Liquid Metal Embrittlement Susceptibility of Hot Formed Zn-Al-Mg Coated Steel with Eutectic Coating Microstructure
Abstract
1. Introduction
2. Experimental and Calculations
2.1. Design of Zn-Al-Mg Coating Alloys
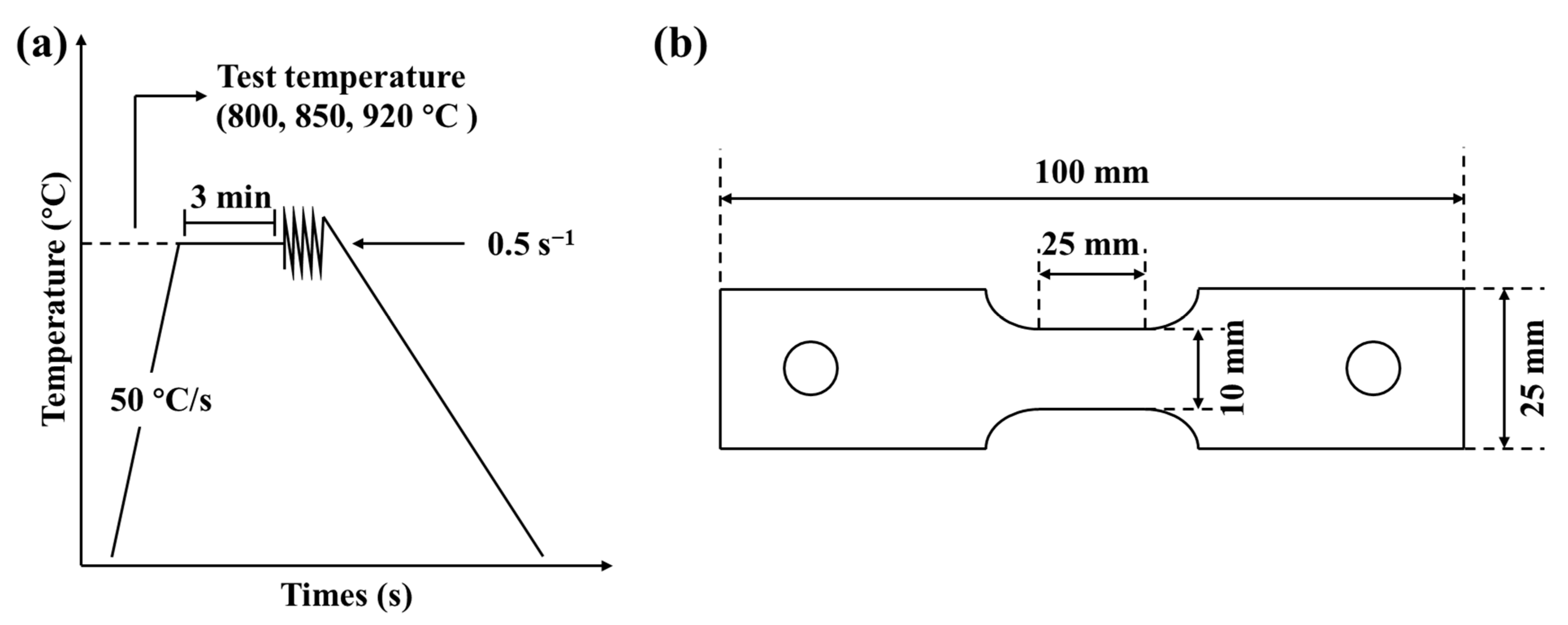
2.2. Materials and Test Methods
3. Results and Discussion
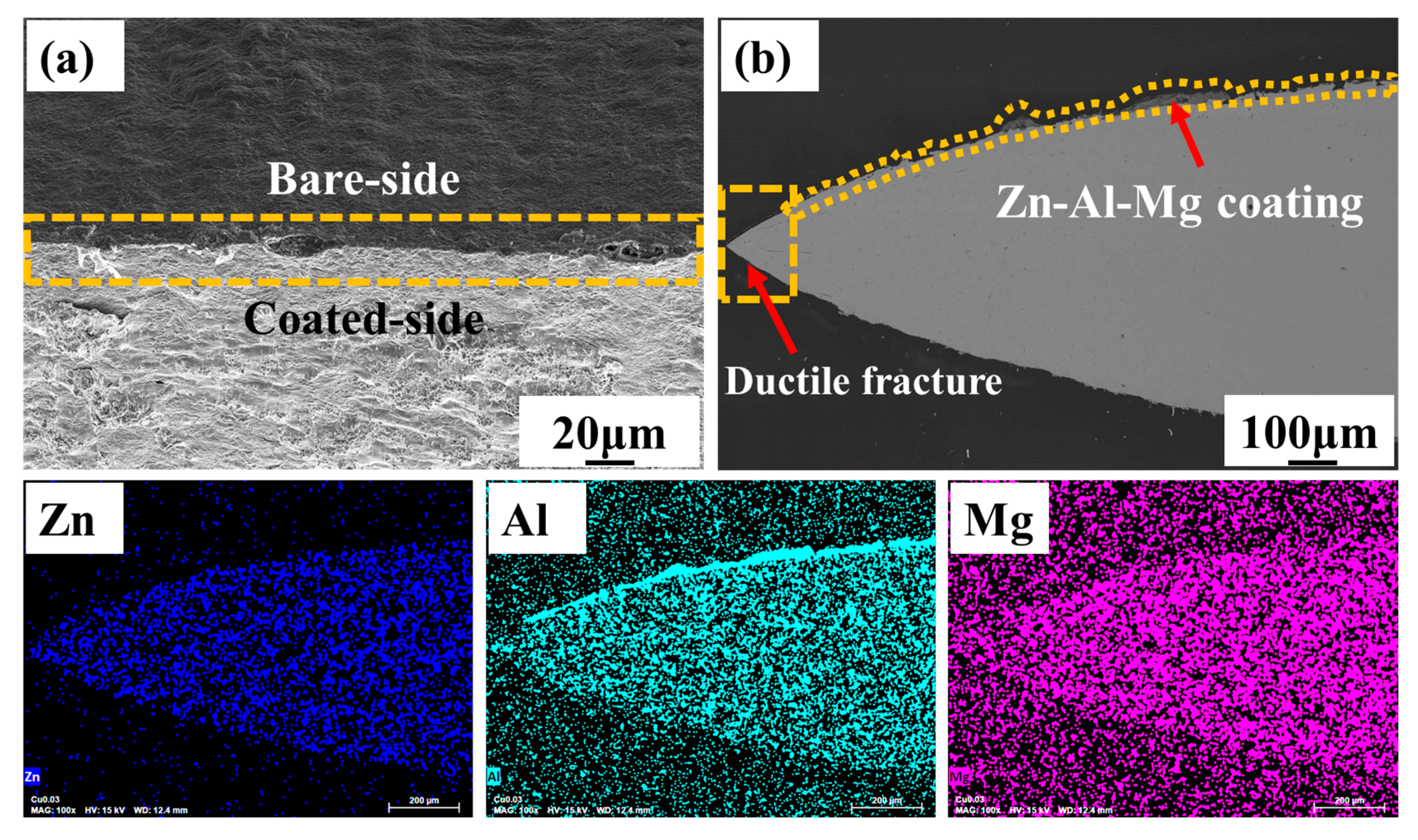
3.1. Microstructural Analysis of Zn-Al-Mg Coated Steel
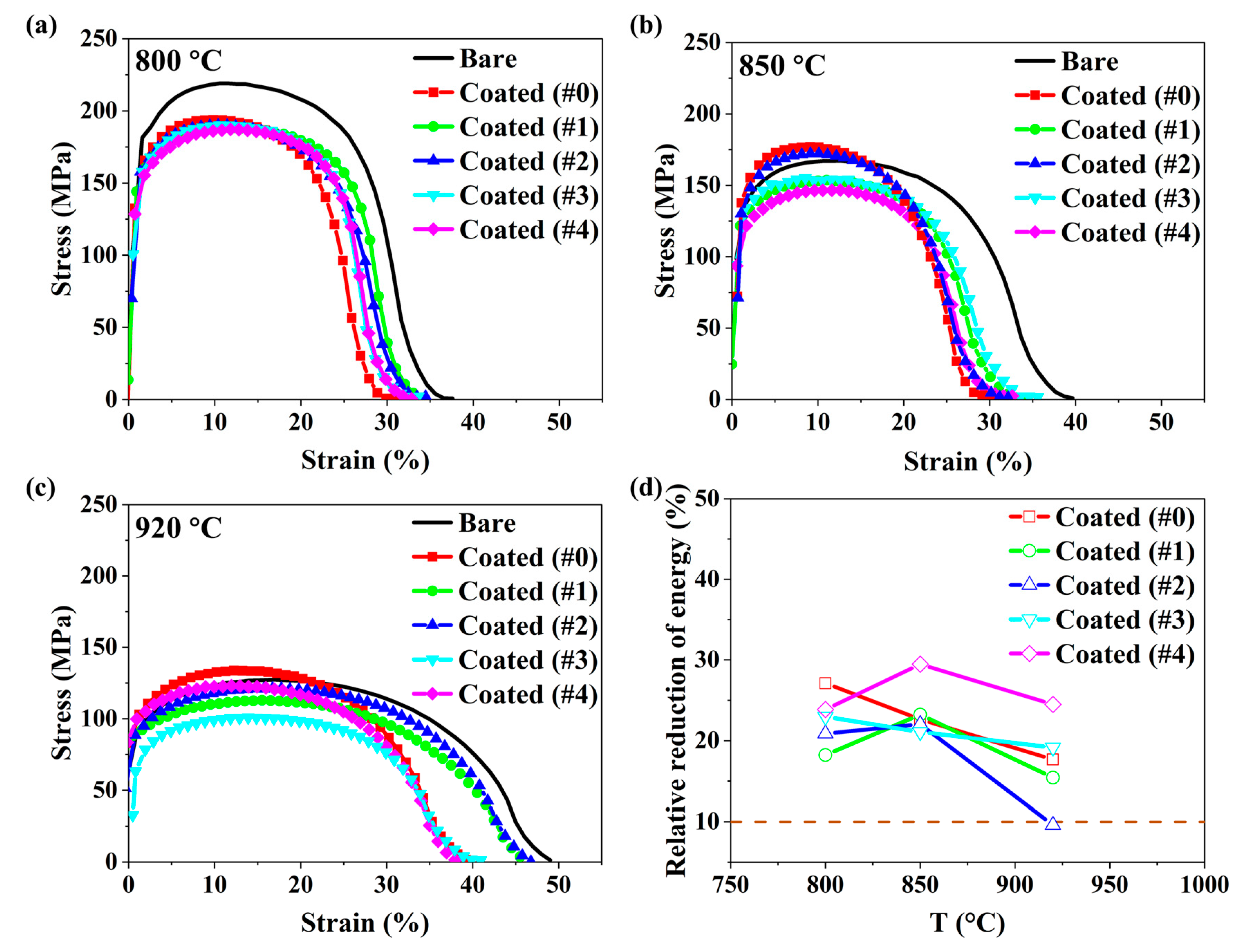
3.2. The LME Behavior of Zn-Al-Mg Coated Steel
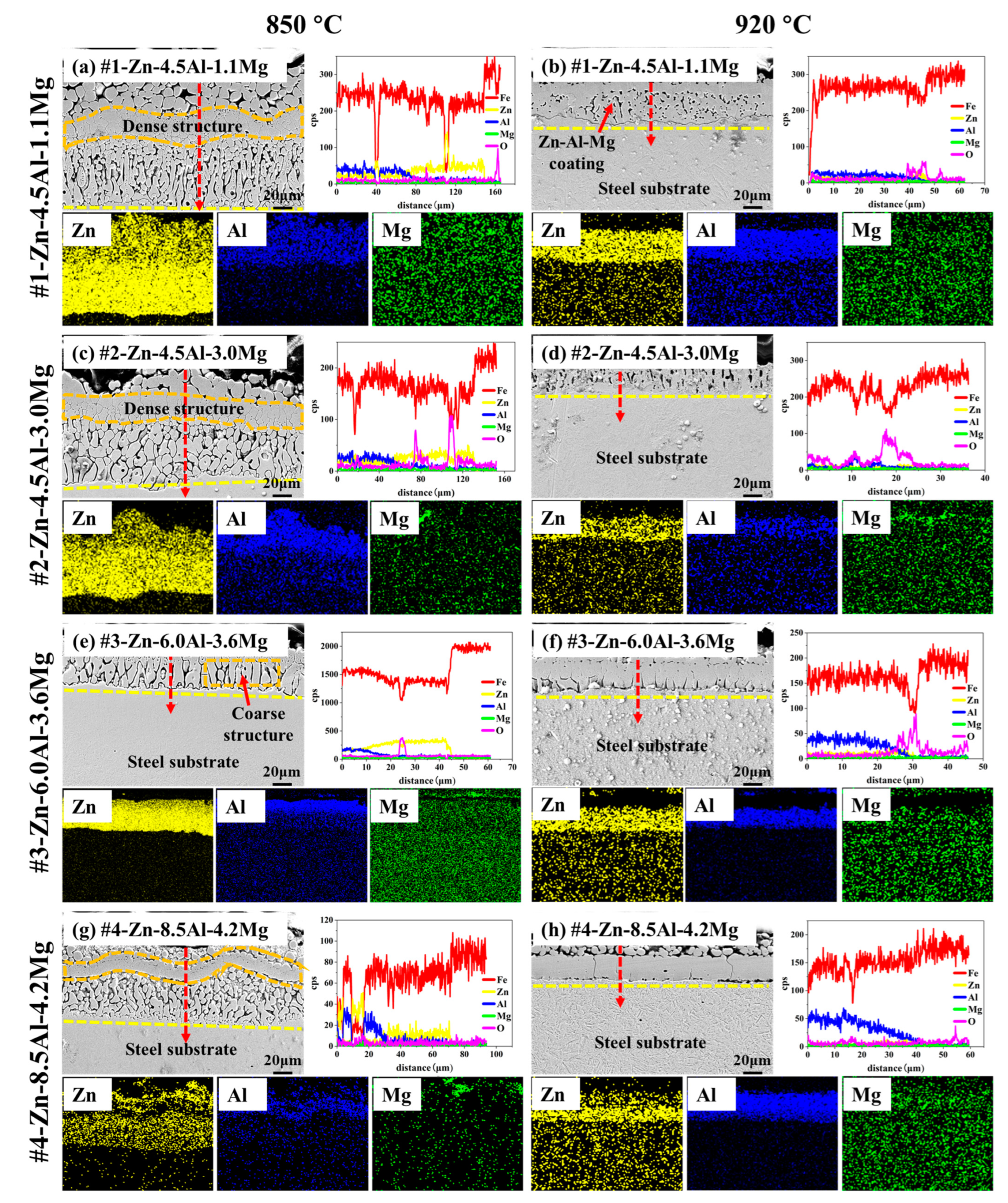
3.3. Microstructure during High-Temperature Heat Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.; Kang, J.-H.; Kim, S.-J. Heating rate effect on liquid Zn-assisted embrittlement of high Mn austenitic steel. Surf. Coat. Technol. 2018, 347, 157–163. [Google Scholar] [CrossRef]
- Ghatei-Kalashami, A.; Ghassemali, E.; DiGiovanni, C.; Goodwin, F.; Zhou, N.Y. Occurrence of liquid-metal-embrittlement in a fully ferritic microstructure. Materialia 2021, 15, 101036. [Google Scholar] [CrossRef]
- Ghatei-Kalashami, A.; Ghassemali, E.; DiGiovanni, C.; Goodwin, F.; Zhou, N. Liquid metal embrittlement cracking behavior in iron-zinc (Fe/Zn) couple: Comparison of ferritic and austenitic microstructures. Mater. Lett. 2022, 324, 132780. [Google Scholar] [CrossRef]
- Kim, J.; Murugan, S.; Kim, J.; Wan, Y.; Lee, C.Y.; Ji, C.; Jeon, J.; Park, Y.-D. Liquid metal embrittlement during the resistance spot welding of galvannealed steels: Synergy of liquid Zn, α-Fe(Zn) and tensile stress. Sci. Technol. Weld. Join. 2021, 26, 196–204. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Cho, L.; van der Aa, E.; Pichler, A.; Pottore, N.; Ghassemi-Armaki, H.; Findley, K.O.; Speer, J.G. Influence of the starting microstructure of an advanced high strength steel on the characteristics of Zn-Assisted liquid metal embrittlement. Mater. Sci. Eng. A 2021, 804, 140391. [Google Scholar] [CrossRef]
- Ikeda, Y.; Yuan, R.; Chakraborty, A.; Ghassemi-Armaki, H.; Zuo, J.M.; Maaß, R. Early stages of liquid-metal embrittlement in an advanced high-strength steel. Mater. Today Adv. 2022, 13, 100196. [Google Scholar] [CrossRef]
- Kang, J.-H.; Hong, S.-H.; Kim, J.; Kim, S.-J. Zn-induced liquid metal embrittlement of galvanized high-Mn steel: Strain-rate dependency. Mater. Sci. Eng. A 2020, 793, 139996. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Cho, L.; Marshall, D.; Walker, M.; van der Aa, E.; Pichler, A.; Ghassemi-Armaki, H.; Findley, K.O.; Speer, J.G. Liquid metal embrittlement susceptibility of two Zn-Coated advanced high strength steels of similar strengths. Mater. Sci. Eng. A 2021, 823, 141569. [Google Scholar] [CrossRef]
- Chen, H.; Xu, W.; Luo, Q.; Li, Q.; Zhang, Y.; Wang, J.; Chou, K.-C. Thermodynamic prediction of martensitic transformation temperature in Fe-C-X (X=Ni, Mn, Si, Cr) systems with dilatational coefficient model. J. Mater. Sci. Technol. 2022, 112, 291–300. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Chen, H.; Luo, Q.; Li, Q. Icosahedral quasicrystal structure of the Mg40Zn55Nd5 phase and its thermodynamic stability. Int. J. Miner. Metall. Mater. 2022, 29, 1543–1550. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Z.; Wu, G.; Mao, X. Titanium microalloying of steel: A review of its effects on processing, microstructure and mechanical properties. Int. J. Miner. Metall. Mater. 2022, 29, 645–661. [Google Scholar] [CrossRef]
- Cai, R.; Wang, W.J.; Zhao, N.; Wang, F.Y.; Duan, J.L.; Hu, H.J.; Yu, Q.H.; Cheng, G.-X. Corrosion resistance of Cr2O3 coating formed by in-situ oxidation on 2205 duplex stainless steel in different pH solutions. Rare Met. 2023, 42, 2189–2196. [Google Scholar] [CrossRef]
- Mahieu, J.; De Cooman, B.C.; Claessens, S. Galvanizability of high-strength steels for automotive applications. Metall. Mater. Trans. A 2001, 32, 2905–2908. [Google Scholar] [CrossRef]
- Chun, E.J.; Lim, S.S.; Kim, Y.T.; Nam, K.S.; Kim, Y.M.; Park, Y.W.; Murugan, S.P.; Park, Y.D. Influence of heat-treated Al–Si coating on the weldability and microstructural inhomogeneity for hot stamped steel resistance nut projection welds. Met. Mater. Int. 2019, 25, 179–192. [Google Scholar] [CrossRef]
- Ma, Z.; Peng, W.; Ding, C.; Wu, G.; Zhang, J. Study on Cracking Behavior of High-strength Steels by Liquid Zinc Induced Embrittlement. Steel Res. Int. 2021. [Google Scholar] [CrossRef]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Razmpoosh, M.H.; DiGiovanni, C.; Zhou, Y.N.; Biro, E. Pathway to understand liquid metal embrittlement (LME) in Fe-Zn couple: From fundamentals toward application. Prog. Mater. Sci. 2021, 121, 100798. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Cho, L.; van der Aa, E.; Ghassemi-Armaki, H.; Pichler, A.; Findley, K.O.; Speer, J.G. Transgranular cracking in a liquid Zn embrittled high strength steel. Scr. Mater. 2020, 175, 49–54. [Google Scholar] [CrossRef]
- DiGiovanni, C.; Ghatei Kalashami, A.; Biro, E.; Zhou, N.Y. Liquid metal embrittlement transport mechanism in the Fe/Zn system: Stress-assisted diffusion. Materialia 2021, 18, 101153. [Google Scholar] [CrossRef]
- Ghatei-Kalashami, A.; Khan, M.S.; Lee, M.-Y.; Zhou, Y.N. High-temperature phase evolution of the ZnAlMg coating and its effect on mitigating liquid-metal-embrittlement cracking. Acta Mater. 2022, 229, 117836. [Google Scholar] [CrossRef]
- Hua, Y.X.; Song, K.X.; Liu, H.T.; Wang, J.W.; Zhang, C.M.; Zhou, Y.J.; Pang, B.; Song, J.T.; He, J.L.; Zhao, H.L. Role of grain boundary character on Bi segregation-induced embrittlement in ultrahigh-purity copper. J. Mater. Sci. Technol. 2023, 159, 52–61. [Google Scholar] [CrossRef]
- Jung, G.; Woo, I.S.; Suh, D.W.; Kim, S.-J. Liquid Zn assisted embrittlement of advanced high strength steels with different microstructures. Met. Mater. Int. 2016, 22, 187–195. [Google Scholar] [CrossRef]
- Murugan, S.P.; Kim, J.; Kim, J.; Wan, Y.; Lee, C.; Jeon, J.B.; Park, Y.-D. Role of liquid Zn and α-Fe(Zn) on liquid metal embrittlement of medium Mn steel: An ex-situ microstructural analysis of galvannealed coating during high temperature tensile test. Surf. Coat. Technol. 2020, 398, 126069. [Google Scholar] [CrossRef]
- Lee, H.; Jo, M.C.; Sohn, S.S.; Kim, S.-H.; Song, T.; Kim, S.-K.; Kim, H.S.; Kim, N.J.; Lee, S. Microstructural evolution of liquid metal embrittlement in resistance-spot-welded galvanized TWinning-Induced Plasticity (TWIP) steel sheets. Mater. Charact. 2019, 147, 233–241. [Google Scholar] [CrossRef]
- Razmpoosh, M.H.; Macwan, A.; Biro, E.; Chen, D.L.; Peng, Y.; Goodwin, F.; Zhou, Y. Liquid metal embrittlement in laser beam welding of Zn-coated 22MnB5 steel. Mater. Des. 2018, 155, 375–383. [Google Scholar] [CrossRef]
- Pang, J.-L.; Zhu, Z.-L.; Zhang, J.-Y.; Chen, Q.; Zhou, J.; Meng, Y.; Sugiyama, S. Thermal forming properties of a Cr-Mn-Si-Ni alloyed naval steel under different forming conditions by different constitutive models. Rare Met. 2022, 41, 3515–3529. [Google Scholar] [CrossRef]
- Rajagopalan, M.; Bhatia, M.A.; Tschopp, M.A.; Srolovitz, D.J.; Solanki, K.N. Atomic-scale analysis of liquid-gallium embrittlement of aluminum grain boundaries. Acta Mater. 2014, 73, 312–325. [Google Scholar] [CrossRef]
- Kolman, D.G. A Review of Recent Advances in the Understanding of Liquid Metal Embrittlement. Corrosion 2018, 75, 42–57. [Google Scholar] [CrossRef]
- Gordon, P.; An, H.H. The mechanisms of crack initiation and crack propagation in metal-induced embrittlement of metals. Metall. Trans. A 1982, 13, 457–472. [Google Scholar] [CrossRef]
- Razmpoosh, M.H.; Langelier, B.; Marzbanrad, E.; Zurob, H.S.; Zhou, N.; Biro, E. Atomic-scale Investigation of Liquid-Metal-Embrittlement Crack-path: Revealing Mechanism and Role of Grain Boundary Chemistry. Acta Mater. 2021, 204, 116519. [Google Scholar] [CrossRef]
- Peng, W.; Peng, H.; Wu, G.; Zhang, J. Effect of zinc-doping on tensile strength of Σ5 bcc Fe symmetric tilt grain boundary. Comput. Mater. Sci. 2020, 171, 109204. [Google Scholar] [CrossRef]
- Glickman, E.E. Grain Boundary Grooving Accelerated by Local Plasticity as a Possible Mechanism of Liquid Metal Embrittlement. Interface Sci. 2003, 11, 451–459. [Google Scholar] [CrossRef]
- Alpas, A.T.; Inagaki, J. Effect of Microstructure on Fracture Mechanisms in Galvannealed Coatings. ISIJ Int. 2000, 40, 172–181. [Google Scholar] [CrossRef][Green Version]
- Razmpoosh, M.H.; Macwan, A.; Goodwin, F.; Biro, E.; Zhou, Y. Suppression of liquid-metal-embrittlement by twin-induced grain boundary engineering approach. Materialia 2020, 11, 100668. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, C.; Wu, G.; Zhang, J. Role of aluminum on liquid metal embrittlement susceptibility for Zn–Al–Mg/Sn coated hot-formed steels. J. Mater. Res. Technol. 2022, 19, 747–764. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.Z.; Luo, Q.; Chen, S.L.; Zhang, J.-Y.; Chou, K.-C. Experimental study and phase diagram calculation in Al–Zn–Mg–Si quaternary system. J. Alloys Compd. 2010, 501, 282–290. [Google Scholar] [CrossRef]
- Beal, C.; Kleber, X.; Fabregue, D.; Bouzekri, M. Embrittlement of a zinc coated high manganese TWIP steel. Mater. Sci. Eng. A 2012, 543, 76–83. [Google Scholar] [CrossRef]

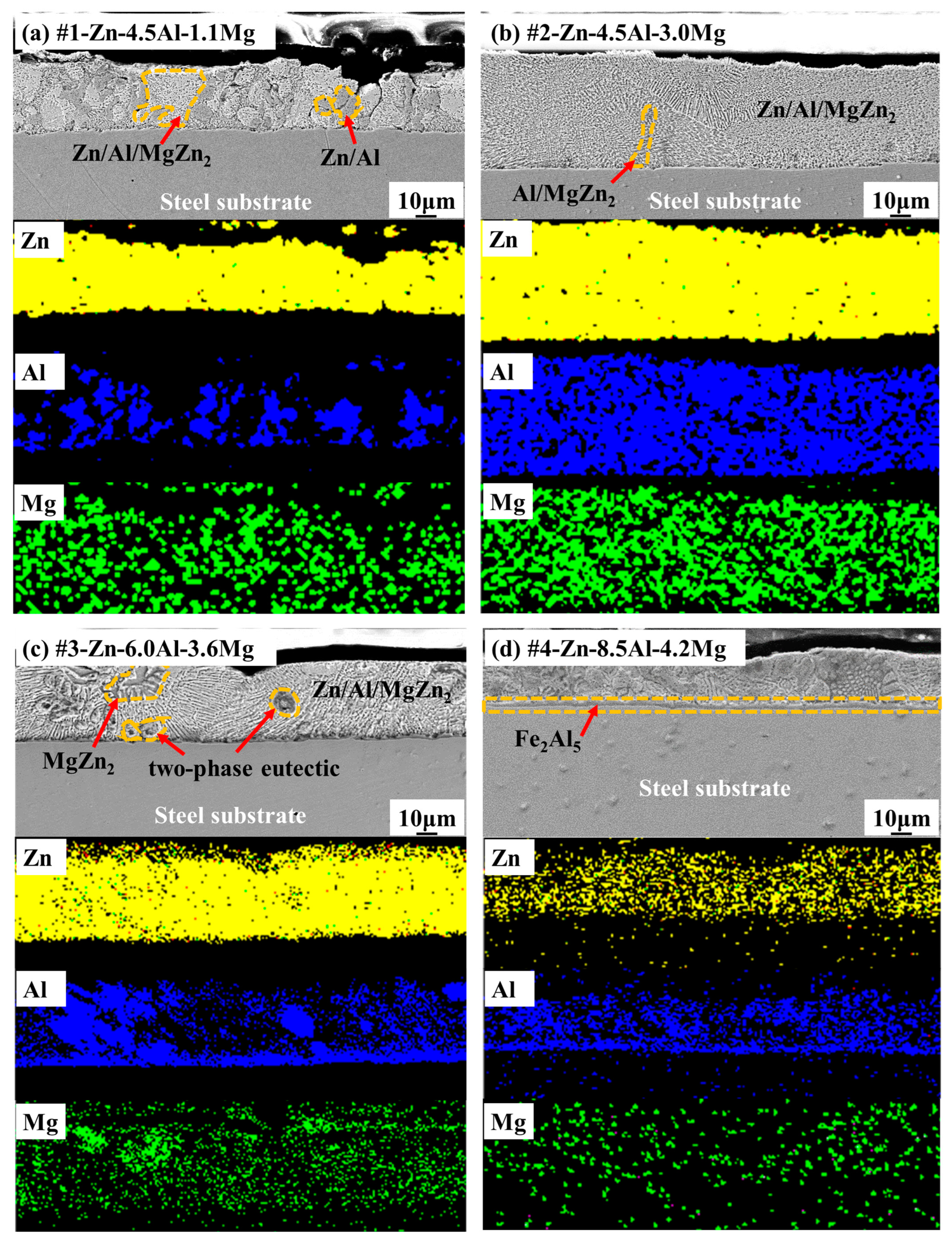
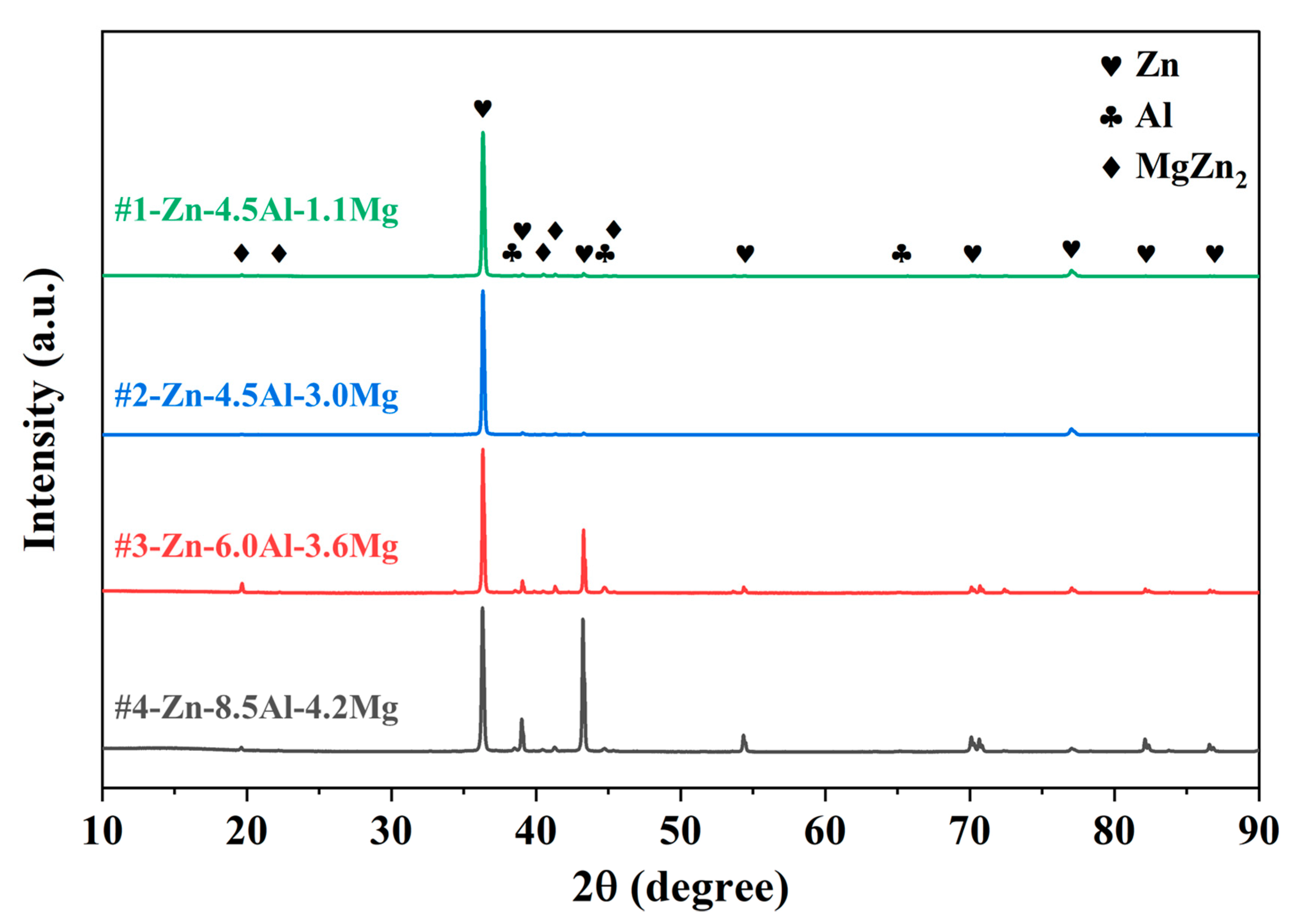
| Structure | HCP-Zn/FCC-Al | FCC-Al/MgZn2 | FCC-Al/Mg2Zn11 | HCP-Zn/FCC-Al/Mg2Zn11 | |
|---|---|---|---|---|---|
| Samples | |||||
| #1 | 56 | \ | \ | 42 | |
| #2 | \ | \ | 25 | 75 | |
| #3 | \ | 15 | 22 | 62 | |
| #4 | \ | 30 | 18 | 52 | |
| C | Si | Mn | P | S | Al | Ti | V | N | B |
|---|---|---|---|---|---|---|---|---|---|
| 0.33 | 0.10 | 1.45 | ≤0.020 | ≤0.004 | 0.080 | 0.020 | 0.18 | ≤0.0040 | 0.0020 |
| Coating | Region | Element | |||
|---|---|---|---|---|---|
| Zn | Al | Mg | Fe | ||
| #1-Zn-4.5Al-1.1Mg | Binary eutectic | 55.1 | 33.9 | 0.4 | 0.3 |
| Ternary eutectic | 88.6 | 3.3 | 0 | 1.0 | |
| #2-Zn-4.5Al-3.0Mg | Three-phase eutectic | 75.9 | 13.8 | 3.7 | 0.5 |
| #3-Zn-6.0Al-3.6Mg | Single phase | 53.8 | 3.7 | 29.7 | 0.3 |
| Two-phase eutectic | 28.4 | 55.1 | 0.9 | 0.2 | |
| Three-phase eutectic | 73.0 | 10.8 | 10.0 | 0.4 | |
| #4-Zn-8.5Al-4.2Mg | Two-phase eutectic | 36.0 | 57.1 | 0 | 0.9 |
| Three-phase eutectic | 67.5 | 7.0 | 4.3 | 1.0 | |
| Intermetallic compound | 4.6 | 67.9 | 0 | 23.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Fu, Y.; Wu, G.; Liu, H.; Chen, Y.; Luo, Q.; Li, Q. Liquid Metal Embrittlement Susceptibility of Hot Formed Zn-Al-Mg Coated Steel with Eutectic Coating Microstructure. Metals 2023, 13, 1523. https://doi.org/10.3390/met13091523
Yang Y, Fu Y, Wu G, Liu H, Chen Y, Luo Q, Li Q. Liquid Metal Embrittlement Susceptibility of Hot Formed Zn-Al-Mg Coated Steel with Eutectic Coating Microstructure. Metals. 2023; 13(9):1523. https://doi.org/10.3390/met13091523
Chicago/Turabian StyleYang, Yubo, Yu Fu, Guangxin Wu, Hongliang Liu, Yu Chen, Qun Luo, and Qian Li. 2023. "Liquid Metal Embrittlement Susceptibility of Hot Formed Zn-Al-Mg Coated Steel with Eutectic Coating Microstructure" Metals 13, no. 9: 1523. https://doi.org/10.3390/met13091523
APA StyleYang, Y., Fu, Y., Wu, G., Liu, H., Chen, Y., Luo, Q., & Li, Q. (2023). Liquid Metal Embrittlement Susceptibility of Hot Formed Zn-Al-Mg Coated Steel with Eutectic Coating Microstructure. Metals, 13(9), 1523. https://doi.org/10.3390/met13091523





