Abstract
The dehydration of titanium dioxide, which is the carrier for denitration catalysts, is a crucial control step in the preparation of functional materials and has an impact on the performance of the product. In this study, the kinetics of the dehydration behavior and reaction mechanism of titanium dioxide were investigated under different atmospheres by measuring the thermal analysis curve of titanium dioxide at different heating rates. The results indicate that the dehydration behavior of the catalyst carrier titanium dioxide is closely related to the calcination atmosphere. The dehydration rate differed for oxygen and no-oxygen atmospheres. Dehydration began quickly in an oxygenated atmosphere and then slowed down towards the end of the reaction, completing slowly in an oxygen-free atmosphere. Kinetic calculations were carried out using modeless and mode function methods. The results show that dehydration of titanium dioxide is consistent with the Avrami–Erofeev equation in an oxygen-containing atmosphere and with the power function rule in an oxygen-free atmosphere, with the process of dehydration being influenced by the formation and growth of crystal nuclei.
1. Introduction
Titanium dioxide is not only an excellent white pigment [1,2] but also an important n-type semiconductor material [3]. It exhibits special photocatalytic oxidation performance [4], optical effects [5], and ultraviolet shielding effects [6] as a functional material. These properties have made it a research hotspot in areas such as catalytic oxidation, flue gas denitration, wastewater treatment, and photoelectric conversion [7,8]. Titanium dioxide has promising applications, significant market value, and outstanding performance. The preparation methods of functional materials using titanium dioxide as a carrier for denitration catalysts include liquid-phase, gas-phase, and laser methods [9,10]. Liquid-phase methods can be divided into three main categories: sol-gel, precipitation, and microemulsion methods [11]. Gas-phase methods also consist of three types: gas-phase oxidation, gas-phase hydrolysis, and gas-phase pyrolysis methods [12,13].
Typically, the denitration catalyst carrier titanium dioxide is prepared using the liquid-phase method [14] because this method allows for the preparation of titanium dioxide by the chemical pathway of hydrolysis using titanium compounds (titanium sulphate, titanium oxy-sulphate, or a mixture of the two, as commonly used in industry) [15]. Subsequent steps such as washing, impregnation, calcination, and dehydration allow for the production of titanium dioxide as a denitration catalyst carrier for different applications [16,17,18]. In the production process of titanium dioxide using the sulfate method, the intermediate product, metatitanic acid (H2TiO3), contains a relatively high amount of water, typically ranging from 40% to 50% and sometimes even up to 60% to 70% [19,20]. This directly affects the calcination and dehydration process of the titanium dioxide catalyst support, which in turn influences the energy consumption, cost, and other economic indicators of the production. Additionally, the calcination and dehydration processes also have a decisive impact on key quality parameters such as particle size distribution, morphology, and crystalline structure [21,22]. Wang et al. [23] utilized granular activated carbon as a catalyst support and metatitanic acid as the titanium source to prepare supported TiO2 photocatalysts through an impregnation hydrothermal method. The resulting samples were subjected to dehydration and thermal treatment in the range of 300 to 800 °C. It was observed that the sample calcined at 600 °C exhibited the best photocatalytic performance. Rui et al. [24] prepared rutile-type TiO2 by microwave calcination using metatitanic acid. The effects of different process parameters on the conversion of rutile TiO2 were studied, among which only calcination temperature and time had significant effects. Chen et al. [21] investigated the influence of sulfate ions on the phase transformation and crystal growth of TiO2 during the calcination of metatitanic acid. The results of their study showed that in the desulfurization calcination process, the metatitanic acid samples with added sulfate ions exhibited solid-state crystallization and a transition from anatase to rutile at a temperature 50 °C lower compared to the untreated samples. This indicates that reducing the calcination temperature can effectively mitigate the sintering tendency of TiO2 and facilitate the preparation of uniformly sized TiO2 crystals. Wu et al. [25] conducted a study on the calcination temperature and particle growth of TiO2 during the calcination of metatitanic acid. They also observed that the transformation of TiO2 from anatase to rutile generally occurs at temperatures above 600 °C. Lu et al. [26] investigated the role of mixed salts (ZnO and P2O5) in the calcination of metatitanic acid to address the problems of high calcination temperature and long calcination time. Komarkova et al. [27] investigated the thermal degradation behavior of amine-containing amorphous (peroxo)titanate in air and argon atmospheres. They found that calcination in an air atmosphere leads to the oxidation of organic components in the reactants, which affects the performance of the product.
The above studies illustrate that the dehydration process of denitration catalyst carrier titanium dioxide has a significant influence on the particles, morphology, and particle size distribution of the prepared titanium dioxide; however, they do not discuss the dehydration kinetics of denitration catalyst carrier TiO2. In this study, titanium dioxide carrier for denitration catalyst was prepared from the titanium dioxide intermediate product in a process using sulfuric acid as raw material, whereby the kinetics of the dehydration process of titanium dioxide carrier for denitration catalyst were investigated using the thermogravimetric analysis method under different heating rates and different atmospheres, thereby providing kinetic reference guidance on the dewatering process of rotary kilns in the industrial production of titanium dioxide carrier for denitration catalyst.
2. Experiment
2.1. Raw Materials
The raw material of the experiment was a denitration catalyst titanium dioxide sample dried at room temperature for 48 h and produced by Shandong Doguide Group Co., Ltd. In this experiment, the labels of the denitration catalyst carrier titanium dioxide were SA90, SA100, and SA200. The standard sample of anatase titanium dioxide in the control group was labeled BA01-01.
The SA90, SA100, and SA200 samples were prepared with the liquid-phase method. The raw material was metatitanic acid after the second washing in the production process of titanium dioxide by sulfuric acid, in which the concentration of total titanium (measured by TiO2 mass) was 466 g/L and the content of iron (measured by Fe2O3 mass) was less than 0.005%. The liquid-phase method consists of two processes: chemical synthesis of the denitration catalyst titanium dioxide samples and calcination dehydration. The first process involved taking a certain volume of metatitanic acid, ionized water, and ammonia and stirring them at 30~50 °C for 30 min. During the stirring, the concentration of ammonia water (based on the mass of NH3·H2O) was controlled between 10% and 15%, which was related to the sulfate group controlled in the final product. By filtration and washing, when the pH of the filtrate water was between 7 and ~8, the chemical synthesis of the sample was completed. In the process of calcination dehydration, the sample was calcined in a rotary kiln, and the calcination was carried out by three-stage heating and holding. Finally, after the calcined sample was cooled to room temperature, the denitration catalyst titanium dioxide samples were obtained after grinding.
For the SA90 sample, the specific calcination was as follows: heating from room temperature to 150 °C at 5 °C/min and holding for 60 min; then, heating to 300 °C at 3 °C/min and holding for 60 min; then, the final heating to 400 °C at 2 °C/min and holding for 60 min. To improve the activity of the catalyst carrier, the SA100 sample was doped with tungsten oxide before the calcination of the SA90 sample described above. Similarly, a three-stage calcination was used with the final stage, requiring a uniform temperature increase to 500 °C at 3 °C/min and a holding time of 100 min. Similar to the SA100 sample, to improve the strength of the catalyst carrier, the SA200 sample was obtained by adding ultra-fine silica to the preparation base of the SA90 sample. The final stage of the calcination temperature required a uniform temperature increase to 550 °C at 4 °C/min and a hold time of 100 min. Table 1 shows the main chemical composition of the SA90, SA100, and SA200 samples. To prevent sulfur poisoning of the catalyst, a small amount of sulfate was retained, as shown in Table 1. For SA100 and SA200 samples, during the doping process, the concentration of tungsten oxide and silica were in the range of 3.5 to 4.0.

Table 1.
Main chemical composition of samples, wt %.
2.2. Experimental Procedure
The thermal analysis instrument (STA449C-QMS403C) used in the experiment was produced by Netzsch, Germany, as shown in Figure 1. This instrument can measure the quality of the sample with changing temperature for an in-depth analysis of the thermal stability, decomposition behavior, and composition of the material. The furnace body has a vacuum-sealing design. The instrument has a top-loading configuration—that is, the sample is weighed on top of the balance. The atmosphere is characterized by bottom-to-top flow. When the furnace body is opened, the sample support separates from the weighing system, which is conducive to the protection of the weighing system.

Figure 1.
Schematic diagram of thermogravimetric analysis experimental device.
Before the experiment began, about 20 mg of the denitration catalyst titanium dioxide sample was loaded with an alumina crucible (6.8 × 4 mm) and placed in the TGA instrument. The thermal analysis experiment of the samples was conducted under air and nitrogen atmospheres, with both air and nitrogen flow rates set at 30 mL/min. After the balance reading stabilized, the temperature was increased at rates of 5 °C/min, 10 °C/min, 15 °C/min, and 20 °C/min. Finally, after the end of the experiment, the thermogravimetric change (TG) data of the samples were measured, and the kinetic analysis was carried out based on these data.
2.3. Kinetic Theory
For non-isothermal kinetic analysis, to avoid the error caused by solving for the activation energy of the kinetic mode function, the Flynn–Wall–Ozawa method for the mode-free function method was adopted as follows:
where β is the heating rate (K/min), T is the absolute temperature (K), A is the pre-exponential factor for the thermal reaction (min−1), R is the gas constant (J·mol−1·K−1), E is the apparent activation energy (kJ/mol), and G(α) is the mechanism function.
In this experiment, the dehydration reaction of denitration catalyst carrier titanium dioxide is a decomposition reaction, and the reaction formula is expressed as follows:
with the kinetic formula as in Equation (3):
where f(α) is the reaction mechanism function.
TiO(OH)2(s)→TiO2(s) + H2O(g)
In the thermal analysis test, there is a linear relationship between temperature and heating rate, which can be expressed as in Equation (4):
By substituting Equation (4) into Equation (3), Equation (5) can be obtained:
By integrating Equation (5), a dynamic equation in integral form can be obtained, as shown in Equation (6):
where G(α) is the integral form of the mechanism function f(α). Both can be derived using Equation (7).
According to the TGA experimental curves, the conversion ratio (α) is calculated as in Equation (8):
where m0 is the initial mass of the sample in mg, mt is the mass in mg at time t, and m is the mass of the sample in mg after complete reaction termination.
3. Results and Discussion
3.1. TG Analysis
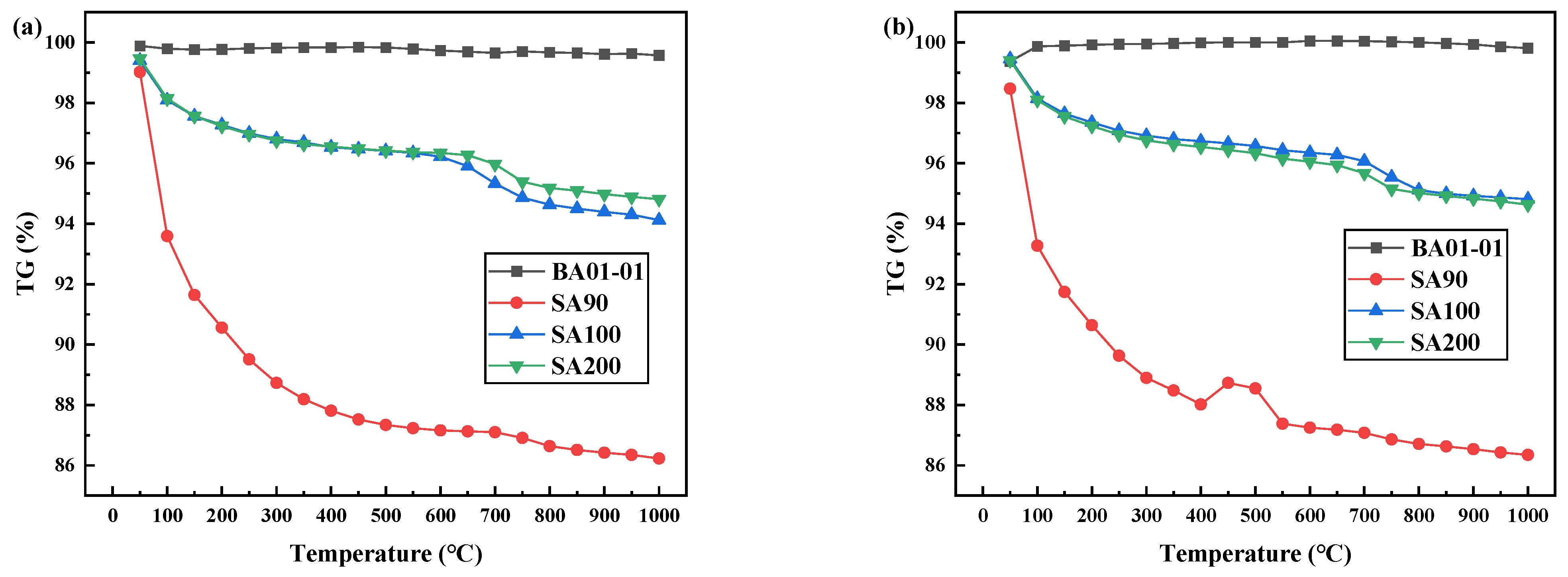
The TG curves of denitration catalyst carrier titanium dioxide, dehydrated under different atmospheres, are shown in Figure 2. As shown in Figure 2a, significant weight loss was observed for the denitration catalyst carrier titanium dioxide (SA90, SA100, and SA200) in the air atmosphere. This observation suggests that dehydration occurred in SA90, SA100, and SA200 as temperature increased. Similarly, as can be seen in Figure 1b, SA90, SA100, and SA200 also exhibited obvious dehydration with increasing temperature under the nitrogen atmosphere. The loss rates of SA90, SA100, and SA200 were 12.33%, 3.55%, and 3.56%, respectively, under the air atmosphere, whereas the loss rates under the nitrogen atmosphere were 12.18%, 3.36%, and 3.60%, respectively, which were similar to the theoretical values. In Figure 2, it can be observed that the TG curves of SA100 and SA200 overlapped significantly. This is attributed to the similar dehydration rates resulting from the similar TiO2 carrier preparation processes of SA100 and SA200, which involved further impregnation of metal salt into SA90. In addition, since the calcination temperature during the preparation of S100 and SA200 was higher than that of SA90, more water was dehydrated during the calcination process, resulting in lower weight loss for the TG curve. The sample (BA01-01) was a titanium dioxide specimen, which was treated by high-temperature calcination. The graph is basically a straight line, and the mass loss rate was within 0.5%.
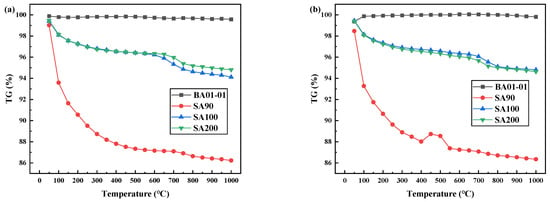
Figure 2.
Dehydration TG curve of titanium dioxide as denitration catalyst carrier under different atmospheres: (a) air and (b) nitrogen.
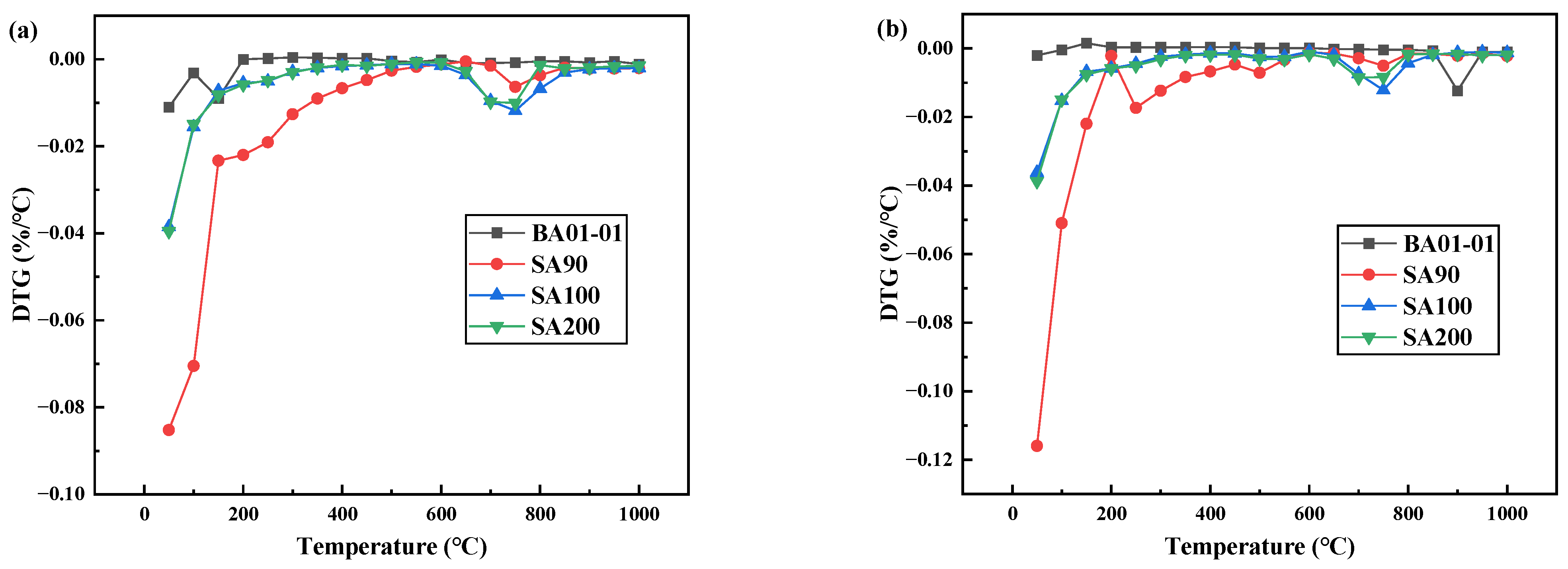
The DTG curves of the dehydration of the denitration catalyst titanium dioxide under different atmospheric conditions are displayed in Figure 3. As shown in Figure 3a, in the dehydration process of SA90 in the air atmosphere, the mass change occurred twice. The first time, the mass change occurred at 60–650 °C, and the thermogravimetric change was very obvious during this process. The second time, the mass change occurred at 650–850 °C, and the thermogravimetric change was weak. The dehydration process of SA100 also underwent two mass changes. The first mass change occurred at 40–600 °C, and the thermogravimetric change was obvious in this process, and the second mass change was from 600–800 °C, also with a very obvious thermogravimetric change. The dehydration process quality of SA200 was similar to that of SA100. The weight loss rate of the standard titanium dioxide sample (BA01-01) did not change significantly. Figure 3b shows that the weight loss change of denitration catalyst titanium dioxide under a nitrogen atmosphere was similar to that of Figure 3a.
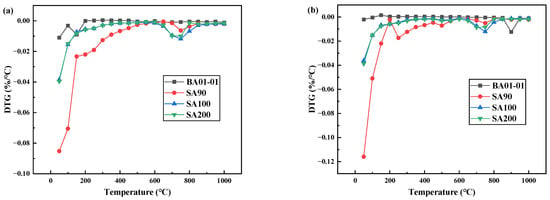
Figure 3.
Dehydration DTG curve of titanium dioxide as denitration catalyst carrier under different atmospheres: (a) air and (b) nitrogen.
It is worth noting that the dehydration reaction of the denitration catalyst titanium dioxide was basically completed at about 650 °C. The subsequent change in mass loss was caused by the decomposition of impurities such as sulfur and phosphorus in titanium dioxide and the transformation into crystal form. With a further increase in temperature, the denitration catalyst titanium dioxide began to undergo dehydration, desulfurization, and crystal transformation, gradually changing from amorphous to stable anatase and rutile. At this temperature, along with a weak dehydration process, there were also desulfurization and crystal phase conversion processes.
3.2. Dynamic Analysis of Dehydration Behavior
For different heating rates of 5, 10, 15, and 20 °C/min, values with 5% to 90% dehydration reactions were considered for the kinetic calculation, and the data are shown in Table 2. The activation energy was calculated using the Flynn–Wall–Ozawa method, and the results are shown in Figure 4.

Table 2.
α-T data of the dehydrating reaction of denitration catalyst carrier titanium dioxide in different atmospheres.
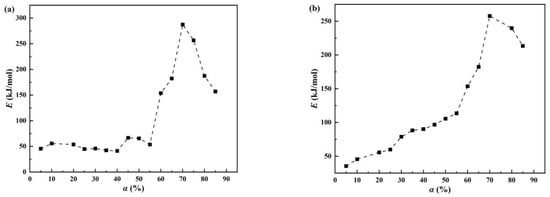
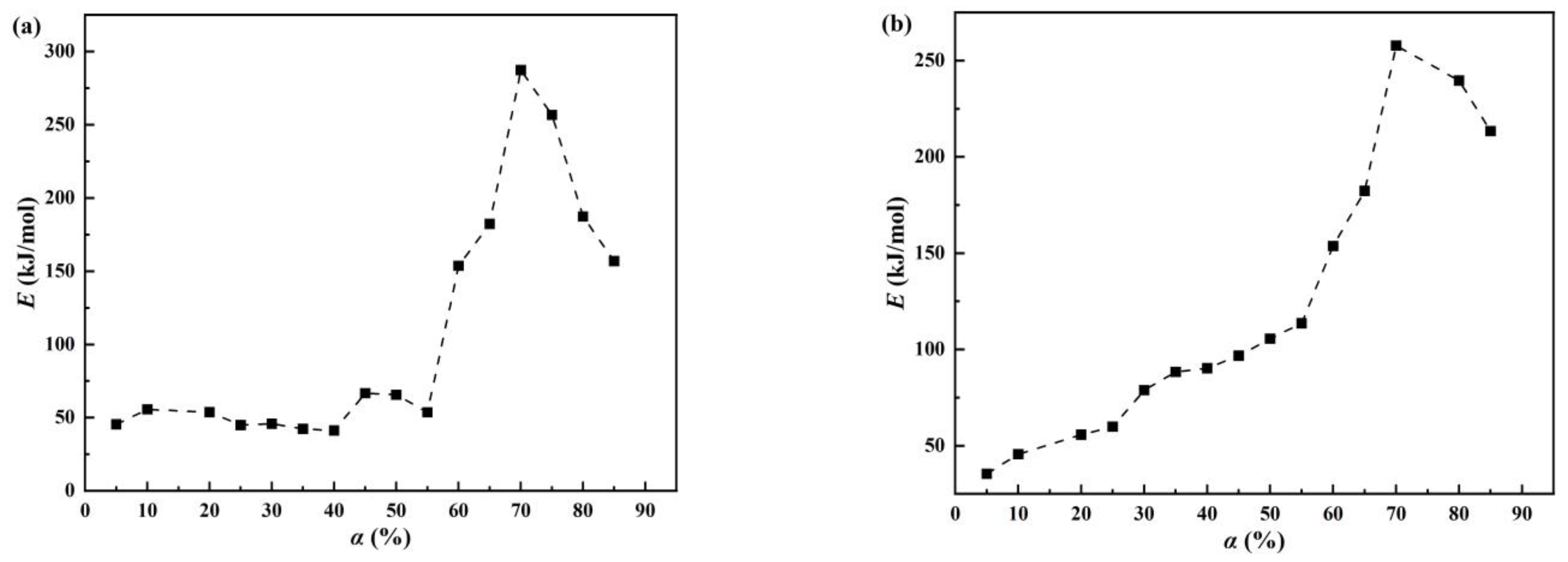
Figure 4.
Activation energy (E) of α in the dehydration process of denitration catalyst titanium dioxide under different atmospheres: (a) air and (b) nitrogen.
As seen in Figure 4a, the denitration catalyst carrier titanium dioxide experienced three stages of activation energy change in the air atmosphere. In the first stage (α = 5–55%), there were small activation energy fluctuations without a significant increase. In the second stage (α = 55–70%), the activation energy increased rapidly. In the third stage (α = 70–85%), the activation energy started to decrease. According to the characteristics of titanium dioxide crystalline transformation, the crystalline-phase transformation from amorphous to anatase and then to rutile requires energy absorption. In the second and third stages, the overall activity was higher than in the first stage, which may be because of the removal of impurities such as sulfur and phosphorus and the conversion process of the titanium dioxide crystal phase. Based on the change in activation energy and the above analysis, the first stage was mainly regarded as a dehydration process. Therefore, the first stage (α = 5–55%) under the air atmosphere was selected as the calculation range of dehydration. As shown in Figure 4b, the activation energy change of denitration catalyst carrier titanium dioxide under the nitrogen atmosphere can also be roughly divided into two stages. In the range of 5% to 70%, the activation energy displayed an overall gradual increase, followed by a decrease in the range of 70–85%. Similarly, based on the crystallographic transformation characteristics of titanium dioxide and the change in activation energy, the dehydration process was accompanied by the crystallographic transformation. Consequently, the calculation range of the dehydration process was chosen to be 5% to 70%. In addition, the third-stage activation energy in the air atmosphere was higher than the activation energy of the second stage under the nitrogen atmosphere because the sample was dehydrated quickly in the air and there were reactions that can remove impurities, such as sulfur and phosphorus, thus requiring more energy.
A higher activation energy indicates that a higher energy is required for the reaction. At the same temperature, an activation energy decrease indicates that the reaction rate has accelerated. Therefore, in Figure 4, it can be seen that the denitration catalyst carrier titanium dioxide dehydrated rapidly at 55% in the air atmosphere and then slowly until the dehydration reaction was completed. Under the nitrogen atmosphere, the denitration catalyst carrier titanium dioxide was dehydrated at a gradually slower rate, which manifested as an overall increase in activation energy.
3.3. Kinetic Mechanism Analysis
The α-T data obtained above for different warming rates were calculated by the general integration method. The Flynn–Wall–Ozawa method was used, and the mechanism function with the highest correlation was selected as the most probable mechanism function, which combined 47 common mechanism functions [28,29,30]. The most probable functions for dehydration of the denitration catalyst carrier titanium dioxide in 5% to 85% were numbered 12, 18, and 25. The differential and integral equations of these functions are shown in Table 3.

Table 3.
Integral and differential forms of mechanism functions.
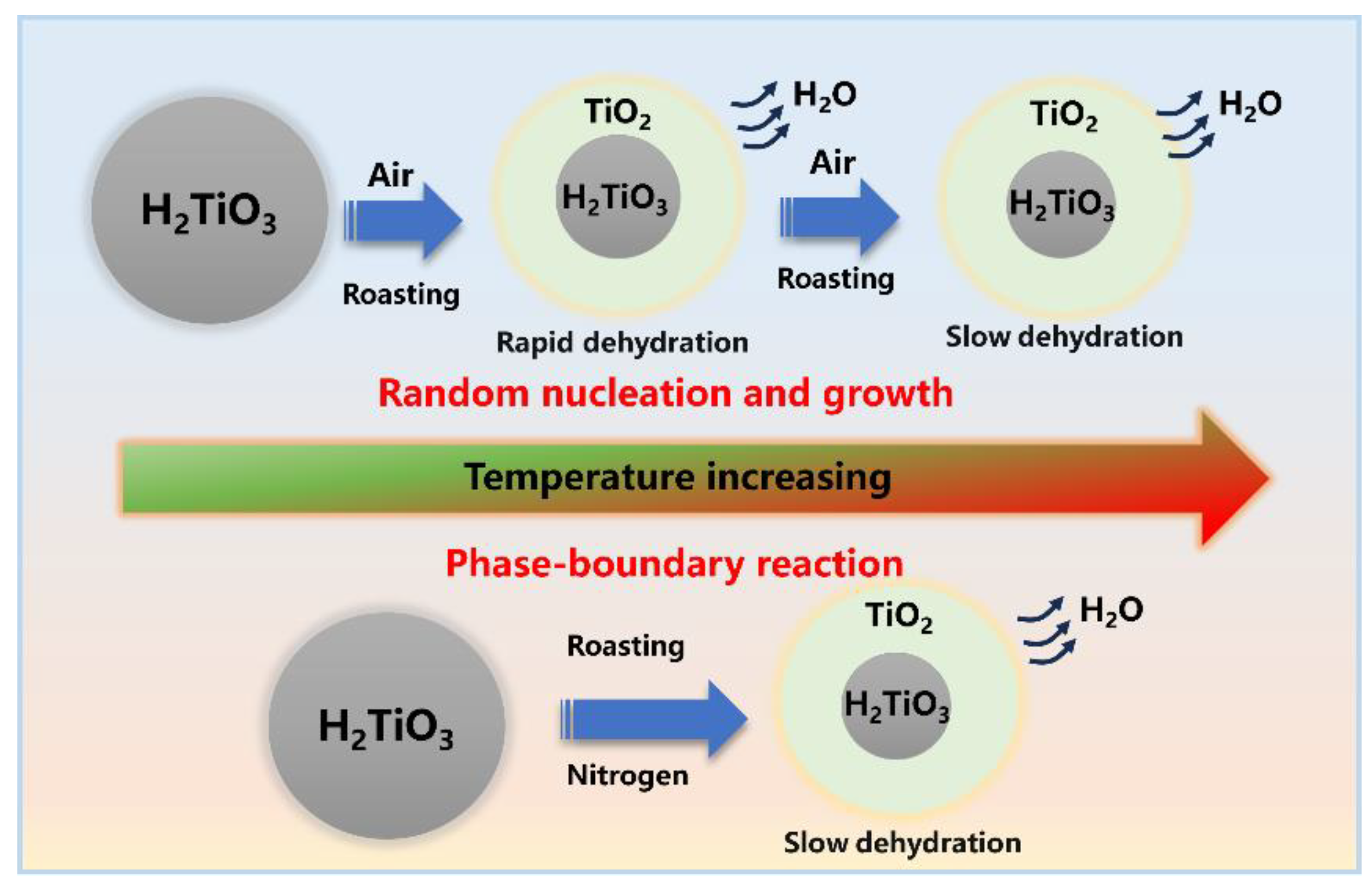
The kinetic results for different atmospheres were calculated by the 12, 18, and 25 functions exhibiting relatively high linear correlation, as shown in Table 4, where the dehydration behavior of the denitration catalyst titanium dioxide is associated with the atmosphere, with the atmosphere influencing the mechanism of the dehydration behavior. Under the air atmosphere, the linear correlation coefficient of the activation energy calculated by the mechanism function 12 was the largest (about 98%), indicating that the most probable mechanism function of the dehydration reaction was G(α) = [−ln(1−α)]2/5. Similarly, based on the calculated correlation coefficient of activation energy, the most probable mechanism function of the dehydration reaction under the nitrogen atmosphere was G(α) = α. To further determine the most probable mechanism function for the dehydration reaction, the activation energies obtained for the air and nitrogen atmospheres were calculated using the Flynn–Wall–Ozawa method. The results of the activation energy under the air and nitrogen atmospheres were 25.69 kJ/mol and 28.88 kJ/mol, respectively, and the absolute deviations from the general integral method were 26% and 14%, respectively. As the factors influencing the calculation of the mean values of the kinetics of the dehydration process under different atmospheres are complex, the deviations introduced are acceptable. Figure 5 shows a schematic diagram of the reaction mechanism of dehydration kinetics. As can be seen, the dehydration process of titanium dioxide in an air atmosphere was controlled by the formation and growth of crystal nuclei in accordance with the Avrami–Erofeev equation, whereas the dehydration process under the nitrogen atmosphere was a one-dimensional phase-boundary reaction in accordance with the Mampel power law.

Table 4.
Kinetics of the dehydration reaction under different atmospheres.
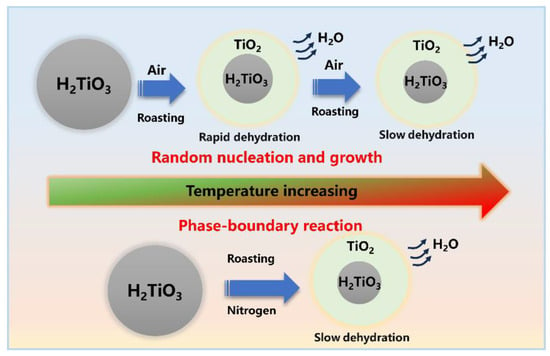
Figure 5.
Schematic reaction mechanism of dehydration kinetics.
By substituting the average value of each parameter calculated by the general integral method into Equation (5), the kinetic equation for the dehydration behavior of denitration catalyst titanium dioxide under air and nitrogen can be obtained:
where Equations (9) and (10) are the kinetic equations for dehydration under air and nitrogen atmospheres, respectively.
Hence, the dehydration behavior of the denitration catalyst carrier titanium dioxide was strongly influenced by air (oxygen content in the air), and this result can be used as the primary basis for the control of the atmosphere of the rotary kiln in the process of calcining and preparing titanium dioxide for denitration catalysts.
3.4. Composition Anazlysis of Carrier Titanium Dioxide
The dehydration process of TiO2 is a complex physical and chemical process. Based on the above experimental analysis, from room temperature to 150 °C, the main weight loss was caused by the removal of water. The increase in temperature initiated the desulfurization reaction, which was completed at approximately 600 °C. The dehydration and desulfurization of TiO2 with different denitration catalyst carriers are shown in Table 5.

Table 5.
Analysis of weight loss of TiO2 as denitration catalyst carrier.
According to the data in Table 5, equations can be obtained by inferring the chemical composition of the denitration catalyst carrier TiO2 as follows:
From calculations based on Equations (11)–(16), the value of x is ~0.2 and the value of y is ~0.06. As a result, the chemical composition of the denitration catalyst carrier TiO2 was characterized as TiO2·0.2H2O·0.06SO3. The denitration catalyst carrier TiO2 was processed from metatitanic acid and calcined at low temperature, and ultimately a portion of the free hydroxyl group and SO42− bridging connection of hydrated TiO2 had to be maintained to meet the requirements of the carrier TiO2. Thus, the calcination control of the denitration catalyst support TiO2 is very complicated and needs to be further explored. On the 10,000 ton demonstration line, the chemical composition (TiO2·0.2H2O·0.06SO3) can be accurately controlled, whereby the prepared products fully meet the needs of domestic and foreign customers, indicating that the composition of the chemical formula is reasonable.
4. Conclusions
- (1)
- The dehydration behavior of denitration catalyst carrier titanium dioxide is simple and belongs to the general decomposition reaction. The heating rate is positively correlated with dehydration speed such that the higher the rate of temperature increase, the more favorable the dehydration reaction is.
- (2)
- The dehydration reaction of denitration catalyst carrier titanium dioxide is related to the atmosphere. In an air atmosphere (oxygen-enriched atmosphere), dehydration occurred quickly, followed by slow dehydration until the reaction was fully realized. In an oxygen-free ambient atmosphere (nitrogen atmosphere), the reaction proceeded slowly.
- (3)
- Kinetic calculations of the dehydration behavior of denitration catalyst carrier titanium dioxide were carried out using the mode-free and mode function methods. The analysis revealed that the dehydration behavior of denitration catalyst carrier titanium dioxide under the air atmosphere conformed to the Avrami–Erofeev equation. Dehydration behavior was also affected by crystalline phase transformation and grain nucleation growth of denitration catalyst carrier titanium dioxide at higher-temperature environments. The chemical composition of denitration catalyst carrier TiO2 was characterized as TiO2·0.2H2O·0.06SO3.
Author Contributions
Conceptualization, H.L. and Y.L.; methodology, H.L. and Y.L.; software, Y.L.; validation, G.Q.; investigation, H.L. and X.L.; data curation, G.Q., X.L., and Y.L.; writing—original draft, H.L.; writing—review and editing, Y.L.; visualization, X.L.; supervision, G.Q.; funding acquisition, G.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (52074052).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (52074052) for the financial support of this research.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Gesenhues, U. Calcination of metatitanic acid to titanium dioxide white pigments. Chem. Eng. Technol. 2001, 24, 685–694. [Google Scholar] [CrossRef]
- Lee, H.J.; Shim, J.W.; Lee, J.J.; Lee, W.J. The Encapsulation of Natural Organic Dyes on TiO2 for Photochromism Control. Int. J. Mol. Sci. 2023, 24, 7860. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, K.; Rajapakse, R.M.G.; Tennakone, K.; Kumara, G.R.A. Stability and efficiency improvement of TiO2-based dye-sensitized solar cells by surface modification of MgO. J. Solid State Electrochem. 2023. [Google Scholar] [CrossRef]
- Li, G.J.; Huang, R.; Zhu, C.Y.; Jia, G.; Zhang, S.L.; Zhong, Q. Effect of oxygen vacancies and its quantity on photocatalytic oxidation performance of titanium dioxide for NO removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126156. [Google Scholar] [CrossRef]
- Wu, X.W.; Wu, D.J.; Liu, X.J. Optical investigation on sulfur-doping effects in titanium dioxide nanoparticles. Appl. Phys. A Mater. Sci. Process. 2009, 97, 243–248. [Google Scholar] [CrossRef]
- Man, Y.K.; Mu, L.Y.; Wang, Y.; Lin, S.H.; Rempel, G.L.; Pan, Q.M. Synthesis and Characterization of Rutile Titanium Dioxide/Polyacrylate Nanocomposites for Applications in Ultraviolet Light-Shielding Materials. Polym. Compos. 2015, 36, 8–16. [Google Scholar] [CrossRef]
- Kang, X.L.; Liu, S.H.; Dai, Z.D.; He, Y.P.; Song, X.Z.; Tan, Z.Q. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Li Puma, G.L.; Bono, A.; Krishnaiah, D.; Collin, J.G. Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: A review paper. J. Hazard. Mater. 2008, 157, 209–219. [Google Scholar] [CrossRef]
- Hu, J.G.; Tang, H.B.; Lin, X.D.; Luo, Z.K.; Cao, H.Q.; Li, Q.W.; Liu, Y.; Long, J.H.; Wang, P. Doped Titanium Dioxide Films Prepared by Pulsed Laser Deposition Method. Int. J. Photoenergy 2012, 2012, 758539. [Google Scholar] [CrossRef]
- Ragadhita, R.; Nandiyanto, A.B.D.; Maulana, A.C.; Oktiani, R.; Sukmafitri, A.; Machmud, A.; Surachman, E. Techo-economic analysis for the production of titanium dioxide nanoparticle produced by liquid-phase synthesis method. J. Eng. Sci. Technol. 2019, 14, 1639–1652. [Google Scholar]
- Zanardo, D.; Ghedini, E.; Menegazzo, F.; Cattaruzza, E.; Manzoli, M.; Cruciani, G.; Signoretto, M. Titanium Dioxide-Based Nanocomposites for Enhanced Gas-Phase Photodehydrogenation. Materials 2019, 12, 3093. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Seo, W.S.; Koumoto, K. Selective deposition and micropatterning of titanium dioxide on self-assembled monolayers from a gas phase. Langmuir 2001, 17, 4876–4880. [Google Scholar] [CrossRef]
- Li, Y.T.; Yi, H.H.; Tang, X.L.; Liu, X.; Wang, Y.; Cui, B.C.; Zhao, S.Z. Study on the performance of simultaneous desulfurization and denitrification of Fe3O4-TiO2 composites. Chem. Eng. J. 2016, 304, 89–97. [Google Scholar] [CrossRef]
- Tian, C.X. Hydrothermal preparation of high purity TiO2 from industrial metatitanic acid by response surface methodology. Sci. Rep. 2022, 12, 20164. [Google Scholar] [CrossRef]
- Gao, Y.F.; Masuda, Y.; Seo, W.S.; Ohta, H.; Koumoto, K. TiO2 nanoparticles prepared using an aqueous peroxotitanate solutions. Ceram. Int. 2004, 30, 1365–1368. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, L.N.; Xue, T.Y.; Qi, T. Preparation of Rutile Titanium Dioxide White Pigment via Doping and Calcination of Metatitanic Acid Obtained by the NaOH Molten Salt Method. Ind. Eng. Chem. Res. 2010, 49, 7693–7696. [Google Scholar] [CrossRef]
- Song, Y.W.; Wang, H.R.; Wang, R.; Zhou, J.C. Novel approach for high-efficiency recovery of titanium dioxide, hydrochloric acid, and organic solvents from titanium white waste acid. J. Clean. Prod. 2021, 315, 128105. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, C.R.; Yuan, Z.G. Precipitation and growth behaviour of metatitanic acid particles from titanium sulfate solution. Powder Technol. 2017, 315, 31–36. [Google Scholar] [CrossRef]
- Lu, R.F.; Liu, C.; Wu, J.C.; Sun, W.; Sun, Q.; Dong, L.C. Process optimization of the extra-adding seeded hydrolysis of TiOSO4 to H2TiO3 by using the unenriched solution for the manufacture of TiO2 pigment. J. Cryst. Growth 2021, 572, 126268. [Google Scholar] [CrossRef]
- Chen, K.; Yan, X.H.; Wu, P.S.; Wang, Z.N.; Wu, B.; Lin, F.R. Effect of sulfate on crystal phase transition and crystal growth of titanium dioxide in metatitanic acid calcination. Phase Transit. 2021, 94, 353–365. [Google Scholar] [CrossRef]
- Tian, C.X. Orthogonal interactions and synergistic effects of salt treatment and calcination on rutile titanium dioxide pigment preparation. Mater. Chem. Phys. 2020, 249, 123125. [Google Scholar] [CrossRef]
- Wang, X.J.; Hu, Z.H.; Chen, Y.J.; Liu, Y.F.; Wen, Z.B.; Zhao, G.H. High Performance Supported Photocatalyst of Nano-TiO2/Activated Carbon from Metatitanic Acid. Acta Chim. Sin. 2008, 66, 2445–2450. [Google Scholar]
- Rui, B.; Liu, B.G.; Ting, Z.; Wu, B.J.; Dong, E.H.; Chao, Y.W. Rutile TiO2 Production: Optimization of Microwave Calcination of Metatitanic Acid Using Response Surface Methodology. Chem. Eng. Technol. 2022, 45, 1826–1834. [Google Scholar] [CrossRef]
- Wu, X.P.; Liu, Y. Phase change and crystal growth of TiO2 in metatitanic acid. Ceram. Int. 2023, 49, 4607–4613. [Google Scholar] [CrossRef]
- Lu, R.F.; Liu, C.; Wu, J.C.; Wu, Y.X.; Zhang, Q.; Sun, Q. Investigation on the structure evolution of rutile TiO2 during calcination of mixed-salt-treated metatitanic acid. J. Cryst. Growth 2023, 602, 126985. [Google Scholar] [CrossRef]
- Komarkova, B.; Motlochova, M.; Slovak, V.; Ecorchard, P.; Bavol, D.; Subrt, J.; Bezdicka, P. Effect of amines on (peroxo)titanates: Characterization and thermal decomposition. J. Therm. Anal. Calorim. 2022, 147, 5009–5022. [Google Scholar] [CrossRef]
- Huang, L.; Chen, Y.C.; Liu, G.; Li, S.N.; Liu, Y.; Gao, X. Non-isothermal pyrolysis characteristics of giant reed (Arundo donax L.) using thermogravimetric analysis. Energy 2015, 87, 31–40. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.B.; Qin, Q.; Lei, W. Kinetics of CO2/Coal Gasification in Molten Blast Furnace Slag. Ind. Eng. Chem. Res. 2012, 51, 15872–15883. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.B.; Xie, H.Q.; Qin, Q.; Wang, K. CO2 Gasification Rate Analysis of Datong Coal Using Slag Granules as Heat Carrier for Heat Recovery from Blast Furnace Slag by Using a Chemical Reaction. Energy Fuels 2013, 27, 4810–4817. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).