Abstract
The unique bicontinuous porous structure and superior electrical conductivity of nanoporous gold (NPG) make it a highly promising material for energy storage and conversion. Although the number of articles on the study of NPG-based materials in energy fields has increased significantly in recent years, the collation and review of these articles are still lacking. Herein, we address this gap by reviewing recent research activities on dealloyed NPG for energy storage and conversion applications. Firstly, the typical dealloying process for forming NPG is introduced. Subsequently, NPG-based composite catalysts used to catalyze water splitting and fuel cells electrode reactions are presented. Afterward, the applications of NPG for different types of electrodes of supercapacitors (SCs) and batteries are discussed. Finally, the studies on NPG for catalyzing CO2 reduction reaction (CO2RR) are reviewed. In a word, the recent research progress of NPG-based materials is reviewed and the future research directions are outlined, laying the cornerstone for the preparation of more advanced energy storage and conversion devices in the future.
1. Introduction
Nanoporous gold (NPG) is a gold-based reticulated porous body, usually made by selective dissolution of non-gold components in Au alloys, that is, dealloying [1,2]. By controlling the preparation process of dealloying, NPG with adjustable ligament size can be obtained. The continuous porous mesh structure of NPG endows it with a high specific surface area and a large number of positive and negative bending curvatures, thus possessing a large number of highly active sites. In addition, NPG has a stable structure and good electrical conductivity, making it an ideal substrate for the fabrication of high-performance composites. Based on the above advantages, NPG presents a wide range of applications, covering catalysis [3,4,5,6,7], energy storage and conversion [8,9,10,11,12], sensing [13,14,15], etc.
In recent years, the number of research articles in the field of energy storage and conversion has increased significantly, and the corresponding review studies have also increased, involving semiconductor materials such as Si [16], Ge [17], Sn [18], transition metals (Fe, Co, Ni, Mn, etc.) oxides [19,20,21] and other precious metals Pd, Ir [22], etc. Regarding NPG, the published review articles mostly study the preparation methods of NPG and their applications in catalysis and sensing fields [23,24,25,26]. Although the number of articles on the application of NPG to the energy storage field is also high, the corresponding review articles are scarce, some of which are of an earlier vintage and some of which are not comprehensive enough [10,27,28,29,30]. Therefore, it is necessary for us to summarize and reflect on the latest research results and point out the current challenges to inspire future research efforts.
In this review, we mainly discuss the characterizations and energy storage applications of dealloyed NPG and its composites. Firstly, we introduce the typical dealloying process for preparing NPG. Secondly, we review the applications of NPG in water splitting and fuel cells, starting from the relevant half-reactions. Water splitting includes hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), and fuel cell electrode reaction consists of fuel oxidation and oxygen reduction reaction (ORR). Finally, the applications of NPG in supercapacitors (SCs), batteries and CO2 reduction reaction (CO2RR) are reviewed. In summary, this review aims to provide readers with a comprehensive understanding of the characterizations and energy storage applications of dealloyed NPG and its composites. Furthermore, we hope that the review can contribute to the development and optimization of energy storage and conversion technologies.
2. Formation of NPG by Dealloying
Dealloying is a corrosion process that selectively removes less noble metals from the precursor alloy through nanoscale galvanic corrosion mechanisms. This can be achieved either by free etching or electrochemical dealloying, depending on the proximity of the constituent metals in the galvanic series [31]. Through experiments and simulation calculations, Jonah Erlebacher et al. [32] proposed a continuum model for the evolution of pores during dealloying. The evolution process of NPG pores begins with the selective dissolution of less noble metal atoms, creating vacancies and exposing high-energy surface sites. The vacancies act as diffusion paths for more noble metal atoms, which migrate and accumulate at the edges, forming islands and clusters. As the dissolution continues, the islands undergo undercutting, leading to the development of interconnected pores and a highly porous structure.
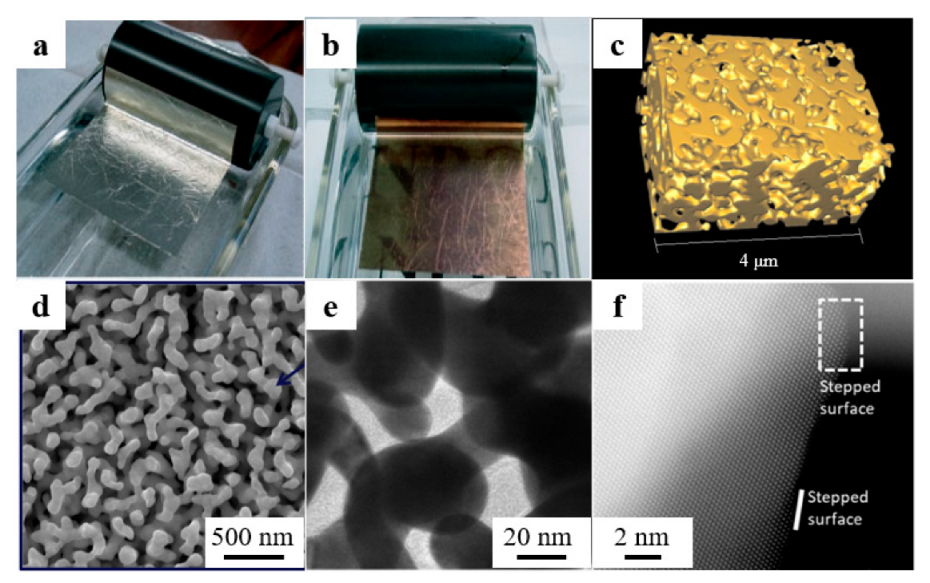
Figure 1a,b [33] presents the Au thin film sampled before and after dealloying. The color change of the sample, from silvery white to rose gold, can be clearly seen. Figure 1c [34] shows the three-dimensional model of a series of two-dimensional projection images. The model exhibits the internal pores network clearly. It shows that NPG has a bicontinuous 3D structure and most of the gold ligaments have curved surfaces. A typical scanning electron microscope (SEM) image of dealloyed NPG is shown in Figure 1d [35]. This fine microstructure consisting of pores and ligaments is formed by the dissolution of inert elements in the precursors and the diffusion of gold adatoms along the solid/electrolyte interface. Figure 1e [36] shows a transmission electron microscope (TEM) image of NPG at low magnification, where it can be observed that the gold ligaments and nanopores are continuous and smooth. However, revealed by high-resolution transmission electron microscopy (HRTEM), we can see that the curved surfaces of NPG are not completely smooth. There are a large number of atomic steps and kinks (Figure 1f), and these surface defects are one of the reasons for the remarkable catalytic activity of NPG [37]. Brunauer–Emmett–Teller (BET) method is commonly used to detect the specific surface area of the dealloyed nanoporous materials. In the future, BET tests can be carried out toward samples taken at different dealloying reaction times to better understand the transformation of porous structure and the kinetics of the dealloying process.
Figure 1.
Characteristics of dealloyed NPG. Optical images of white-gold leaf (a) before and (b) after dealloying (reproduced from [33], with permission from Wiley, 2023.); (c) 3D tomographic reconstruction by x-ray nanotomography (reproduced from [34], with permission from AIP Publishing, 2023.); (d) SEM (reproduced from [35], with permission from Elsevier, 2023.), (e) TEM (reproduced from [36], with permission from Elsevier, 2023.) and (f) HAADF-STEM (reproduced from [37], with permission from American Chemical Society, 2023.) images of NPG.
Gold nanoparticles (Au NPs) typically exhibit regular shapes and concave-convex surface curvatures, whereas NPG possesses complex 3D nanostructures with positive, negative, and saddle point curvatures [38]. The unique structure of NPG results in a high density of surface defects, such as steps and kinks, which serve as ideal sites for catalytic reactions. The undercoordinated surface atoms at step edges and kink corners play a crucial role in electrochemical CO2RR, while the high-coordinating atoms serve as excellent catalytic sites for other reactions. This makes NPG highly effective in catalyzing CO2RR and enhances its activity and selectivity. Moreover, the surface defects of NPG exhibit greater kinetic stability compared to nanoparticle surface defects. Despite the higher thermodynamic surface energy caused by the presence of non-coordinating surface atoms, the overall system of NPG with its hyperbolic metallic ligament shape remains in a low-energy state compared to gold nanoparticles of the same size, indicating its remarkable thermal stability.
3. Application in Energy Storage and Conversion
3.1. Water Splitting
With the advantages of high energy density and environmentally friendly production, hydrogen presents a promising application in the green energy economy. Hydrogen production by water splitting as a key technology for obtaining hydrogen fuel has been closely followed by scientists.
3.1.1. HER
The key step in hydrogen production by water splitting is HER. However, due to the high thermodynamic overpotential of HER, appropriate catalysts are needed to reduce the overpotential and increase the reaction rate. Pt and its alloy are considered to be the best catalyst for HER. However, Pt has defects of low natural abundance and high cost. Therefore, looking for the replacement of the Pt catalyst has become an important research direction. Over the last decade, there have been many reports about transition metal dihalide semiconductors and metal oxide semiconductors as ideal alternatives to Pt. Studies have shown that the edge sites of nanosheets are catalytic active sites for HER, so designing reasonable structures to expose more edge active sites is an effective way to improve HER efficiency. Due to the widespread problem of low electrical conductivity in semiconductors, they are often combined with other materials to improve electrical conductivity. NPG is a good choice for this purpose.
Figure 2a [39] shows the SEM image of the monolayer MoS2@NPG composite with a pore size of ~100 nm. Monolayer MoS2 was grown on the inner surface of the highly curved NPG, naturally inheriting the three-dimensional curvature of the NPG. In contrast to the previous belief that the electrocatalytic activity of MoS2 comes from the edge sites of 2H sulfide compounds, the catalytic active sites of this material are induced by the strain of the curved MoS2 lattice. The conductive NPG provides a short path for fast charge transfer and transport. Ge et al. [40] prepared NPG-based amorphous molybdenum sulfide composites (MoS2.7@NPG). HRTEM image (Figure 2b) shows that the MoS2.7 layer is well combined with the NPG substrate. Moreover, no crystal lattice can be found in the molybdenum sulfide layer, which indicates that the molybdenum sulfide has amorphous structure after plating. Fast Fourier transform (FFT) patterns obtained from the plated layer (inset of Figure 2b) further confirm the amorphous characteristics of MoS2.7. Amorphous molybdenum sulfide has not only edge sites, but also intrinsic defect sites, which can improve HER catalytic activity in a wide range of pH (acidic and neutral media). Bak et al. [41] demonstrated that α-Fe2O3/NPG structures have fast electron transfer channels and can reduce electron and hole recombination. The maximum photocurrent density of the composite is 1.6 mA cm−2, which is two times higher than that of the unmodified α-Fe2O3. Nanosheets grown uniformly along the vertical orientation on the substrate can expose more edge sites for HER catalytic. Meanwhile, Jin et al. found that the phase transition of MoS2 from 2H to 1T is another way that can effectively improve HER efficiency. Combining the two points, Huan et al. [42] prepared 1T-TaS2 nanosheets grown on NPG substrate with an average thickness of ~30 nm and an average diagonal length of ~5 μm. Based on the nanoporous structure of NPG, it can provide more nucleation sites for TaS2 growth and promote chemical vapor deposition (CVD) growth process for rapid sulfurization. More importantly, the curved surface of NPG is a key factor for the growth of TaS2 nanosheets along the vertical orientation. In addition, the researchers discussed the effect of phase state on the catalytic activity of HER by combining theoretical calculations and experimental results.
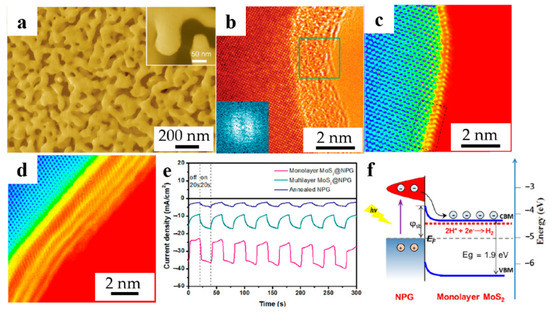
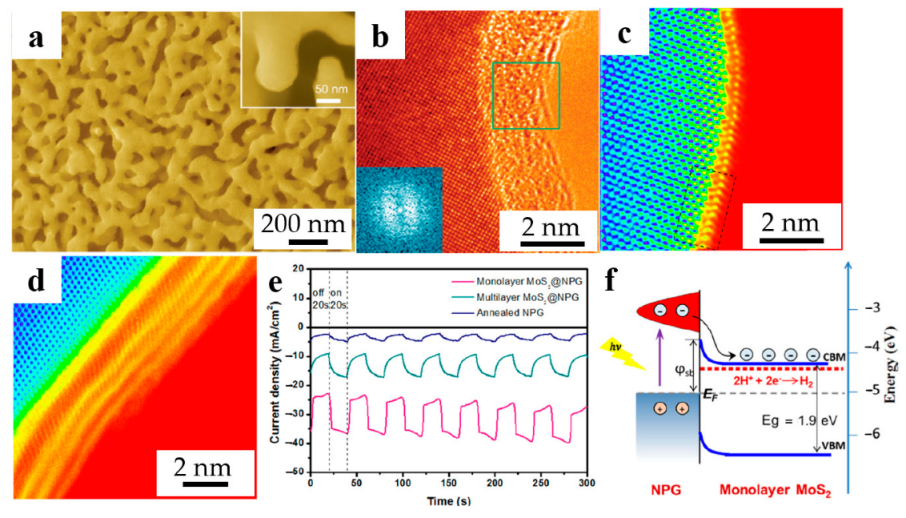
Figure 2.
Dealloyed NPG-based materials for HER. (a) SEM image of the monolayer MoS2@NPG (reproduced from [39], with permission from Wiley, 2023.); (b) HRTEM image of MoS2.7@NPG. (inset: FFT pattern taken from the labeled square area) (reproduced from [40], with permission from Wiley, 2023); HAADF-STEM images of (c) monolayer MoS2@NPG, (d) multilayer MoS2@NPG (marked by square in (c)); (e) I-t curves with light on and off every 20 s at the bias of −200 mV; (f) Band diagram and mechanism of interfacial hot electrons transfer process (reproduced from [43], with permission from Elsevier, 2023).
In recent years, plasma meta-enhanced electrochemical hydrogen production has attracted interest. Zhang et al. [43] combined the plasma material NPG with the HER catalyst MoS2 to prepare MoS2@NPG for HER under visible light, which is expected to further enhance kinetics of the HER process. The results show that the photocatalytic activity of the monolayer MoS2@NPG hybrid structure for HER is higher under visible light irradiation with an onset potential of −70 mV vs. RHE and a Tafel slope of 38 mV dec−1. As shown in Figure 2c,d, both monolayer and multilayer MoS2 were coated on the ligament of NPG. However, the distance between NPG and MoS2 monolayer is much slower than the multilayer, indicating the beneficial influence of direct contact. Further photoresponsivity was shown in Figure 2e, illustrating the photocurrent response of monolayer MoS2@NPG is higher than multilayer MoS2@NPG. In addition, the schematic diagram of the underlying mechanism of hot electron capture reveals the excellent ability which can transfer the electron to the catalytically active sites between NPG and monolayer MoS2, contributing to the enhanced HER catalytic activity, as shown in Figure 2f. In addition, Lu et al. [44] investigated the effect of SnOx-modified NPG on the catalytic activity and durability of HER in neutral media, which provides guidance for the future design of high-performance HER catalysts in more environmentally friendly neutral media. The application of dealloyed NPG-based materials for HER is summarized in Table 1.

Table 1.
Dealloyed NPG-based materials for HER.
3.1.2. OER
The OER occurs at the anode of the water splitting reaction and is limited by the slow reaction kinetics and high overpotential. Therefore, it is essential to find catalysts with enhanced intrinsic activity and reasonable geometric structure to accelerate the reaction process and reduce the overpotential. In acidic media, precious metals have higher catalytic activity in OER reaction, especially Ru, Ir, and their oxides. Wang et al. [45] prepared NPG@Pt/Ir composites. It was shown that the large specific surface area of NPG is beneficial to enhance the catalytic activity of OER, while there is a synergistic catalytic effect between NPG and Pt/Ir, which has better catalytic activity than Pt/Ir alone. However, the scarcity of precious metals still prevents the NPG@Pt/Ir electrodes from wider applications. At the same time, the study of non-precious metal catalysts to enhance the catalytic activity of OER in alkaline medium has made some achievements, which opens new ideas for finding alternatives to precious metal catalysts.
Considering the positive effect of chromium doping, NPG-supported Cr-doped NiFe oxyhydroxides (noted by NP Au/Cr-NiFe) were prepared via Sun et al. [46]. The precursor Au/Cr-NiFe hydroxide was shown in Figure 3a. Noticeably, the layered (oxy)hydroxide nanosheet was quasi-vertically grown on the NPG skeleton, leading to abundant active edge sites and optimized electron transfer pathway (Figure 3b), which are better than layered nanostructures (Figure 3c). Subsequent characterizations and density functional theory plus Hubbard U calculations further highlight the significance of the doped Cr which not only increases the intrinsic catalytic activity but lowers the overpotential of the hybrid electrode, as shown in Figure 3d,e.
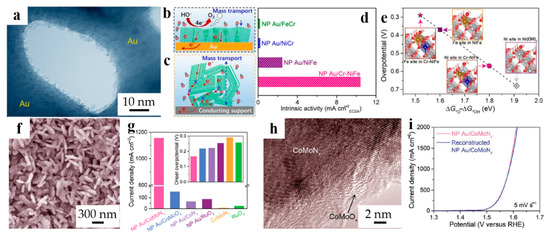
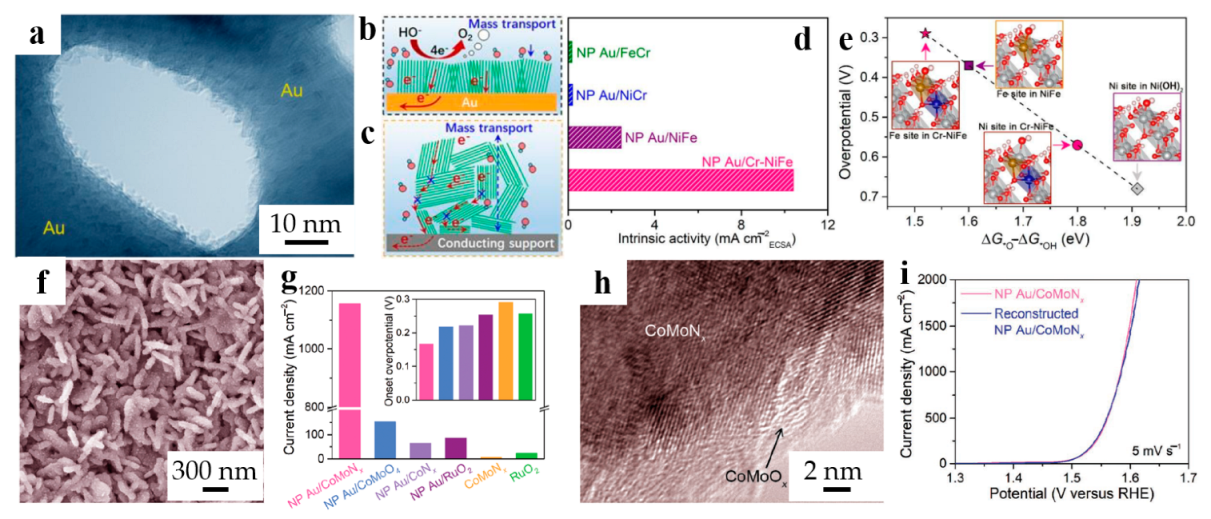
Figure 3.
Dealloyed NPG-based materials for OER. (a) TEM image for NP Au/Cr-NiFe hydroxide hybrid electrode; Schematic diagrams of layered nanocatalysts (b) deposited on Au ligaments in a quasi-vertical orientation, (c) randomly stacked on conductive materials; (d) Comparison of the intrinsic catalytic activity of NP Au/Cr-NiFe hydroxide electrodes with other electrodes at an overpotential of 0.340 V; (e) Theoretical overpotentials of *O and *OH adsorption Gibbs energy difference functions at different sites (reproduced from [46], with permission from Royal Society of Chemistry, 2023.); (f) SEM image of NP Au/CoMoNx; (g) Comparison of current densities for different electrocatalysts (inset: The onset overpotentials of OER); (h) HRTEM image of CoMoNx nanosheets in the NP Au/CoMoNx electrode after durability measurement for 50 h; (i) Comparison of the OER polarization curves for the initial and reconstructed NP Au/CoMoNx (reproduced from [47], with permission from Wiley, 2023.).
Toward higher activity and more robust electrocatalysts than commercial ones, the cobalt-molybdenum nitride nanosheets integrated on nanoporous gold were reported by Yao et al. [47], which synthesized through hydrothermal method together with annealing treatment. The as-prepared NP Au/CoMoNx (Figure 3f) showed a more outstanding catalytic ability than any other combination of the constituent elements (Figure 3g), emphasizing the importance of both Mo and NPG. It has been found that, with the proceeding of catalytic reaction, the NP Au/CoMoNx gradually keeps the CoMoOx surface layer, as shown in Figure 3h, which is the precursor of the as-prepared catalysts. Hence, a facile recycling method has been proposed and the catalytic activity of reconstructed NP Au/CoMoNx electrode is comparable to the initial one, as shown in Figure 3i, uncovering its excellent recyclability.
Recently, NPG-based plasmon-enhanced water splitting has been demonstrated by Graf et al. [48] The current response of NPG under a broad wavelength range satisfies the basic requirements of photocatalytic. In addition, to achieve in-situ characterization of the photochemical reaction products, a second laser is focused on the same spot to trigger the photochemical reaction while acquiring the spectrum, denoted by 2L-SERS. The 2L-SER spectra prove that the formation of additional Au-OH species was caused by illumination. Further density functional theory (DFT) calculation and transfer mechanism indicate an additional energetic state that affects the photon absorption. The application of dealloyed NPG-based materials for OER is shown in Table 2.

Table 2.
Dealloyed NPG-based materials for OER.
3.2. Fuel Cells
Fuel cells are devices that convert the stored chemical energy in redox reactions into electrical energy through an external circuit, generating an electric current. They have received considerable attention in research over the past few decades, particularly in the development of highly active and low-cost electrocatalysts to drive electrochemical reactions. Among the many catalysts, Pt nanoparticles have obvious advantages, but their high-cost issues cannot be ignored. Meanwhile, Pt nanoparticles are often deactivated by particle agglomeration and have poor stability. To avoid Pt agglomeration as much as possible, an excessive loading (usually carbon) is often used to disperse Pt nanoparticles, but this forms a thick catalyst layer, which impedes the transport of substances and increases the internal resistance. However, NPG-based alloy catalysts designed based on the structural advantages of NPG itself and its good adaptability with Pt are expected to solve the above problems.
3.2.1. Fuel Oxidation
Numerous studies have shown the excellent catalytic performance of Pt modified NPG in fuel oxidation, and these catalysts can work in a range of fuels, for example, hydrogen [49], methanol [50] and formic acid [51]. In addition, other NPG-based alloys, such as NP AuPd alloys, are usually employed as the catalysts of ethanol oxidation [52]. Moreover, owing to the substantial specific surface area and distinctive electronic properties of graphene, NPG/RGO has also been used in methanol electro-oxidation [53].
Wang et al. [54] have emphasized the importance of surface structure in enhancing catalytic performance. They modified the initial dealloyed NPG (i-NPG) (Figure 4a) with different CV scan rates to construct {111}-rich NPG (l-NPG) and {100}-rich NPG (h-NPG). The different facets percentage of these NPG is presented in Figure 4b. Further CV curves (Figure 4c) and chronoamperometric results (Figure 4d) of ethanol oxidation indicate that with the growing share of {111} facet, to a lesser extent, {100} facet, the catalytic activities of NPG are gradually increasing, which directly confirmed the result that the catalytic activities of these three facets of Au catalysts in alkaline ethanol oxidation reaction obey the order: {111} > {100} > {110}.
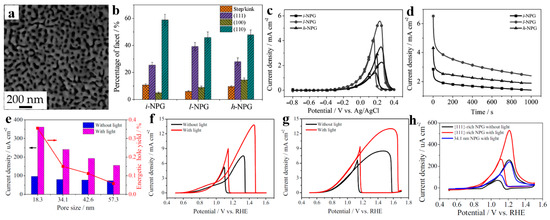
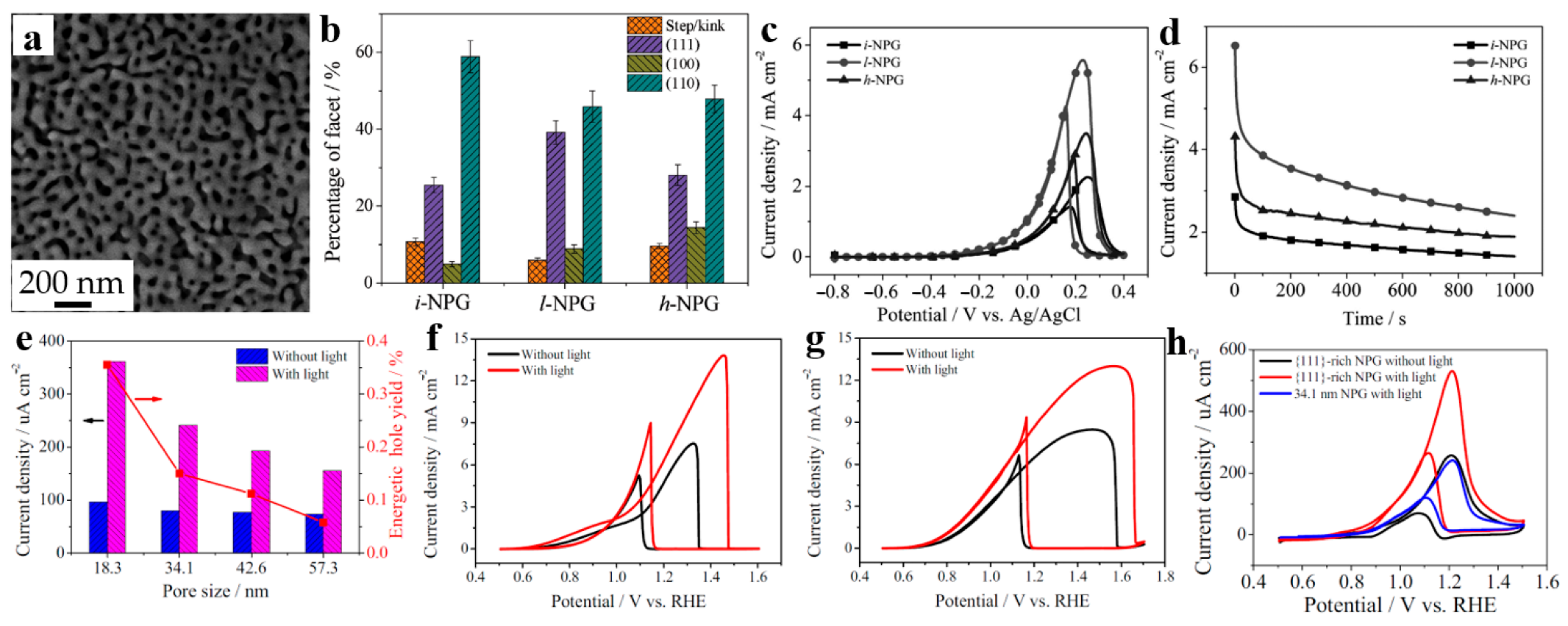
Figure 4.
Dealloyed NPG-based materials for fuel oxidation. (a) SEM image of initial NPG (i-NPG); (b) The percentages of different facets in i-NPG, l-NPG (50 cycles at 5 mV s−1), and h-NPG (50 cycles at 50 mV s−1); (c) CV curves, (d) Chronoamperometric results of the ethanol oxidation reaction on i-NPG, l-NPG, and h-NPG (reproduced from [54], with permission from Wiley, 2023.); (e) Peak current densities and energetic hole yields under light illumination versus the nanopores size of NPG; CV curves of 18.3 nm NPG in 0.5 M KOH electrolyte solutions containing (f) 1.0 M isopropanol, and (g) 0.25 M ethylene glycol; (h) CV curves of MOR on {111}-rich NPG and 34.1 nm NPG in a 0.5 M KOH/1.0 M methanol solution (reproduced from [55], with permission from Elsevier, 2023.).
Wang et al. [55] investigated the impact of localized surface plasmon resonance (LSPR) excitation on alcohol molecules by constructing a series of NPG with different ligament/nanopore sizes. In Figure 4e, it can be seen that with the rising of the ligament/nanopore size, the peak current density as well as energetic hole yield gradually decrease. Hence, 18.3 nm NPG with best LSPR extinction was selected to enhance catalytic activity and evaluated their catalytic ability of different alcohol molecules such as ethanol and isopropanol (Figure 4f) together with ethylene glycol (Figure 4g). Additionally, in order to further explore the performance of plasmonic enhanced NPG, {111}-rich NPG were synthesized via surfactant modified dealloying method, and their catalytic activities were shown in Figure 4h, which draw the same conclusion mentioned above.
A nanoporous ternary Au-Cu-Pt alloy thin films fabricated via co-electrodeposition as well as Cu partial selective removal was reported by Xie et al. [56]. The SEM image shows a refined ligament/pore size compared to conventional NPG, attributed to the lower diffusivity of Pt which impedes the diffusion of Au. The CV curves of 2.6% Pt-loaded NP Au-Cu-Pt at 93 mC and 43.5 mC total deposition charge, respectively, corresponding to two films at thicknesses of 70 nm and 33 nm. The cyclic test results suggest that the 70 nm thicker film exhibits better catalytic durability, which may be due to the fact that under the same test conditions, the thicker NP Au-Cu-Pt contains a larger total amount of Pt. In addition, no obvious CO oxidation peak is observed in these curves, indicating that samples possess good resistance to CO poisoning. The synergistic effect among Au, Cu, and Pt is one of the reasons for the high catalytic activity of NP Au-Cu-Pt. The application of dealloyed NPG-based materials for fuel oxidation is revealed in Table 3.

Table 3.
Dealloyed NPG-based materials for fuel oxidation.
3.2.2. Oxygen Reduction Reaction (ORR)
Fuel cells rely on the oxidation reaction of fuel and the reduction reaction of oxygen to generate electricity. However, the kinetics of ORR in fuel cells are much slower than fuel oxidation. Furthermore, existing ORR catalysts have poor durability problems that need to be solved urgently. As a result, the improvement of the catalyst activity and durability is the primary focus of current studies in this field.
Li et al. [57] reported a core-shell structure NPG-Pd-Pt electrode fabricated via Cu underpotential deposition (UPD) that exhibits higher activity and better stability than commercial Pt/C electrodes. In brief, the fresh NPG-Pd-Pt electrode demonstrates 4 times and 12 times higher specific and mass activities than the fresh Pt/C electrode, respectively. In addition, the study shows that the improvement of specific activities and stability of long-term cycling may be related to the stable and uniform Pt-Pd-Au trimetallic shell.
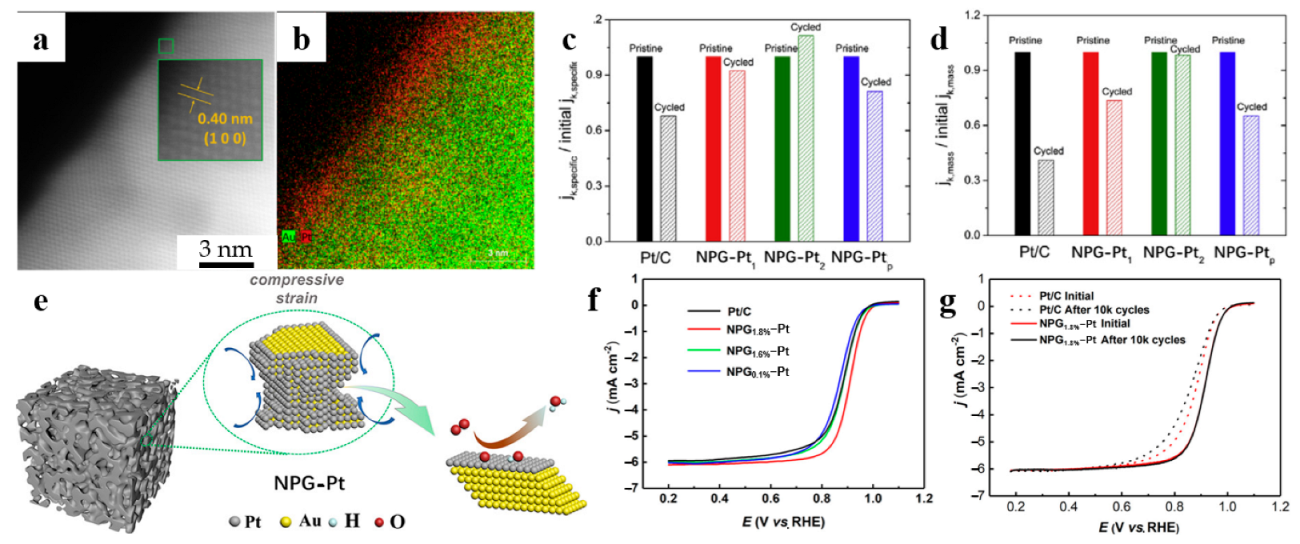
Similar methodology was used in the catalyst reported by Yu et al. [36] and Zhang et al. [58] Through controlling the number of copper UPD and Pt galvanic replacement cycles, Yu et al. synthesized NPG-Pt with monolayer Pt (NPG-Pt1) and two-layer Pt (NPG-Pt2). The atomic structure of the NPG-Pt2 surface region, where the Pt layer is in a single-crystalline region with NPG substrate, is shown in Figure 5a,b. Compared to other Pt loading catalysts shown in Figure 5c,d, NPG-Pt2 has the best stability performance. The reason why NPG-Pt2 is more stable than NPG-Pt1 is that it has a thicker Pt atomic layer, so Au atoms are less likely to diffuse to the surface. However, the increase in the number of Pt atomic layers leads to a decrease in the specific surface area of Pt, which reduces the catalytic activity. Therefore, achieving a balance between catalytic efficiency and stability requires a reasonable design of Pt atomic layers.
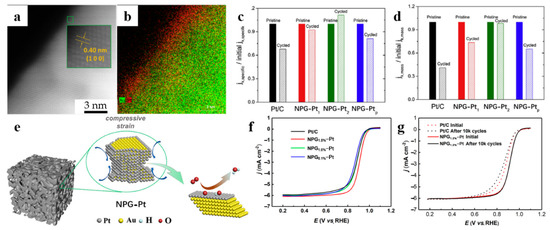
Figure 5.
Dealloyed NPG-based materials for ORR. (a) HAADF, and (b) EDS mapping image of NPG-Pt with two-layer Pt shell (NPG-Pt2); (c) Specific, and (d) mass activities of NPG-Pt and Pt/C catalysts before and after the stability tests (reproduced from [36], with permission from Elsevier, 2023); (e) Illustration of the compressive strain of the monolayer Pt on NPG; (f) ORR polarization curves of NPG-Pt and Pt/C; (g) ORR polarization curves of the NPG1.8%-Pt and Pt/C before and after the ADT test (reproduced from [58], with permission from American Chemical Society, 2023).
Another major factor affecting ORR activity is lattice strain. Zhang et al. [58] obtained ORR catalysts with enhanced catalytic activity and cycling durability by designing the surface strain of NPG-Pt, which provides a novel research direction. Figure 5e shows the accelerated ORR process occurring on the compressively strained Pt layer. Figure 5f shows the ORR polarization curves of NPG-Pt samples compared with commercial Pt/C. The diffusion-limiting current densities are close to the theoretical value of 6 mA cm−2. Among the prepared NPG substrates with compressive strain of 0.1%, 1.6% and 1.8%, respectively, NPG-1.8%-Pt shows the best apparent ORR activity with a half-wave potential of 0.91 V. From the NPG1.8%-Pt and Pt/C cycling durability test results (Figure 5g), it can be seen that after 10 thousand cycles, the half-wave potential of NPG1.8%-Pt was relatively unchanged and exhibited higher durability than that of Pt/C. The application of dealloyed NPG-based materials for ORR is presented in Table 4.

Table 4.
Dealloyed NPG-based materials for ORR.
3.3. Supercapacitors
In 2011, Lang and co-workers applied dealloying and electroplating techniques to fabricate NPG/MnO2 composite films (100 nm thick) as active charge storage electrodes for simple supercapacitor devices [59]. Subsequent electrochemical studies, including a 2 M Li2SO4 aqueous solution as the electrolyte, led to a specific capacitance of the constituent MnO2 of ~1145 F g−1, which is close to the theoretical value. This result illustrated the excellent electronic transport of MnO2 at the abundant Au/MnO2 interfaces provided by the NPG. Similar electrochemical deposition strategy was also used in the fabrication of nonaqueous supercapacitors electrodes. To achieve high electrochemical stability and electrical conductivity, the MnO2@NPG composites with several electrolytes (including one organic electrolyte and two ionic liquids) were assembled into a nonaqueous supercapacitor [60]. The result of HRTEM analysis indicates that MnO2 is epitaxially grown on the surface of gold ligaments to form a chemically bound metal/oxide interface. The highest specific capacitance of the MnO2@NPG supercapacitor reached ~160 F g−1 at a discharge current density of 8 A g−1 in EMI-DCA (1-ethyl-3-methylimidazolium dicyanamide) and maintain ~130 F g−1 at the high current density of 52 A g−1. In order to further increase the MnO2 loading amount and film thickness, Kang et al. [61] designed a novel sandwich electrode. The MnO2/NPG/MnO2 sandwich electrode was prepared by controlling the time of electrochemical deposition, leading the nanostructured MnO2 sprouts from the nanopore channels and symmetrically grows on the two outer surfaces of NPG sheet. The porous structure ensures efficient electrolyte transport throughout the electrode, maximizing the capacitance of MnO2. Recently, Prabhin et al. [62] synthesized a hybrid electrode through simultaneously electrodepositing cobalt oxide and manganese oxide nanoparticles on NPG substrate. Different from multi-layered electrode, the hybrid electrode apparently showed a mesoporous nature with shallow and deep traps, which are vital in increasing the kinetics of the process of charge transfer. Without surprisingly, the hybrid electrode exhibits a high capacitance of 1334 F g−1 at 5 mV s−1. Other metal oxides, such as RuO2 [63], were also used in NPG-based supercapacitor electrodes. In addition, conducting polymers have been used to modify the electrodes of NPG-based supercapacitors. Lang et al. [64] prepared NPG/PANI thin films. The resulting supercapacitors based on NPG/PANI demonstrated exceptional volumetric capacitance (~1500 F cm−3) and energy density (~0.078 Wh cm−3), surpassing electrolytic capacitors of the same power density by seven and four orders of magnitude, respectively.
After that, asymmetric metal hydroxide supercapacitors, such as RuO2-NPG//Co(OH)2-NPG asymmetric pseudocapacitor reported by Chen et al. [65], and NP Au/VA Ni(OH)2 electrode reported by Hou et al. [66], reached a specific capacitance of 1800 F g−1 and ~2416 F g−1, respectively. Kim et al. [67] developed a hydrothermal deposition method to deposit Ni(OH)2 directly on the NPG electrode. In Figure 6a, Ni(OH)2 active materials were observed to be deposited on both region A (top of the NPG electrode) and the region B (inner surface of the NPG electrode). Compared to other studies, the thickness of region A and region B were balanced, aiming to achieve the best capacitance value. However, it is worth noting that samples with higher thickness exhibited good volumetric capacity but poor kinetic properties and uncontrolled deposition after exceeding 30 min, thus limiting their overall performance. Therefore, considering comprehensively, the thickness of Ni(OH)2 and NPG were chosen to be 270 nm and 870 nm, respectively. As shown in Figure 6b,c, the capacitance of the optimized Ni(OH)2/NPG (270 nm/870 nm) electrode reached 3168 F g−1 at 5 A g−1, retained 70% capacitance even at 500 A g−1, and maintained 90% capacitance after 30,000 cycles at 200 A g−1.
In order to enhance the flexibility and reduce the weight of the device, polymers such as polypyrrole [68,69] have been used in the supercapacitor electrodes. Additionally, RGO was also combined with polypyrrole, as reported by Purkait et al. [70] and Yang et al. [71]. These supercapacitor devices exhibited excellent cycling stability with retention of 85.9% and 91% compared to the initial capacitance after 10,000 and 2000 galvanostatic charge–discharge cycles, respectively. Other polymers such as polyaniline (PANI) and graphitic carbon nitride (g-C3N4) were also applied in supercapacitors. Lee et al. [72] developed a PANI/NPG electrode via coating PANI on dealloyed Au20Mg80 alloy. Taking advantage of perchloric acid instead of sulfuric acid in electro-polymerization made a more stable PANI layer on NPG. To distinguish the PANI layer from epoxy layer, a thin chromium layer of approximately 40 nm was coated on the PANI/NPG electrode. Subsequent electrochemical studies confirmed these optimizations, i.e., the usage of perchloric acid in electrodeposition can increase the stability of the PANI/NPG electrode. Chen et al. [73] reported on the construction of NPG/g-C3N4 heterostructures, as shown in Figure 6d, a layer of 2D g-C3N4 nanosheets was covered on the surface of 3D-NPG, and the TEM result (Figure 6e) showed that g-C3N4 was uniformly coated covering the pores and walls of NPG with a thickness of about 10 nm. The authors also investigated the effect of bending angle on the performance of supercapacitor devices, as depicted in Figure 6f. It is noteworthy that both the volumetric and mass specific capacitance illustrate a spectacular increase, as shown in Figure 6g, reaching 2031 F cm−3 and 2295 F g−1 at 90° bending angle, respectively. These values are much higher than the initial capacitance (440 F g−1 without bending). The striking improvement of capacitance was attributed to the exposure of active sites resulting from bending deformation, which provides a novel strategy for optimizing the performance of supercapacitance. The application of dealloyed NPG-based materials for supercapacitor is shown in Table 5.
In general, NPG is mainly used as a support and a current collector in supercapacitors. Metal oxides and conductive polymers are coated on the surface of NPG to form a stable interface structure. With the help of the structural advantages of NPG itself, the efficient transmission of electrons, ions, and electrolytes is ensured, thereby improving capacitance and electrode stability.
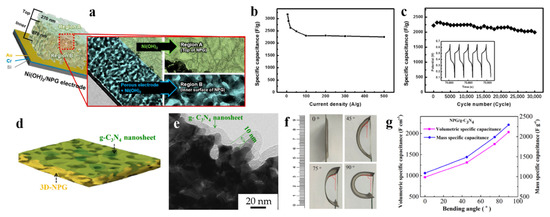
Figure 6.
Dealloyed NPG-based materials for supercapacitor. (a) Illustration of Ni(OH)2 in two separate regions of NPG; (b) Kinetic property, and (c) Cycle stability of the optimized Ni(OH)2/NPG electrode (reproduced from [67], with permission from Elsevier, 2023); (d) Schematic illustration of the 2D-3D structure; (e) TEM image of NPG/g-C3N4 prepared at 200 CV cycles (NPG/g-C3N4-200); (f) Pictures of NPG/g-C3N4-200 after bending different angles; (g) Volumetric and Mass specific capacitance of NPG/g-C3N4-200 with bending angle (reproduced from [73], with permission from Elsevier, 2023).
Figure 6.
Dealloyed NPG-based materials for supercapacitor. (a) Illustration of Ni(OH)2 in two separate regions of NPG; (b) Kinetic property, and (c) Cycle stability of the optimized Ni(OH)2/NPG electrode (reproduced from [67], with permission from Elsevier, 2023); (d) Schematic illustration of the 2D-3D structure; (e) TEM image of NPG/g-C3N4 prepared at 200 CV cycles (NPG/g-C3N4-200); (f) Pictures of NPG/g-C3N4-200 after bending different angles; (g) Volumetric and Mass specific capacitance of NPG/g-C3N4-200 with bending angle (reproduced from [73], with permission from Elsevier, 2023).
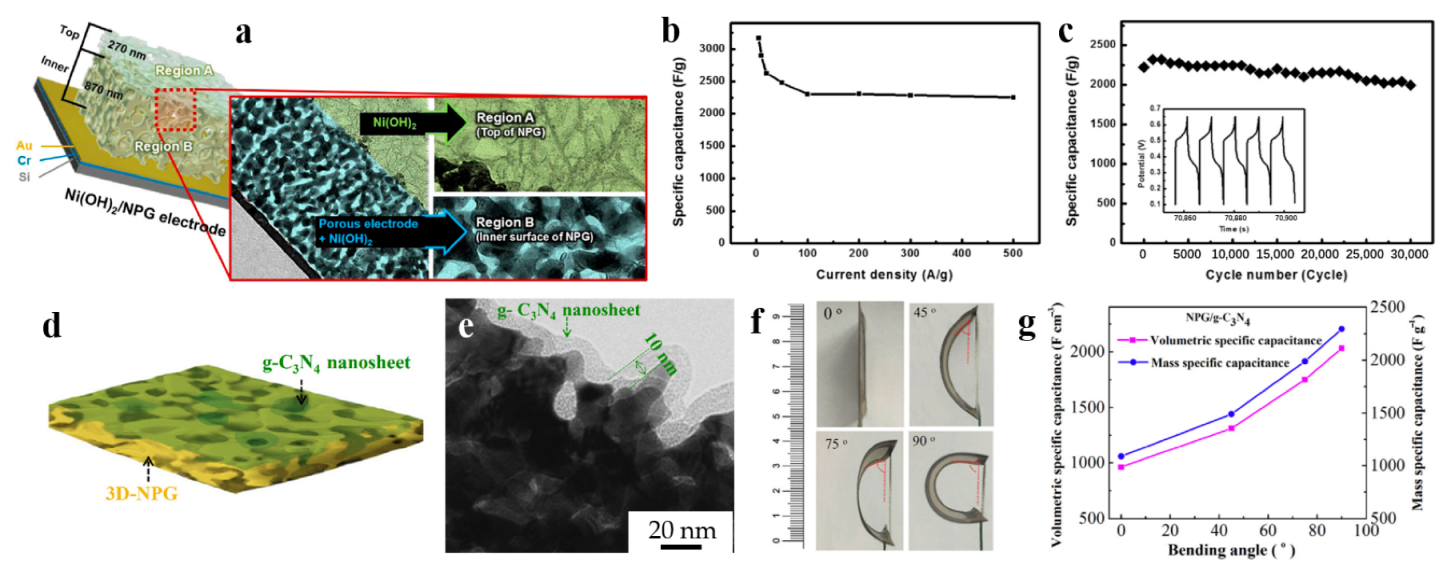

Table 5.
Dealloyed NPG-based materials for supercapacitor.
Table 5.
Dealloyed NPG-based materials for supercapacitor.
| Electrode | Specific Capacitance (Scan Rate or Current Density) | Capacitance Retention (Cycles, Scan Rate or Current Density) | Energy Density | Power Density | Function of NPG | Ref. |
|---|---|---|---|---|---|---|
| NPG/MnO2 | 1145 F/g | - | - | - | Increase electrical conductivity and ion diffusion rate, act as a double layer capacitor | [59] |
| nonaqueous MnO2@NPG | 160 F/g | - | - | - | Increase specific capacitance | [60] |
| MnO2/NPG/MnO2 | 841 F/g (5 mV/s) | 97.1% (3000, 50 mV/s) | - | - | Support and current collector | [61] |
| MnO2/graphene | 310 F/g (2 mV/s) | - | - | - | - | |
| Co3O4–MnO2–Au layered electrode | 1215 F/g (5 mV/s) | 83% (2000, 100 mV/s) | 24.37 Wh/kg | 1403 W/kg | Improve ionic conductivity and increase contact area | [62] |
| Co3O4+MnO2—Au hybrid electrode | 1334 F/g (5 mV/s) | 85% (2000, 100 mV/s) | 48.1 Wh/kg | 2524 W/kg | - | |
| RuO2@NPG | 1500 F/g (10 mV/s) | - | 50.3 Wh/kg | 84 kW/kg | Support and current collector | [63] |
| NPG/PANI | 1500 F/cm3 | - | 0.078 Wh/cm3 | - | - | [64] |
| RuO2–NPG//Co(OH)2–NPG | 350 F/g | 78% (3000) | 120 Wh/kg | - | - | [65] |
| NPG/VA Ni(OH)2 | 2911 F/cm3 | - | 31.4 Wh/kg | 100 kW/kg | - | [66] |
| Ni(OH)2/NPG | 2223 F/cm3 (5 A/g) | 90% (30,000, 500 A/g) | 98 Wh/kg | 50 kW/kg | Current Collector | [67] |
| PPy–NPG//MnO2–NPG | 193 F/g | 85% (2000, 100 mV/s) | 86 Wh/kg | 25 kW/kg | Support and current collector | [68] |
| PPy–3DrGO/NPG | 245.34 F/cm3 | 85.9% (10,000) | 98.48 mWh/cm3 | 19.68 W/cm3 | - | [70] |
| rGO-PPy/NPG | 55.6 F/cm3 | 91% (2000) | 238.8 mWh/cm3 | - | - | [71] |
| PANI/NPG | 6.54 mF/cm2 | - | 9.11 mWh/cm2 | 1.56 W/cm2 | - | [72] |
| NPG/g–C3N4 | 440 F/g (2 A/g) | 98% (10,000) | - | - | - | [73] |
3.4. Battery
Over the past three decades, lithium-ion batteries (LIBs) have been widely applied in daily life owing to their high energy density [74,75]. However, there are some problems that need to be further settled: for example, volume variation [9]. Given the stabilization and interaction between Au and Li, NPG serves as an excellent model for a current collector and alloy-type anode. Yu et al. [76] introduced an alternative anode for rechargeable LIBs consisting of a dealloyed NPG-supported nanocrystalline thin tin foil. The special nanoporous structure of NPG offers ample space to accommodate big volume variations during charge–discharge cycling, thereby preventing the loss of specific mass in long-term cycling. This design results in a remarkable reversible capacity of 620 mAh g−1 after 140 cycles at 0.1 C. The SEM image of the Sn intact structure after 140 cycles indicates that metal-based current collectors with nanoporous structures are promising. The performance of LIB electrodes is significantly affected by several interconnected thermodynamics and kinetics processes related to structural design [77]. Ye et al. [78] conducted a comprehensive study about the effects of length scale on electrochemical performance on the model of 3D NPG/TiO2 core/shell electrodes. The rate performance of these 3D core/shell models could be greatly optimized by adjusting the pore size of current collector and the thickness of deposition layer. Moreover, NPG was utilized as a classic model to investigate the degradation and substantial volume variation in alloy-type LIB anodes [9]. A new understanding of the degradation mechanisms of the solid-electrolyte interphase (SEI) and its morphology has been proposed. These mechanisms include the pulverization of material caused by volume expansion, the implantation of active material nanoparticles in a thick SEI layer, the formation of a bimodal and hierarchical nanoporous morphology, and the delamination of SEI and nanoparticles created by volume contraction.
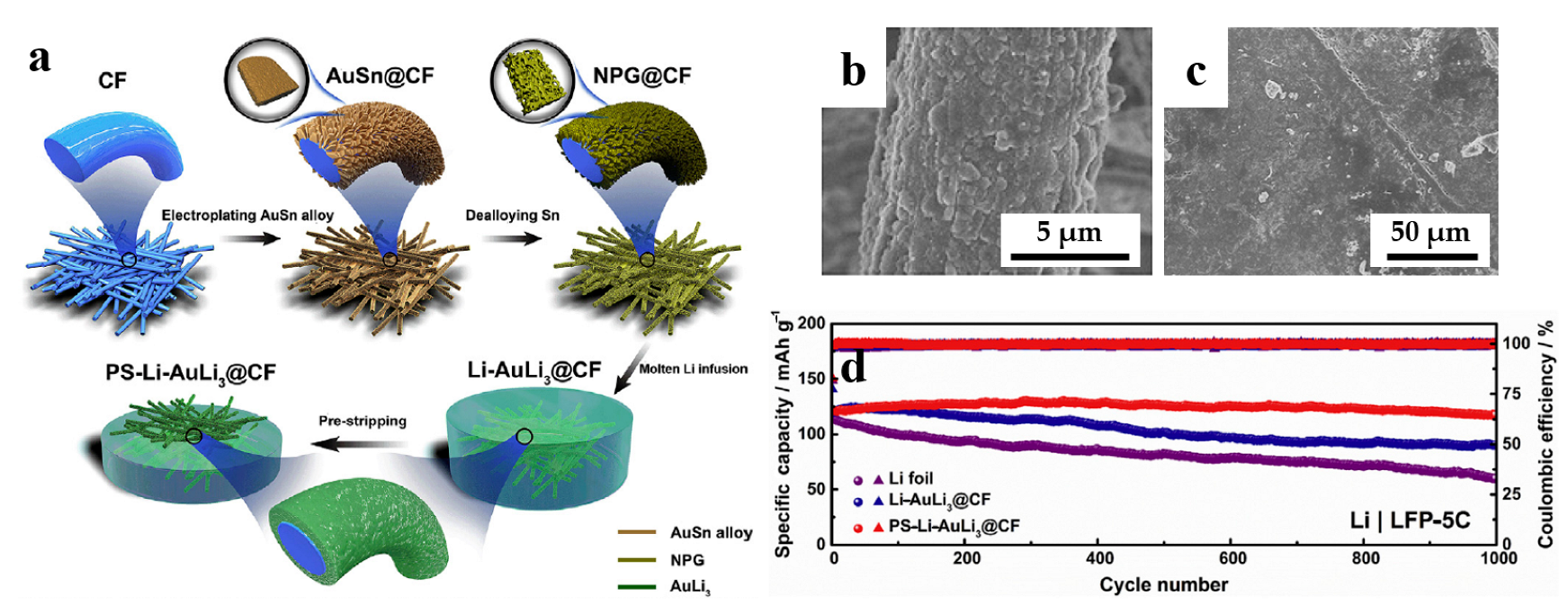
With years of development, LIBs are gradually approaching their energy density limits. As a result, Li metal has been widely considered as a promising alternative anode material, owing to its high specific capacity (3860 mAh g−1), low redox potential (−3.040 V versus standard hydrogen electrode) and small gravimetric density (0.534 g cm−3) [79]. However, there are many technical issues that hinder the commercialization of the lithium metal anodes (LMAs), particularly related to low coulombic efficiency and SEI stability issues induced by dendrite growth during Li plating/stripping, which raises safety concerns [80,81]. To address these challenges, many strategies have been proposed to extend the lifespan of lithium metal anodes, for example, facilitating SEI formation through manipulating the electrolyte additives [82], constructing an artificial protective SEI via chemical reaction [83], and improving lithiophilicity to reduce lithium nucleation overpotential [84]. Nevertheless, these strategies are primarily effective at low current densities and cycling capacities, and further advancements are needed to achieve high-rate and long-lifespan LMAs. Chen et al. [85] proposed a strategy, which is infusing molten Li into the NPG-coated carbon fiber (CF) to form AuLi3@CF scaffold, and a treatment process called “pre-stripping” to expose the buried AuLi3@CF scaffold in Li metal layer, enabling a lower Li nucleation overpotential and suppression of Li dendrite growth. As shown in Figure 7a, the pre-stripping AuLi3@CF scaffold (PS-AuLi3@CF) (Figure 7b) shows an excellent lithiophilicity of NPG, and the scaffold with a large surface area can significantly reduce local current densities during cycling and retard Li dendrite formation. Figure 7c shows the PS-AuLi3@CF electrodes after cycling for 150 cycles. The Li deposit appears smooth and has uniform morphology, revealing the structural advantages of PS-Li-AuLi3@CF composites in maintaining electrode integrity. As illustrated in Figure 7d, the electrochemical performance of the PS-Li-AuLi3@CF electrode was compared with other electrodes in a full battery configuration with a LiFePO4 (LFP) cathode. The PS-Li-AuLi3@CF|LFP electrode exhibited a specific capacity of approximately 122 mAh/g in the initial cycle, and after 1000 cycles, it retained a capacity of 117 mAh/g, resulting in a capacity retention rate of 96.1%. Moreover, the PS-Li-AuLi3@CF|LFP electrode demonstrated enhanced rate capability compared to the other electrodes. Because Au is regarded as one of the most lithiophilic substrates [86], the Ni foam (NF) with the modified nanoporous AuLi3 (NPAuLi3) nanosheets (NPAuLi3@NF) was designed for use in LMAs [84]. This composite current collector exhibits a structure of triporosity, which can not only regulate ions transportation but also accommodate volume change during Li plating/stripping, while the strong Au–Li interaction can benefit the reducing of Li nucleation overpotential and inducing homogeneous Li nuclei growth.
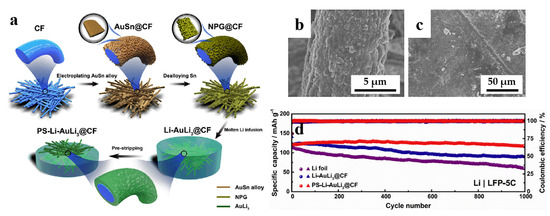
Figure 7.
Dealloyed NPG-based materials for batteries. (a) Schematic illustration of the fabrication process of PS-Li-AuLi3@CF electrodes; SEM images of (b) Li–AuLi3@CF after pre-stripping (PS-Li-AuLi3@CF), (c) PS-Li-AuLi3@CF electrode after cycling for 150 cycles; (d) Cycling performance of Li foil, Li–AuLi3@CF and PS-Li-AuLi3@CF electrodes in full cells with LiFePO4 (LFP) cathode at 5C (reproduced from [85], with permission from Elsevier, 2023).
Li-O2 battery is considered as a promising battery due to its expected specific energy, which is f times higher than that of LIB. Chen et al. [87] prepared NPG/RuO2 composite catalyst for solving the problem of the high overpotential of Li2O2 oxygen evolution reaction. The HRTEM image of NPG/RuO2 shows that RuO2 is uniformly coated on the NPG substrate with a thickness of 3~5 nm and well bonded to the Au interface. The pores of NPG provide enough space to accommodate the discharge products and minimize volume change of the electrode during charging and discharging process. The TEM image of the discharged RuO2-NPG cathode demonstrates that the discharge products are evenly distributed within the composite’s pores without obstructing the channel networks. Also noteworthy is the rechargeable Al-CO2 battery, which enables both effective utilization of the greenhouse gas CO2 and relatively safe energy storage and conversion. Ma et al. [88] prepared an Al-CO2 battery using aluminum foil as the anode and NPG@Pd as the cathode, demonstrating the broad applicability of Al-CO2 battery. The NPG@Pd cathode formed a single crystal structure. This is because Au and Pd have the similar crystal structure (fcc) and lattice parameters, which make an easy bonding. To evaluate the cyclability of the cell, a discharge/charge cycle test was performed at a constant current density of 333 mA g−1 with 1 h time interval. The results indicate that the Al-CO2 battery with NPG@Pd cathode exhibited lower charging voltage and smaller discharge/charge voltage gap compared to the battery with NPG cathode. NPG films are also applied to the fabrication of perovskite solar cells (PSCs). Yang et al. [89] introduce NPG thin film as a contact electrode in PSCs. NPG electrode can be recycled more than 12 times, which reduces the manufacturing cost. Moreover, NPG/PSCs have the advantages of high-power conversion efficiency (19%) (PCE) and bending durability (98.5%). The application of dealloyed NPG-based materials for batteries is uncovered in Table 6.

Table 6.
Dealloyed NPG-based materials for batteries.
3.5. CO2 Reduction Reaction
CO2RR converts the greenhouse gas CO2 into other fuels for reuse, which is an important way to solve its excessive emissions. However, the key challenge remains the development of suitable catalysts. Fortunately, the research on NPG and its alloys brings new hope for finding efficient CO2RR catalysts.
To optimize the transport of reactant, ion together with electron at the same time, a 3D hierarchically porous gold with nanoporous/macroporous structure (N/M-Au) was synthesized through the electroplated metal into a template and subsequent dealloying [93]. The unique template was made by an advanced lithography technique, denoted by proximity-field nanopatterning, and was removed by a plasma etcher after plating. As shown in Figure 8a,b, the interconnected macroporous channels contribute to the mass transport of reactant, while the nanoporous structure having high-index Au surface is conductive to the high CO selectivity and a large electrochemical active area. Thus, both specific current density and relative mass activity of the N/M-Au are better than Au structures that are only nanoporous or macroporous, as shown in Figure 8c,d, respectively.
Lu et al. [37] conducted a comparison of the electrochemical CO2 reduction performance between NPG prepared by electrochemical dealloying with potential cycling (pc-NPG) and chemical dealloying (cd-NPG). The results showed that pc-NPG had better activity against CO at all tested potentials, which may result from its highly curved internal surfaces with numerous step/kink sites. Moreover, to achieve long-term catalytic activity, a reactivation method was proposed, i.e., potential cycling. On one hand, the restored shares of step/kink sites after potential cycling imply the regeneration of active sites, on the other hand, the vanishing signals of zinc and copper indicate the removal of metal impurities from the electrolyte. The author also presented a nanoporous Au–Sn catalyst with minor Sn atoms in CO2RR [94]. The incorporation of Sn atoms induced lattice strain, enhancing the efficiency of CO generation, while also preventing ligament coarsening and improving electrochemical stability.
Bimetallic catalysts are promising CO2RR catalysts. The doping of other elements such as Sn and Cu can effectively improve the catalytic performance of NPG catalysts. For instance, Ma et al. prepared Au3Cu [95] and AuCu3@Au [96] for electrochemical carbon dioxide reduction, respectively. Figure 8e reveals that the surface of AuCu3@Au ligaments exhibit a high density of atomic steps and kinks, which are typically active sites for catalytic reactions. To explore practical applications, the researchers fabricated bulk nanoporous AuCu3@Au electrodes with dimensions of 23 cm in length and 0.15 cm in width. At a potential of −0.7 V (versus RHE), the effective current reaches 37.2 mA, with a corresponding current density of 10.78 mA cm−2 (Figure 8f), indicating the potential for achieving high CO Faradaic efficiency. Computational investigations were also conducted to study the CO2 reduction reaction mechanism on the surfaces of Au{311} and AuCu3@Au{311} catalysts. The results demonstrate that the free energy change for the formation of *COOH intermediates on the AuCu3@Au{311} catalyst is significantly lower than that on the Au{311} catalyst. This difference arises from the enhanced interaction between the metals Au and Cu with the *COOH intermediate in the AuCu3@Au{311} catalyst, whereas only one Au atom in Au{311} interacts with the *COOH intermediate (Figure 8h,i). The bidentate adsorption in AuCu3@Au effectively improves the stability of the *COOH intermediate, enhancing the adsorption of *COOH on the AuCu3@Au{311} surface and favoring the formation of CO. Additionally, Liu et al. [97] conducted a study showcasing the capability of nanoporous AuCu in converting CO2 into CO, CH4, and HCOOH. The application of dealloyed NPG-based materials for CO2 reduction reaction is presented in Table 7.
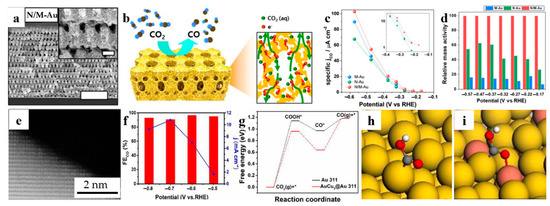
Figure 8.
Dealloyed NPG-based materials for CO2 reduction reaction. (a) SEM images of hierarchically porous gold (N/M-Au) (Scale bar, 5 μm; Inset scale bar, 500 nm); (b) Schematic illustration (Left) and the cross-sectional view with the expected reaction pathway (Right) for the N/M-Au electrode, NPG induced CO2RR is indicated by an arrow; (c) Electrochemical performance for CO2 reduction electrolysis to CO on different Au nanostructured electrodes; (d) Relative mass activity comparing between M-, N-, and N/M-Au (reproduced from [93], with permission from PNAS, 2023.); (e) HAADF image of AuCu3@Au nanoporous alloy; (f) Determination of the FE of CO at high current density of AuCu3@Au; (g) Gibbs free changes of the intermediates of CO2RR on Au{311} and AuCu3@Au{311}; Adsorption structure of *COOH on (h) Au{311} and (i) AuCu3@Au{311} (reproduced from [96], with permission from Royal Society of Chemistry, 2023).
Figure 8.
Dealloyed NPG-based materials for CO2 reduction reaction. (a) SEM images of hierarchically porous gold (N/M-Au) (Scale bar, 5 μm; Inset scale bar, 500 nm); (b) Schematic illustration (Left) and the cross-sectional view with the expected reaction pathway (Right) for the N/M-Au electrode, NPG induced CO2RR is indicated by an arrow; (c) Electrochemical performance for CO2 reduction electrolysis to CO on different Au nanostructured electrodes; (d) Relative mass activity comparing between M-, N-, and N/M-Au (reproduced from [93], with permission from PNAS, 2023.); (e) HAADF image of AuCu3@Au nanoporous alloy; (f) Determination of the FE of CO at high current density of AuCu3@Au; (g) Gibbs free changes of the intermediates of CO2RR on Au{311} and AuCu3@Au{311}; Adsorption structure of *COOH on (h) Au{311} and (i) AuCu3@Au{311} (reproduced from [96], with permission from Royal Society of Chemistry, 2023).
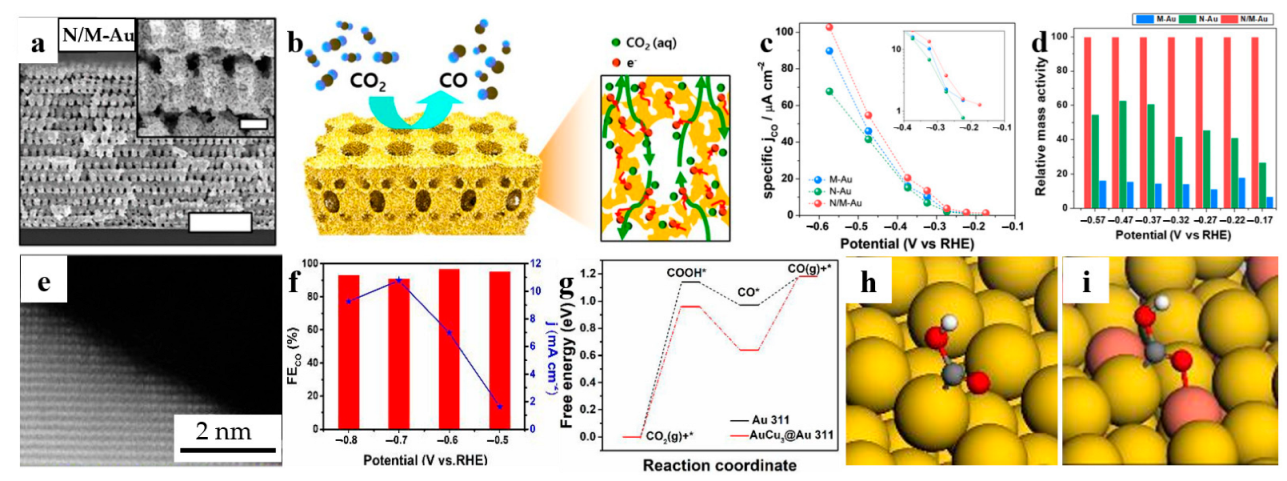

Table 7.
Dealloyed NPG-based materials for CO2 reduction reaction.
Table 7.
Dealloyed NPG-based materials for CO2 reduction reaction.
| Catalyst | jCO (mA/cm2) | Overpotential (mV) | Faradaic Efficiency | Current Densities (mA/cm2) | Tafel Slope (mV/Decade) | Ref. |
|---|---|---|---|---|---|---|
| pc-NPG | - | 390 | 98% | - | 52 | [37] |
| cd-NPG | - | - | - | - | 70 | |
| N-Au | 0.759 | - | - | −13.2 | 106 | [93] |
| M-Au | - | - | - | −12.6 | 120 | |
| N/M-Au | 0.891 | - | - | −19 | 107 | |
| NP Au-Sn | - | - | 86.5% at −0.8 V | - | - | [94] |
| NP Au3Cu | - | - | 98.12% at −0.7 V vs. RHE | 12.77 | - | [95] |
| NP AuCu3@Au | - | - | 97.27% at −0.6 V vs. RHE | 10.78 | - | [96] |
4. Summary and Outlook
In this review, we introduce the typical formation process of dealloyed NPG. Subsequently, we focus our attention on exploring the wide-ranging applications of NPG in energy storage and conversion, encompassing water splitting, fuel cells, supercapacitors, battery and CO2RR.
- About water splitting, researchers make use of the advantages of the geometric structure of NPG and good electrical conductivity, combined with non-precious metal catalysts, to design low-cost, high-activity catalysts that can significantly reduce HER and OER overpotential and accelerate the reaction kinetics process.
- Regarding fuel cells, studies have focused on reducing Pt loading and improving fuel oxidation and ORR catalyst stability, and the good adaptability of NPG to Pt makes it a better loading material than carbon. To this end, the effects of NPG surface structure, localized surface plasmon resonance excitation and synergistic effects between Au and Pt, Pd and Cu on fuel oxidation and ORR catalytic activity have been mainly investigated.
- For supercapacitors, various structures of NPG hybrid electrodes have been designed to improve capacitance and cycling stability. Among them, the electrodes compounded with NPG and conducting polymers are lightweight and flexible, which have broad application prospects in wearable devices.
- In battery research, on the one hand, NPG is used as a classical model to study the existing problems of volume variation and stability of alloy-type LIB anodes; On the other hand, NPG is employed as an electrode material in various types of lithium batteries and metal-air cells to improve cell performance, such as reducing volume change, inhibiting lithium dendrite growth, extending electrode lifespan, etc.
- For CO2RR, the works mainly revolve around the preparation methods of NPG catalysts capable of efficient mass transport and CO selectivity and the catalytic mechanism of NPG-based alloy catalysts.
In a word, the research on the application of NPG in energy storage and conversion has made significant progress. However, there are still some pressing challenges that need to be addressed, there are three main points:
- High cost: The limited global reserves of gold contribute to its status as a precious metal, resulting in a relatively high cost. The production of NPG involves the use of gold alloys as raw materials and specialized manufacturing techniques, which further contribute to the higher production costs associated with NPG.
- Complex manufacturing process: To produce NPG-based materials with desired functionalities, it is necessary to finely tune the pore size and porosity of NPG. This involves careful control of various reaction conditions during the dealloying process, including selection of precursors, electrolytes, applied potentials, temperature, and duration. Additionally, factors such as coarsening after dealloying, surface adsorption, and surface coating treatment play a role in achieving the desired properties. As a result, the process of obtaining NPG with specific characteristics is intricate and requires attention to multiple variables and steps.
- Environmental hazard: Dealloying processes typically involve the use of strong acid or strong alkali solutions, which possess a certain degree of toxicity. Improper handling of these reagents can lead to environmental pollution and pose health risks to operators. Therefore, it is crucial to address the issue of waste liquid treatment and expedite the development of dealloying technologies that can be conducted under neutral and mild conditions.
The most prominent problem is the limited availability of gold reserves and its high cost, which hinders the commercialization of NPG. Therefore, there is an urgent need to find an effective alternative to gold or to minimize the usage of gold while maintaining the superior performance of NPG. One approach is to develop other nanoporous materials, such as transition group non-precious metals [98] or the group IVA materials [99,100], which offer advantages in terms of chemical structure stability and low cost. Additionally, nanoporous gold-based composites can be designed through rational structural engineering, such as thin films, layered, or core-shell structures. While improving the material properties and Au utilization, it is also important to consider whether the designed structures can be robustly integrated into real systems. Furthermore, the utilization of 3D printing technology [101] offers the possibility of fabricating NPG-based composites that precisely meet our design requirements. This advanced manufacturing technique is not confined to component-level fabrication but can also enable the printing of complete NPG-based electrode devices. This will provide a more convenient way to study NPG from the perspective of practical applications. In recent years, the electrochemical reduction of nitrogen has attracted interest as an alternative to the Haber–Bosch process for the synthesis of NH3. NPG has demonstrated excellent catalytic activity for nitrogen reduction reactions (NRR) at room temperature and pressure [102], and more research on NPG for catalyzing NRR is expected in the future. In addition, the research of NPG in the energy storage field can be extended to broader applications such as sodium ion batteries, solar cells, bio-cells, etc. In the future, the application of dealloyed NPG materials in different fields will shine dazzling light.
Author Contributions
Conceptualization, C.Q. and Z.W.; formal analysis, M.Y. and X.W.; investigation, M.Y. and X.W.; writing—original draft preparation, M.Y. and X.W.; writing—review and editing, Z.W.; visualization, M.Y. and C.Q.; supervision, C.Q. and Z.W.; funding acquisition, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the financial support from the Natural Science Foundation of Hebei Province, China (E2020202071), and the Hebei Higher Education Teaching Reform Research and Practice Project, China (2021GJJG050).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- An, Y.; Tian, Y.; Wei, C.; Tao, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Dealloying: An effective method for scalable fabrication of 0D, 1D, 2D, 3D materials and its application in energy storage. Nano Today 2021, 37, 101094. [Google Scholar] [CrossRef]
- Fu, J.; Welborn, S.S.; Detsi, E. Dealloyed air- and water-sensitive nanoporous metals and metalloids for emerging energy applications. ACS Appl. Energy Mater. 2022, 5, 6516–6544. [Google Scholar] [CrossRef]
- Xi, W.; Wang, K.; Shen, Y.; Ge, M.; Deng, Z.; Zhao, Y.; Cao, Q.; Ding, Y.; Hu, G.; Luo, J. Dynamic co-catalysis of Au single atoms and nanoporous Au for methane pyrolysis. Nat. Commun. 2020, 11, 1919. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tang, C.; Chen, G.; He, Z.; Wang, T.; He, X.; Yi, T.; Liu, Y.; Zhang, L.; Du, K. Toward the limitation of dealloying: Full spectrum responsive ultralow density nanoporous gold for plasmonic photocatalytic SERS. ACS Appl. Mater. Interfaces 2021, 13, 7735–7744. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, X.; Chen, Y.; Chen, J.; Yuan, S.; Qian, L.; Lu, X.; Liu, P.; Lei, P.; Li, X. Intrinsic contribution of mass transport within nanoscale channels of nanoporous gold for CO2 electrochemical reduction. Adv. Mater. Interfaces 2022, 9, 2200895. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Li, F.; Su, X.; Zhang, Q.; Guan, G.; Hu, F.; Zhang, J.; Wang, Q.; Jiang, Y. Modulating hydrogen adsorption via charge transfer at the semiconductor–metal heterointerface for highly efficient hydrogen evolution catalysis. Adv. Mater. 2023, 35, 2207114. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, X.; Chen, Y.; Chen, J.; Yuan, S.; Zhang, Z.; Qian, L.; Li, S. Robust catalysis of hierarchically nanoporous gold for CO2 electrochemical reduction. Electrochim. Acta 2023, 437, 141537. [Google Scholar] [CrossRef]
- Cheng, C.; Lührs, L. Robust metallic actuators based on nanoporous gold rapidly dealloyed from gold–nickel precursors. Adv. Funct. Mater. 2021, 31, 2107241. [Google Scholar] [CrossRef]
- Corsi, J.S.; Welborn, S.S.; Stach, E.A.; Detsi, E. Insights into the degradation mechanism of nanoporous alloy-type Li-ion battery anodes. ACS Energy Lett. 2021, 6, 1749–1756. [Google Scholar] [CrossRef]
- Kim, S.H. Nanoporous gold for energy applications. Chem. Rec. 2021, 21, 1199–1215. [Google Scholar] [CrossRef]
- Wang, M.; Meng, A.C.; Fu, J.; Foucher, A.C.; Serra-Maia, R.; Stach, E.A.; Detsi, E.; Pikul, J.H. Surface facet engineering in nanoporous gold for low-loading catalysts in aluminum-air batteries. ACS Appl. Mater. Interfaces 2021, 13, 13097–13105. [Google Scholar] [CrossRef]
- Li, J.; Li, L.Y.; Jia, P.; Okulov, I.V. Electrochemical behavior of nanoporous gold/polypyrrole supercapacitor under deformation. Nanomaterials 2022, 12, 2149. [Google Scholar] [CrossRef]
- Downs, A.M.; Gerson, J.; Hossain, M.N.; Ploense, K.; Pham, M.; Kraatz, H.B.; Kippin, T.; Plaxco, K.W. Nanoporous gold for the miniaturization of in vivo electrochemical aptamer-based sensors. ACS Sens. 2021, 6, 2299–2306. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, W.; Xu, L.; Hao, C.; Ma, W.; Sun, M.; Wu, X.; Qin, X.; Colombari, F.M.; de Moura, A.F.; et al. Polarization-sensitive optoionic membranes from chiral plasmonic nanoparticles. Nat. Nanotechnol. 2022, 17, 408–416. [Google Scholar] [CrossRef]
- Tortolini, C.; Cass, A.E.G.; Pofi, R.; Lenzi, A.; Antiochia, R. Microneedle-based nanoporous gold electrochemical sensor for real-time catecholamine detection. Mikrochim. Acta 2022, 189, 180. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Ma, W.; Zhou, K.; Cao, Y.; Liu, X.; Ma, R. Advanced silicon nanostructures derived from natural silicate minerals for energy storage and conversion. Green Energy Environ. 2022, 7, 205–220. [Google Scholar] [CrossRef]
- Ng, S.; Pumera, M. 2D functionalized germananes: Synthesis and applications. Adv. Mater. 2022, 35, 2207196. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lan, X.; Hu, R.; Yao, Y.; Yu, Y.; Zhu, M. Tin-based anode materials for stable sodium storage: Progress and perspective. Adv. Mater. 2022, 34, 2106895. [Google Scholar] [CrossRef]
- Jiang, M.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. A review of degradation mechanisms and recent achievements for Ni-rich cathode-based Li-ion batteries. Adv. Energy Mater. 2021, 11, 2103005. [Google Scholar] [CrossRef]
- Lan, X.; Xiong, X.; Liu, J.; Yuan, B.; Hu, R.; Zhu, M. Insight into reversible conversion reactions in SnO2-based anodes for lithium storage: A review. Small 2022, 18, 2201110. [Google Scholar] [CrossRef]
- Lichchhavi; Kanwade, A.; Shirage, P.M. A review on synergy of transition metal oxide nanostructured materials: Effective and coherent choice for supercapacitor electrodes. J. Energy Storage 2022, 55, 105692. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, Y. Iridium-based electrocatalysts toward sustainable energy conversion. EcoMat 2022, 4, e12176. [Google Scholar] [CrossRef]
- Du, R.; Jin, X.; Hübner, R.; Fan, X.; Hu, Y.; Eychmüller, A. Engineering self-supported noble metal foams toward electrocatalysis and beyond. Adv. Energy Mater. 2019, 10, 1901945. [Google Scholar] [CrossRef]
- Koya, A.N.; Zhu, X.; Ohannesian, N.; Yanik, A.A.; Alabastri, A.; Proietti Zaccaria, R.; Krahne, R.; Shih, W.C.; Garoli, D. Nanoporous metals: From plasmonic properties to applications in enhanced spectroscopy and photocatalysis. ACS Nano 2021, 15, 6038–6060. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, P.; Lingden, D.; Bhattarai, J.K.; Demchenko, A.V.; Stine, K.J. Applications of nanoporous gold in therapy, drug delivery, and diagnostics. Metals 2022, 13, 78. [Google Scholar] [CrossRef]
- Pan, J.; Xu, W.; Li, W.; Chen, S.; Dai, Y.; Yu, S.; Zhou, Q.; Xia, F. Electrochemical aptamer-based sensors with tunable detection range. Anal. Chem. 2023, 95, 420–432. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.M. Nanoporous metals: Fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, Y.; Chen, M. Nanoporous metal by dealloying for electrochemical energy conversion and storage. MRS Bull. 2018, 43, 43–48. [Google Scholar] [CrossRef]
- Wu, X.; He, G.; Ding, Y. Dealloyed nanoporous materials for rechargeable lithium batteries. Electrochem. Energy Rev. 2020, 3, 541–580. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Kumar, A.; da Silva, M.I.; Toma, H.E.; Martins, P.R.; Araki, K.; Bertotti, M.; Angnes, L. Nanoporous gold-based materials for electrochemical energy storage and conversion. Energy Technol. 2021, 9, 2000927. [Google Scholar] [CrossRef]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of Nanoporosity in Dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Kim, Y.J.; Erlebacher, J. Nanoporous Gold Leaf: “Ancient Technology”/Advanced Material. Adv. Mater. 2004, 16, 1897–1900. [Google Scholar] [CrossRef]
- Chen, Y.c.K.; Chu, Y.S.; Yi, J.; McNulty, I.; Shen, Q.; Voorhees, P.W.; Dunand, D.C. Morphological and topological analysis of coarsened nanoporous gold by x-ray nanotomography. Appl. Phys. Lett. 2010, 96, 043122. [Google Scholar] [CrossRef]
- Scaglione, F.; Alladio, E.; Damin, A.; Turci, F.; Baggiani, C.; Giovannoli, C.; Bordiga, S.; Battezzati, L.; Rizzi, P. Functionalized nanoporous gold as a new biosensor platform for ultra-low quantitative detection of human serum albumin. Sens. Actuators B Chem. 2019, 288, 460–468. [Google Scholar] [CrossRef]
- Yu, Q.; Yin, S.; Zhang, J.; Yin, H. Structure dependent activity and durability towards oxygen reduction reaction on Pt modified nanoporous gold. Electrochim. Acta 2019, 298, 599–608. [Google Scholar] [CrossRef]
- Lu, X.; Yu, T.; Wang, H.; Qian, L.; Lei, P. Electrochemical fabrication and reactivation of nanoporous gold with abundant surface steps for CO2 reduction. ACS Catal. 2020, 10, 8860–8869. [Google Scholar] [CrossRef]
- Sang, Q.; Hao, S.; Han, J.; Ding, Y. Dealloyed nanoporous materials for electrochemical energy conversion and storage. EnergyChem 2022, 4, 100069. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, P.; Chen, L.; Cong, W.; Ito, Y.; Han, J.; Guo, X.; Tang, Z.; Fujita, T.; Hirata, A.; et al. Monolayer MoS2 films supported by 3D nanoporous metals for high-efficiency electrocatalytic hydrogen production. Adv. Mater. 2014, 26, 8023–8028. [Google Scholar] [CrossRef]
- Ge, X.; Chen, L.; Zhang, L.; Wen, Y.; Hirata, A.; Chen, M. Nanoporous metal enhanced catalytic activities of amorphous molybdenum sulfide for high-efficiency hydrogen production. Adv. Mater. 2014, 26, 3100–3104. [Google Scholar] [CrossRef]
- Bak, C.H.; Kim, K.; Jung, K.; Kim, J.B.; Jang, J.H. Efficient photoelectrochemical water splitting of nanostructured hematite on a three-dimensional nanoporous metal electrode. J. Mater. Chem. A 2014, 2, 17249–17252. [Google Scholar] [CrossRef]
- Huan, Y.; Shi, J.; Zou, X.; Gong, Y.; Zhang, Z.; Li, M.; Zhao, L.; Xu, R.; Jiang, S.; Zhou, X.; et al. Vertical 1T-TaS2 synthesis on nanoporous gold for high-performance electrocatalytic applications. Adv. Mater. 2018, 30, 1705916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, J.; Luo, R.; Wang, Z.; Wang, Z.; Han, J.; Liu, P.; Fujita, T.; Xue, Q.; Chen, M. 3D bicontinuous nanoporous plasmonic heterostructure for enhanced hydrogen evolution reaction under visible light. Nano Energy 2019, 58, 552–559. [Google Scholar] [CrossRef]
- Lu, X.; Yu, T.; Wang, H.; Luo, R.; Liu, P.; Yuan, S.; Qian, L. Self-supported nanoporous gold with gradient tin oxide for sustainable and efficient hydrogen evolution in neutral media. J. Renew. Mater. 2020, 8, 133–151. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, J. Oxygen evolution reaction on nanoporous gold modified with Ir and Pt: Synergistic electrocatalysis between structure and composition. Electroanalysis 2019, 31, 1026–1033. [Google Scholar] [CrossRef]
- Sun, J.S.; Zhou, Y.T.; Yao, R.Q.; Shi, H.; Wen, Z.; Lang, X.Y.; Jiang, Q. Nanoporous gold supported chromium-doped NiFe oxyhydroxides as high-performance catalysts for the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 9690–9697. [Google Scholar] [CrossRef]
- Yao, R.Q.; Shi, H.; Wan, W.B.; Wen, Z.; Lang, X.Y.; Jiang, Q. Flexible Co-Mo-N/Au electrodes with a hierarchical nanoporous architecture as highly efficient electrocatalysts for oxygen evolution reaction. Adv. Mater. 2020, 32, 1907214. [Google Scholar] [CrossRef]
- Graf, M.; Vonbun-Feldbauer, G.B.; Koper, M.T.M. Direct and broadband plasmonic charge transfer to enhance water oxidation on a gold electrode. ACS Nano 2021, 15, 3188–3200. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M.W.; Erlebacher, J. Metallic mesoporous nanocomposites for electrocatalysis. J. Am. Chem. Soc. 2004, 126, 6876–6877. [Google Scholar] [CrossRef]
- Ge, X.B.; Wang, R.Y.; Liu, P.P.; Ding, Y. Platinum-decorated nanoporous gold leaf for methanol electrooxidation. Chem. Mater. 2007, 19, 5827–5829. [Google Scholar] [CrossRef]
- Wen, X.; Yin, S.; Yin, H.; Ding, Y. A displacement dealloying route to dilute nanoporous PtAu alloys for highly active formic acid electro-oxidation. Electrochim. Acta 2021, 373, 137884. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chen, N.; Hou, Y.; Wang, Z.C.; Lv, S.H.; Fujita, T.; Jiang, J.H.; Hirata, A.; Chen, M.W. Geometrically controlled nanoporous PdAu bimetallic catalysts with tunable Pd/Au ratio for direct ethanol fuel cells. ACS Catal. 2013, 3, 1220–1230. [Google Scholar] [CrossRef]
- Xu, H.; Liu, S.; Pu, X.; Shen, K.; Zhang, L.; Wang, X.; Qin, J.; Wang, W. Dealloyed porous gold anchored by in situ generated graphene sheets as high activity catalyst for methanol electro-oxidation reaction. RSC Adv. 2020, 10, 1666–1678. [Google Scholar] [CrossRef]
- Wang, Z.; Ning, S.; Liu, P.; Ding, Y.; Hirata, A.; Fujita, T.; Chen, M. Tuning surface structure of 3D nanoporous gold by surfactant-free electrochemical potential cycling. Adv. Mater. 2017, 29, 1703601. [Google Scholar] [CrossRef]
- Wang, Z.; Du, J.; Zhang, Y.; Han, J.; Huang, S.; Hirata, A.; Chen, M. Free-standing nanoporous gold for direct plasmon enhanced electro-oxidation of alcohol molecules. Nano Energy 2019, 56, 286–293. [Google Scholar] [CrossRef]
- Xie, Y.; Dimitrov, N. Ultralow Pt loading nanoporous Au-Cu-Pt thin film as highly active and durable catalyst for formic acid oxidation. Appl. Catal. B Environ. 2020, 263, 118366. [Google Scholar] [CrossRef]
- Li, J.; Yin, H.M.; Li, X.B.; Okunishi, E.; Shen, Y.L.; He, J.; Tang, Z.K.; Wang, W.X.; Yücelen, E.; Li, C.; et al. Surface evolution of a Pt–Pd–Au electrocatalyst for stable oxygen reduction. Nat. Energy 2017, 2, 17111. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, S.; Yin, H.M. Strain engineering to enhance the oxidation reduction reaction performance of atomic-layer Pt on nanoporous gold. ACS Appl. Energy Mater. 2020, 3, 11956–11963. [Google Scholar] [CrossRef]
- Lang, X.; Hirata, A.; Fujita, T.; Chen, M. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 2011, 6, 232–236. [Google Scholar] [CrossRef]
- Chen, L.Y.; Kang, J.L.; Hou, Y.; Liu, P.; Fujita, T.; Hirata, A.; Chen, M.W. High-energy-density nonaqueous MnO2@nanoporous gold based supercapacitors. J. Mater. Chem. A 2013, 1, 9202–9207. [Google Scholar] [CrossRef]
- Kang, J.; Chen, L.; Hou, Y.; Li, C.; Fujita, T.; Lang, X.; Hirata, A.; Chen, M. Electroplated thick manganese oxide films with ultrahigh capacitance. Adv. Energy Mater. 2013, 3, 857–863. [Google Scholar] [CrossRef]
- Prabhin, V.S.; Jeyasubramanian, K.; Benitha, V.S.; Veluswamy, P.; Cho, B.J. Fabrication and evaluation of hybrid supercapacitor consisting of nano cobalt oxide and manganese oxide deposited electrochemically on nanoporous Au-Electrode. Electrochim. Acta 2020, 330, 135199. [Google Scholar] [CrossRef]
- Chen, L.Y.; Hou, Y.; Kang, J.L.; Hirata, A.; Fujita, T.; Chen, M.W. Toward the theoretical capacitance of RuO2 reinforced by highly conductive nanoporous gold. Adv. Energy Mater. 2013, 3, 851–856. [Google Scholar] [CrossRef]
- Lang, X.; Zhang, L.; Fujita, T.; Ding, Y.; Chen, M. Three-dimensional bicontinuous nanoporous Au/polyaniline hybrid films for high-performance electrochemical supercapacitors. J. Power Sources 2012, 197, 325–329. [Google Scholar] [CrossRef]
- Chen, L.Y.; Hou, Y.; Kang, J.L.; Hirata, A.; Chen, M.W. Asymmetric metal oxide pseudocapacitors advanced by three-dimensional nanoporous metal electrodes. J. Mater. Chem. A 2014, 2, 8448–8455. [Google Scholar] [CrossRef]
- Hou, C.; Lang, X.Y.; Wen, Z.; Zhu, Y.-F.; Zhao, M.; Li, J.C.; Zheng, W.-T.; Lian, J.S.; Jiang, Q. Single-crystalline Ni(OH)2 nanosheets vertically aligned on a three-dimensional nanoporous metal for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 23412–23419. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, S.W.; Jung, K.; Kim, J.B.; Jang, J.H. Ideal nanoporous gold based supercapacitors with theoretical capacitance and high energy/power density. Nano Energy 2016, 24, 17–24. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, L.; Liu, P.; Kang, J.; Fujita, T.; Chen, M. Nanoporous metal based flexible asymmetric pseudocapacitors. J. Mater. Chem. A 2014, 2, 10910–10916. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, J.; Qian, L.; Yuan, S.; Wang, S.; Lei, P. Planar integration of flexible micro-supercapacitors with ultrafast charge and discharge based on interdigital nanoporous gold electrodes on a chip. J. Mater. Chem. A 2016, 4, 9502–9510. [Google Scholar] [CrossRef]
- Purkait, T.; Singh, G.; Kamboj, N.; Das, M.; Dey, R.S. All-porous heterostructure of reduced graphene oxide–polypyrrole–nanoporous gold for a planar flexible supercapacitor showing outstanding volumetric capacitance and energy density. J. Mater. Chem. A 2018, 6, 22858–22869. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zhang, H.; Hou, Y.; Guo, J. High-performance three-dimensional nanoporous gold based electrodes for flexible all-solid-state supercapacitors. J. Porous Mater. 2020, 27, 1309–1317. [Google Scholar] [CrossRef]
- Lee, K.U.; Byun, J.Y.; Shin, H.J.; Kim, S.H. A high-performance supercapacitor based on polyaniline-nanoporous gold. J. Alloys Compd. 2019, 779, 74–80. [Google Scholar] [CrossRef]
- Chen, A.Y.; Zhang, T.T.; Qiu, Y.J.; Wang, D.; Wang, P.; Li, H.J.; Li, Y.; Yang, J.H.; Wang, X.Y.; Xie, X.F. Construction of nanoporous gold/g-C3N4 heterostructure for electrochemical supercapacitor. Electrochim. Acta 2019, 294, 260–267. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, Y.; Zhang, Y.; Chen, Y.; Peng, X.; Wang, X.; Zhao, W.; Qin, C.; Liu, Q.; Liu, X.; et al. Single-atomic Co-B2N2 sites anchored on carbon nanotube arrays promote lithium polysulfide conversion in lithium–sulfur batteries. Carbon Energy 2023, e306. [Google Scholar] [CrossRef]
- Yu, Y.; Gu, L.; Lang, X.; Zhu, C.; Fujita, T.; Chen, M.; Maier, J. Li storage in 3D nanoporous Au-supported nanocrystalline tin. Adv. Mater. 2011, 23, 2443–2447. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Ye, J.C.; Baumgaertel, A.C.; Wang, Y.M.; Biener, J.; Biener, M.M. Structural optimization of 3D porous electrodes for high-rate performance lithium ion batteries. ACS Nano 2015, 9, 2194–2202. [Google Scholar] [CrossRef]
- Yang, C.; Fu, K.; Zhang, Y.; Hitz, E.; Hu, L. Protected lithium-metal anodes in batteries: From liquid to solid. Adv. Mater. 2017, 29, 1701169. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, G.; Kim, Y.U.; Kim, J.H.; Park, C.M.; Sohn, H.J. Metallic anodes for next generation secondary batteries. Chem. Soc. Rev. 2013, 42, 9011–9034. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Engelhard, M.H.; Mei, D.; Jiao, S.; Polzin, B.J.; Zhang, J.-G.; Xu, W. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2017, 2, 17012. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Wang, X.G.; Wang, C.; Chen, Y.N.; Xie, Z.; Zhou, Z. An extremely simple method for protecting lithium anodes in Li-O2 batteries. Angew. Chem. Int. Ed. Engl. 2018, 57, 12814–12818. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Ke, X.; Zhang, Z.; Wu, W.; Lin, G.; Zhou, Z.; Shi, Z. Coupling of triporosity and strong Au–Li interaction to enable dendrite-free lithium plating/stripping for long-life lithium metal anodes. J. Mater. Chem. A 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Chen, Y.; Ke, X.; Cheng, Y.; Fan, M.; Wu, W.; Huang, X.; Liang, Y.; Zhong, Y.; Ao, Z.; Lai, Y.; et al. Boosting the electrochemical performance of 3D composite lithium metal anodes through synergistic structure and interface engineering. Energy Storage Mater. 2020, 26, 56–64. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Chen, L.Y.; Guo, X.W.; Han, J.H.; Liu, P.; Xu, X.D.; Hirata, A.; Chen, M.W. Nanoporous metal/oxide hybrid materials for rechargeable lithium–oxygen batteries. J. Mater. Chem. A 2015, 3, 3620–3626. [Google Scholar] [CrossRef]
- Ma, W.; Liu, X.; Li, C.; Yin, H.; Xi, W.; Liu, R.; He, G.; Zhao, X.; Luo, J.; Ding, Y. Rechargeable Al-CO2 batteries for reversible utilization of CO2. Adv. Mater. 2018, 30, 1801152. [Google Scholar] [CrossRef]
- Yang, F.; Liu, J.; Lu, Z.; Dai, P.; Nakamura, T.; Wang, S.; Chen, L.; Wakamiya, A.; Matsuda, K. Recycled utilization of a nanoporous au electrode for reduced fabrication cost of perovskite solar cells. Adv. Sci. (Weinh) 2020, 7, 1902474. [Google Scholar] [CrossRef]
- Peng, Z.Q.; Freunberger, S.A.; Chen, Y.H.; Bruce, P.G. A Reversible and Higher-Rate Li-O2 Battery. Science 2012, 337, 563–566. [Google Scholar] [CrossRef]
- Guo, X.; Han, J.; Liu, P.; Chen, L.; Ito, Y.; Jian, Z.; Jin, T.; Hirata, A.; Li, F.; Fujita, T.; et al. Hierarchical nanoporosity enhanced reversible capacity of bicontinuous nanoporous metal based Li-O2 battery. Sci. Rep. 2016, 6, 33466. [Google Scholar] [CrossRef]
- Yang, H.; Xia, J.; Bromberg, L.; Dimitrov, N.; Whittingham, M.S. Electrochemically synthesized nanoporous gold as a cathode material for Li-O2 batteries. J. Solid State Electrochem. 2016, 21, 463–468. [Google Scholar] [CrossRef]
- Hyun, G.; Song, J.T.; Ahn, C.; Ham, Y.; Cho, D.; Oh, J.; Jeon, S. Hierarchically porous Au nanostructures with interconnected channels for efficient mass transport in electrocatalytic CO2 reduction. Proc. Natl. Acad. Sci. USA 2020, 117, 5680–5685. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, T.; Wang, H.; Qian, L.; Luo, R.; Liu, P.; Yu, Y.; Liu, L.; Lei, P.; Yuan, S. Nanoporous Au-Sn with solute strain for simultaneously enhanced selectivity and durability during electrochemical CO2 reduction. J. Mater. Sci. Technol. 2020, 43, 154–160. [Google Scholar] [CrossRef]
- Ma, X.; Shen, Y.; Yao, S.; Shu, M.; Si, R.; An, C. Self-supported nanoporous Au3Cu electrode with enriched gold on surface for efficient electrochemical reduction of CO2. Chemistry 2019, 26, 4143–4149. [Google Scholar] [CrossRef]
- Ma, X.; Shen, Y.; Yao, S.; An, C.; Zhang, W.; Zhu, J.; Si, R.; Guo, C.; An, C. Core–shell nanoporous AuCu3@Au monolithic electrode for efficient electrochemical CO2 reduction. J. Mater. Chem. A 2020, 8, 3344–3350. [Google Scholar] [CrossRef]
- Liu, Z.; Hossain, M.N.; Wen, J.; Chen, A. Copper decorated with nanoporous gold by galvanic displacement acts as an efficient electrocatalyst for the electrochemical reduction of CO2. Nanoscale 2021, 13, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Liu, X.; Zhang, W.; Zhang, Y.; Li, Y.; Qin, C.; Zhao, W.; Bakenov, Z. Dual-network nanoporous NiFe2O4/NiO composites for high performance Li-ion battery anodes. Chem. Eng. J. 2020, 388, 124207. [Google Scholar] [CrossRef]
- Liu, S.; Pang, F.; Zhang, Q.; Guo, R.; Wang, Z.; Wang, Y.; Zhang, W.; Ou, J. Stable nanoporous Sn/SnO2 composites for efficient electroreduction of CO2 to formate over wide potential range. Appl. Mater. Today 2018, 13, 135–143. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; Zhang, Y.; Qin, C.; Bakenov, Z.; Wang, Z. Improving the cycling stability of three-dimensional nanoporous Ge anode by embedding Ag nanoparticles for high-performance lithium-ion battery. J. Colloid Interface Sci. 2021, 592, 103–115. [Google Scholar] [CrossRef]
- Tan, H.W.; Choong, Y.Y.C.; Kuo, C.N.; Low, H.Y.; Chua, C.K. 3D printed electronics: Processes, materials and future trends. Prog. Mater. Sci. 2022, 127, 100945. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Z.; Wang, Z. Electroreduction of nitrogen to ammonia on nanoporous gold. Nanoscale 2021, 13, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).