Structure and Properties of Ti-Al Intermetallic Coatings Reinforced with an Aluminum Oxide Filler
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
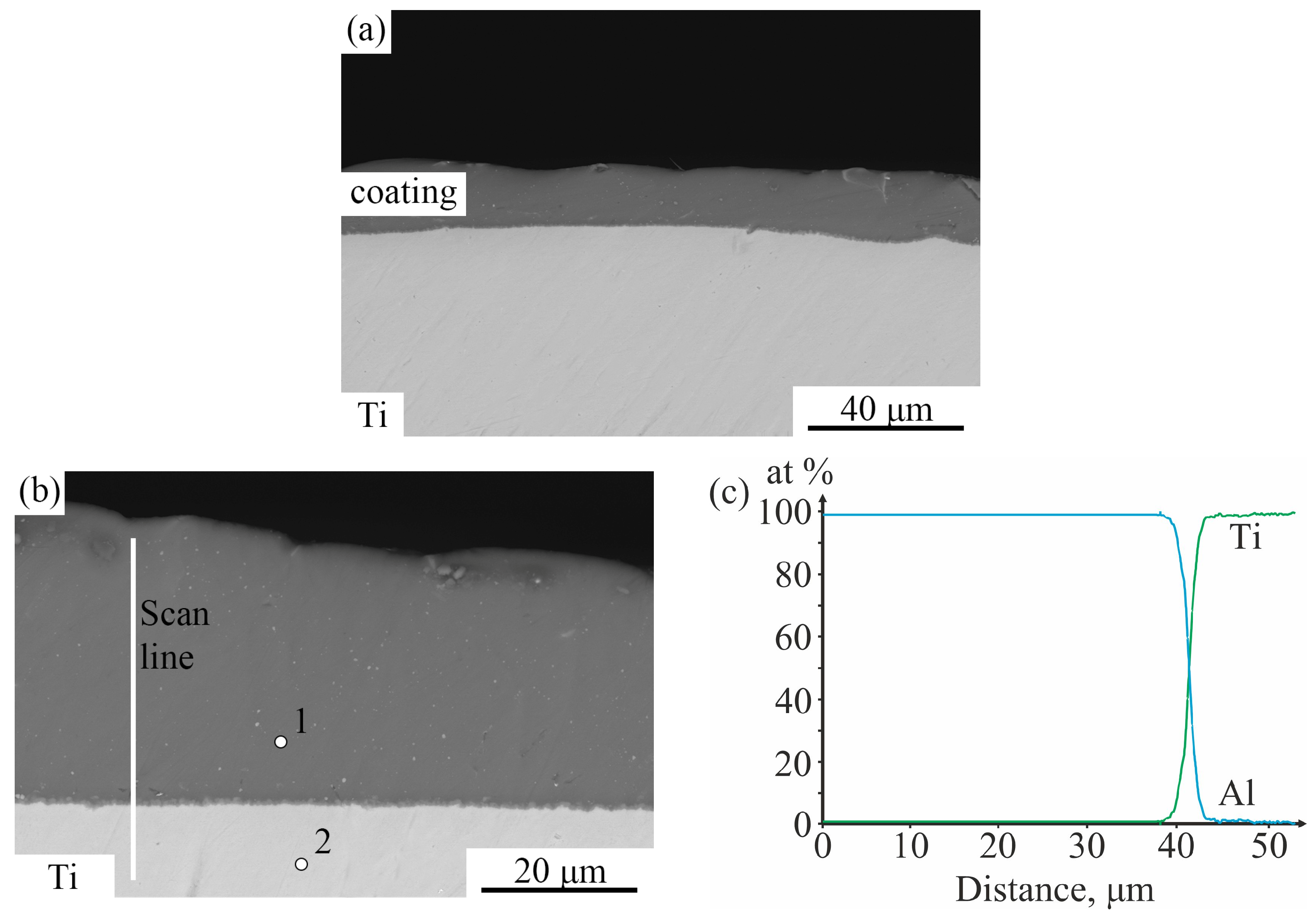
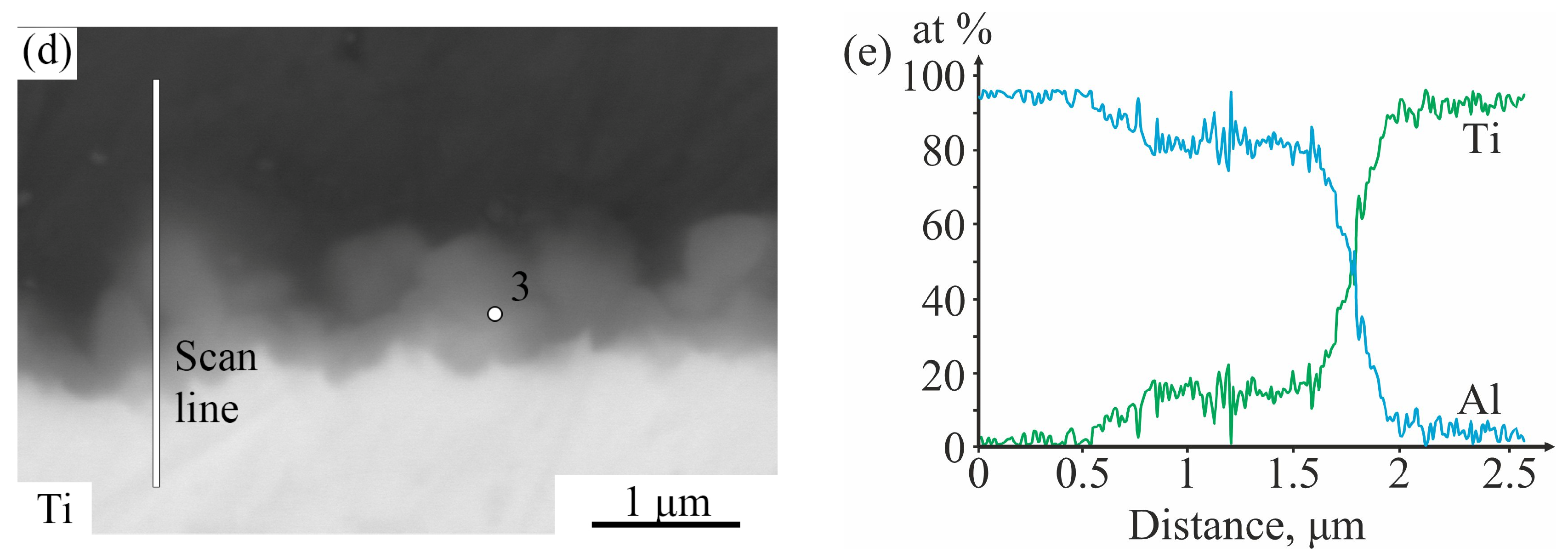
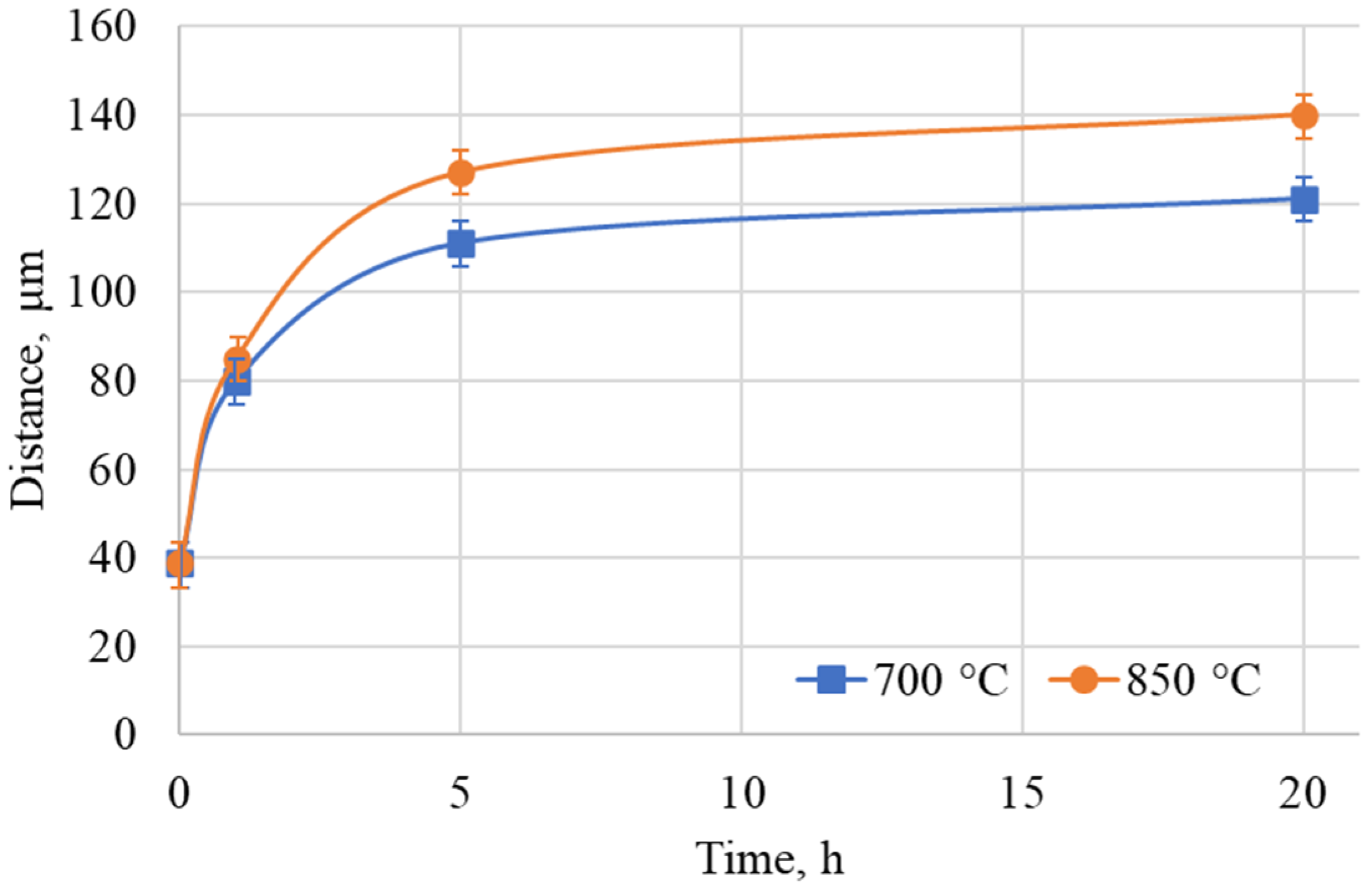
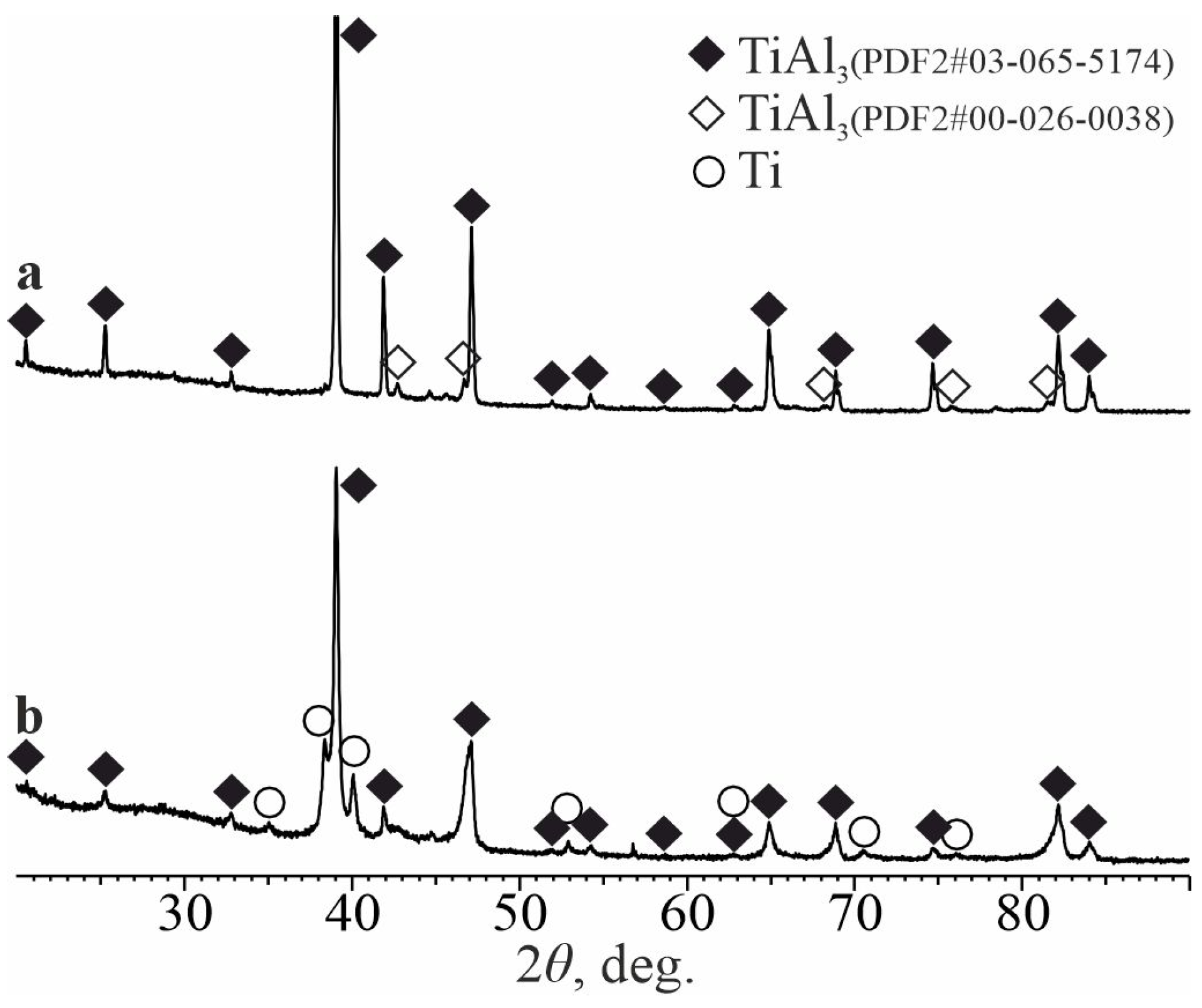
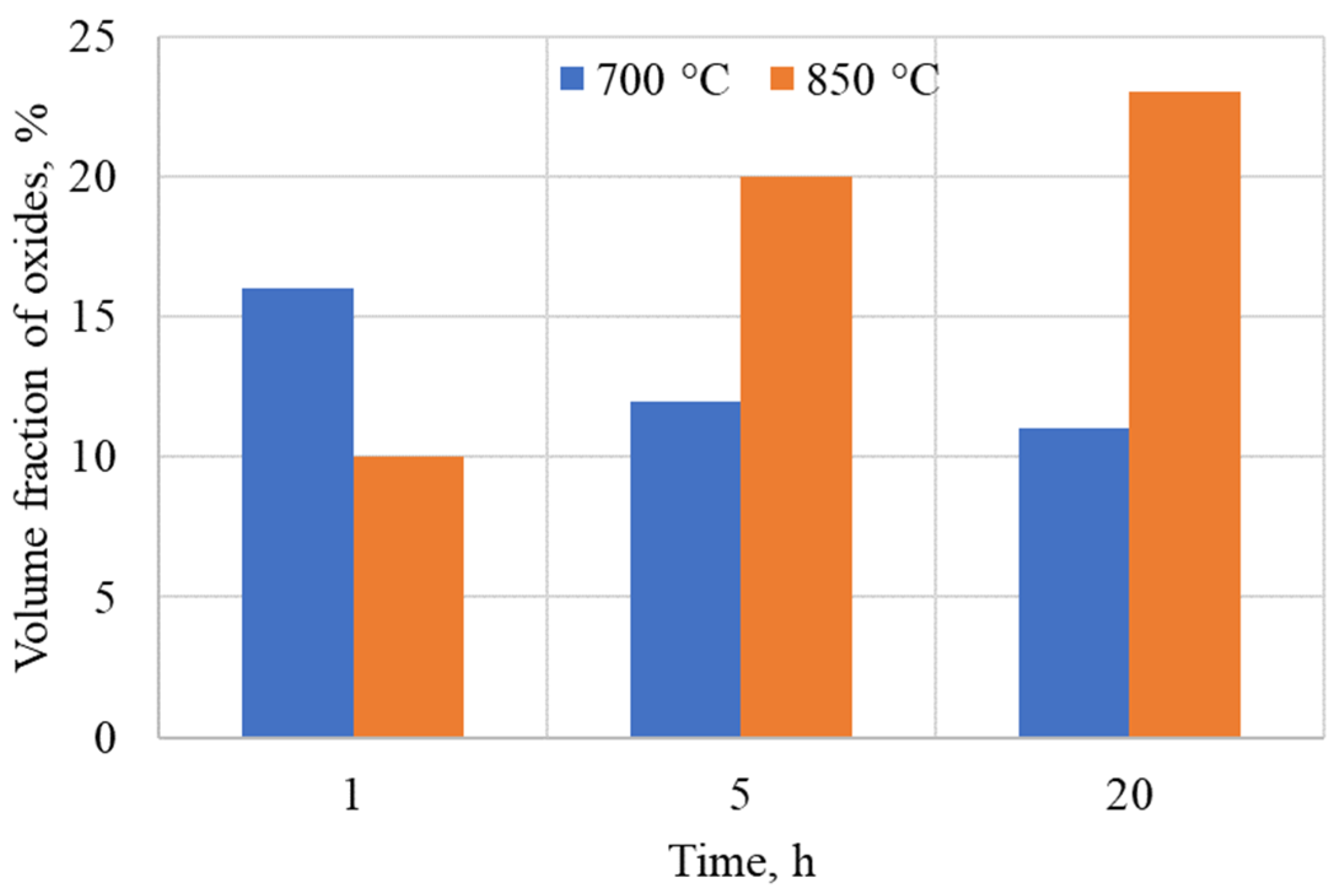
3.1. As-Aluminized State
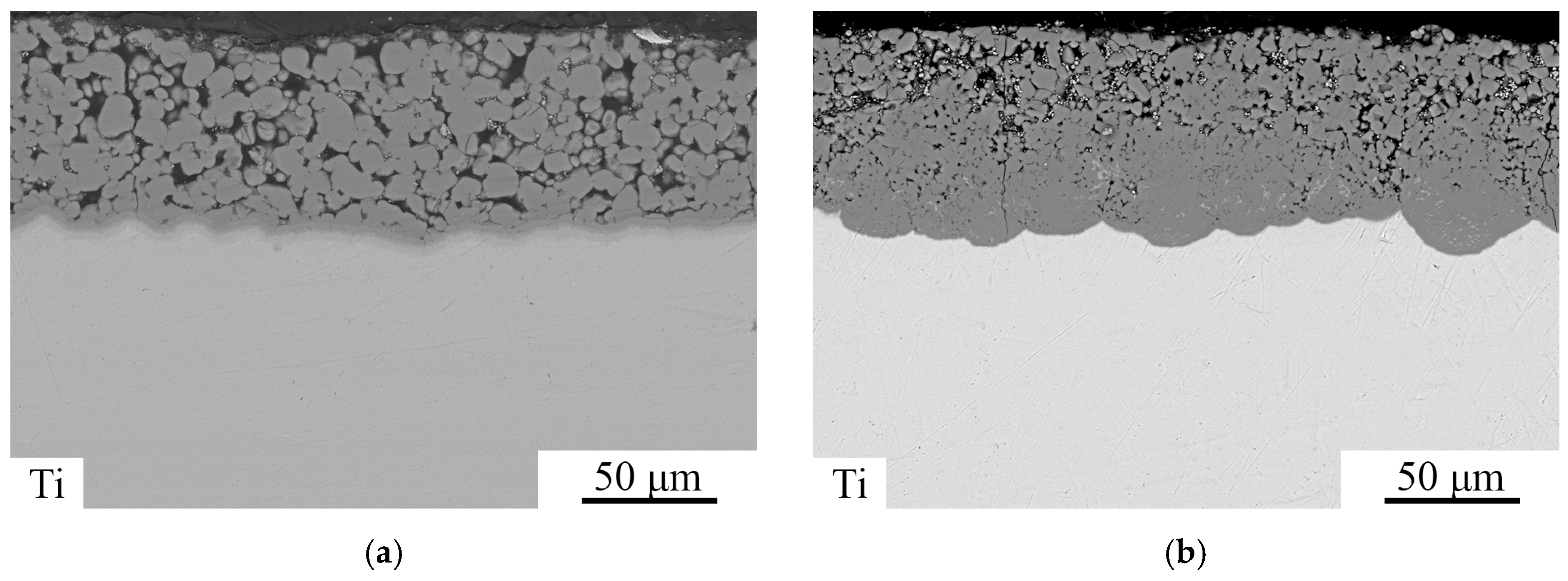
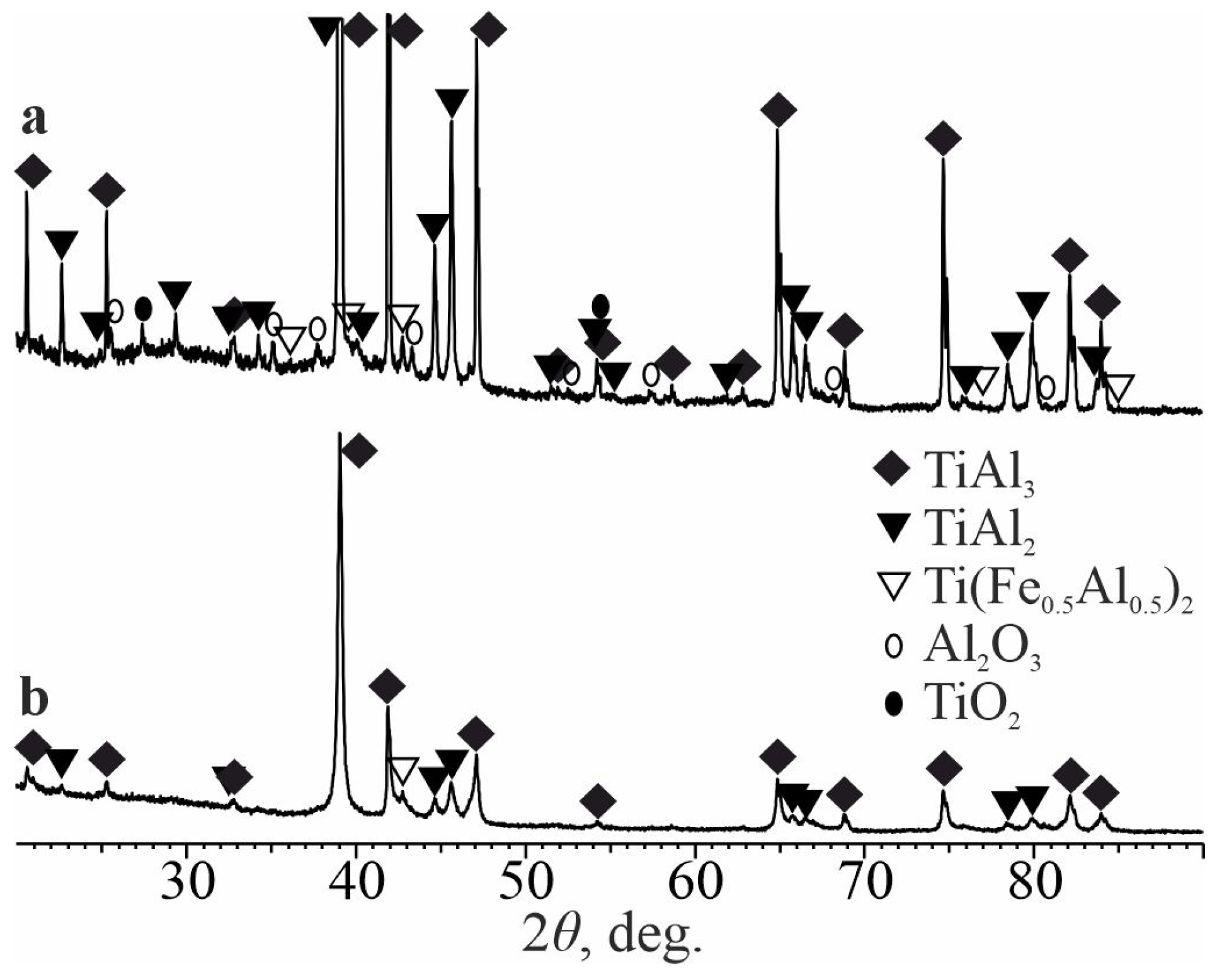
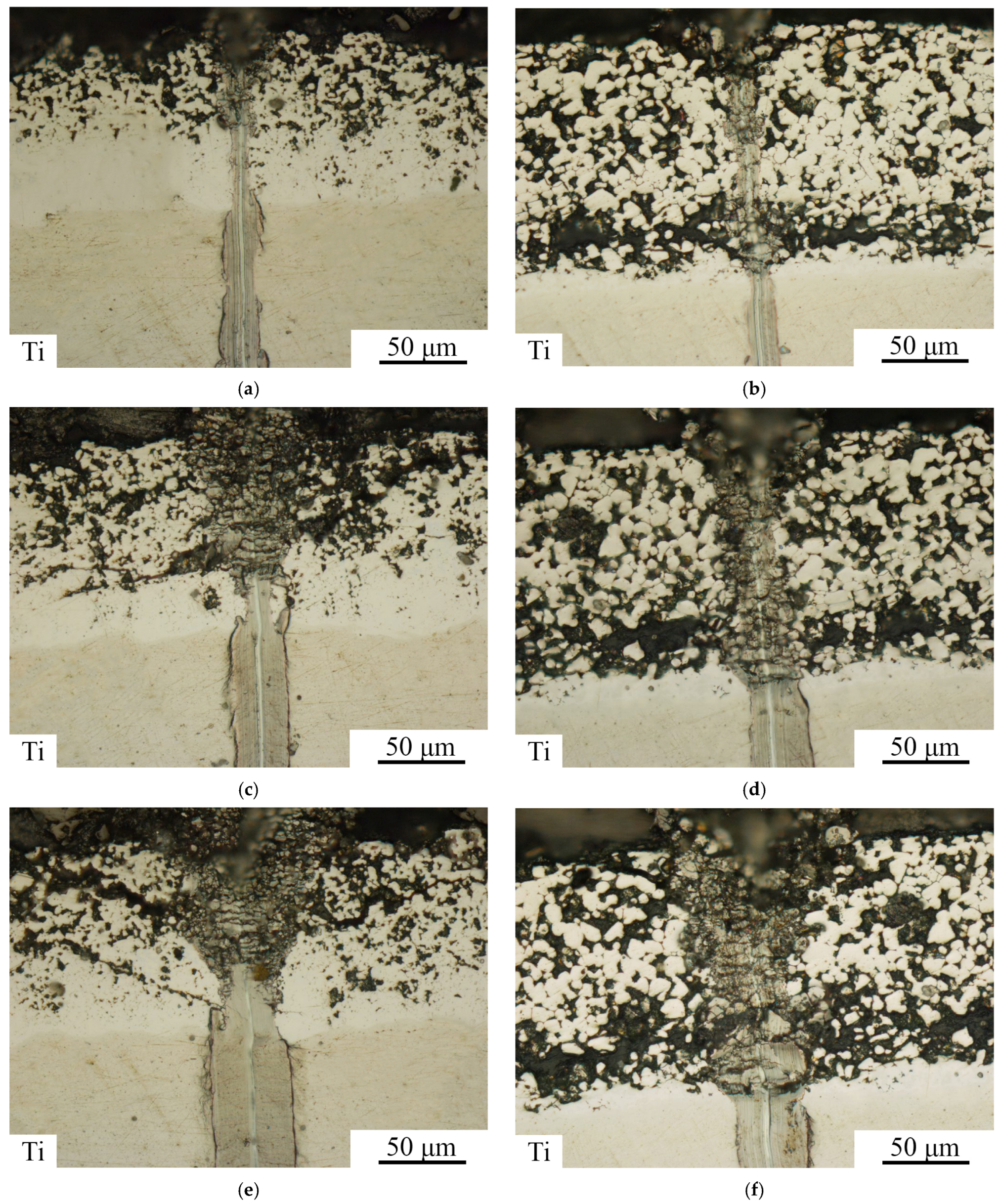
3.2. Heat-Treated State
3.3. Mechanism of Coating Structure Formation
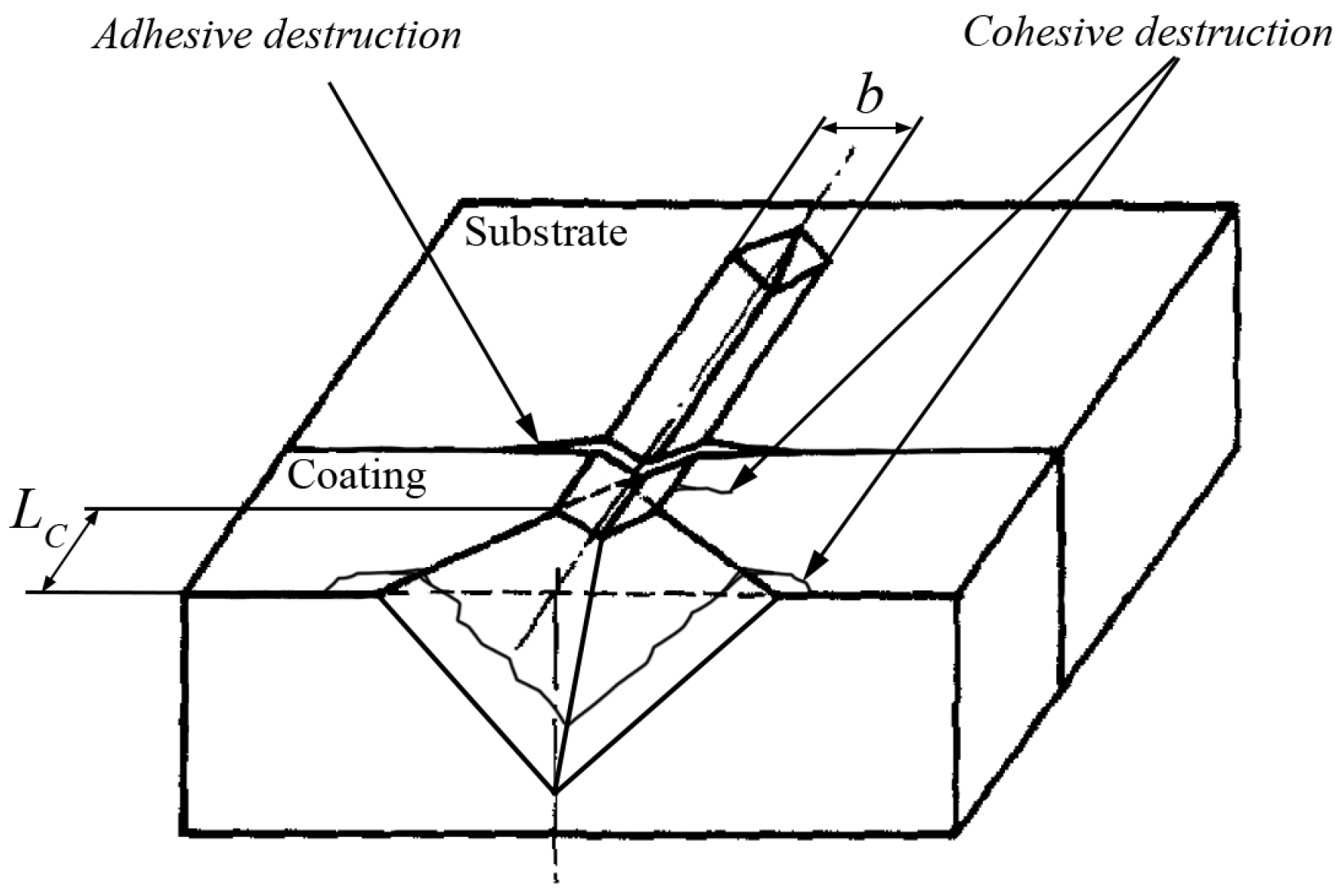
3.4. Scratch Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Titanium and Titanium Alloys: Fundamentals and Applications; Leyens, C., Peters, M., Eds.; John Wiley & Sons: Weinheim, Germany; Chichester, UK, 2003; ISBN 978-3-527-30534-6. [Google Scholar]
- Ivanov, A.S.; Potekhin, A.R.; Tomsinskii, V.S. Wear-Resistant Coating on Titanium Alloys. Met. Sci. Heat Treat. 1990, 32, 38–40. [Google Scholar] [CrossRef]
- Srinath, M.K.; Prasad, M.S.G. Wear and Corrosion Resistance of Titanium Carbo-Nitride Coated Al-7075 Produced through PVD. Bull. Mater. Sci. 2020, 43, 108. [Google Scholar] [CrossRef]
- Gurrappa, I.; Gogia, A.K. Development of Oxidation Resistant Coatings for Titanium Alloys. Mater. Sci. Technol. 2001, 17, 581–587. [Google Scholar] [CrossRef]
- Cizek, J.; Man, O.; Roupcova, P.; Loke, K.; Dlouhy, I. Oxidation Performance of Cold Spray Ti–Al Barrier Coated γ-TiAl Intermetallic Substrates. Surf. Coat. Technol. 2015, 268, 85–89. [Google Scholar] [CrossRef]
- Kong, L.Y.; Shen, L.; Lu, B.; Yang, R.; Cui, X.Y.; Li, T.F.; Xiong, T.Y. Preparation of TiAl3-Al Composite Coating by Cold Spray and Its High Temperature Oxidation Behavior. J. Therm. Spray Tech. 2010, 19, 1206–1210. [Google Scholar] [CrossRef]
- Sienkiewicz, J.; Kuroda, S.; Molak, R.M.; Murakami, H.; Araki, H.; Takamori, S.; Kurzydłowski, K.J. Fabrication of TiAl Intermetallic Phases by Heat Treatment of Warm Sprayed Metal Precursors. Intermetallics 2014, 49, 57–64. [Google Scholar] [CrossRef]
- Sasaki, T.; Yagi, T.; Watanabe, T.; Yanagisawa, A. Aluminizing of TiAl-Based Alloy Using Thermal Spray Coating. Surf. Coat. Technol. 2011, 205, 3900–3904. [Google Scholar] [CrossRef]
- Deqing, W.; Ziyuan, S.; Yingli, T. Microstructure and Oxidation of Hot-Dip Aluminized Titanium at High Temperature. Appl. Surf. Sci. 2005, 250, 238–246. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Peng, Y.P.; Mao, Y.L.; Pang, C.J.; Lu, L.Y. Effect of Hot-Dip Aluminizing on the Oxidation Resistance of Ti–6Al–4V Alloy at High Temperatures. Corros. Sci. 2012, 55, 187–193. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Li, M.; Cao, J. Enhancing High-Temperature Oxidation Resistance of Ti6Al4V Alloy by Simple Surface Aluminization. Corros. Sci. 2021, 192, 109810. [Google Scholar] [CrossRef]
- Kung, S.-C. High-Temperature Coating for Titanium Aluminides Using the Pack-Cementation Technique. Oxid. Met. 1990, 34, 217–228. [Google Scholar] [CrossRef]
- Szkliniarz, W.; Moskal, G.; Szkliniarz, A.; Swadźba, R. The Influence of Aluminizing Process on the Surface Condition and Oxidation Resistance of Ti–45Al–8Nb–0.5(B, C) Alloy. Coatings 2018, 8, 113. [Google Scholar] [CrossRef]
- Zarchi, H.R.K.; Soltanieh, M.; Aboutalebi, M.R.; Guo, X. Thermodynamic Study on Pack Aluminizing Systems of Pure Titanium and Nickel. Trans. Nonferrous Met. Soc. China 2013, 23, 1838–1846. [Google Scholar] [CrossRef]
- Pyachin, S.A.; Burkov, A.A.; Komarova, V.S. Formation and Study of Electrospark Coatings Based on Titanium Aluminides. J. Synch. Investig. 2013, 7, 515–522. [Google Scholar] [CrossRef]
- Yumoto, A.; Hiroki, F.; Shiota, I.; Niwa, N. In Situ Synthesis of Titanium-Aluminides in Coating with Supersonic Free-Jet PVD Using Ti and Al Nanoparticles. Surf. Coat. Technol. 2003, 169–170, 499–503. [Google Scholar] [CrossRef]
- Abdulrahman, K.O.; Akinlabi, E.T.; Mahamood, R.M.; Pityana, S.; Tlotleng, M. Laser Metal Deposition of Titanium Aluminide Composites: A Review. Mater. Today Proc. 2018, 5, 19738–19746. [Google Scholar] [CrossRef]
- Hoosain, S.E.; Pityana, S.; Freemantle, C.S.; Tlotleng, M. Heat Treatment of In Situ Laser-Fabricated Titanium Aluminide. Metals 2018, 8, 655. [Google Scholar] [CrossRef]
- Maliutina, I.N.; Si-Mohand, H.; Sijobert, J.; Bertrand, P.; Lazurenko, D.V.; Bataev, I.A. Structure and Oxidation Behavior of γ-TiAl Coating Produced by Laser Cladding on Titanium Alloy. Surf. Coat. Technol. 2017, 319, 136–144. [Google Scholar] [CrossRef]
- Sujata, M.; Bhargava, S.; Sangal, S. Microstructural Features of TiAl3 Base Compounds Formed by Reaction Synthesis. ISIJ Int. 1996, 36, 255–262. [Google Scholar] [CrossRef]
- Suzuki, A.; Miyake, S.; Naruse, W.; Takata, N.; Kobashi, M. Synthesis of Porous Al/Al3Ti Composite with Hierarchical Open–Cell Structure for Combining with Phase Change Material. J. Alloys Compd. 2019, 770, 1100–1111. [Google Scholar] [CrossRef]
- Shahzad, A.; Zadorozhnyy, V.Y.; Pavlov, M.D.; Zheleznyi, M.V.; Chirkov, A.M.; Zagrebin, D.S.; Semenov, D.V.; Khasenova, R.S.; Kaloshkin, S.D. Deposition of the Ti-Al Coatings on Different Metallic Substrates by Mechanical Alloying and Subsequent Laser Treatment. J. Alloys Compd. 2018, 731, 1295–1302. [Google Scholar] [CrossRef]
- Mizuta, N.; Matsuura, K.; Kirihara, S.; Miyamoto, Y. Titanium Aluminide Coating on Titanium Surface Using Three-Dimensional Microwelder. Mater. Sci. Eng. A 2008, 492, 199–204. [Google Scholar] [CrossRef]
- Mridha, S.; Baker, T.N. Oxidation Protection Behaviour of Titanium Aluminide Coatings Developed by TIG Technique. Adv. Mater. Process. Technol. 2015, 1, 453–464. [Google Scholar] [CrossRef]
- Wei, X.; Yong, L.; Jingfei, B.; Hu, D.; Qing, S.; RuTao, X.; Hong, L.; Dan, Y. Effect of Pressure on Microstructure and Properties of Ti–Al Coating on Titanium Alloy Surface. AIP Adv. 2024, 14, 075308. [Google Scholar] [CrossRef]
- Hu, C.-J.; Chiu, P.-H. Wear and Corrosion Resistance of Pure Titanium Subjected to Aluminization and Coated with a Microarc Oxidation Ceramic Coating. Int. J. Electrochem. Sci. 2015, 10, 4290–4302. [Google Scholar] [CrossRef]
- Van Loo, F.J.J.; Rieck, G.D. Diffusion in the Titanium-Aluminium System—I. Interdiffusion between Solid Al and Ti or Ti-Al Alloys. Acta Metall. 1973, 21, 61–71. [Google Scholar] [CrossRef]
- Gurevich, L.; Pronichev, D.; Trunov, M. Structure Formation Mechanisms during Solid Ti with Molten Al Interaction. IOP Conf. Ser. Mater. Sci. Eng. 2016, 116, 012011. [Google Scholar] [CrossRef]
- Gurevich, L.M.; Shmorgun, V.G. Intermetallic Compound Formation During Reaction of Molten Aluminum with Titanium. Metallurgist 2016, 59, 1221–1227. [Google Scholar] [CrossRef]
- Buschow, K.H.J. Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 2001; ISBN 978-0-08-043152-9. [Google Scholar]
- Phase Diagrams of Binary Titanium Alloys; Murray, J.L., Ed.; Monograph series on alloy phase diagrams; 2. print.; ASM International: Metals Park, OH, USA, 1990; ISBN 978-0-87170-248-7. [Google Scholar]
- Kattner, U.R.; Lin, J.-C.; Chang, Y.A. Thermodynamic Assessment and Calculation of the Ti-Al System. Met. Trans A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the Binary Aluminum-Titanium Phase Diagram. JPED 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Braun, J.; Ellner, M. Phase Equilibria Investigations on the Aluminum-Rich Part of the Binary System Ti-Al. Met. Mater Trans A 2001, 32, 1037–1047. [Google Scholar] [CrossRef]
- Karpets, M.V.; Milman, Y.V.; Barabash, O.M.; Korzhova, N.P.; Senkov, O.N.; Miracle, D.B.; Legkaya, T.N.; Voskoboynik, I.V. The Influence of Zr Alloying on the Structure and Properties of Al3Ti. Intermetallics 2003, 11, 241–249. [Google Scholar] [CrossRef]
- Liang, Z.; Kattner, U.; Choudharry, K.; Tavazza, F.; Campbell, C. Thermodynamic Assessments of Ti-Al, Ti-Fe, and Ti-Al-Fe Systems with Four-Sublattice Description of Ordered Body-Centered Cubic Phase and Density Functional Theory Data. J. Phase Equilib. Diffus. 2024, 45, 732–756. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances; VCH: Weinheim, Germany; New York, NY, USA, 1989; ISBN 978-0-89573-866-0. [Google Scholar]
- Yan, X.; Chen, X.-Q.; Grytsiv, A.; Witusiewicz, V.T.; Rogl, P.; Podloucky, R.; Pomjakushin, V.; Giester, G. Site Preference, Thermodynamic, and Magnetic Properties of the Ternary Laves Phase Ti(Fe1–xAlx)2 with the Crystal Structure of the MgZn2-Type. Int. J. Mater. Res. 2006, 97, 450–460. [Google Scholar] [CrossRef]
- Pukenas, A.; Chekhonin, P.; Scharnweber, J.; Chulist, R.; Oertel, C.-G.; Freudenberger, J.; Skrotzki, W. TiAl-Based Semi-Finished Material Produced by Reaction Annealing of Ti/Al Layered Composite Sheets. Mater. Today Commun. 2022, 30, 103083. [Google Scholar] [CrossRef]
- Slama, G.; Vignes, A. Coating of Niobium and Niobium Alloys with Aluminium. J. Less Common Met. 1971, 24, 1–21. [Google Scholar] [CrossRef]
- Malkin, A.I.; Popov, D.A. The Rehbinder Effect in Fracturing of Metals and Rocks. Phys. Metals Metallogr. 2022, 123, 1234–1244. [Google Scholar] [CrossRef]
- Chikova, O.A.; Tsepelev, V.S.; Shmakova, K.Y. On Spontaneous Dispersion as a Cause of Microstratification of Metal Melts. Materials 2024, 17, 2215. [Google Scholar] [CrossRef]
- Mthisi, A.; Popoola, A.P.I.; Popoola, O.M. Tribological Behaviour of Laser Synthesized Ti-Al2O3 Coatings on Ti-6Al-4V Alloy. Int. J. Adv. Manuf. Technol. 2019, 103, 655–664. [Google Scholar] [CrossRef]
- Shmorgun, V.G.; Bogdanov, A.I.; Arisova, V.N.; Trykov, Y.P. Growth Kinetics of the Diffusion Zone at the Interface of the Explosion-Welded Nickel–Aluminium Composite. Weld. Int. 2016, 30, 625–629. [Google Scholar] [CrossRef]
- Taube, A.O.; Shmorgun, V.G.; Bogdanov, A.I.; Storozheva, E.I.; Solovev, D.B. Estimation of the Service Lifetime of Layered Heat-Resistant Ni-Al and Ni-Cr-Al Coatings. IOP Conf. Ser. Earth Environ. Sci. 2020, 459, 062113. [Google Scholar] [CrossRef]
- Shmorgun, V.G.; Bogdanov, A.I.; Kulevich, V.P.; Iskhakova, L.D.; Taube, A.O. Microstructure and Phase Composition of Diffusion Coating Formed in NiCr Alloys by Hot-Dip Aluminizing. Surf. Interfaces 2021, 23, 100988. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]






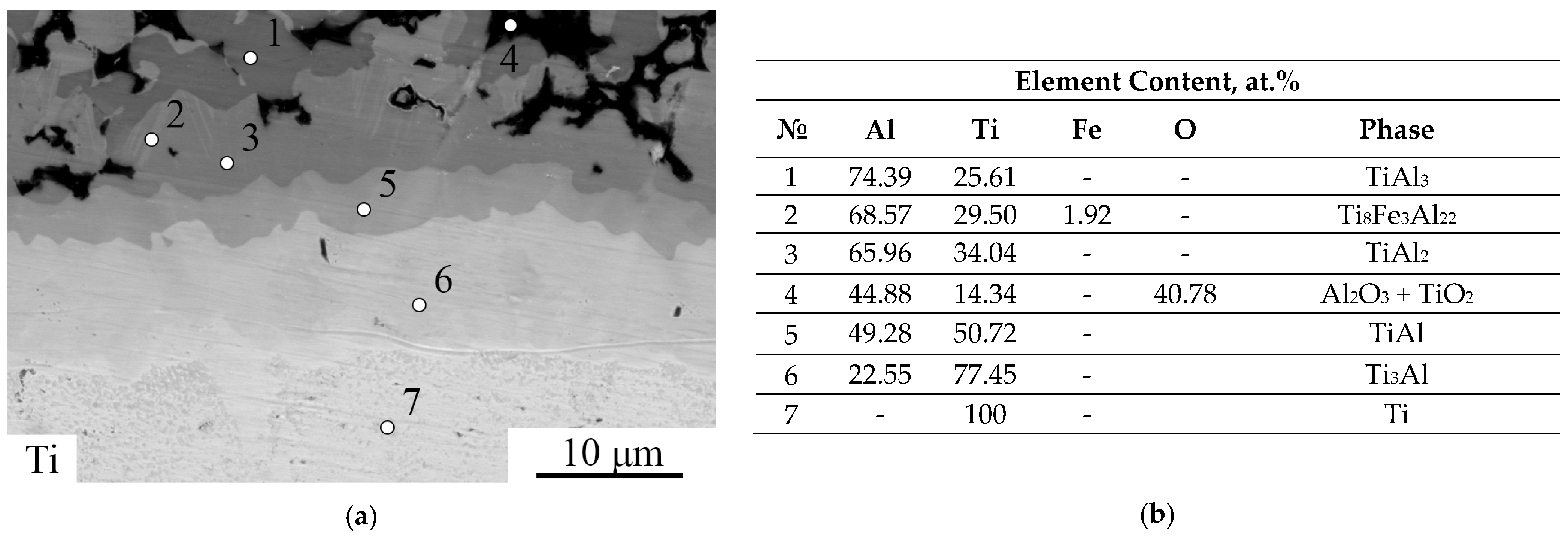

| Ti | Fe | Si | N | O | H | C | Others |
|---|---|---|---|---|---|---|---|
| 99.24–99.70 | ≤0.25 | ≤0.10 | ≤0.04 | ≤0.2 | ≤0.01 | ≤0.07 | <0.3 |
| Al | Mg | Si | Mn | Fe | Zn | Cu | Cr | Ti |
|---|---|---|---|---|---|---|---|---|
| 97.50–99.35 | 0.45–0.90 | 0.2–0.6 | ≤0.10 | ≤0.35 | ≤0.10 | ≤0.10 | ≤0.10 | ≤0.10 |
| Element | Content, at.% | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Al | 100 | - | 83.35 |
| Ti | - | 100 | 16.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, A.I.; Kulevich, V.P.; Shmorgun, V.G.; Gurevich, L.M. Structure and Properties of Ti-Al Intermetallic Coatings Reinforced with an Aluminum Oxide Filler. Metals 2024, 14, 1336. https://doi.org/10.3390/met14121336
Bogdanov AI, Kulevich VP, Shmorgun VG, Gurevich LM. Structure and Properties of Ti-Al Intermetallic Coatings Reinforced with an Aluminum Oxide Filler. Metals. 2024; 14(12):1336. https://doi.org/10.3390/met14121336
Chicago/Turabian StyleBogdanov, Artem Igorevich, Vitaliy Pavlovich Kulevich, Victor Georgievich Shmorgun, and Leonid Moiseevich Gurevich. 2024. "Structure and Properties of Ti-Al Intermetallic Coatings Reinforced with an Aluminum Oxide Filler" Metals 14, no. 12: 1336. https://doi.org/10.3390/met14121336
APA StyleBogdanov, A. I., Kulevich, V. P., Shmorgun, V. G., & Gurevich, L. M. (2024). Structure and Properties of Ti-Al Intermetallic Coatings Reinforced with an Aluminum Oxide Filler. Metals, 14(12), 1336. https://doi.org/10.3390/met14121336









