1. Introduction
Titanium and its alloys have become indispensable in a multitude of engineering applications, where the demand for materials with superior mechanical and chemical attributes is paramount. Renowned for their low density, remarkable mechanical strength, and exceptional corrosion resistance, titanium alloys have spurred extensive research efforts aimed at enhancing their mechanical performance for structural purposes. Originating in aerospace structures, the appeal of titanium and its alloys has transcended into diverse industrial sectors, including chemical, automotive, and biomedical fields. The exceptional properties of titanium alloys have proven instrumental in advancing technological frontiers and fueling innovation across these industries [
1,
2,
3].
The industrial applicability of titanium is impeded by severe oxidation challenges, despite its exceptional mechanical properties. Titanium exhibits a propensity to form a diverse array of oxides, including TiO
2, Magnéli phases (Ti
nO
2n−1, n ≥ 4), Ti
3O
5, Ti
2O
3, TiO, Ti
2O, Ti
3O, and others [
4,
5], with TiO
2 being the most encountered, in phases such as anatase, rutile, and brookite [
6]. Elevated temperatures above 500–520 °C prompt titanium to readily absorb oxygen, resulting in the formation of non-protective Rutilium (TiO
2). This oxide layer facilitates oxygen diffusion, exacerbating oxidation rates and the development of fragile surface layers [
7,
8]. Non-stoichiometric TiO
2 compounds exhibit structural defects such as oxygen vacancies and interstitial Ti cations, promoting rapid oxygen and metal ion diffusion. Increased TiO
2 concentrations in surface layers render them more flexible and prone to defects [
8], with a greater transformation of anatase into rutile leading to diminished corrosion resistance [
9].
Oxidation effects occur predominantly on the surface of materials, and for this reason surface modification is a promising approach to improving the surface properties of titanium and its alloys [
10]. Recent studies on the high-temperature behavior of titanium alloys have focused on the use of coatings deposited by the PVD technique. PVD processes provide the advantage of selecting the alloy constituents as binary or ternary compositions with controlled composition and high purity; they can also be deposited on different types of substrates [
11]. The PVD technique has been used to produce thin TiSi [
12,
13], AlTi [
14,
15], and WTi [
16,
17] coatings and analyze their behavior when subjected to high temperatures. Research shows that the oxides formed from Si, Al, and W help to counteract the negative effects of titanium oxide by forming oxides that are stable and adherent on the coating surfaces, which causes an improvement in wear resistance and resistance to oxidation at high temperatures in these systems [
18].
TiSi coatings obtained by laser alloying have shown attractive properties for industrial applications, such as improved resistance to oxidation and wear, high hardness, low relative density, and a high melting point, showing that laser alloying with Si on the surface of Ti or on titanium alloys was beneficial for substrate modification [
19]. From the point of view of the stable formation of oxides on the surface of the TiSi coating, it has been inferred that titanium oxide TiO
2 and silicon oxide SiO
2 can form simultaneously on the exposed surface, in which this oxide (SiO
2) could provide an improved corrosion resistance [
20].
Aluminum is an important and effective alloying element for improving the high-temperature oxidation resistance of titanium and its alloys. At high temperature conditions, sufficient aluminum can be selectively oxidized into a stable, compact, and continuous Al
2O
3 aluminum oxide layer. This oxide can effectively delay the diffusion of titanium and oxygen and, therefore, improve the high temperature corrosion resistance for this type of material [
21]. Dai manufactured AlTi coatings on Ti6Al4V titanium alloy fabricated by laser surface alloying. The produced coatings showed excellent oxidation resistance at 900 °C for 1000 h. [
22]. In AlTi coatings, titanium oxide TiO
2 and aluminum oxide Al
2O
3 are usually formed. The formation of other types of titanium and aluminum oxides would delay the generation of the Al
2O
3 protective layer, and the temperature, composition, and constituents of the high-temperature medium would affect the formation of the initial oxides and the subsequent formation of the protective layer [
23]. An oxide layer with large proportions of TiO
2 will have poor resistance to high-temperature corrosion; conversely, large proportions of Al
2O
3 oxide will offer adequate resistance to corrosion and wear [
24].
WTi coatings present some notable properties, such as their electrical resistance, thermal stability, oxidation resistance, chemical stability, and good adhesion to the substrate, including a low friction coefficient and good wear resistance [
25]. WTi coatings have been studied as anticorrosive protection [
26]. The presence of titanium in the alloy reduces the accumulation of holes at the alloy/oxide interface, causing the modification of the microstructure of the surface oxide, improving corrosion resistance, and increasing the coating adhesion. Titanium also improves the diffusion barrier performance of tungsten due to its affinity for nitrogen and oxygen [
27]. The diffusion barrier properties of the WTi coating depend principally on the concentration of titanium. The most frequently used stoichiometry is W:Ti (90:10%), where tungsten is used as a diffusion barrier because the atomic diffusivity of the metals in tungsten is low [
27].
As mentioned, TiSi, AlTi, and WTi coatings produced by PVD can present improved corrosion resistances as a consequence of the effect of the alloy’s elements, which reduce the formation of non-protective titanium oxides. In this research, we wish to identify the mechanisms of high-temperature corrosion and the growth of oxides on the surface of the TiSi, AlTi, and WTi coatings produced by PVD on a Ti6Al4V substrate. Complementary surface analysis techniques are being applied, and they provide information about the predominant species and surface distribution of corrosion products.
2. Materials and Methods
For the development of this research, samples of TiSi, TiAl, and TiW coatings deposited using an RF magnetron sputtering technique were used. The synthesis of the coatings was carried out using Alcatel type HS 2000 (Alcatel Vacuum Technology, Annecy, France) commercial equipment, following the procedure presented by Gordillo in [
25]. The coatings were deposited on Titanium Aluminum Vanadium (Ti6Al4V) substrates (5.82 Al% by weight, 3.82% by weight of V, and balance of Ti). The Ti6Al4V substrates used had a size of 1.5 cm × 1.5 cm × 0.6 cm. Targets of Tungsten-titanium W:Ti (90:10%), Aluminum-Titanium Al:Ti (50:50%), and Titanium-Silicon Ti:Si (81:19%) with 99.99% purity were used.
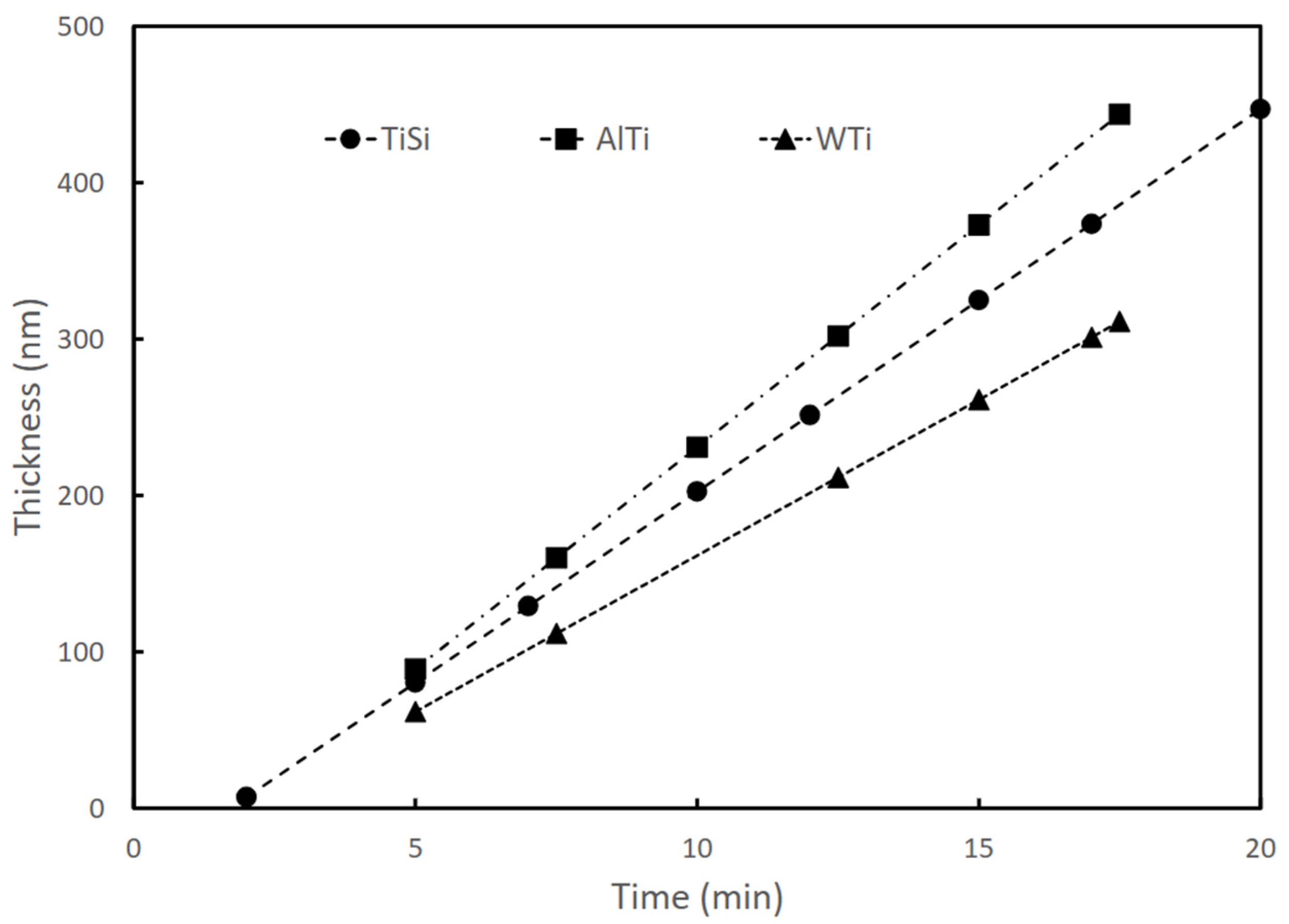
Table 1 presents the configuration parameters of the equipment for the coating’s deposition parameters. The thickness of the coatings was approximately 200 nm. The deposition rate was approximately 20 nm/min in all cases and remained constant during the sputtering process, as shown in
Figure 1. The elemental composition (% by weight) was determined by energy dispersive X-ray spectroscopy (EDS) using an X-MAX 50/Oxford Instruments probe (Oxford Instruments, Abingdon, UK); the results are shown in
Table 2, as reported in [
25].
The coated samples were subjected to an oxidation test in hot air at a temperature of 600 °C for a period of 100 h. The test was carried out in an EQUIFAR (Equifar, Bogotá Colombia) muffle furnace in a horizontal arrangement.
The samples were analyzed before and after the oxidation treatment. The morphology of the coatings was analyzed by scanning electron microscopy (SEM) (Tescan/Mira 3) (Tescan Mira 3, Tescan, Brno, The Czech Republic). The roughness in the samples was measured with a Bruker contour GT-K vertical scanning interferometer (Bruker Contour GTK, Bruker Nano Surfaces Division, Tucson, AZ, USA). Each sample underwent three distinct roughness measurements. Microstructural analyses were performed with X-pert Pro Panalytical X-ray (Panalytical, Almelo, The Netherlands) diffraction equipment in grazing beam mode at 5°, with the monochromatic line Kα of copper (1.540998 Å) working at 45 kV and 40 mA.
In order to carry out a semiquantitative analysis of the corrosion products on the surfaces of the coatings, Raman spectroscopy analyses were performed on the oxidized samples. A Horiba Scientific LabRAM HR Evolution Raman microscope (Horiba Jobin Yvon, Piscataway, NJ, USA) with an Ar+ laser with a wavelength of 514 nm was used. The microscope mapping tool was used to scan the surface in an array of 100 analysis points for the TiSi/AlTi coatings and 350 points for the WTi coating, in an a × b array. The Raman spectra obtained were in the range [50–1200 cm
−1] (Vis NIR). In order to determine the presence of oxides on the surface of the coatings, a semiquantitative analysis methodology was applied. Each spectrum obtained was considered a response vector, and all spectra were organized in a matrix. Reference Raman spectra of the oxides TiO
2 anatase phase, TiO
2 rutilium phase, and tungsten oxide WO
3 were obtained from the RRUFF database and organized in a reference matrix Y. The response and reference matrices were processed applying the analysis method multivariate MCR-ALS “multivariate resolution of alternating least squares curves” according to the methodology proposed in the literature [
28] and using the software “MatLab R 2017b, MCR-ALS toolbox 2” (MathWorks, Inc., Natick, MA, USA). “Non-negativity” was used as an input restriction for concentrations and for constitutive spectra. From the analysis, a vector was obtained, the values of which are an indicator of the presence and concentration of each of the oxides detected by this technique. The values were plotted on a surface diagram that allows for identifying the presence of oxides.
Additionally, the oxidized coatings were analyzed using the X-ray photoelectron spectroscopy technique (XPS). Kratos Axis Ultra equipment (Kratos. Analytical, Manchester, UK) with an X-ray source of 1486.5 eV, 150 W, and a measurement resolution of 0.58 eV was used for the development of the tests. A scan of each of the samples was performed in survey mode, followed by localized scans in the W4f, Si2p, and Ti2p regions, corresponding to the locations of the tungsten, silicon, and titanium peaks. CasaXPS software (Casa Software Ltd., Wilmslow, Cheshire, UK) was used to analyze the spectra obtained.
Author Contributions
Conceptualization, O.P. and J.O.; methodology, O.P. and O.G.; validation, O.G., O.P. and J.O.; data curation, formal analysis, software, writing—original draft preparation, O.G.; investigation, O.G., W.S.H., J.E.A. and G.C.; resources, V.T.-A.; writing—review and editing, O.P., J.O. and V.T.-A.; visualization, O.G.; and supervision, O.P., J.O., W.S.H., J.E.A., G.C. and V.T.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding author.
Acknowledgments
To the Universidad Nacional de Colombia and the Instituto Nacional de Pesquisas Espaciais (Associated Laboratory of Sensors and Materials) in Brazil for the use of their laboratories and facilities in the development of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saurabh, A.; Meghana, C.M.; Singh, P.K.; Verma, P.C. Titanium-based materials: Synthesis, properties, and applications. Mater. Today Proc. 2022, 56, 412–419. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2024, 17, 114. [Google Scholar] [CrossRef]
- Williams, J.C.; Boyer, R.R. Opportunities and Issues in the Application of Titanium Alloys for Aerospace Components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Surface modification of a Ti–6Al–4V alloy by thermal oxidation. Surf. Coat. Technol. 2005, 192, 164–170. [Google Scholar] [CrossRef]
- Shvets, P.; Maksimova, K.; Demin, M.; Dikaya, O.; Goikhman, A. Cathodic arc sputtering of functional titanium oxide thin films, demonstrating resistive switching. Phys. B. Condens. Matter 2017, 513, 15–20. [Google Scholar] [CrossRef]
- Duarte, L.T.; Bolfarini, C.; Biaggio, S.R.; Rocha-Filho, R.C.; Nascente, P.A. Growth of aluminum-free porous oxide layers on titanium and its alloys Ti-6Al-4V and Ti-6Al-7Nb by micro-arc oxidation. Mater. Sci. Eng. C 2014, 41, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.P.; Casagrande, A.; Sambogna, G. Effect of Ni, Si and Cr in the structural formation of diffusion aluminide coatings on commercial-purity titanium. Surf. Coat. Technol. 2006, 201, 230–242. [Google Scholar] [CrossRef]
- Ebach-Stahl, A.; Eilers, C.; Laska, N.; Braun, R. Cyclic oxidation behaviour of the titanium alloys Ti-6242 and Ti-17 with Ti–Al–Cr–Y coatings at 600 and 700 °C in air. Surf. Coat. Technol. 2013, 223, 24–31. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Hu, T.; Leicht, P.; Liu, Y. Oxidation resistance and thermal stability of Ti (C, N) and Ti (C, N, O) coatings deposited by chemical vapor deposition. Int. J. Refract. Met. Hard Mater. 2016, 54, 295–303. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, R.; Sun, J.; Liu, C. Oxidation mechanism of biomedical titanium alloy surface and experiment. Int. J. Corros. 2020, 1678615. [Google Scholar] [CrossRef]
- Gudla, V.C.; Bordo, K.; Engberg, S.; Rechendorff, K.; Ambat, R. High frequency pulse anodising of magnetron sputtered Al–Zr and Al–Ti Coatings. Mater. Des. 2016, 95, 340–347. [Google Scholar] [CrossRef]
- Mitoraj-Królikowska, M.; Godlewska, E. Silicide coatings on Ti-6Al-1Mn (at.%) alloy and their oxidation resistance. Surf. Coat. Technol. 2018, 334, 491–499. [Google Scholar] [CrossRef]
- Bouzakis, K.D.; Bouzakis, E.; Kombogiannis, S.; Paraskevopoulou, R.; Skordaris, G.; Makrimallakis, S.; Andersson, J.M. Effect of silicon content on PVD film mechanical properties and cutting performance of coated cemented carbide inserts. Surf. Coat. Technol. 2013, 237, 379–389. [Google Scholar] [CrossRef]
- Lauwerens, W.; De Boeck, A.; Thijs, M.; Claessens, S.; Van Stappen, M.; Steenackers, P. PVD Al–Ti and Al–Mn coatings for high temperature corrosion protection of sheet steel. Surf. Coat. Technol. 2001, 146, 27–32. [Google Scholar] [CrossRef]
- Sanchette, F.; Loi, T.H.; Billard, A.; Frantz, C. Structure—Properties relationship of metastable Al-Cr and Al-Ti alloys deposited by rf magnetron sputtering: Role of nitrogen. Surf. Coat. Technol. 1995, 74, 903–909. [Google Scholar] [CrossRef]
- Louro, C.; Cavaleiro, A. Thermal Oxidation of Tungsten-Based Sputtered Coatings. J. Electrochem. Soc. 1997, 144, 259–266. [Google Scholar] [CrossRef]
- Arvizua, M.A.; Triana, C.A.; Stefanov, B.I.; Granqvist, C.G.; Niklasson, G.A. Electrochromism in sputter-deposited W–Ti oxide films: Durability enhancement due to Ti. Sol. Energy Mater. Sol. Cells 2014, 125, 184–189. [Google Scholar] [CrossRef]
- Chang, C.-L.; Lee, J.-W. Microstructure, corrosion and tribological behaviors of TiAlSiN coatings deposited by cathodic arc plasma deposition. Thin Solid Film. 2009, 517, 5231–5236. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, A.H.; Zhang, Z.; Zheng, R.R.; Xia, H.B.; Wang, Y.N. Laser alloying of Ti–Si compound coating on Ti–6Al–4V alloy for the improvement of bioactivity. Appl. Surf. Sci. 2014, 305, 16–23. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Middleton, H. Effect of silicon on corrosion resistance of Ti–Si alloys. Mater. Sci. Eng. B 2011, 176, 79–86. [Google Scholar] [CrossRef]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation protection of γ-TiAl-based alloys—A review. Intermetallics 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, F.; Wang, A.; Yu, H.; Chen, C. Microstructure and properties of Ti-Al coating and Ti-Al-Si system coatings on Ti-6Al-4V fabricated by laser surface alloying. Surf. Coat. Technol. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Garbacz, H.; Pouquet, J.M.; García-Lecina, E.; Díaz-Fuentes, M.; Wieciński, P.; Martin, R.H.; Kurzydlowski, K.J. Microstructure, fatigue and corrosion properties of the Ti–Al intermetallic layers. Surf. Coat. Technol. 2011, 205, 4433–4440. [Google Scholar] [CrossRef]
- Du, H.L.; Datta, P.K.; Hu, D.; Wu, X. High temperature corrosion mechanisms of certain new TiAl-based inter-metallic alloys in an aggressive H2/H2O/H2S environment at 850 °C. Corros. Sci. 2007, 49, 2406–2420. [Google Scholar] [CrossRef]
- Gordillo, O.; Hincapie, W.; Piamba, O.; Olaya, J.; Trava-Airoldi, V. Study of Adhesive Wear Test on TiSi, AlTi, and WTi Coatings. Coatings 2022, 12, 1370. [Google Scholar] [CrossRef]
- Petrović, S.; Peruško, D.; Gaković, B.; Mitrić, M.; Kovač, J.; Zalar, A.; Milosavljević, M. Effects of thermal annealing on structural and electrical properties of sputtered W–Ti thin films. Surf. Coat. Technol. 2010, 204, 2099–2102. [Google Scholar] [CrossRef]
- Petrović, S.; Gaković, B.; Peruško, D.; Trtica, M.; Radak, B.; Panjan, P.; Miljanić, Š. Surface modification of a WTi thin film on Si substrate by nanosecond laser pulses. Appl. Surf. Sci. 2008, 254, 4013–4017. [Google Scholar] [CrossRef]
- Sacré, P.Y.; De Bleye, C.; Chavez, P.F.; Netchacovitch, L.; Hubert, P.; Ziemons, E. Data processing of vibrational chemical imaging for pharmaceutical applications. J. Pharm. Biomed. Anal. 2014, 101, 123–140. [Google Scholar] [CrossRef]
- Smith, G.P.; McGoverin, C.M.; Fraser, S.J.; Gordon, K.C. Raman imaging of drug delivery systems. Adv. Drug Deliv. Rev. 2015, 89, 21–41. [Google Scholar] [CrossRef]
- Azzouz, T.; Tauler, R. Application of multivariate curve resolution alternating least squares (MCR-ALS) to the quantitative analysis of pharmaceutical and agricultural samples. Talanta 2008, 74, 1201–1210. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Lv, Y.H.; Li, J.; Tao, Y.F.; Hu, L.F. High-temperature wear and oxidation behaviors of TiNi/Ti2Ni matrix composite coatings with TaC addition prepared on Ti6Al4V by laser cladding. Appl. Surf. Sci. 2017, 402, 478–494. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Hsiao, C.Y. High temperature oxidation resistance of multicomponent Cr–Ti–Al–Si–N coatings. Surf. Coat. Technol. 2009, 204, 992–996. [Google Scholar] [CrossRef]
- Luthin, J.; Linsmeier, C. Influence of oxygen on the carbide formation on tungsten. J. Nucl. Mater. 2001, 290, 121–125. [Google Scholar] [CrossRef]
- Libardi, J.; Grigorov, K.G.; Massi, M.; da Silva Sobrinho, A.S.; Pessoa, R.S.; Sismanoglu, B. Diffusion of silicon in titanium dioxide thin films with different degree of crystallinity: Efficiency of TiO2 and TiN barrier layers. Vacuum 2016, 128, 178–185. [Google Scholar] [CrossRef]
- Crespo-Villegas, J.; Cavarroc, M.; Knittel, S.; Martinu, L.; Klemberg-Sapieha, J.E. Protective TixSiy coatings for enhanced oxidation resistance of the γ-TiAl alloy at 900 C. Surf. Coat. Technol. 2022, 430, 127963. [Google Scholar] [CrossRef]
- Arunchandran Chenan, J.; Jyothymol, J.; Abirami, S.; Bonu, V.; Barshilia, H.C. Comprehensive Electrochemical Studies on Nanolayered Multilayered Ti/TiN and TiAl/TiAlN Coatings Deposited on Ti6Al4V Substrates. Corrosion 2023, 79, 134–145. [Google Scholar] [CrossRef]
Figure 1.
Dependence of TiSi, AlTi, and WTi coating thicknesses on sputtering time (produced by RF magnetron sputtering).
Figure 2.
X-ray diffraction patterns of thin films before and after the corrosion process: (a) TiSi coating, (b) AlTi coating, and (c) WTi coating.
Figure 3.
Surface images of thin films before and after high-temperature corrosion treatment, captured via profilometry: (a) TiSi before corrosion, (b) TiSi after corrosion, (c) AlTi before corrosion, (d) AlTi after corrosion, (e) WTi before corrosion, and (f) WTi after corrosion.
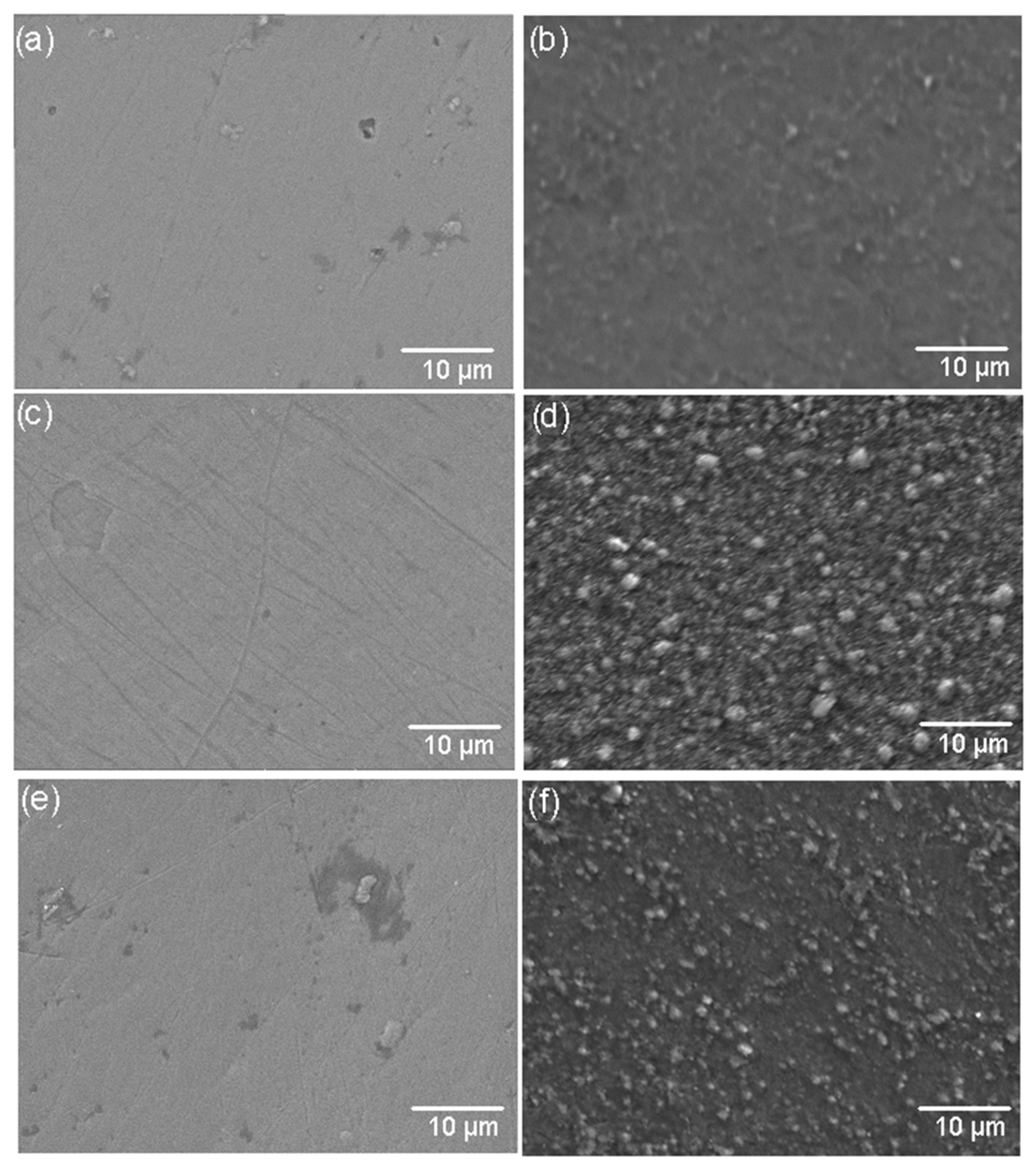
Figure 4.
SEM images of coating surfaces before and after corrosion treatment. (a) TiSi before corrosion, (b) TiSi after corrosion, (c) AlTi before corrosion, (d) AlTi after corrosion, (e) WTi before corrosion, and (f) WTi after corrosion.
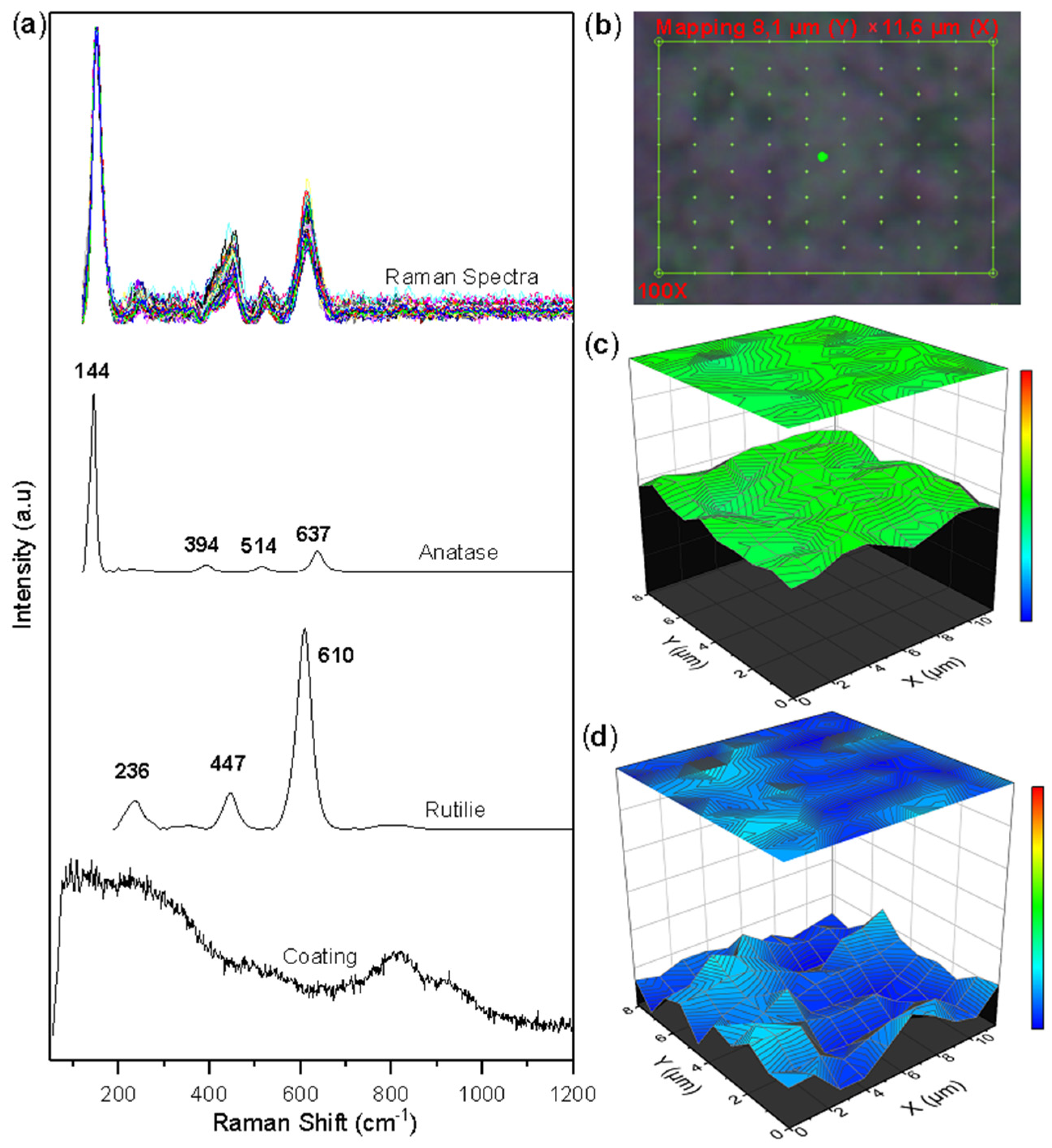
Figure 5.
Raman mapping of corroded TiSi coatings: (a) Measured spectra and reference spectra; (b) Analyzed area; (c) Anatase concentration; (d) Rutile concentration.
Figure 6.
Raman mapping of corroded AlTi coatings: (a) Measured spectra and reference spectra; (b) Analyzed area; (c) Rutile concentration.
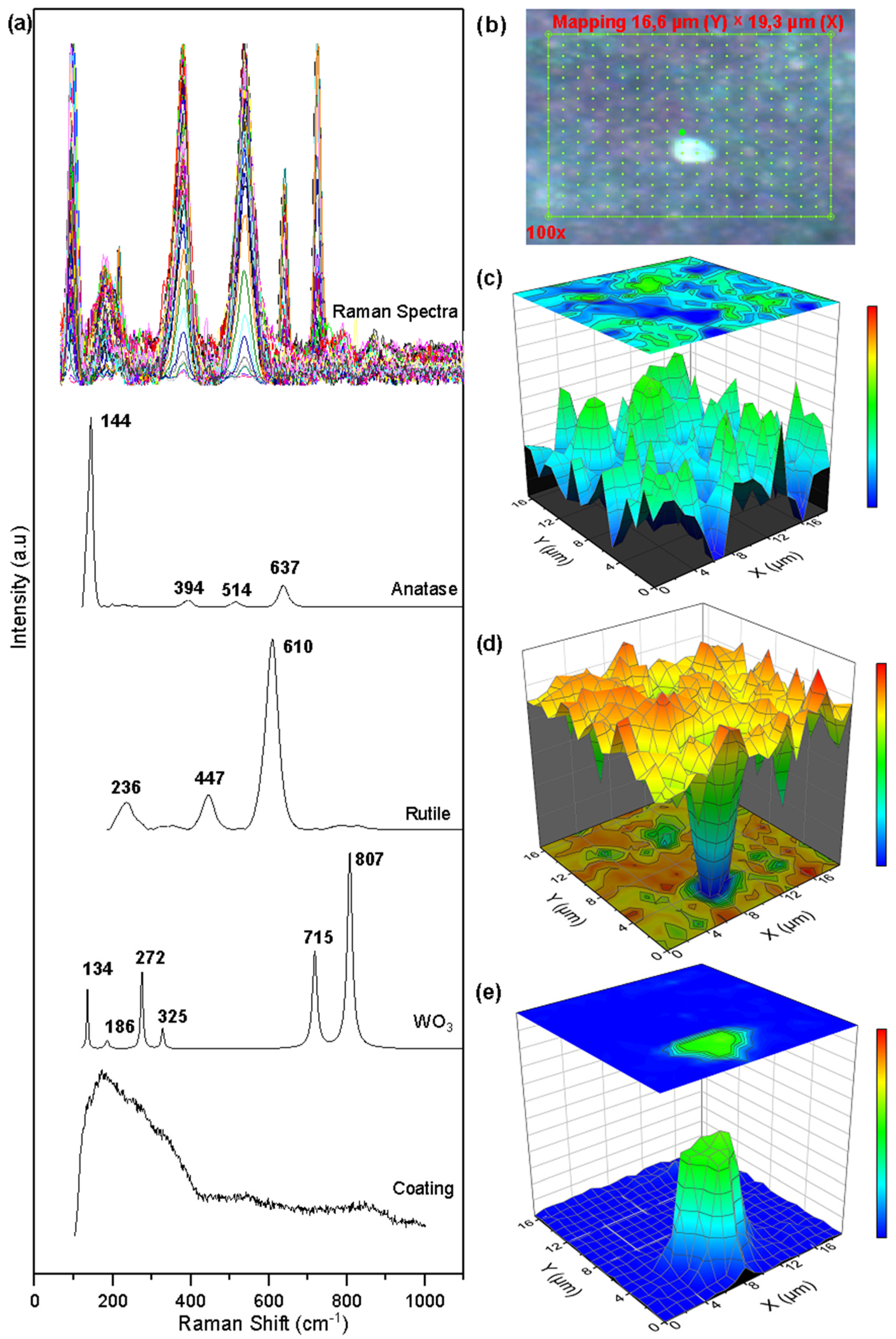
Figure 7.
Raman mapping of corroded WTi coatings: (a) Measured spectra and reference spectra; (b) Analyzed area; (c) Anatase concentration; (d) Rutile concentration; (e) Tungsten oxide.
Figure 8.
XPS spectra of the oxide coatings: (a) TiSi coating, (b) AlTi coating, and (c) WTi coating.
Figure 9.
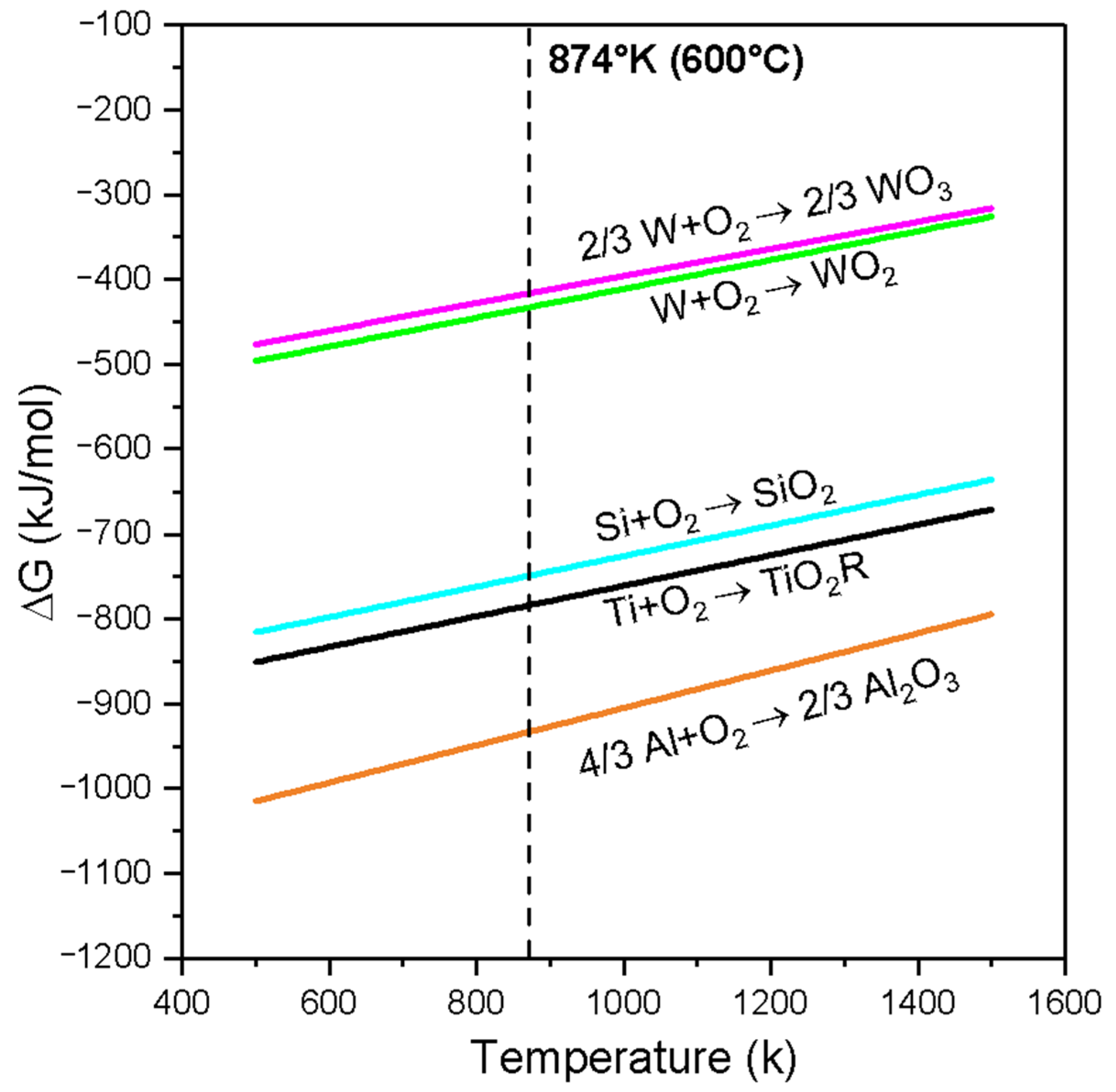
Ellingham diagram for WO₃, WO₂, SiO₂, TiO₂, and Al₂O₃.
Figure 10.
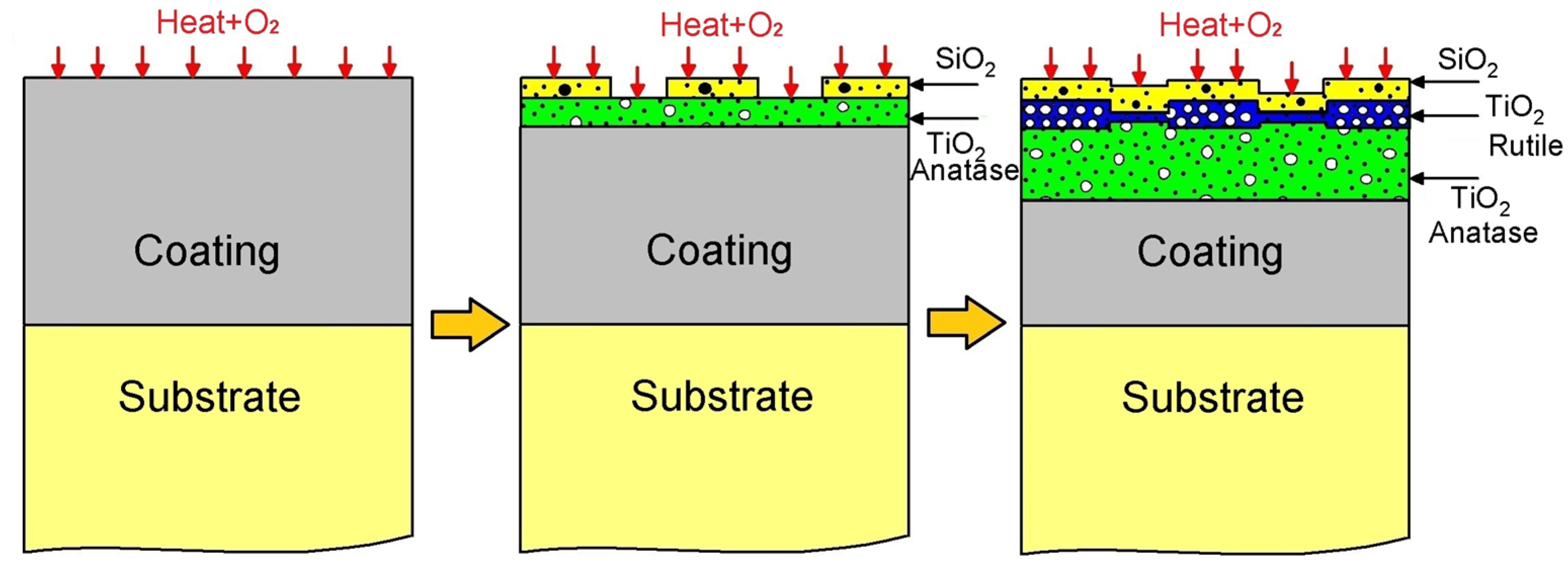
Diagram of mechanism of corrosion of TiSi coating.
Figure 11.
Diagram of mechanism of corrosion of AlTi coating.
Figure 12.
Diagram of mechanism of corrosion of WTi coating.
Table 1.
Summary of deposit conditions for coatings growth.
Vacuum Pressure
Pa | Work Pressure
Pa | Substrate Temperature
K | Discharge Power
W | Target-Substrate Distance
m | Ar Flow
sccm |
|---|
| 5.6 × 10−4 | 4.6 × 10−2 | 290 | 200 | 0.1 | 20 |
Table 2.
The elemental composition (% by weight) of thin films analyzed using EDX (Energy Dispersive X-ray Spectroscopy).
| Coating | Elemental Concentration wt.% |
|---|
| TiSi | 86.4 Ti | 13.6 Si |
| AlTi | 12.7 Ti | 87.3 Al |
| WTi | 21.1 Ti | 78.9 W |
Table 3.
Roughness of coatings TiSi, AlTi, and WTi before and after high-temperature corrosion treatment.
| Coating | Initial Roughness (Ra) | Standard Error | Final Roughness (Ra) | Standard Error |
|---|
| TiSi | 35.9 nm | 7.8 nm | 109.2 nm | 14.8 nm |
| AlTi | 21.1 nm | 1.2 nm | 301.9 nm | 22.3 nm |
| WTi | 40.1 nm | 2.2 nm | 135 nm | 29.2 nm |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).