Revealing the Surface and In-Depth Operational Performances of Oxygen-Evolving Anode Coatings: A Guideline for the Synthesis of Inert Durable Anodes in Metal Electrowinning from Acid Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
2.2. Electrochemical Measurement
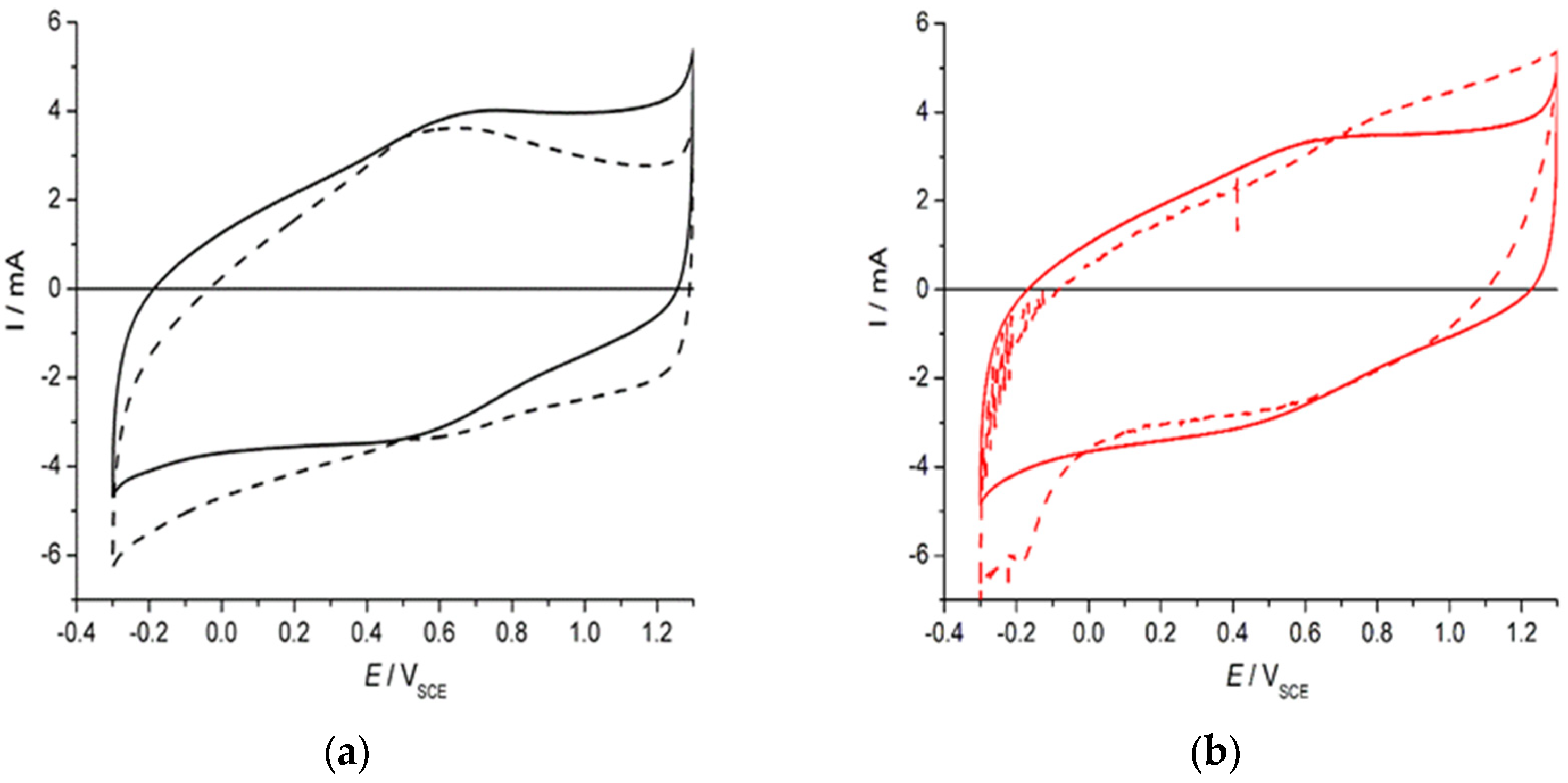
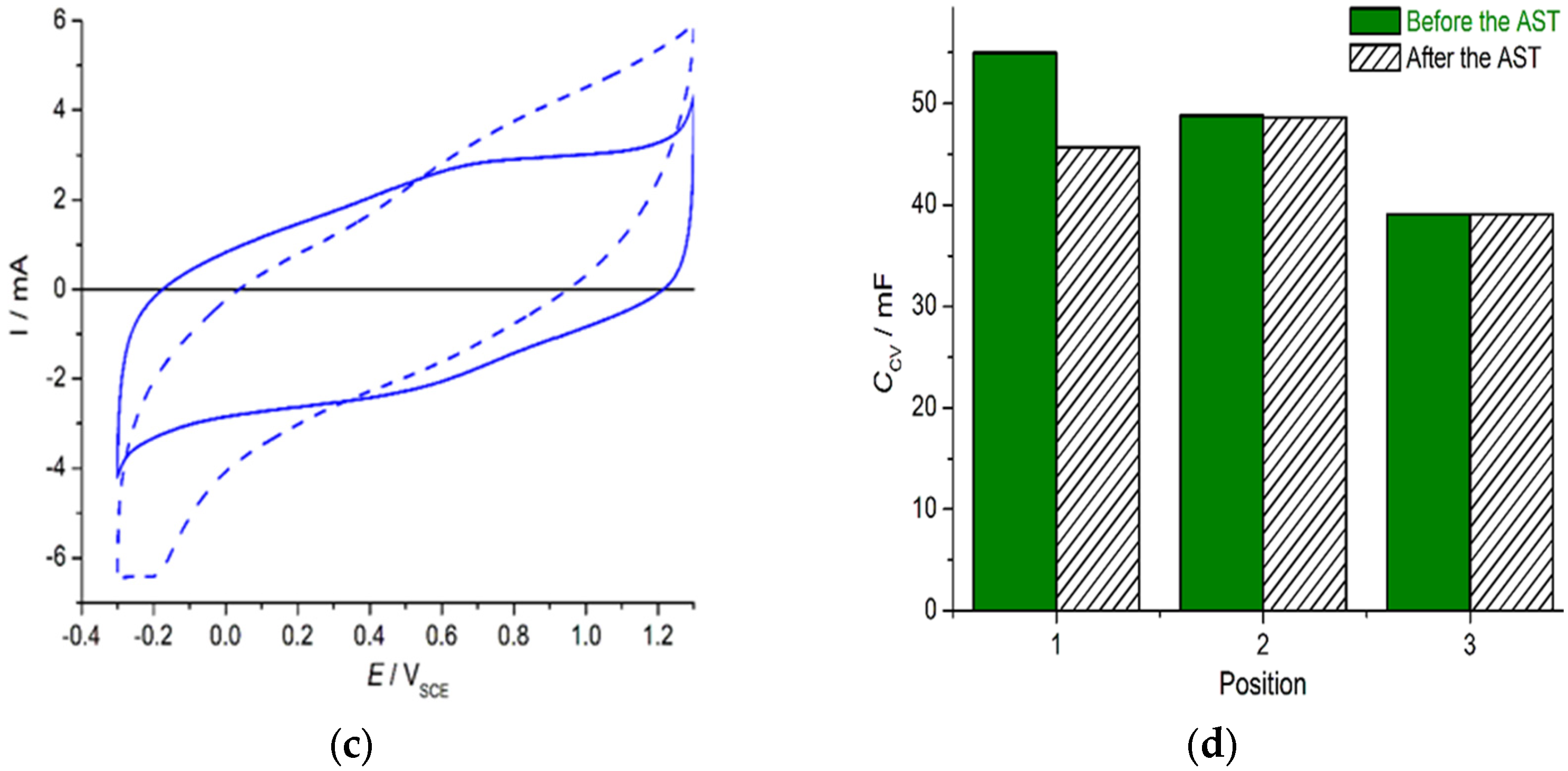
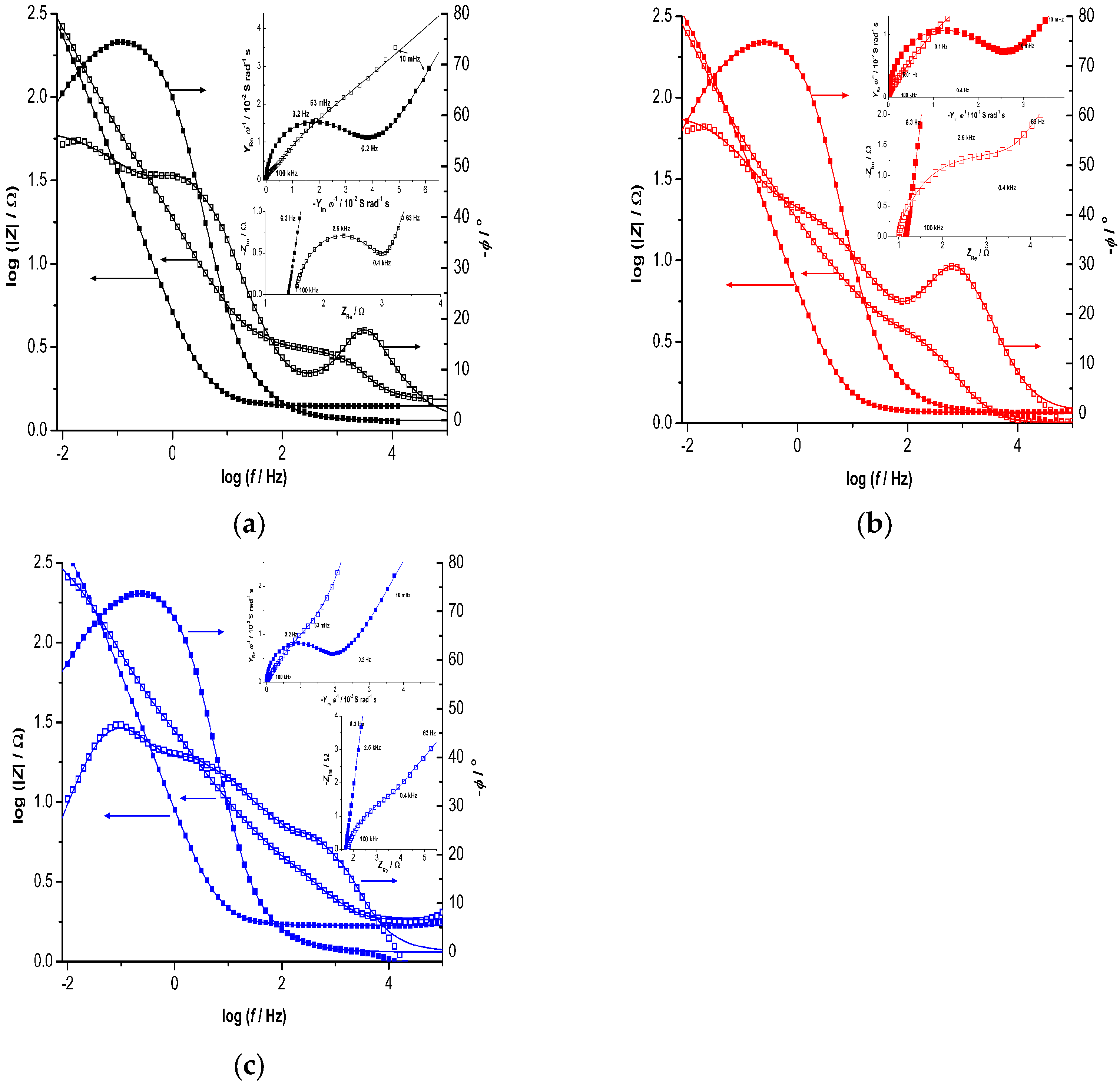
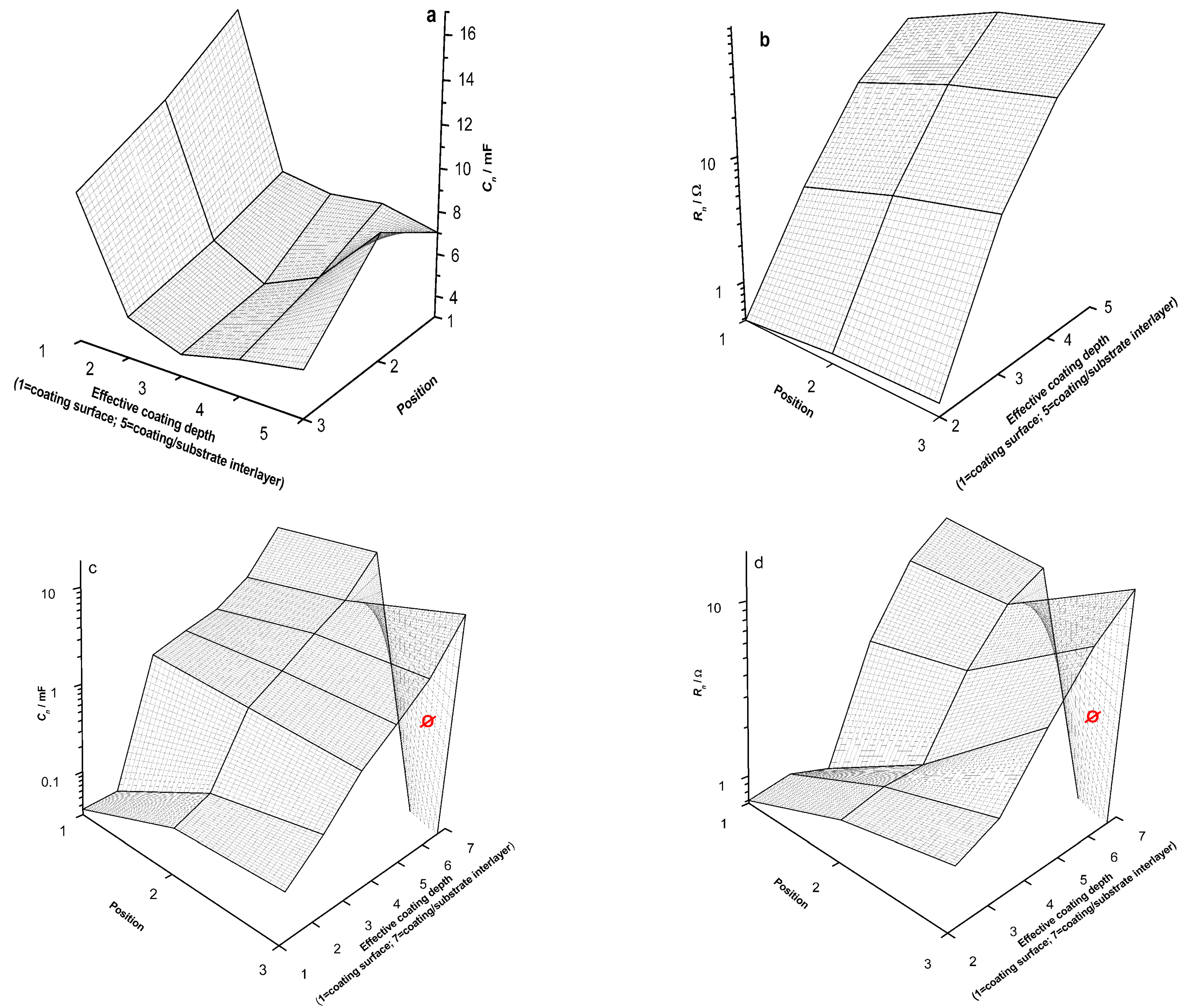
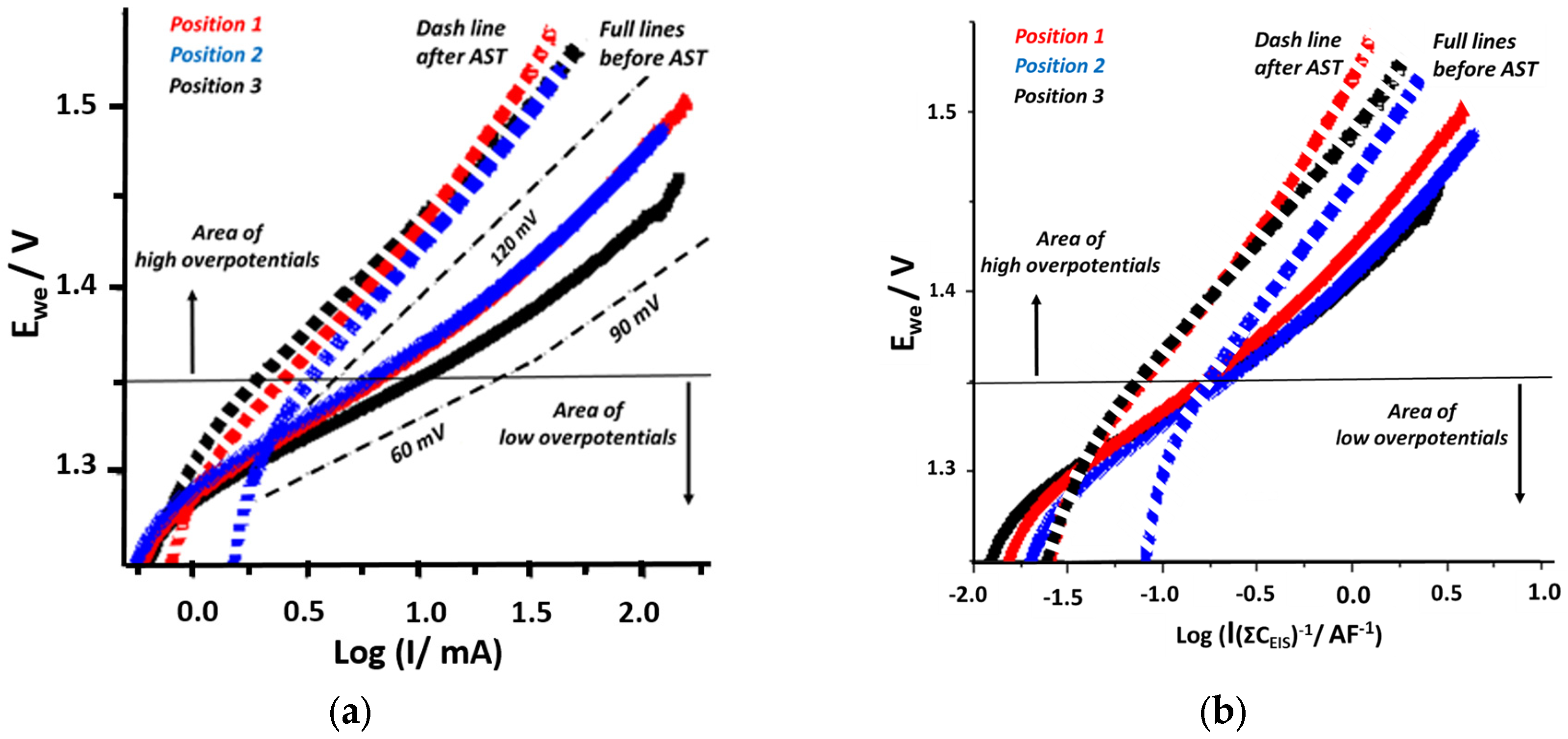
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Belal, R.M.; Zayed, M.A.; El-Sherif, R.M.; Abdel Ghany, N.A. Advanced electrochemical degradation of basic yellow 28 textile dye using IrO2/Ti meshed electrode in different supporting electrolytes. J. Electrochem. Soc. 2021, 882, 114979. [Google Scholar] [CrossRef]
- Ma, R.; Cheng, S.; Zhang, X.; Li, S.; Liu, Z.; Li, X. Oxygen evolution and corrosion behavior of low-MnO2-content Pb-MnO2 composite anodes for metal electrowinning. Hydrometallurgy 2016, 159, 6–11. [Google Scholar] [CrossRef]
- Bebelis, S.; Bouzek, K.; Cornell, A.; Ferreira, M.G.S.; Kelsall, G.H.; Lapicque, F.; de León, C.P.; Rodrigo, M.A.; Walsh, F.C. Highlights during the development of electrochemical engineering. Chem. Eng. Res. Des. 2013, 91, 1998–2020. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, P.; Xu, L.; Kannan, C.S.; Guo, S.; Liu, J.; Koppala, S.; Ju, S. Electrochemical properties of the IrO2-Ta2O5 coated anodes with Al/Ti and Cu/Ti layered composites substrates. J. Alloys Compd. 2018, 769, 210–217. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, L.; Chen, M. Characteristics of anodic TiO2 nanotube arrays mediated IrO2 Active Anode in the Oxygen Evolution Reaction. Int. J. Electrochem. Sci. 2022, 17, 220461. [Google Scholar] [CrossRef]
- Dondapati, J.S.; Thiruppathi, A.R.; Salverda, A.; Chen, A. Comparison of Pt and IrO2-Ta2O5/Ti as a counter electrode in acidic media. Electrochem. commun. 2021, 124, 106946. [Google Scholar] [CrossRef]
- Trasatti, S. Electrodes of Conductive Metallic Oxides, 1st ed.; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1980; p. 702. [Google Scholar]
- Kawaguchi, K.; Morimitsu, M. Reaction Selectivity of IrO2-Based Nano/Amorphous Hybrid Oxide-Coated Titanium Anodes in Acidic Aqueous Solutions: Oxygen Evolution and Lead Oxide Deposition. J. Electrochem. Soc. 2020, 167, 133503. [Google Scholar] [CrossRef]
- Kasian, O.; Li, T.; Mingers, A.M.; Schweinar, K.; Savan, A.; Ludwig, A.; Mayrhofer, K. Stabilization of an iridium oxygen evolution catalyst by titanium oxides. J. Phys. Energy 2021, 3, 034006. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA®. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Kotz, R.; Stucki, S. Stabilization of RuO2, by IrO2, for anodic oxygen evolution in acid media. Electrochim. Acta 1986, 31, 1311–1316. [Google Scholar] [CrossRef]
- Kamegaya, Y.; Sasaki, K.; Oguri, M.; Asaki, T.; Kobayashi, H.; Mitamura, T. Improved durability of iridium oxide coated titanium anode with interlayers for oxygen evolution at high current densities. Electrochim. Acta 1995, 40, 889–895. [Google Scholar] [CrossRef]
- Tang, D.; Wen, S.; Chen, S. Preparation and characterization for a RuO2 60%-TiO2 40% nano-material. Transc. Met. Heat Treat. 2000, 21, 12–16. [Google Scholar]
- Kim, M.; Choi, J.; Lee, W.; Ahn, Y.; Lee, H.; Cho, K.; Lee, J. Performance of Magnéli phase Ti4O7 and Ti3+ self-doped TiO2 as oxygen vacancy-rich titanium oxide anodes: Comparison in terms of treatment efficiency, anodic degradative pathways, and long-term stability. Appl. Catal. B Environ. 2023, 337, 122993. [Google Scholar] [CrossRef]
- Mirseyed, S.F.; Jafarzadeh, K.; Rostamian, A.; Abbasi, H.M.; Ostadhassan, M. A new insight on the mechanisms of corrosion deactivation of a typical Ti/IrO2 + RuO2+TiO2 coating in the presence of Ta2O5 in chlor-alkali medium. Corros. Sci. 2023, 214, 111005. [Google Scholar] [CrossRef]
- Rosestolato, D.; Neodo, S.; Ferro, S.; Battaglin, G.; Rigato, V.; De Battisti, A. A comparison between structural and electrochemical properties of iridium oxide-based electrocatalysts prepared by sol-gel and reactive sputtering deposition. J. Electrochem. Soc. 2014, 161, 151–158. [Google Scholar] [CrossRef]
- Herrada, R.A.; Acosta-Santoyo, G.; Sepúlveda-Guzmán, S.; Brillas, E.; Sirés, I.; Bustos, E. IrO2-Ta2O5| Ti electrodes prepared by electrodeposition from different Ir:Ta ratios for the degradation of polycyclic aromatic hydrocarbons. Electrochim. Acta 2018, 263, 353–361. [Google Scholar] [CrossRef]
- Yang, C.; Shang, S.; Li, X. Oxygen-vacancy-enriched substrate-less SnOx/La-Sb anode for high-performance electrocatalytic oxidation of antibiotics in wastewater. J. Hazard. Mater. 2022, 436, 129212. [Google Scholar] [CrossRef]
- Comninellis, C.; Vercesi, G.P. Characterization of DSA-type oxygen evolving electrodes: Choice of a coating. J. Appl. Electrochem. 1991, 21, 335–345. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, D.K.; Lee, K.; Chang, D. An investigation on the electrochemical characteristics of Ta2O5-IrO2 anodes for the application of electrolysis process. Mater. Sci. Appl. 2011, 2, 237–243. [Google Scholar]
- Xu, W.; Haarberg, G.M.; Seland, F.; Sunde, S.; Ratvik, A.P.; Holmin, S.; Gustavsson, J.; Afvander, Å.; Zimmerman, E.; Åkre, T. The durability of the thermally decomposed IrO2-Ta2O5 coated titanium anode in a sulfate solution. Corros. Sci. 2019, 150, 76–90. [Google Scholar] [CrossRef]
- Herradaa, R.A.; Rodilb, S.E.; Sepúlveda-Guzmánc, S.; Manríqueza, J.; Exnerd, K.S.; Bustosa, E. Characterization of Ti electrodes electrophoretically coated with IrO2-Ta2O5 films with different Ir:Ta molar ratios. J. Alloys Compd. 2021, 862, 158015. [Google Scholar] [CrossRef]
- Xu, L.; Xin, Y.; Wang, J. A comparative study on IrO2-Ta2O5 coated titanium electrodes prepared with different methods. Electrochim. Acta 2009, 54, 1820–1825. [Google Scholar] [CrossRef]
- Herrada, R.A.; Medel, A.; Manríquez, F.; Sirés, I.; Bustos, E. Preparation of IrO2-Ta2O5|Ti electrodes by immersion, painting and electrophoretic deposition for the electrochemical removal of hydrocarbons from water. J. Hazard. Mater. 2016, 319, 102–110. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Morimitsu, M. Effects of oxide composition on structure, surface morphology, and oxygen evolution behaviors of IrO2-Ta2O5/Ti anodes prepared at a high temperature. Electrochemistry 2015, 83, 256–261. [Google Scholar] [CrossRef]
- Xu, W.; Haarberg, G.M.; Sunde, S.; Seland, F.; Ratvik, A.P.; Holmin, S.; Gustavsson, J.; Afvander, Å.; Zimmerman, E.; Åkre, T. Sandblasting effect on performance and durability of Ti based IrO2-Ta2O5 anode in acidic solutions. Electrochim. Acta 2019, 295, 204–214. [Google Scholar] [CrossRef]
- Guzmána, D.; Dubraya, G.; Aguilarb, C.; Rojasc, P.; Guzmána, A.; Soliz, Á.; Sepúlvedaa, R.; Espinozad, R. Mechanochemical processing of IrO2–Ta2O5: An alternative route for synthesizing Ir and Ir(Ta)O2 solid solution. Boletín Soc. Española Cerámica Vidr. 2021, 60, 109–118. [Google Scholar] [CrossRef]
- Martelli, G.; Ornelas, R.; Faita, G. Deactivation mechanisms of oxygen evolving anodes at high current densities. Electrochim. Acta 1994, 39, 1551–1558. [Google Scholar]
- Jansen, H.J.; Mackor, A. Anodes with Extended Service Life and Methods for Their Manufacturing. EPO Patent No. 0538955B1, 1998. [Google Scholar]
- Yan, Z.; Zhang, H.; Feng, Z.; Tang, M.; Yuan, X.; Tan, Z. Promotion of in situ TiNx interlayer on morphology and electrochemical properties of titanium based IrO2-Ta2O5 coated anode. J. Alloys Compd. 2017, 708, 1081–1088. [Google Scholar] [CrossRef]
- Liu, B.; Ma, B.; Chen, Y.; Wang, C. Corrosion mechanism of Ti/IrO2-RuO2-SiO2 anode for oxygen evolution in sulfuric acid solution. Corros. Sci. 2020, 170, 108662. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Xuan, J.; Xin, Y.; Li, Y.; Duan, T.; Liu, F. A comparative study on Ti/IrO2–Ta2O5 anodes prepared by microwave plasma-assisted sintering and conventional thermal decomposition methods. J. Mater. Res. Technol. 2023, 23, 1447–1457. [Google Scholar] [CrossRef]
- Mehdipour, M.; Tabaian, S.H.; Firoozi, S. Effect of IrO2 crystallinity on electrocatalytic behavior of IrO2–Ta2O5/MWCNT composite as anodes in chlor-alkali membrane cell. Ceram. Int. 2019, 45, 19971–19980. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, H.; Xu, W. Electrocatalytic activity of Ti/TiO2 electrodes in H2SO4 solution. Chin. J. Process. Eng. 2003, 3, 356–360. [Google Scholar]
- Ren, Z.; Quan, S.; Gao, J.; Li, W.; Zhu, Y.; Liu, Y.; Chai, B.; Wang, Y. The electrocatalytic activity of IrO2–Ta2O5 anode materials and electrolyzed oxidizing water preparation and sterilization effect. RSC Adv. 2015, 5, 8778–8786. [Google Scholar] [CrossRef]
- Panić, V.; Dekanski, A.; Mišković-Stanković, V.B.; Milonjić, S.; Nikolić, B. On the deactivation mechanism of RuO2–TiO2/Ti anodes prepared by the sol–gel procedure. J. Electroanal. Chem. 2005, 579, 67–76. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science + Business Media, LLC.: New York, NY, USA, 1999; pp. 221–257. [Google Scholar]
- Gileadi, E. Simultaneous two-electron transfer in electrode kinetics. J. Electroanal. Chem. 2002, 532, 181–189. [Google Scholar] [CrossRef]
- Chizmadzhevand, Y.A.; Chirkov, Y.G. Porous Electrodes. In Comprehensive Treatise of Electrchemistry, 1st ed.; Yeager, E., Bockris, J.O.M., Conway, B.E., Sarangapani, S., Eds.; Springer Science + Business Media: New York, NY, USA, 1983; Volume 6, pp. 317–385. [Google Scholar]

| Parameter | Position 1 | Position 2 | Position 3 |
|---|---|---|---|
| Before AST | |||
| ΣCEIS,ap/mF | 48.4 | 38.8 | 27.6 |
| ΣCEIS,ap,S/mF (n ≤ 3(2), Rn < 10(1) Ω) | 33.5 (25.5) | 24.4 (19.3) | 18.2 (14.3) |
| After AST | |||
| ηSurface, % | 69 (53) | 63 (50) | 66 (52) |
| δpc/% | 14 | 26 | 42 |
| ΣCEIS,ex/mF | 25.9 | 31.1 | 18.4 |
| ΣδCEIS,ap-ex/% | 47 | 20 | 33 |
| δpc/% | 76 | 57 | 112 |
| Position | Tafel Slope Before AST (mV dec−1) (TS ± SD) | Tafel Slope After AST (mV dec−1) (TS ± SD) |
|---|---|---|
| 1 | 64 ± 0.1 Low overpotentials * 99 ± 0.5 High overpotentials ** | 123 ± 1 |
| 2 | 78 ± 0.4 Low overpotentials 131 ± 0.7 High overpotentials | 143 ± 1 |
| 3 | 73 ± 0.1 Low overpotentials 126 ± 0.6 High overpotentials | 143 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bošnjaković, J.; Panić, V.; Stevanović, M.; Stopic, S.; Stevanović, J.; Grgur, B.; Šekularac, G. Revealing the Surface and In-Depth Operational Performances of Oxygen-Evolving Anode Coatings: A Guideline for the Synthesis of Inert Durable Anodes in Metal Electrowinning from Acid Solutions. Metals 2024, 14, 1339. https://doi.org/10.3390/met14121339
Bošnjaković J, Panić V, Stevanović M, Stopic S, Stevanović J, Grgur B, Šekularac G. Revealing the Surface and In-Depth Operational Performances of Oxygen-Evolving Anode Coatings: A Guideline for the Synthesis of Inert Durable Anodes in Metal Electrowinning from Acid Solutions. Metals. 2024; 14(12):1339. https://doi.org/10.3390/met14121339
Chicago/Turabian StyleBošnjaković, Jovana, Vladimir Panić, Maja Stevanović, Srecko Stopic, Jasmina Stevanović, Branimir Grgur, and Gavrilo Šekularac. 2024. "Revealing the Surface and In-Depth Operational Performances of Oxygen-Evolving Anode Coatings: A Guideline for the Synthesis of Inert Durable Anodes in Metal Electrowinning from Acid Solutions" Metals 14, no. 12: 1339. https://doi.org/10.3390/met14121339
APA StyleBošnjaković, J., Panić, V., Stevanović, M., Stopic, S., Stevanović, J., Grgur, B., & Šekularac, G. (2024). Revealing the Surface and In-Depth Operational Performances of Oxygen-Evolving Anode Coatings: A Guideline for the Synthesis of Inert Durable Anodes in Metal Electrowinning from Acid Solutions. Metals, 14(12), 1339. https://doi.org/10.3390/met14121339








