Design of Near α-Ti Alloys with Optimized Mechanical and Corrosion Properties and Their Characterizations
Abstract
1. Introduction
2. Alloy Design and Experimental Procedures
3. Results and Discussion
4. Conclusions
- (1)
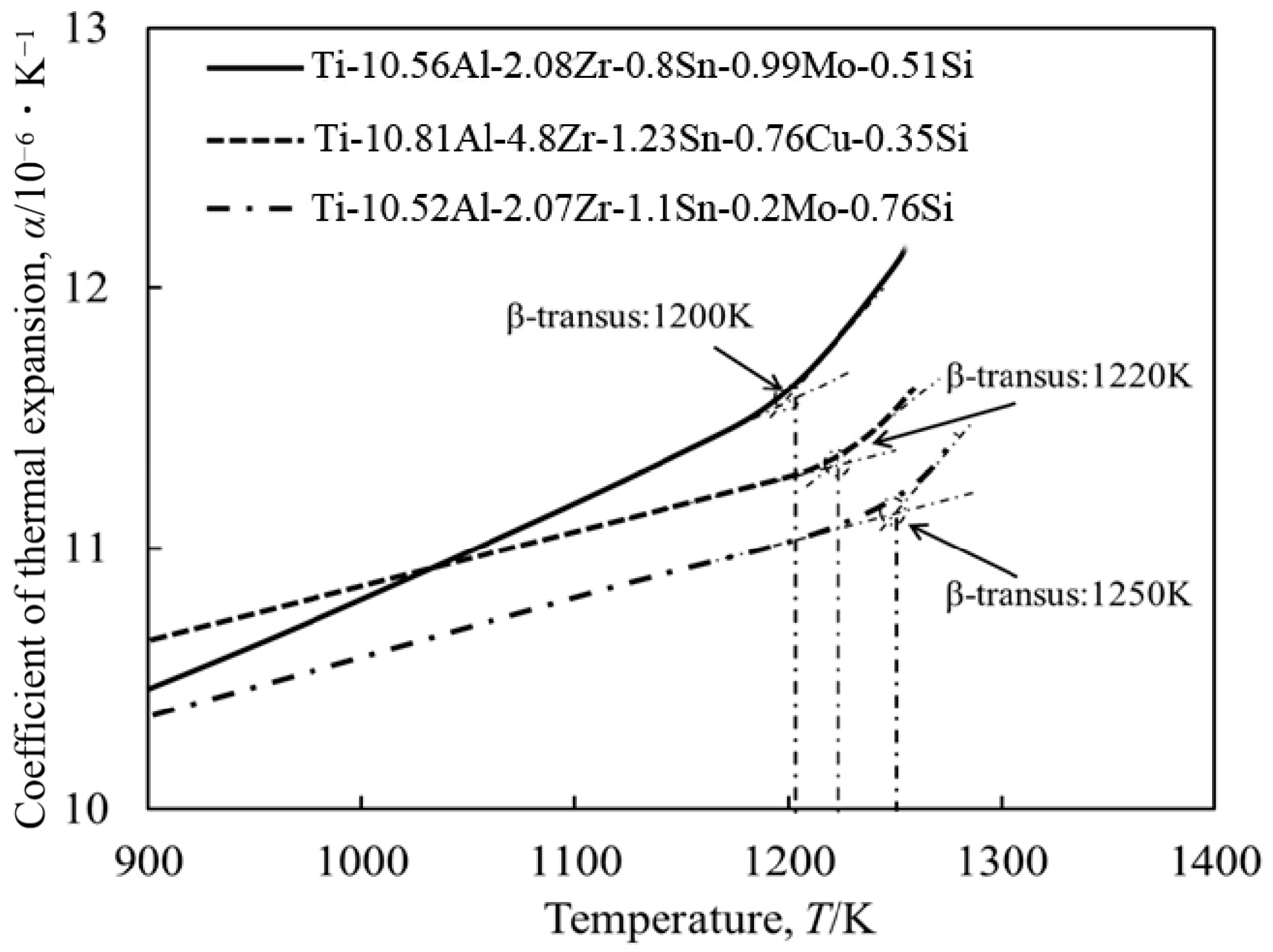
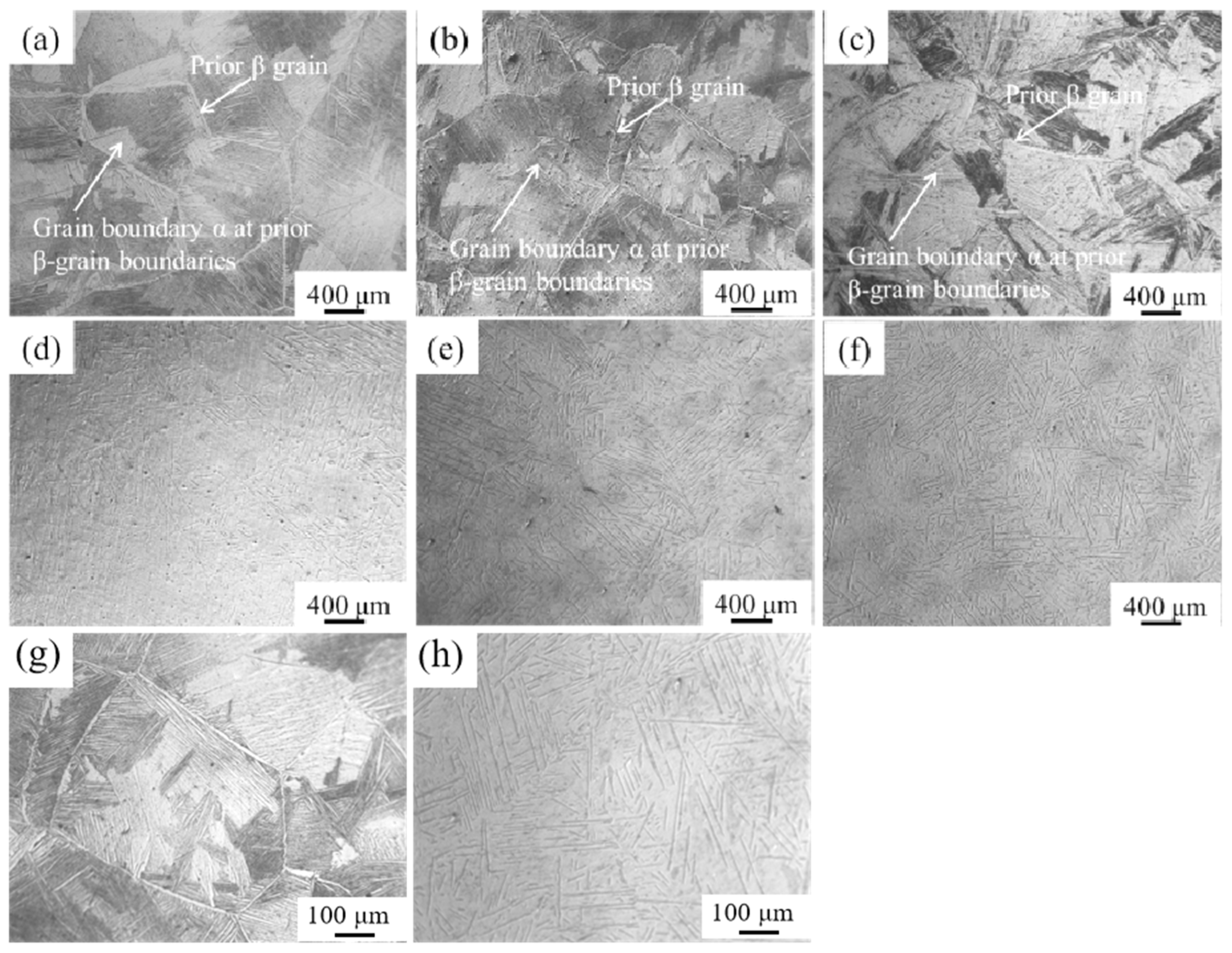
- The mechanical and corrosion properties of the three near α-Ti alloys, including two newly designed and one commercial near α-Ti alloy, were compared by considering their Bot and Mdt values; the three near α-Ti alloys were fabricated by CCLM with the same processing parameters. The three near α-Ti alloys showed thin films of β phase at the α interfaces in the as-cast condition and acicular α or α’ phase after solution treatment.
- (2)
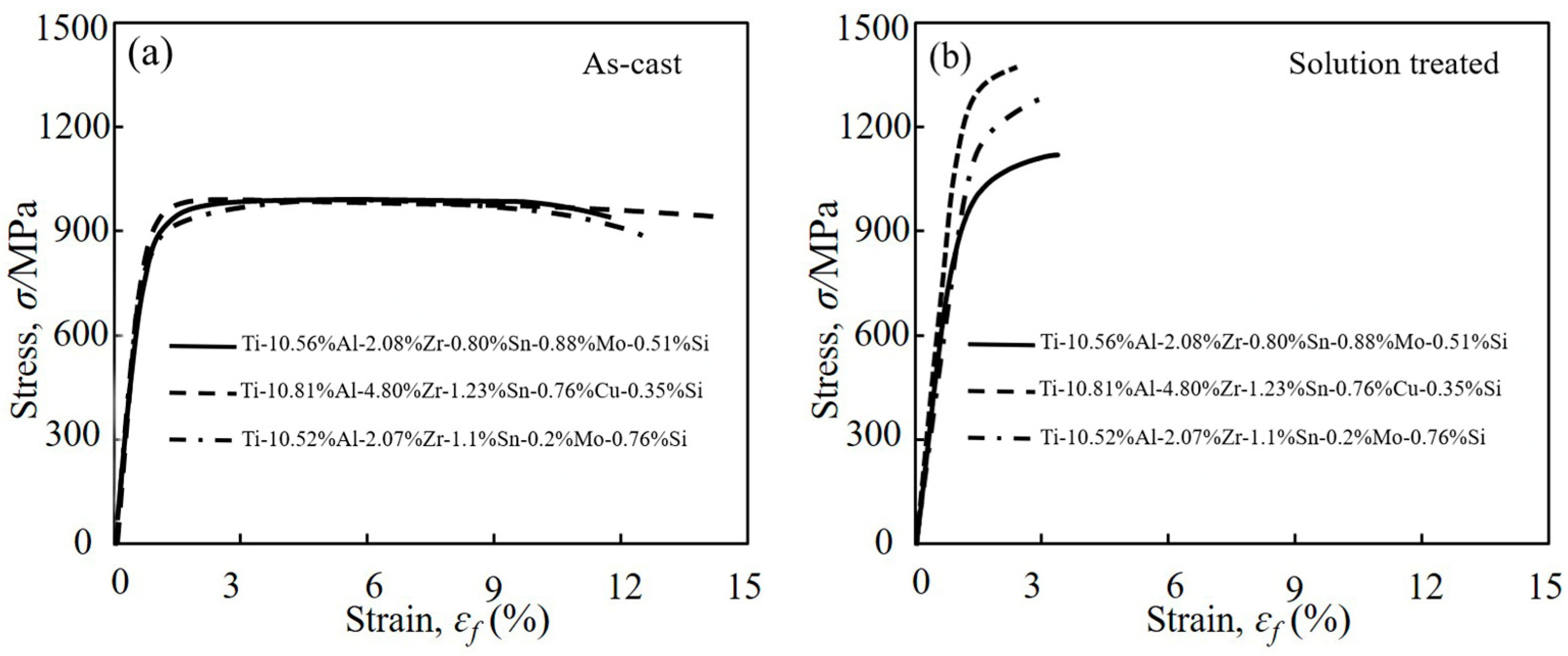
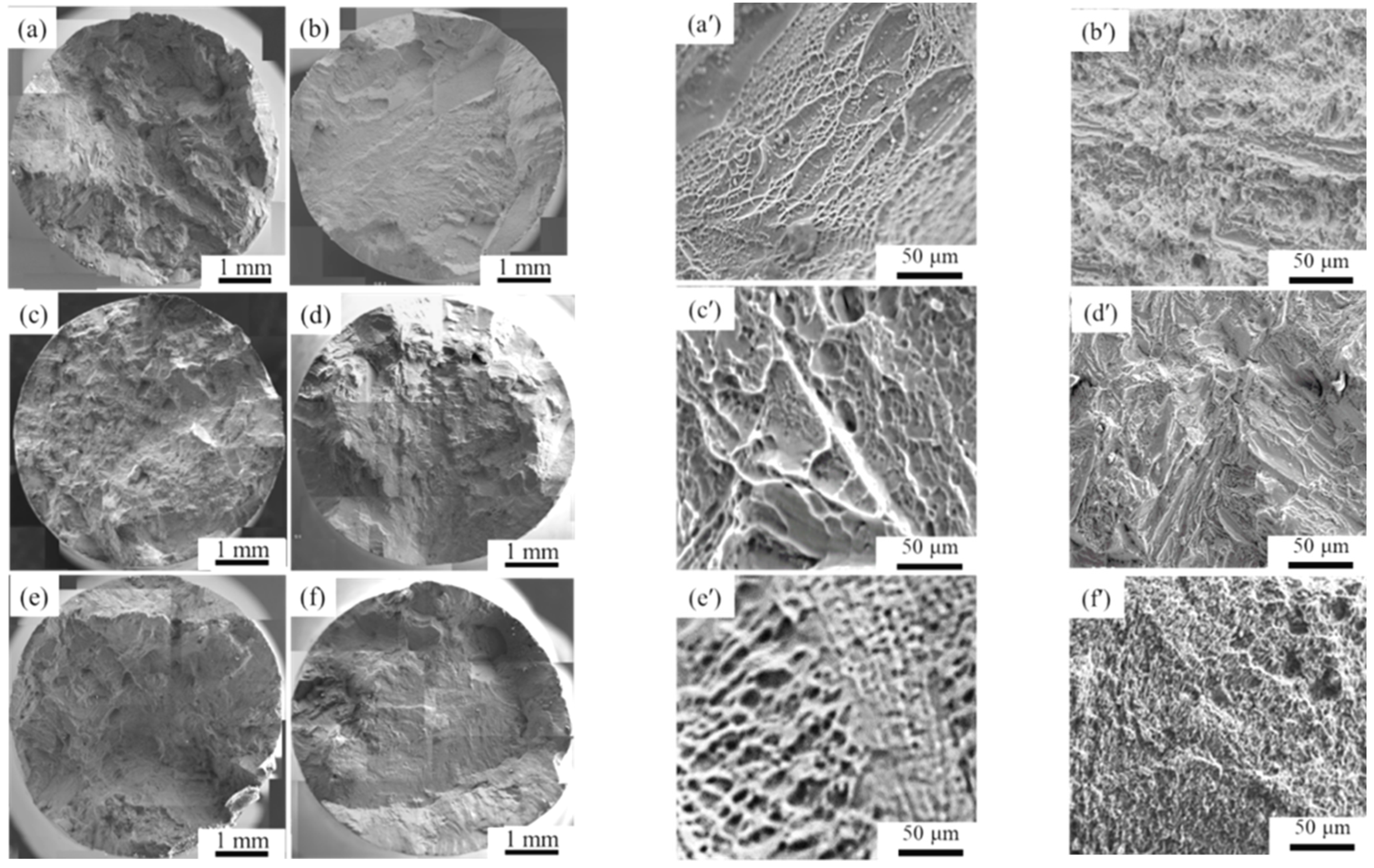
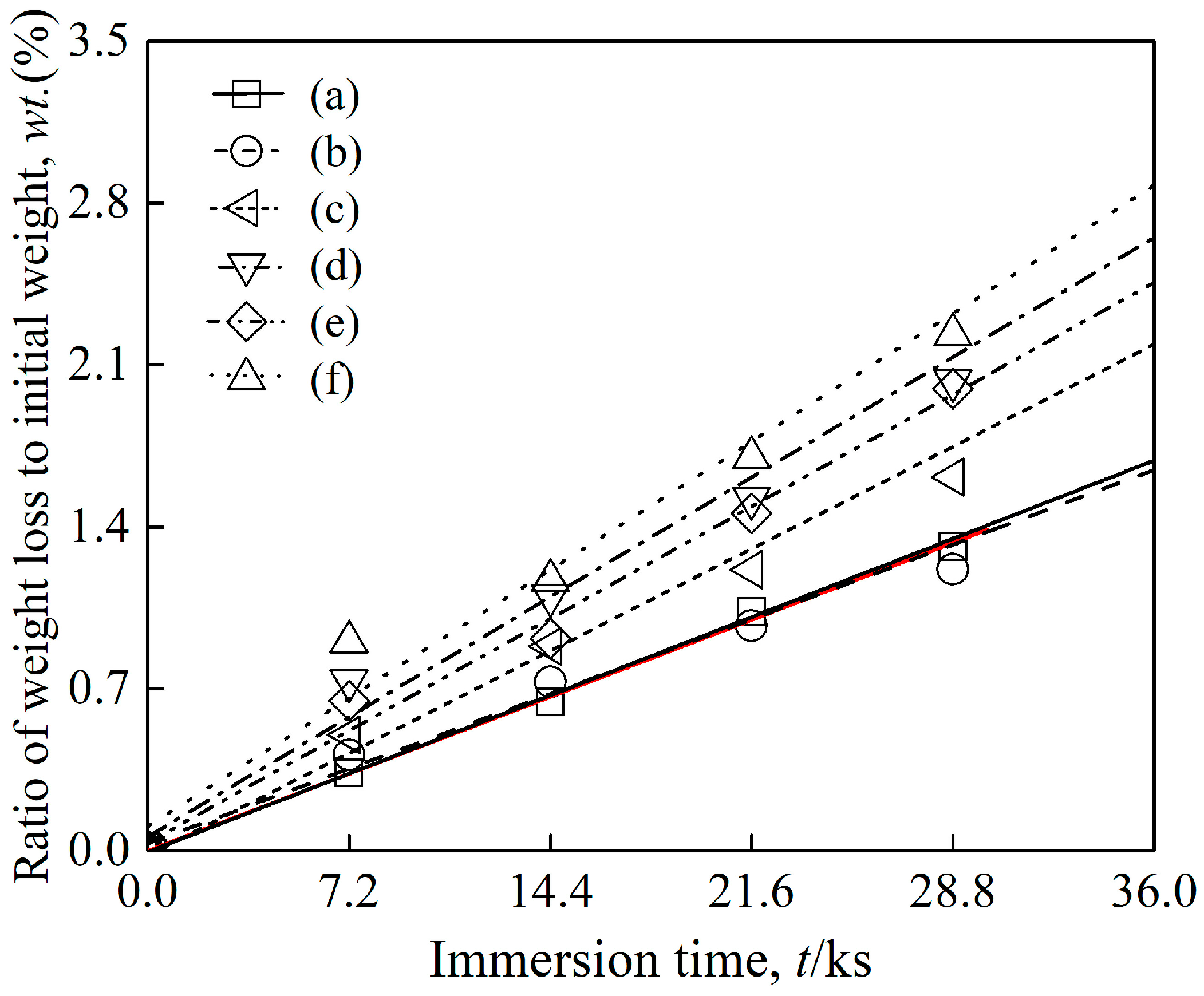
- The tensile results indicated that both the designed and modified near α-Ti alloys exhibited comparable mechanical properties to the reference near α-Ti alloy at both as-cast and after solution treatment conditions. The hot salt corrosion test after 28.8 ks revealed that the designed and modified near α-Ti alloys displayed improved hot salt corrosion resistance ability compared to the reference near α-Ti alloy.
- (3)
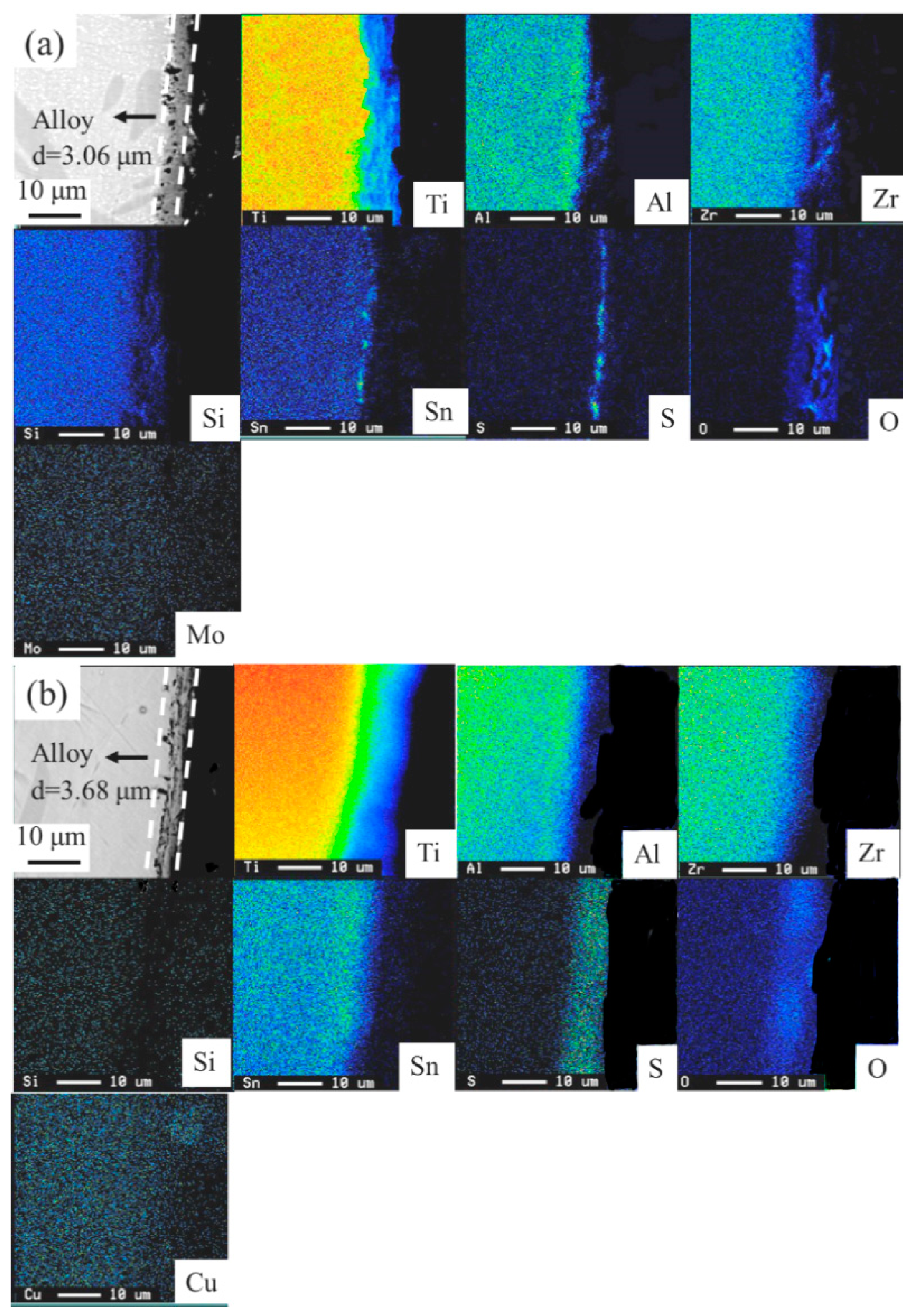
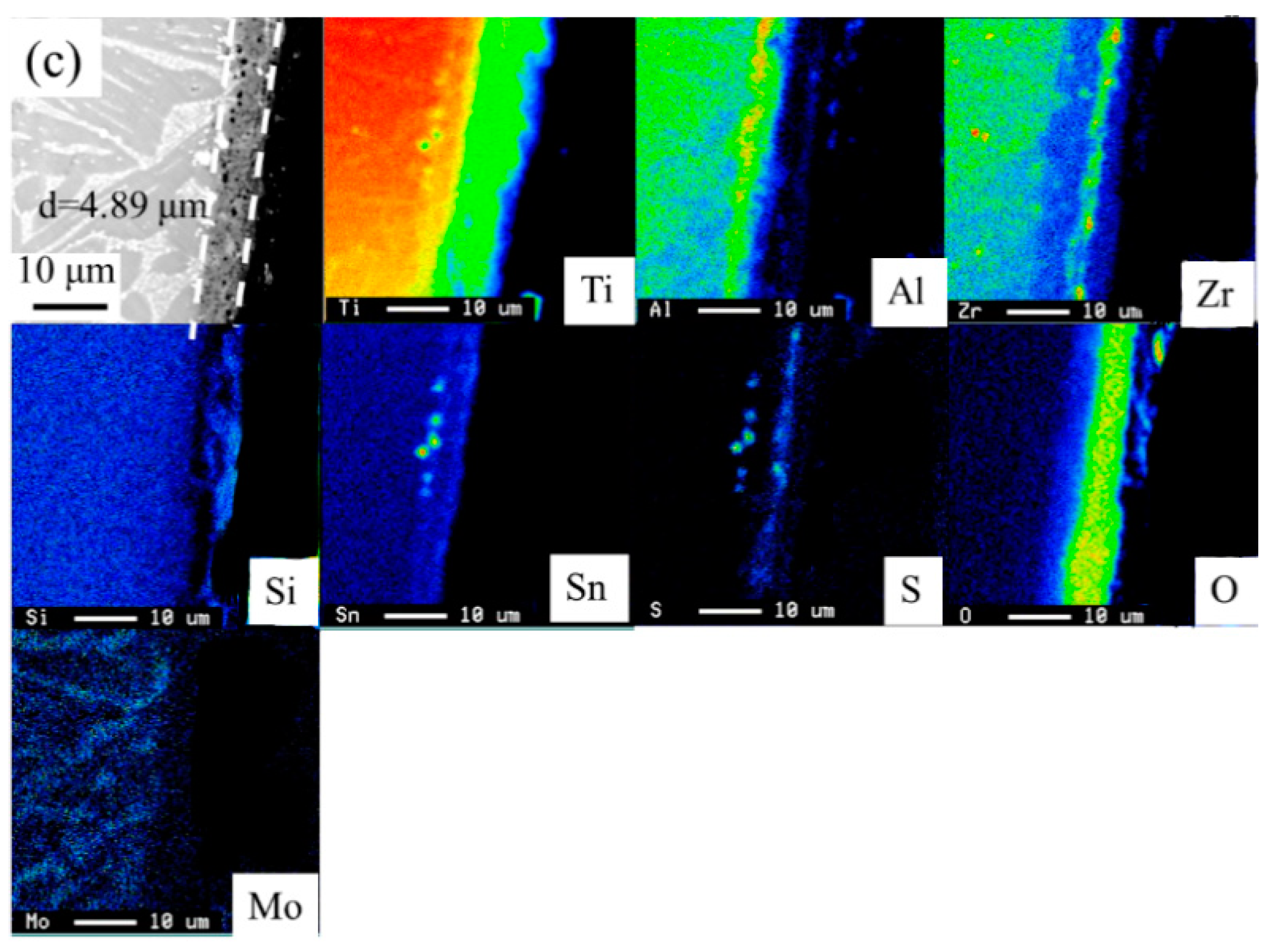
- The consideration of d-electrons was a possible approach for the development of new near α-Ti alloys with optimized tensile properties and hot salt corrosion resistance. The modified alloy Ti-10.81%Al-4.80%Zr-1.23%Sn-0.76%Cu-0.35%Si showed σUTS and ɛf values at both as-cast and solution-treated conditions that were 993 and 1354 MPa higher than those of the reference (commercial) alloy. The thickness of corrosion layers of the solution-treated designed, modified and reference near α-Ti alloys after immersion in hot salts for 28.8 ks were measured at 3.06, 3.68 and 4.89 µm.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and Applications of Titanium Alloys: A Brief Review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. Available online: https://www.researchgate.net/publication/283863116_Properties_and_applications_of_titanium_alloys_A_brief_review (accessed on 23 November 2023).
- Hardt, S.; Maier, H.J.; Christ, H.-J. High-temperature fatigue damage mechanisms in near-α titanium alloy IMI 834. Int. J. Fatigue 1999, 21, 779–789. [Google Scholar] [CrossRef]
- Gurrappa, I. Mechanism of Degradation of Titanium Alloy IMI 834 and its Protection under Hot Corrosion Conditions. Oxid. Met. 2003, 59, 321–332. [Google Scholar] [CrossRef]
- Jinwen, L.; Peng, G.; Yongqing, Z. Recent Development of Effect Mechanism of Alloying Elements in Titanium Alloy Design. Rare Met. Mater. Eng. 2014, 43, 0775–0779. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, R.; Wang, Z.; Cai, J.; Chen, B. High temperature fatigue behavior of a near-alpha titanium alloy. Int. J. Fatigue 2022, 161, 106918. [Google Scholar] [CrossRef]
- Fu, B.; Wang, H.; Zou, C.; Wei, Z. The effects of Nb content on microstructure and fracture behavior of near a titanium alloys. Mater. Des. 2016, 106, 371–379. [Google Scholar] [CrossRef]
- Cai, C.; Song, B.; Xue, P.; Wei, Q.; Yan, C.; Shi, Y. A novel near α-Ti alloy prepared by hot isostatic pressing: Microstructure evolution mechanism and high temperature tensile properties. Mater. Des. 2016, 106, 371–379. [Google Scholar] [CrossRef]
- Akbarpour, M.R.; Mirabad, H.M.; Hemmati, A.; Kim, H.S. Processing and microstructure of Ti-Cu binary alloys: A comprehensive review. Prog. Mater. Sci. 2022, 127, 100933. [Google Scholar] [CrossRef]
- Liang, C.P.; Gong, H.R. Fundamental Influence of Hydrogen on Various Properties of α-Titanium. Int. J. Hydrogen Energy 2010, 35, 3812–3816. [Google Scholar] [CrossRef]
- Joost, W.J.; Ankem, S.; Kuklja, M.M. Interaction between oxygen interstitials and deformation twins in alpha-titanium. Acta Mater. 2016, 105, 44–51. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1359645415301282#:~:text=Oxygen%20%28O%29%20has%20up%20to%2033%20at%25%20solubility,the%20shearing%2Fshuffling%20process%20associated%20with%20twin%20growth%20%5B7%5D (accessed on 20 November 2023). [CrossRef]
- Matsugi, K.; Kashiwagi, T.; Choi, Y. Property-Control of TiNi System Intermetallics and Their Characteristics. Mater. Trans. 2011, 52, 2189–2196. Available online: https://www.jstage.jst.go.jp/article/matertrans/52/12/52_F-M2011827/_article (accessed on 23 November 2023). [CrossRef]
- Ma, X.L.; Matsugi, K.; Xu, Z.F. Possibility of As-Cast Applications on β-Type Titanium Alloys Proposed in the Newly Expanded Area of Bot-Mdt Diagram. Mater. Trans. 2020, 61, 740–749. Available online: https://www.jstage.jst.go.jp/article/matertrans/61/4/61_F-M2020803/_article/-char/en (accessed on 23 November 2023). [CrossRef]
- Morinaga, M.; Yukawa, N.; Maya, T. Theoretical Design Of Titanium-Alloys. In Proceedings of the 6th World Conference on Titanium, TMS, Cannes, France, 6–9 June 1988; pp. 1601–1606. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=3469458852026c21a0fe7a145cb8be3f (accessed on 23 November 2023).
- Ma, X.L.; Matsugi, K.; Xu, Z.F. Applicability of As-Cast on β Type Titanium Alloys Proposed in the Compositional Region with Different Tensile Deformation Types. Mater. Trans. 2019, 60, 2426–2434. Available online: https://www.jstage.jst.go.jp/article/matertrans/60/11/60_F-M2019848/_article/-char/en (accessed on 23 November 2023). [CrossRef]
- Matsugi, K.; Kishimoto, H.; Yamakawa, D.; Xu, Z.F.; Choi, Y.B. Mater. Compositional Optimization of β Type Titanium Alloys with Shape Memory Ability and Their Characteristics. Mater. Trans. 2015, 56, 1747–1755. [Google Scholar] [CrossRef]
- Minghang, L.; Yong, G.; Gang, L.; Ziyan, G.; Xue, R.; Shijiang, X. Uncovering spatiotemporal evolution of titanium in China: A dynamic material flow analysis. Resour. Conserv. Recycl. 2022, 180, 106166. [Google Scholar] [CrossRef]
- Li, C.C.; Xin, C.; Wang, Q.; Ren, J.Q.; Zhao, B.; Wu, J.P.; Pan, X.L.; Lu, X.F. A novel low-cost high-strength β titanium alloy: Microstructure evolution and mechanical behavior. J. Alloys Compd. 2023, 959, 170497. [Google Scholar] [CrossRef]
- Guo, Y.H.; Niu, J.Z.; Cao, J.X.; Sun, Z.G.; Dan, Z.H.; Chang, H. Relative strength of β phase stabilization by transition metals in titanium alloys: The Mo equivalent from a first principles study. Mater. Today Commun. 2023, 35, 106123. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, B.; Wang, L.M.; Li, K.D.; Yin, B.Q.; Zhang, Z.H.; Li, J.H. Investigation of strain partition behavior in the lamellar microstructure of dual-phase titanium alloy based on crystal plasticity simulations. Mater. Sci. Eng. A 2023, 880, 145321. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.-H. A review on alloy design, biological response, and strengthening of β-titanium alloys as biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, C.; Liaw, P.K. Structural Stabilities of β-Ti Alloys Studied Using a New Mo Equivalent Derived from [β/(α+β)] PhaseBoundary Slopes. Metall. Mater. Trans. A 2015, 46, 3440–3447. [Google Scholar] [CrossRef]
- Gouthamraj, K.; Venkatesan, S. A review on β-Ti alloys for biomedical applications: The influence of alloy composition and thermomechanical processing on mechanical properties, phase composition, and microstructure. Sage J. 2022, 237, 1251–1294. [Google Scholar] [CrossRef]
- Boraei, N.F.E.; Ibrahim, M.A.; Rehim, S.S.A.E.; Elshamy, I.H. Electrochemical corrosion behavior of β-Ti alloy in a physiological saline solution and the impact of H2O2 and albumin. J. Solid State Electrochem. 2023. [Google Scholar] [CrossRef]
- Elshamy, I.H.; Ibrahim, M.A.M.; Abdel Rehim, S.S. Electrochemical Characteristics of a Biomedical Ti70Zr20Nb7.5Ta2.5 Refractory High Entropy Alloy in an Artificial Saliva Solution. J. Bio Tribo Corros. 2023, 9, 10. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, K.; Lei, S.; Zhang, D.; Guo, L.; Dai, Y.; Lin, J. A β-type titanium alloy with high strength and low elastic modulus achieved by spinodal decomposition. J. Alloys Compd. 2023, 963, 171270. [Google Scholar] [CrossRef]
- Morinaga, M.; Saito, J.; Morishita, M. Design of titanium alloys by means of a d-electrrons theory. Light Met. 1992, 42, 614–621. [Google Scholar] [CrossRef]
- Masao, M.; Masahiro, C.; Yoshio, A.; Masahiko, M.; Natsuo, Y.; Hirohiko, A. Electronic States of the Cathodes of Titanium-based Alloys in Aqueous Corrosion. Mater. Trans. 1991, 32, 264–271. [Google Scholar] [CrossRef]
- Jiang, X.J.; Zhou, Y.K.; Feng, Z.H. Influence of Zr content on β-phase stability in α-type Ti–Al alloys. Mater. Sci. Eng. A 2015, 639, 407–411. [Google Scholar] [CrossRef]
- Laheurte, P.; Prima, F.; Eberhardt, A. Mechanical properties of low modulus β titanium alloys designed from the electronic approach. J. Mech. Behav. Biomed. Mater. 2010, 3, 565–573. [Google Scholar] [CrossRef]
- Morinaga, M. Alloy Design Based on Molecular Orbital Method. Mater. Trans. 2016, 57, 213–226. [Google Scholar] [CrossRef]
- Ramachandra, C.; Singh, A.K.; Sarma, G.M.K. Microstructural Characterisation of near α-Ti Alloy Ti-6Al-4Sn-4Zr-0.70Nb-0.50Mo-0.40Si. Metall. Trans. A 1993, 24, 1273–1280. [Google Scholar] [CrossRef]
- Sridhar, G.; Arma, D.S. Structure and Properties of a Near-α Titanium Alloy After β Solution Treatment and Aging at 625 °C. Metall. Trans. A 1988, 19, 3025–3033. [Google Scholar] [CrossRef]
- Guo, G.S.; Meng, Q.; Cheng, X.; Zhao, X. α′ martensite Ti–10Nb–2Mo–4Sn alloy with ultralow elastic modulus and High strength. Mater. Lett. 2014, 133, 236–239. [Google Scholar] [CrossRef]
- Eylon, D.; Fujishiro, S.; Postans, P.J.; Froes, F.H. High-Temperature Titanium Alloys-A Review. J. Met. 1984, 11, 55–62. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys. Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 1–36. [Google Scholar]
- Zhao, E.; Sun, S.; Zhang, Y. Recent advances in silicon containing high temperature titanium alloys. J. Mater. Res. Technol. 2021, 14, 3029–3042. [Google Scholar] [CrossRef]
- Weck, A.; Wilkinson, D.S.; Maire, E. Observation of void nucleation, growth and coalescence in a model metal matrix composite using X-ray tomography. Mater. Sci. Eng. A 2008, 488, 435–445. [Google Scholar] [CrossRef]
- Myers, J.R.; Bomberger, H.B.; Froes, F.H.E. Corrosion Behavior and Use of Titanium and Its Alloys. J. Miner. Met. Mater. Soc. 1984, 36, 50–60. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhu, J.Y.; Chen, C.H.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.-L.; Matsugi, K.; Liu, Y. Design of Near α-Ti Alloys with Optimized Mechanical and Corrosion Properties and Their Characterizations. Metals 2024, 14, 81. https://doi.org/10.3390/met14010081
Ma X-L, Matsugi K, Liu Y. Design of Near α-Ti Alloys with Optimized Mechanical and Corrosion Properties and Their Characterizations. Metals. 2024; 14(1):81. https://doi.org/10.3390/met14010081
Chicago/Turabian StyleMa, Xi-Long, Kazuhiro Matsugi, and Ye Liu. 2024. "Design of Near α-Ti Alloys with Optimized Mechanical and Corrosion Properties and Their Characterizations" Metals 14, no. 1: 81. https://doi.org/10.3390/met14010081
APA StyleMa, X.-L., Matsugi, K., & Liu, Y. (2024). Design of Near α-Ti Alloys with Optimized Mechanical and Corrosion Properties and Their Characterizations. Metals, 14(1), 81. https://doi.org/10.3390/met14010081




