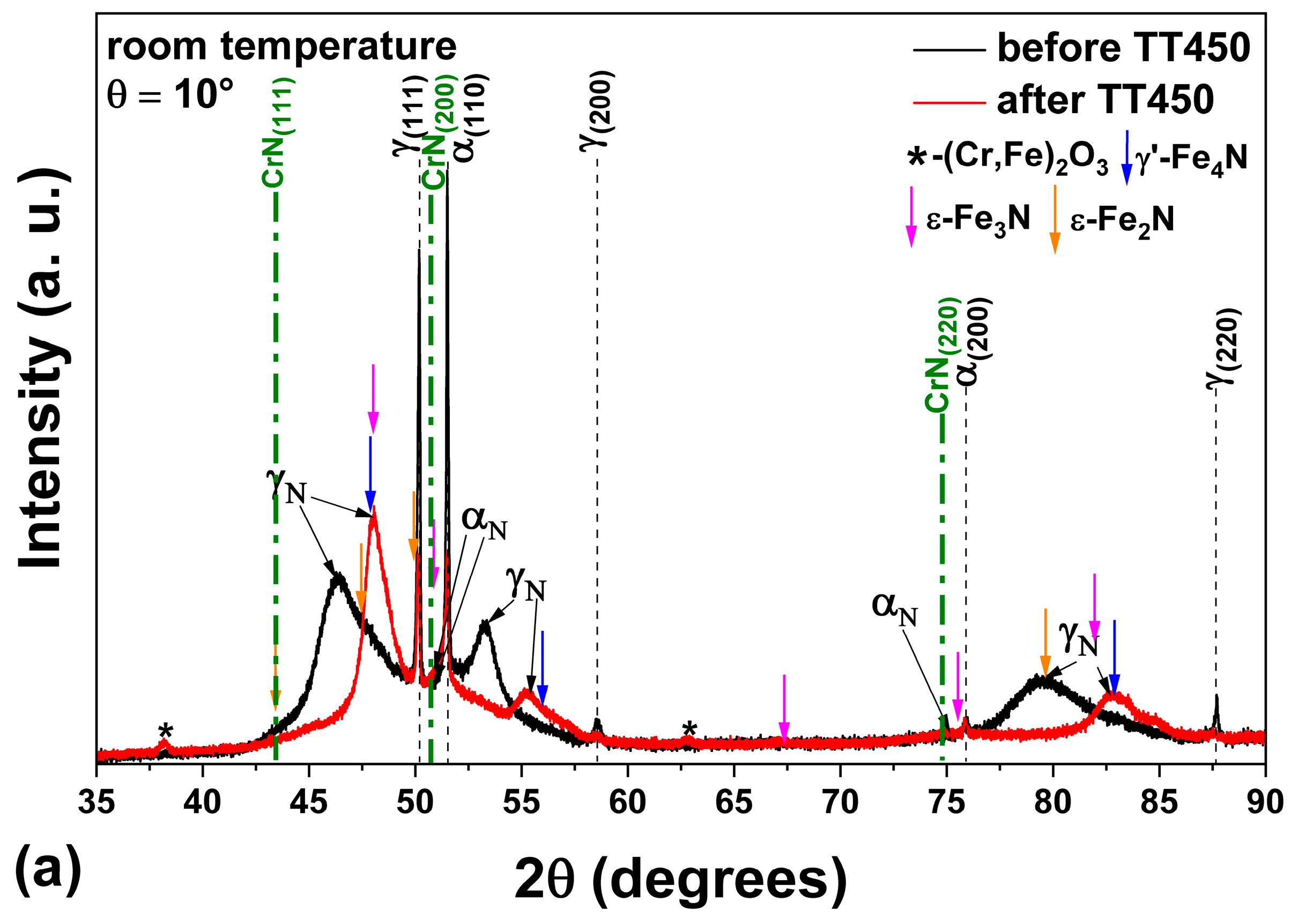
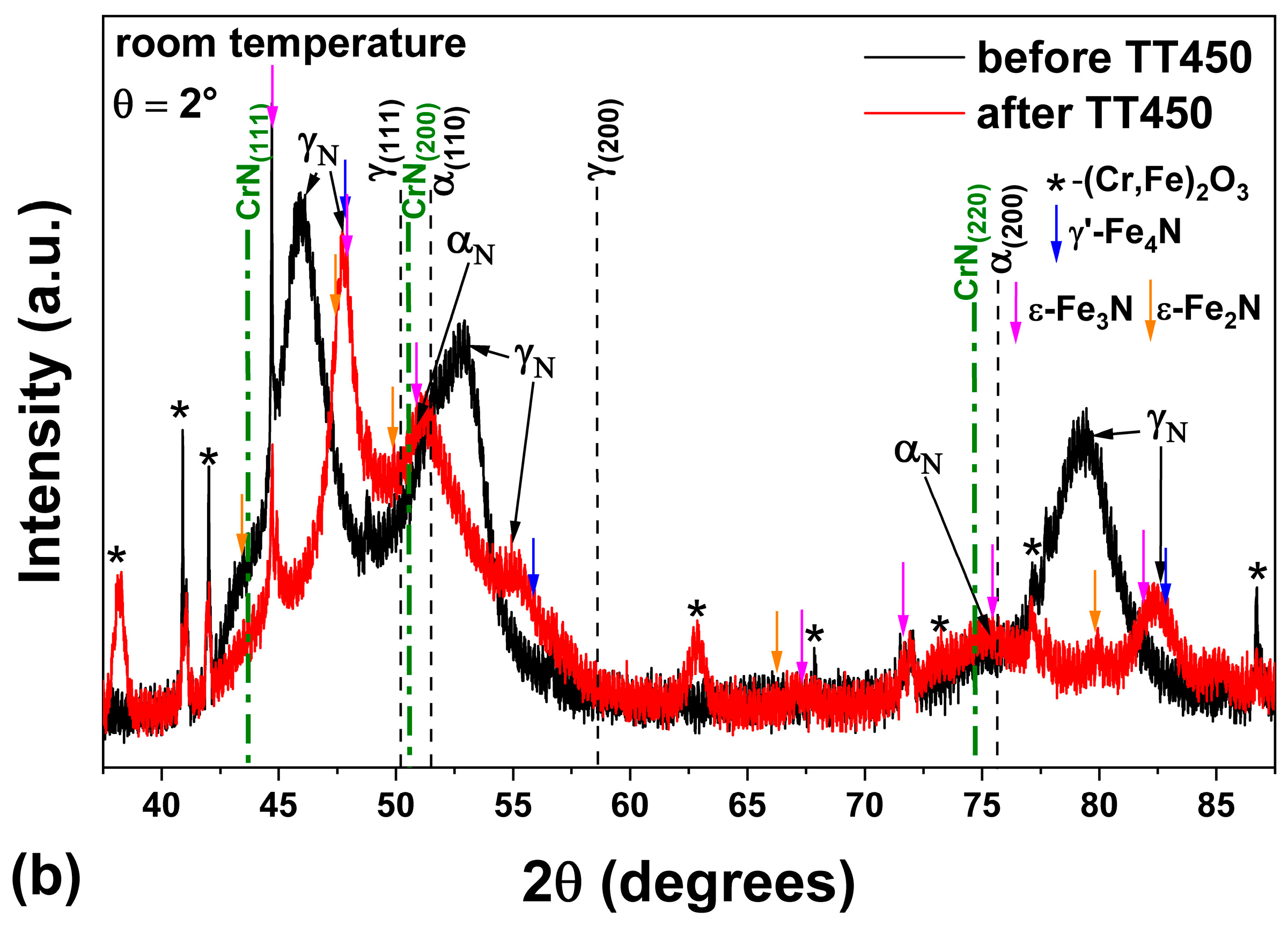
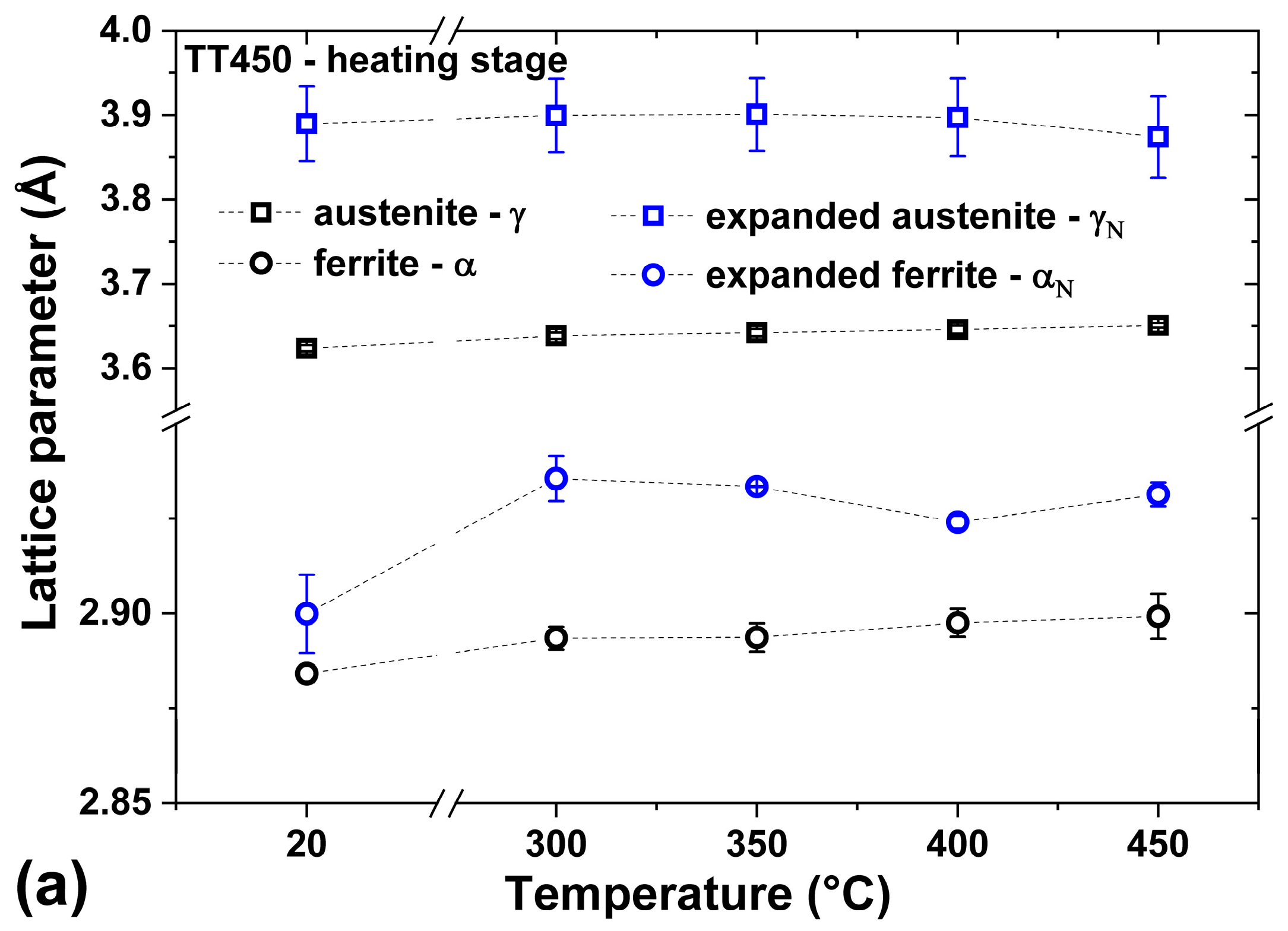
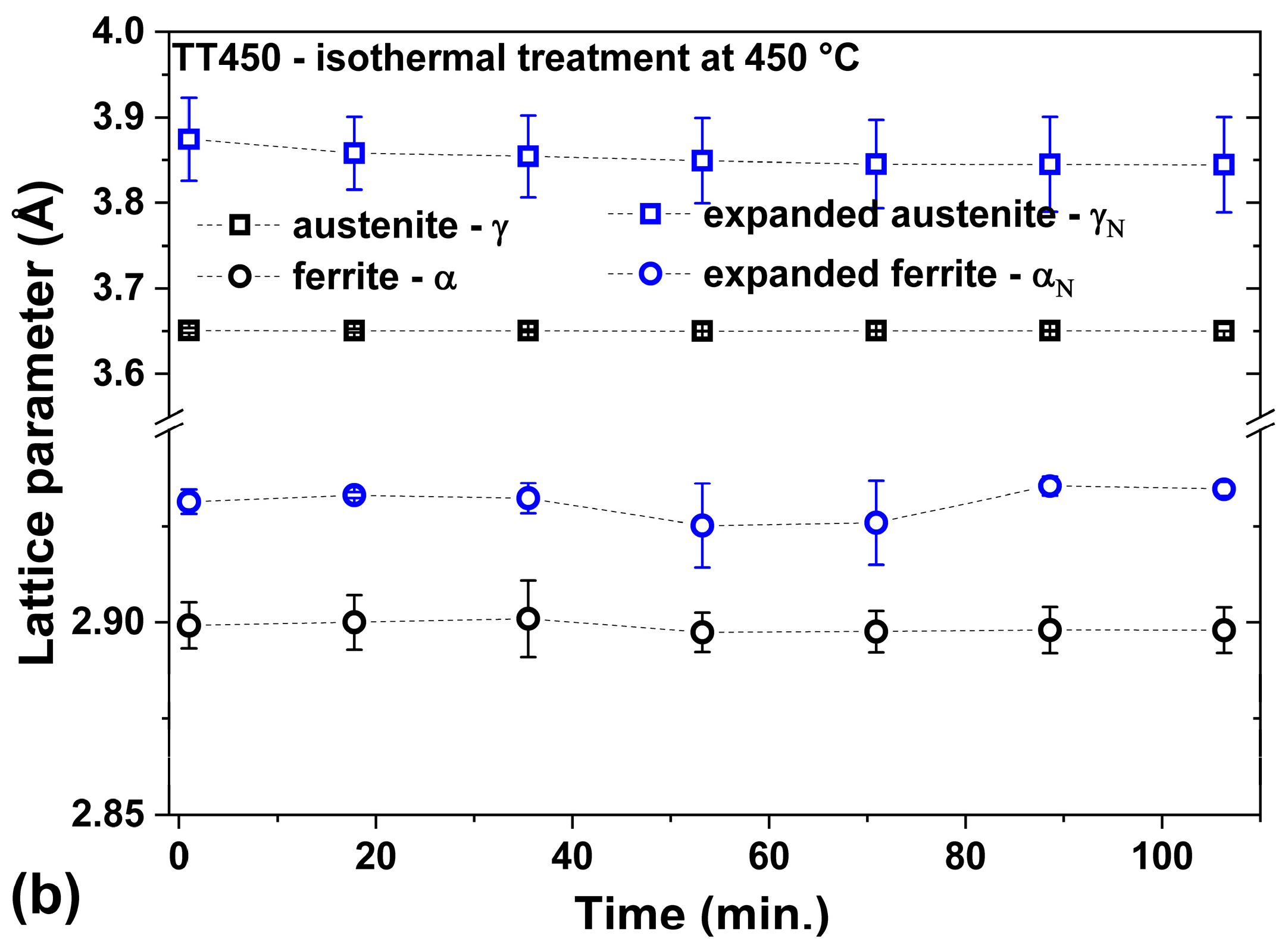
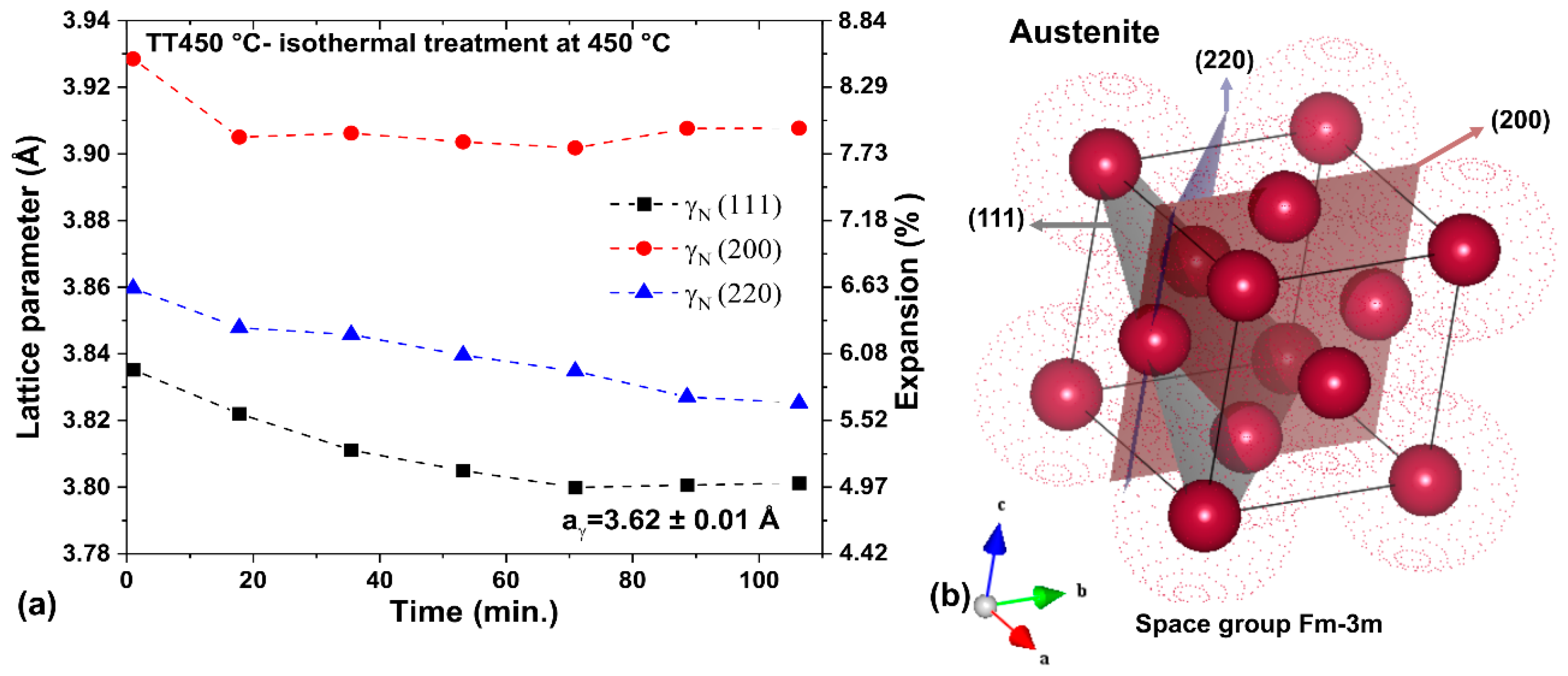
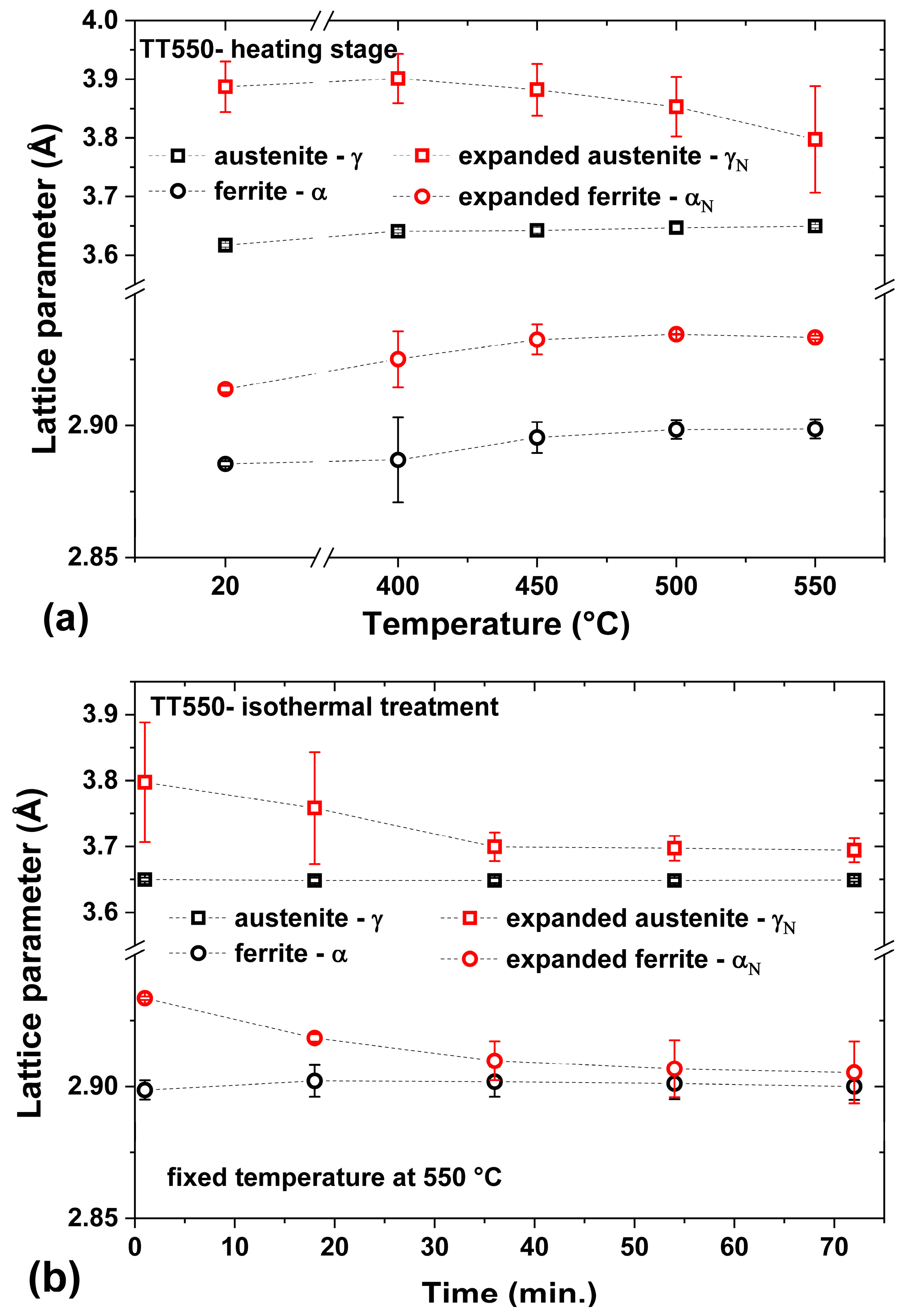
3.3. Heating at 450 °C
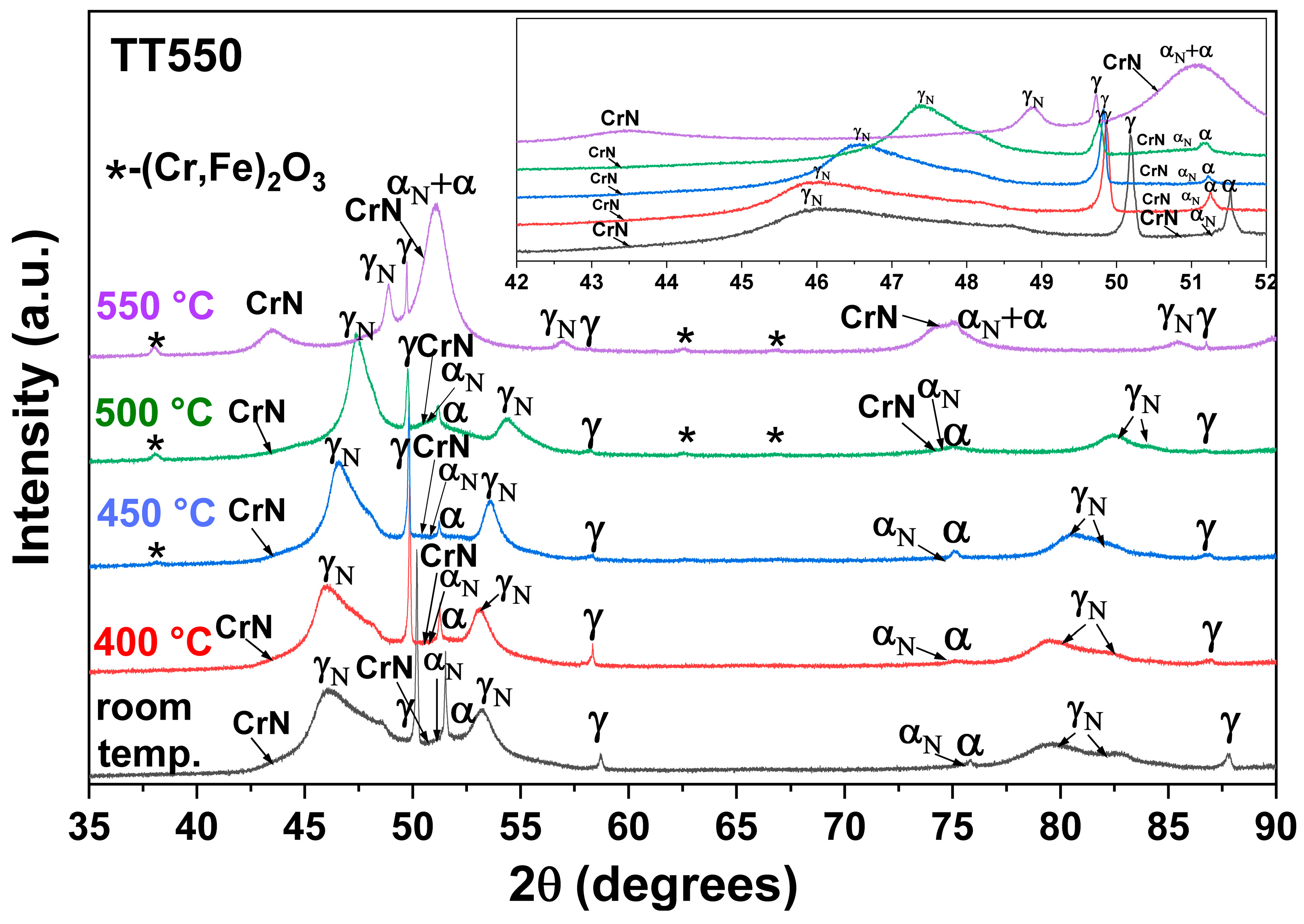
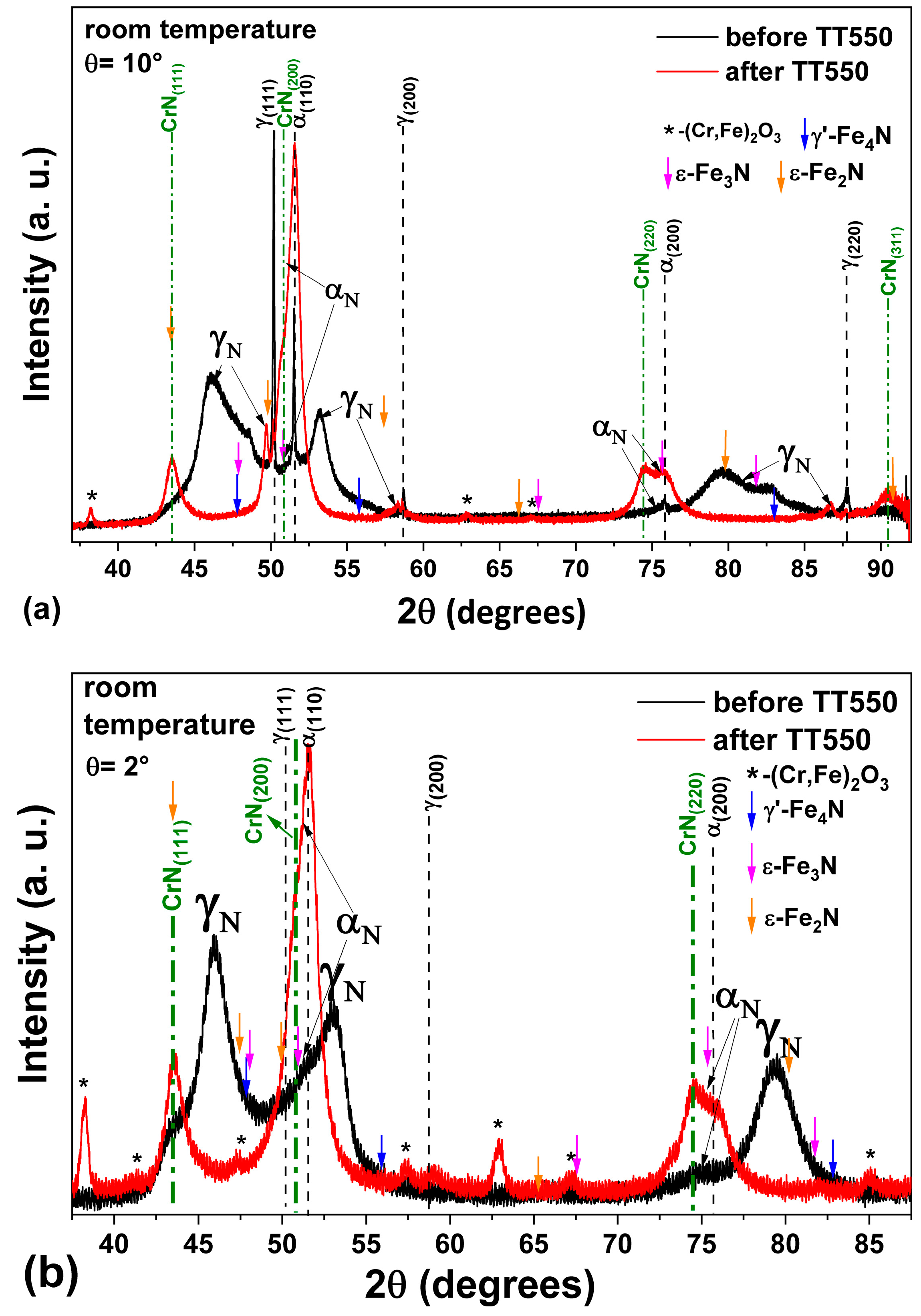
3.4. Heating at 550 °C
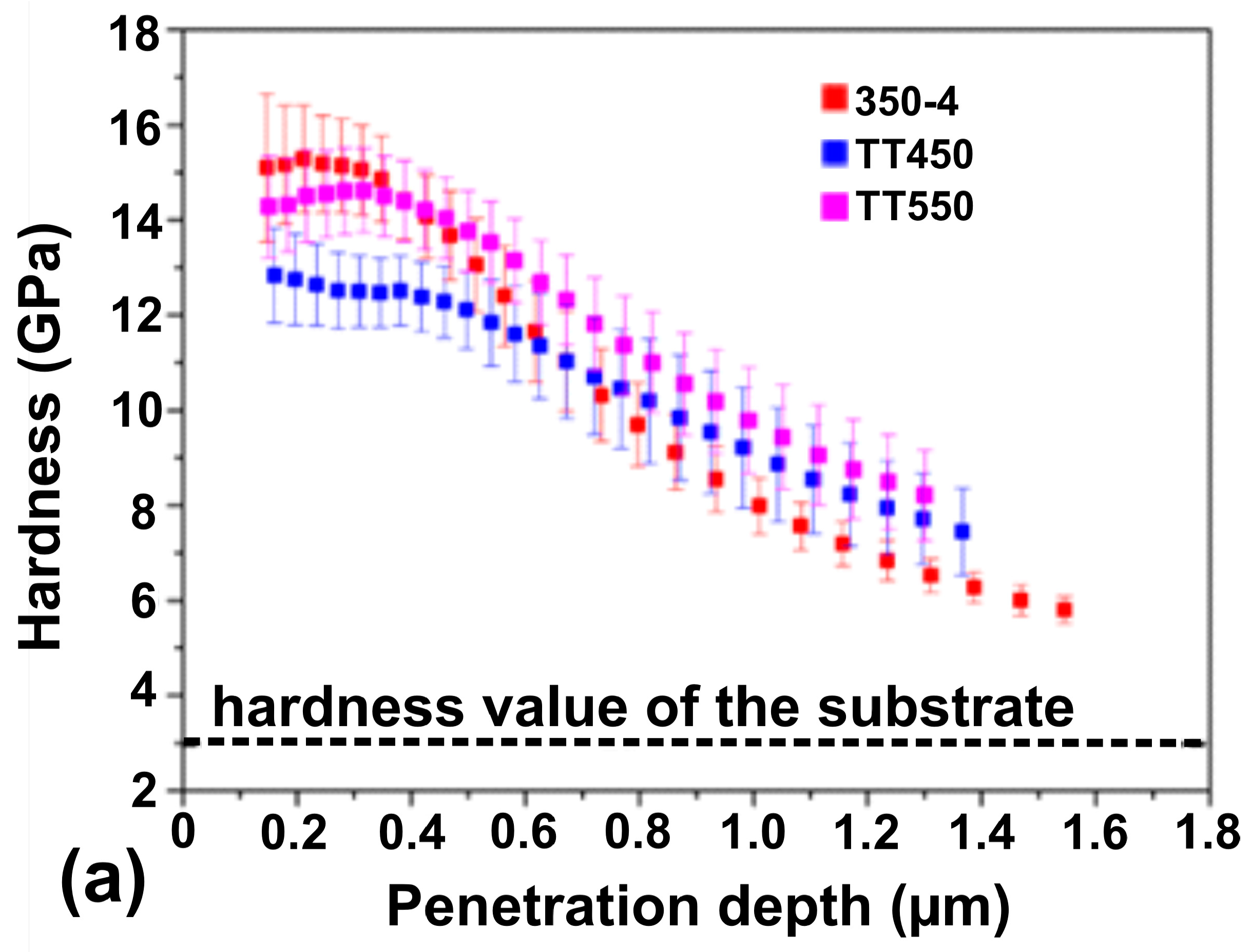
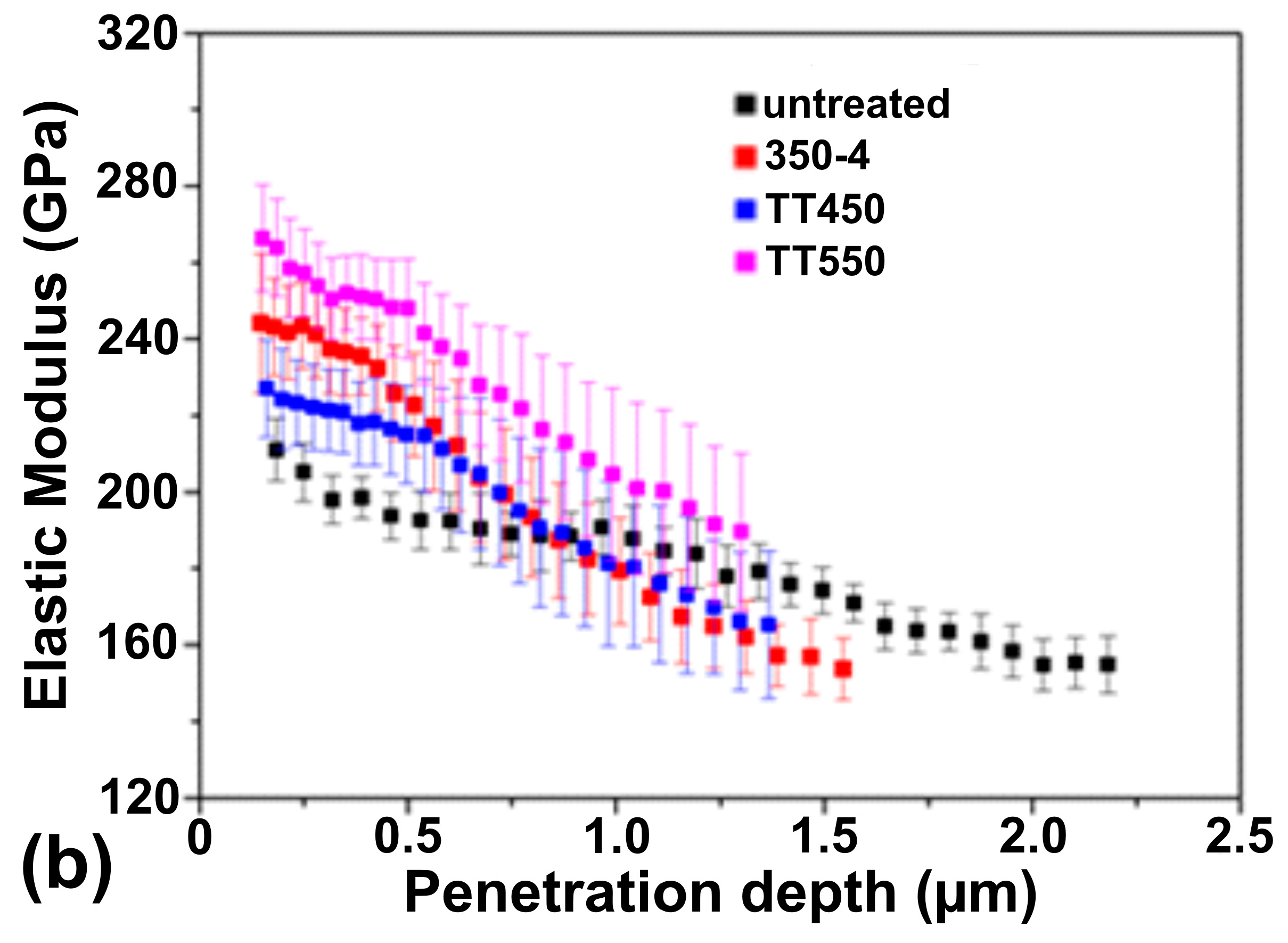
3.5. Mechanical Properties
4. Conclusions
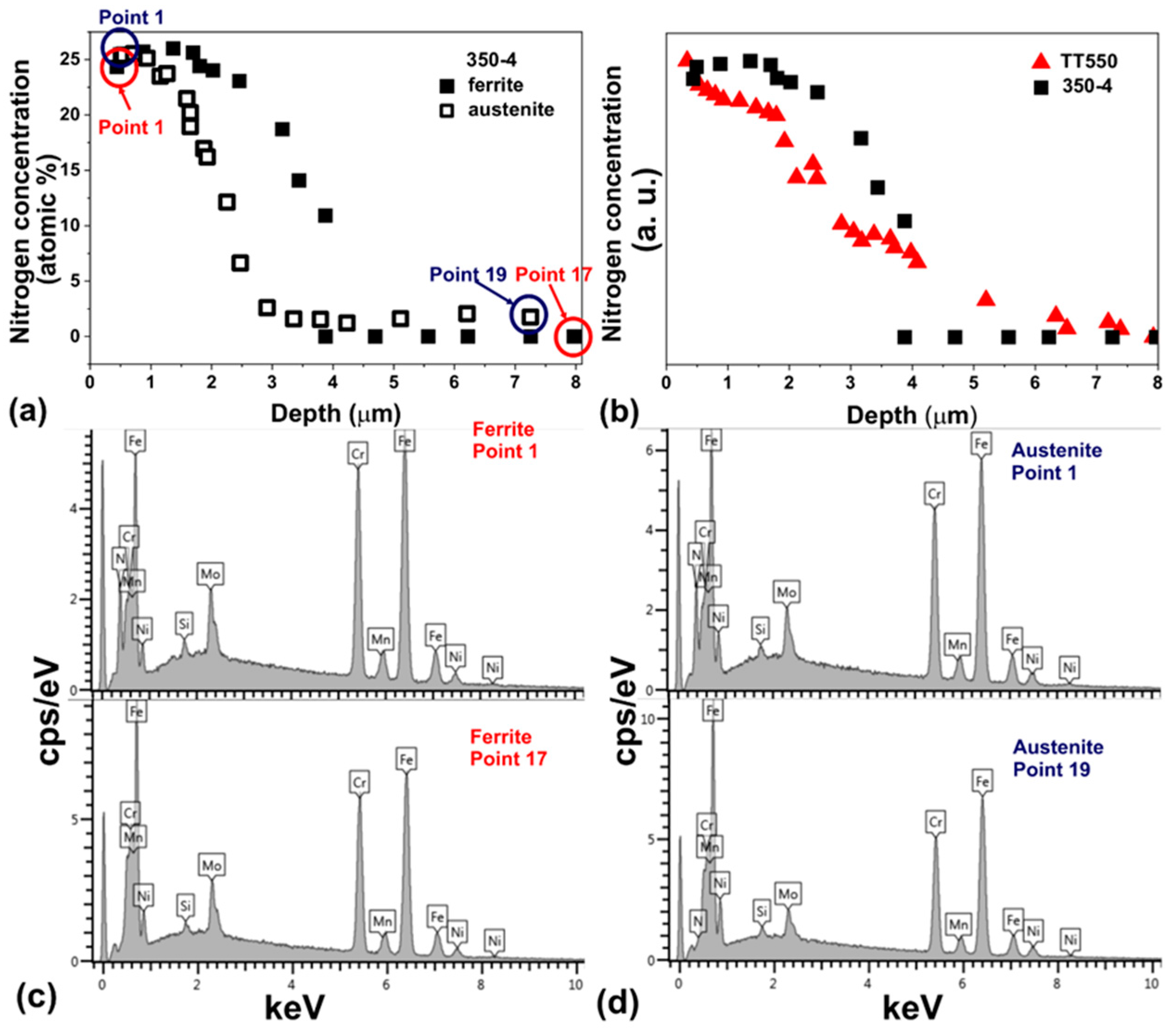
- The nitriding of UNS S32750 steel by PIII at 350 °C for 4 h was sufficient to form a modified layer with varying thicknesses on the ferrite (3.7 ± 0.3) and austenite (2.9 ± 0.4) phases.
- The modified layer was composed of γN and αN with minor contributions of CrN and ε phase observed on the surface of the samples.
- The heat treatment up to 450 °C results an increase in layer thickness, maintaining the microstructure composed predominantly of γN and αN, while the heat treatment at higher temperatures, up to 550 °C, causes the substantive decay/transformation of expanded austenite into CrN, α and αN.
- In both heat treatments, the appearance of chromium nitrides and iron oxides was observed.
- The close monitoring of the phases decay enabled the identification of the reactions involving αN, as well as γN → α + αN + CrN.
- The nitriding process increases the surface hardness of the material by up to four times the value of the untreated sample.
- Heat treatments decrease the hardness near the surface due to nitrogen diffusion while simultaneously increasing it in the deeper regions at a gradual rate with increasing thickness of the modified layer.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manova, D.; Mändl, S.; Neumann, H.; Rauschenbach, B. Formation of Metastable Diffusion Layers in Cr-Containing Iron, Cobalt and Nickel Alloys after Nitrogen Insertion. Surf. Coat. Technol. 2017, 312, 81–90. [Google Scholar] [CrossRef]
- Borgioli, F. The “Expanded” Phases in the Low-Temperature Treated Stainless Steels: A Review. Metals 2022, 12, 331. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T.; Pradelli, G. Influence of the Treatment Atmosphere on the Characteristics of Glow-Discharge Treated Sintered Stainless Steels. Surf. Coat. Technol. 2002, 149, 192–197. [Google Scholar] [CrossRef]
- Dong, H. S-Phase Surface Engineering of Fe-Cr, Co-Cr and Ni-Cr Alloys. Int. Mater. Rev. 2010, 55, 65–98. [Google Scholar] [CrossRef]
- Anders, A. Handbook of Plasma Immersion Ion Implantation and Deposition; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Blawert, C.; Mordike, B.L. Industrial Applications of Plasma Immersion Ion Implantation. Surf. Coat. Technol. 1997, 93, 274–279. [Google Scholar] [CrossRef]
- Schibicheski Kurelo, B.C.E.; de Souza, G.B.; Serbena, F.C.; Lepienski, C.M.; Chuproski, R.F.; Borges, P.C. Improved Saline Corrosion and Hydrogen Embrittlement Resistances of Superaustenitic Stainless Steel by PIII Nitriding. J. Mater. Res. Technol. 2022, 18, 1717–1731. [Google Scholar] [CrossRef]
- Pinedo, C.E.; Varela, L.B.; Tschiptschin, A.P. Low-Temperature Plasma Nitriding of AISI F51 Duplex Stainless Steel. Surf. Coat. Technol. 2013, 232, 839–843. [Google Scholar] [CrossRef]
- Bobadilla, M.; Tschiptschin, A.; Bobadilla, M.; Tschiptschin, A. On the Nitrogen Diffusion in a Duplex Stainless Steel. Mater. Res. 2015, 18, 390–394. [Google Scholar] [CrossRef]
- Tschiptschin, A.P.; Varela, L.B.; Pinedo, C.E.; Li, X.Y.; Dong, H. Development and Microstructure Characterization of Single and Duplex Nitriding of UNS S31803 Duplex Stainless Steel. Surf. Coat. Technol. 2017, 327, 83–92. [Google Scholar] [CrossRef]
- Dib, J.; Gómez, B.; Strubbia, R.; Ares, A.; Méndez, C.; Fuster, V.; Hereñú, S. Characterization of Plasma Nitrided Duplex Stainless Steel: Influence of Prior Shot Peening and Nitriding Atmosphere. J. Mater. Eng. Perform. 2023, 32, 406–414. [Google Scholar] [CrossRef]
- Palma Calabokis, O.; Núñez de la Rosa, Y.; Lepienski, C.M.; Perito Cardoso, R.; Borges, P.C. Crevice and Pitting Corrosion of Low Temperature Plasma Nitrided UNS S32750 Super Duplex Stainless Steel. Surf. Coat. Technol. 2021, 413, 17–20. [Google Scholar] [CrossRef]
- Possoli, F.A.A.; Souza, A.P.N.; Bernardelli, E.A.; Borges, P.C. Tribocorrosion Assessment of Low-Temperature Plasma Nitrided Super Duplex Stainless Steel. Surf. Coat. Technol. 2024, 479, 130572. [Google Scholar] [CrossRef]
- Lima, J.F.V.; Scheuer, C.J.; Brunatto, S.F.; Cardoso, R.P. Kinetics of the UNS S32750 Super Duplex Stainless Steel Low-Temperature Plasma Nitriding. Mater. Res. 2022, 25, 17–28. [Google Scholar] [CrossRef]
- Blawert, C.; Mordike, B.L.; Jirásková, Y.; Schneeweiss, O. Structure and Composition of Expanded Austenite Produced by Nitrogen Plasma Immersion Ion Implantation of Stainless Steels X6CrNiTi1810 and X2CrNiMoN2253. Surf. Coat. Technol. 1999, 116–119, 189–198. [Google Scholar] [CrossRef]
- de Oliveira, W.R.; Kurelo, B.C.E.S.; Ditzel, D.G.; Serbena, F.C.; Foerster, C.E.; de Souza, G.B. On the S-Phase Formation and the Balanced Plasma Nitriding of Austenitic-Ferritic Super Duplex Stainless Steel. Appl. Surf. Sci. 2018, 434, 1161–1174. [Google Scholar] [CrossRef]
- Li, X.; Dou, W.; Tian, L.; Dong, H. Combating the Tribo-Corrosion of LDX2404 Lean Duplex Stainless Steel by Low Temperature Plasma Nitriding. Lubricants 2018, 6, 93. [Google Scholar] [CrossRef]
- Pinheiro, R.S.; Santos, A.R.; Marques, M.; Schnitzler, E.; Signorini, D.; Tomita, R.A. Well Construction Challenges in the Pre-Salt Development Projects. In Proceedings of the Offshore Technology Conference; Offshore Technology Conference, Houston, TX, USA, 4 May 2015; Volume 4–7, pp. 1–13. [Google Scholar]
- Oliveira, J.J.M.; Filho, J.R.B.Z. O Pré-Sal Brasileiro e o Problema Da Corrosão Por CO2. Rev. Pós-Graduação Fac. Cent. Leste 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Maistro, G.; Pérez-García, A.; Norell, M.; Nyborg, L.; Cao, Y. Thermal Decomposition of N-Expanded Austenite in 304L and 904L Steels. Surf. Eng. 2017, 33, 319–326. [Google Scholar] [CrossRef]
- Jirásková, Y.; Blawert, C.; Schneeweiss, O. Thermal Stability of Stainless Steel Surfaces Nitrided by Plasma Immersion Ion Implantation. Phys. Stat. Sol. 1999, 175, 537–548. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. Decomposition Kinetics of Expanded Austenite with High Nitrogen Contents. Int. J. Mater. Res. 2006, 97, 79–88. [Google Scholar] [CrossRef]
- Brink, B.; Ståhl, K.; Christiansen, T.L.; Somers, M.A.J. Thermal Expansion and Phase Transformations of Nitrogen-Expanded Austenite Studied with in Situ Synchrotron X-Ray Diffraction. J. Appl. Crystallogr. 2014, 47, 819–826. [Google Scholar] [CrossRef]
- Tschiptschin, A.P.; Nishikawa, A.S.; Varela, L.B.; Pinedo, C.E. Thermal Stability of Expanded Austenite Formed on a DC Plasma Nitrided 316L Austenitic Stainless Steel. Thin Solid Films 2017, 644, 156–165. [Google Scholar] [CrossRef]
- Schreiber, G.; Rensch, U.; Oettel, H.; Blawert, C.; Mordike, B.L. Thermal Stability of PI 3 Nitrided Surface Layers on Ferritic Steels. Surf. Coat. Technol. 2003, 170, 447–451. [Google Scholar] [CrossRef]
- Schibicheski Kurelo, B.C.E.; Lepienski, C.M.; de Oliveira, W.R.; de Souza, G.B.; Serbena, F.C.; Cardoso, R.P.; Neves, J.C.K.; Borges, P.C. Identification of Expanded Austenite in Nitrogen-Implanted Ferritic Steel through In Situ Synchrotron X-Ray Diffraction Analyses. Metals 2023, 13, 1744. [Google Scholar] [CrossRef]
- Somers, M.A.J.; van der Pers, N.M.; Schalkoord, D.; Mittemeijer, E.J. Dependence of the Lattice Parameter of γ′ Iron Nitride, Fe4N, on Nitrogen Content; Accuracy of the Nitrogen Absorption Data. Metall. Trans. A 1989, 20, 1533–1539. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Tischler, G.; Mitterer, C. Microstructure and Mechanical/Thermal Properties of Cr-N Coatings Deposited by Reactive Unbalanced Magnetron Sputtering. Surf. Coat. Technol. 2001, 142–144, 78–84. [Google Scholar] [CrossRef]
- Chen, H.Y.; Tsai, C.J.; Lu, F.H. The Young’s Modulus of Chromium Nitride Films. Surf. Coat. Technol. 2004, 184, 69–73. [Google Scholar] [CrossRef]
- Bian, L.; Chen, Z.; Wang, L.; Li, F.; Chou, K. Oxidation Resistance, Thermal Expansion and Area Specific Resistance of Fe-Cr Alloy Interconnector for Solid Oxide Fuel Cell. J. Iron Steel Res. Int. 2017, 24, 77–83. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A General-Purpose Peak Fitting Program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- de Oliveira, W.R.; Chuproski, R.F.; Valadão, G.M.; Cintho, O.M.; de Souza, E.C.F.; Serbena, F.C.; de Souza, G.B. Symmetry between the Anisotropic N Behavior in the Lattice under High Pressures and the Formation of Expanded Austenite. J. Alloys Compd. 2021, 871, 159509. [Google Scholar] [CrossRef]
- Manova, D.; Lotnyk, A.; Mändl, S.; Neumann, H.; Rauschenbach, B. CrN Precipitation and Elemental Segregation during the Decay of Expanded Austenite. Mater. Res. Express 2016, 3, 066502. [Google Scholar] [CrossRef]
- Borgioli, F. From Austenitic Stainless Steel to Expanded Austenite-S Phase: Formation, Characteristics and Properties of an Elusive Metastable Phase. Metals 2020, 10, 187. [Google Scholar] [CrossRef]
- Espitia, L.A.; Dong, H.; Li, X.Y.; Pinedo, C.E.; Tschiptschin, A.P. Scratch Test of Active Screen Low Temperature Plasma Nitrided AISI 410 Martensitic Stainless Steel. Wear 2017, 376–377, 30–36. [Google Scholar] [CrossRef]
- Stróz, D.; Psoda, M. TEM Studies of Plasma Nitrided Austenitic Stainless Steel. J. Microsc. 2010, 237, 227–231. [Google Scholar] [CrossRef]
- Che, H.L.; Lei, M.K. Microstructure of Perfect Nitrogen-Expanded Austenite Formed by Unconstrained Nitriding. Scr. Mater. 2021, 194, 113705. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Harcourt College Publishing: San Diego, CA, USA, 1976. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Moskalioviene, T.; Galdikas, A. Kinetic Model of Anisotropic Stress Assisted Diffusion of Nitrogen in Nitrided Austenitic Stainless Steel. Surf. Coat. Technol. 2019, 366, 277–285. [Google Scholar] [CrossRef]
- Martinavičius, A.; Abrasonis, G.; Möller, W. Influence of Crystal Orientation and Ion Bombardment on the Nitrogen Diffusivity in Single-Crystalline Austenitic Stainless Steel. J. Appl. Phys. 2011, 110, 074907. [Google Scholar] [CrossRef]
- Saha, R.; Nix, W.D. Effects of the Substrate on the Determination of Thin Film Mechanical Properties by Nanoindentation. Acta Mater. 2002, 50, 23–38. [Google Scholar] [CrossRef]
| Element | Fe | Cr | Ni | Mo | Mn | Si | Cu | P | C |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 61.84 | 25.60 | 6.45 | 3.88 | 0.76 | 0.28 | 0.16 | 0.03 | 1.00 |
| Sample | Nitriding Temp. (°C) | Nitriding Time (h) | Maximum Temperature During In Situ XRD (°C) | Time at the Maximum Temperature During In Situ XRD (min) | Layer Thickness |
|---|---|---|---|---|---|
| 350-4 | 350 | 4 | - | - | 3.7 ± 0.3 (α) 2.9 ± 0.4 (γ) |
| TT450 | 350 | 4 | 450 | 124 | 5.1 ± 0.2 |
| TT550 | 350 | 4 | 550 | 94 | 5.9 ± 0.7 |
| Amostra | Average Expansion (%) | |
|---|---|---|
| γN in Relation to γ | αN in Relation to α | |
| 350-4 | 7.35 | 0.55 |
| TT450 | 5.37 | 0.84 |
| TT550 | 1.40 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schibicheski Kurelo, B.C.E.; Monteiro, J.F.H.L.; de Souza, G.B.; Serbena, F.C.; Lepienski, C.M.; Cardoso, R.P.; Brunatto, S.F. Thermal Evolution of Expanded Phases Formed by PIII Nitriding in Super Duplex Steel Investigated by In Situ Synchrotron Radiation. Metals 2024, 14, 1396. https://doi.org/10.3390/met14121396
Schibicheski Kurelo BCE, Monteiro JFHL, de Souza GB, Serbena FC, Lepienski CM, Cardoso RP, Brunatto SF. Thermal Evolution of Expanded Phases Formed by PIII Nitriding in Super Duplex Steel Investigated by In Situ Synchrotron Radiation. Metals. 2024; 14(12):1396. https://doi.org/10.3390/met14121396
Chicago/Turabian StyleSchibicheski Kurelo, Bruna Corina Emanuely, João Frederico Haas Leandro Monteiro, Gelson Biscaia de Souza, Francisco Carlos Serbena, Carlos Maurício Lepienski, Rodrigo Perito Cardoso, and Silvio Francisco Brunatto. 2024. "Thermal Evolution of Expanded Phases Formed by PIII Nitriding in Super Duplex Steel Investigated by In Situ Synchrotron Radiation" Metals 14, no. 12: 1396. https://doi.org/10.3390/met14121396
APA StyleSchibicheski Kurelo, B. C. E., Monteiro, J. F. H. L., de Souza, G. B., Serbena, F. C., Lepienski, C. M., Cardoso, R. P., & Brunatto, S. F. (2024). Thermal Evolution of Expanded Phases Formed by PIII Nitriding in Super Duplex Steel Investigated by In Situ Synchrotron Radiation. Metals, 14(12), 1396. https://doi.org/10.3390/met14121396










