Numerical Investigations of Phase Transformations Controlled by Interface Thermodynamic Conditions during Intercritical Annealing of Steels
Abstract
1. Introduction
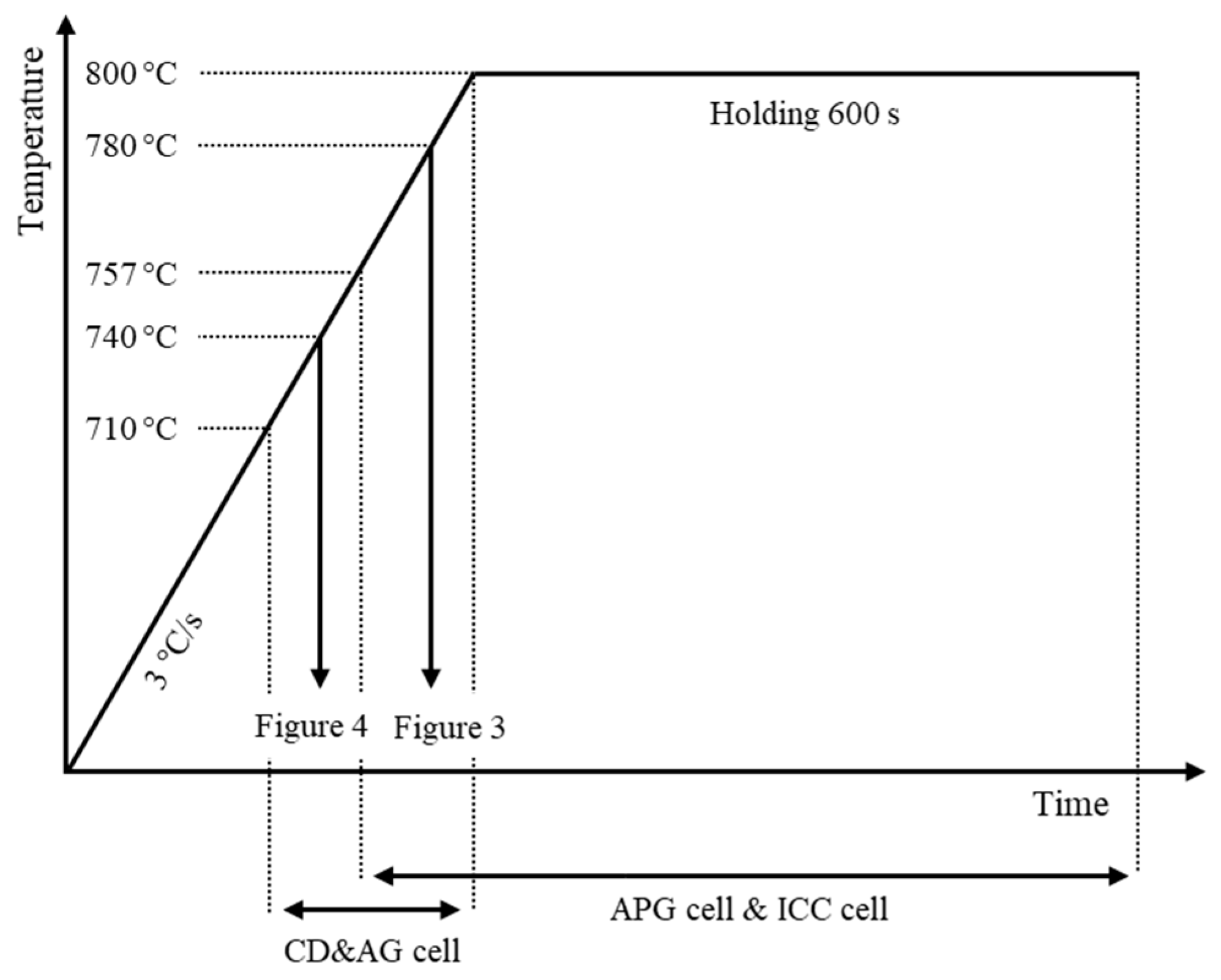
2. Materials and Methods/Simulation Settings
2.1. Simulation Cell for Austenite Planar Growth Investigation (APG Cell)
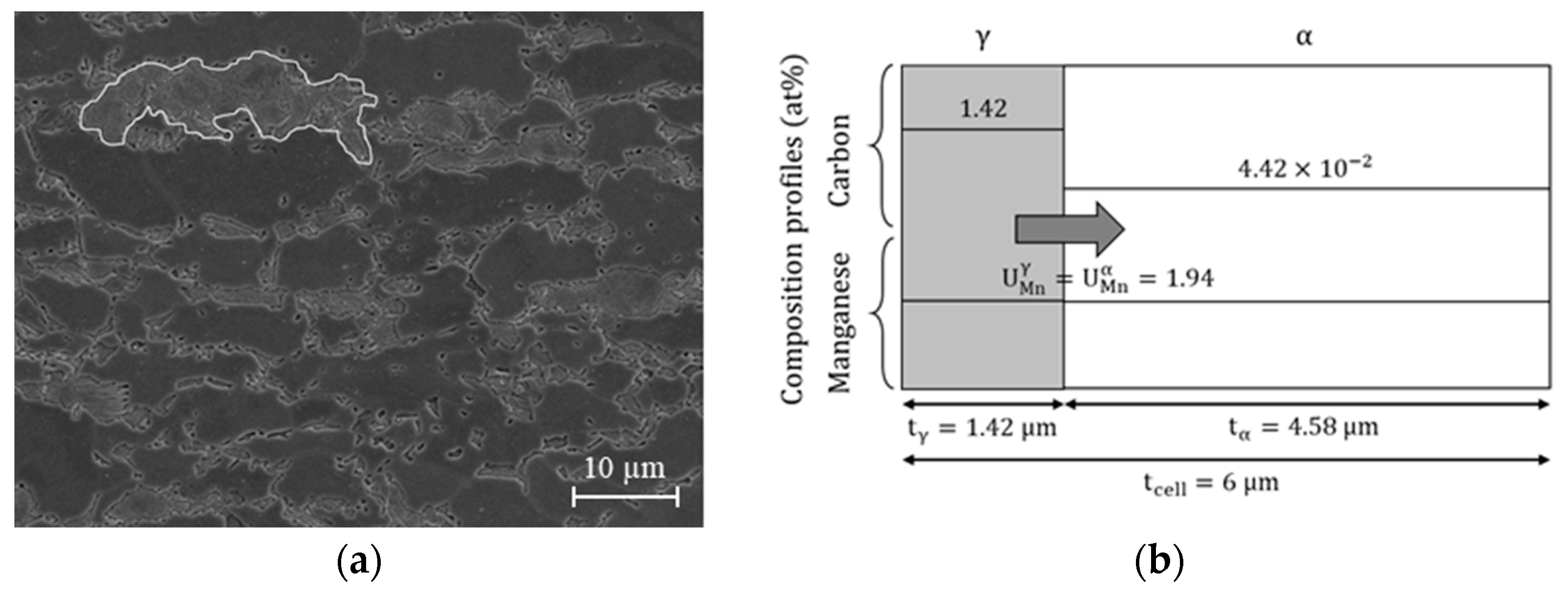
2.2. Simulation Cell for Carbide Dissolution and Austenite Growth Investigation (CD and AG Cell)

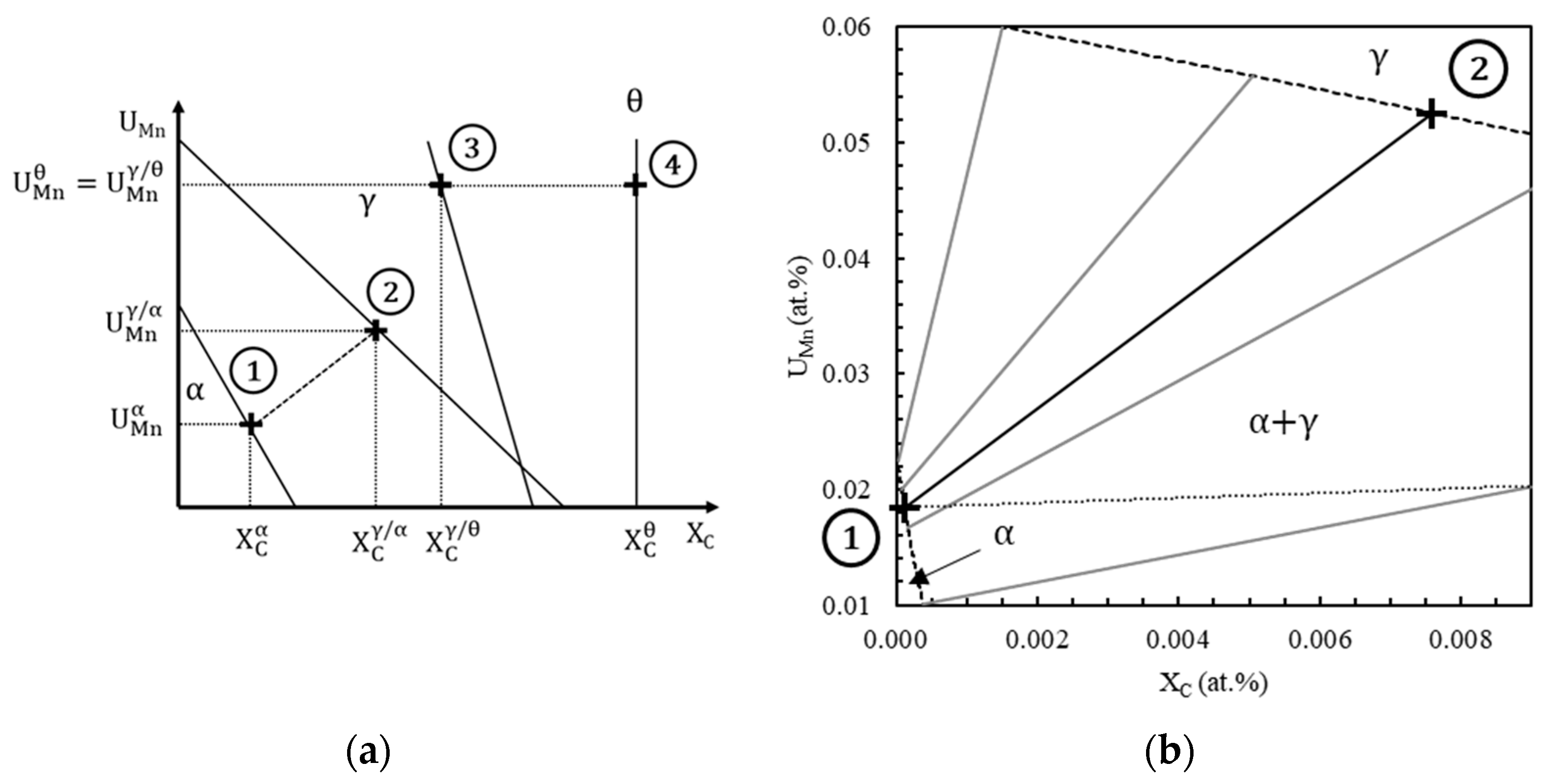
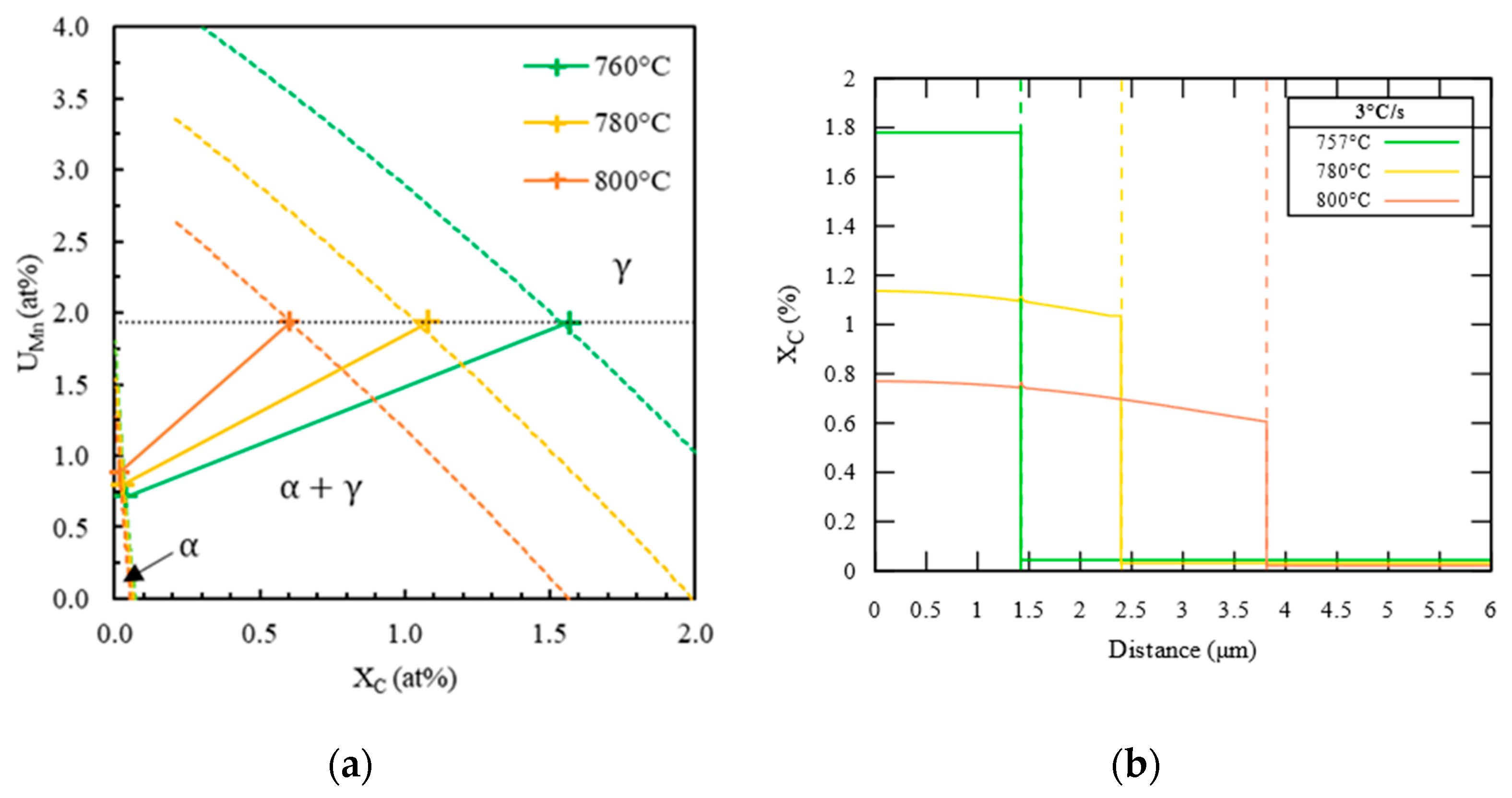
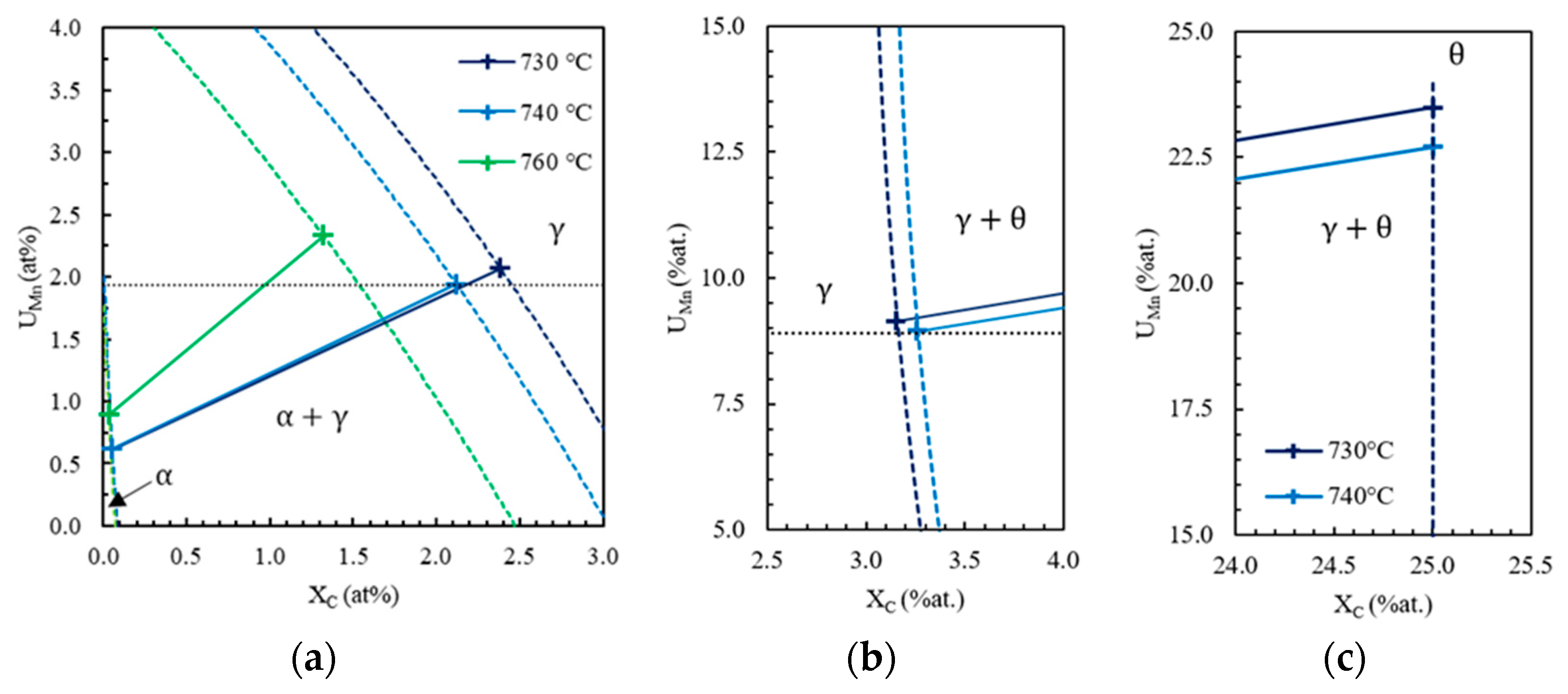
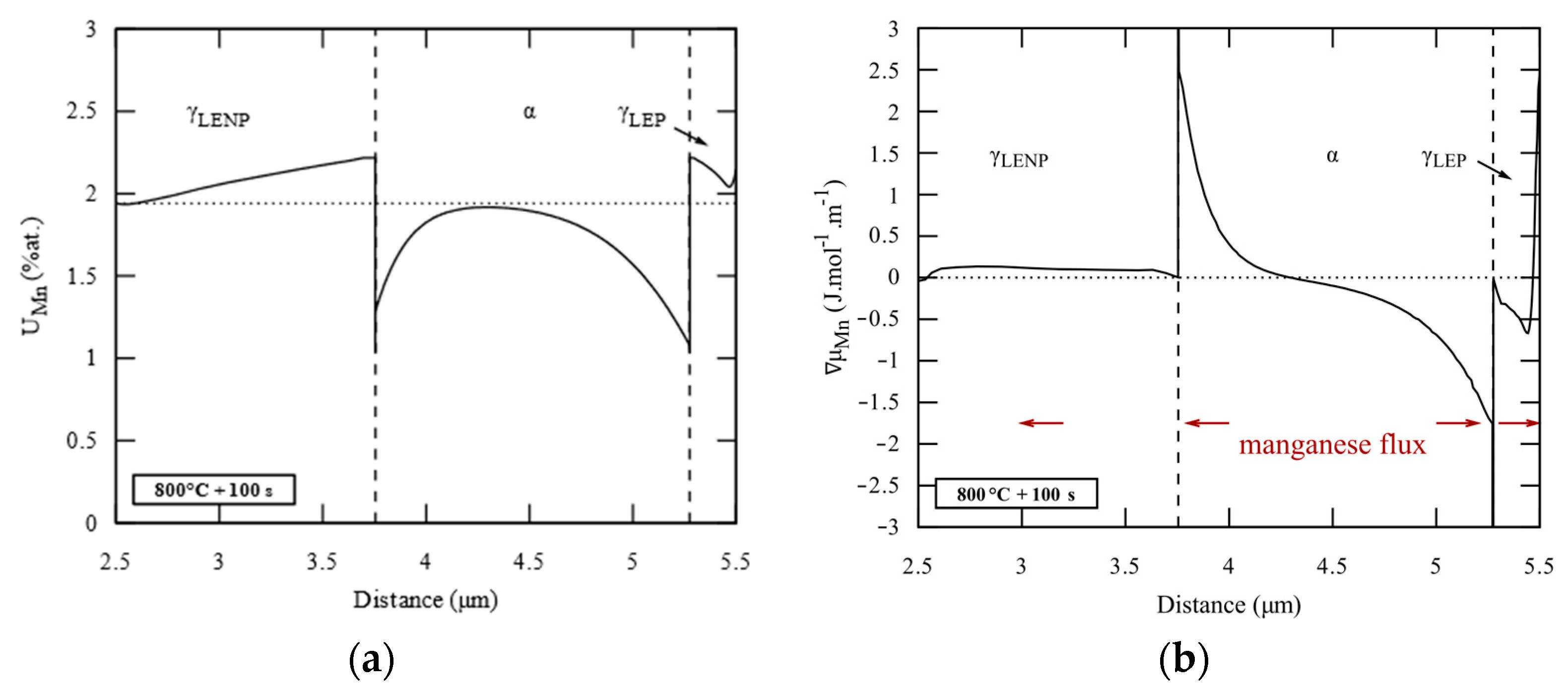
- First, possible ferrite compositions , plotted in Figure 5b with a black dotted line, are computed by carbon mass balance as a function of the cell size such that the simulation cell has the nominal composition in carbon and manganese of the steel.
- Then, Thermo-Calc is used to compute equilibrium operative tie-lines, highlighted in Figure 5b with gray solid lines, at the interface α/γ at 710 °C. There is a single ferrite composition that both belongs to an equilibrium tie-line and makes possible the mass balance. This composition, marked by a cross labeled 1 in Figure 5a,b, is chosen as ferrite bulk composition and interface composition . The chosen tie-line, plotted with a black solid line in Figure 5a,b, gives the austenite composition at the interface γ/α, represented by the cross labeled 2 in Figure 5a,b.
- Finally, the carbon and manganese profiles in austenite are supposed to be linear between interface compositions. The initial carbon and manganese profiles are schematized in Figure 4b.
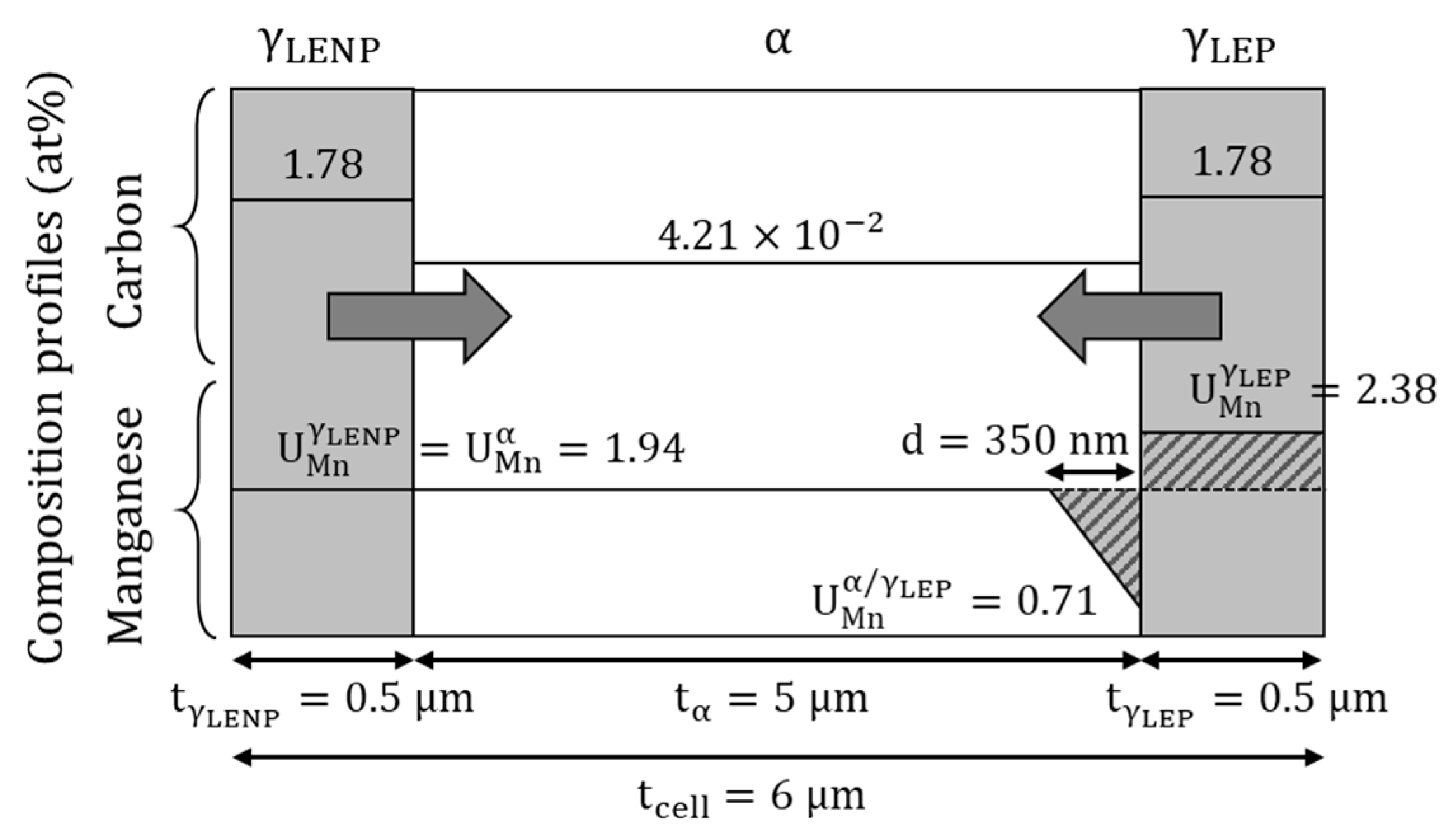
2.3. Simulation Cell for Interface Condition Competition Investigation (ICC Cell)
3. Results and Discussion
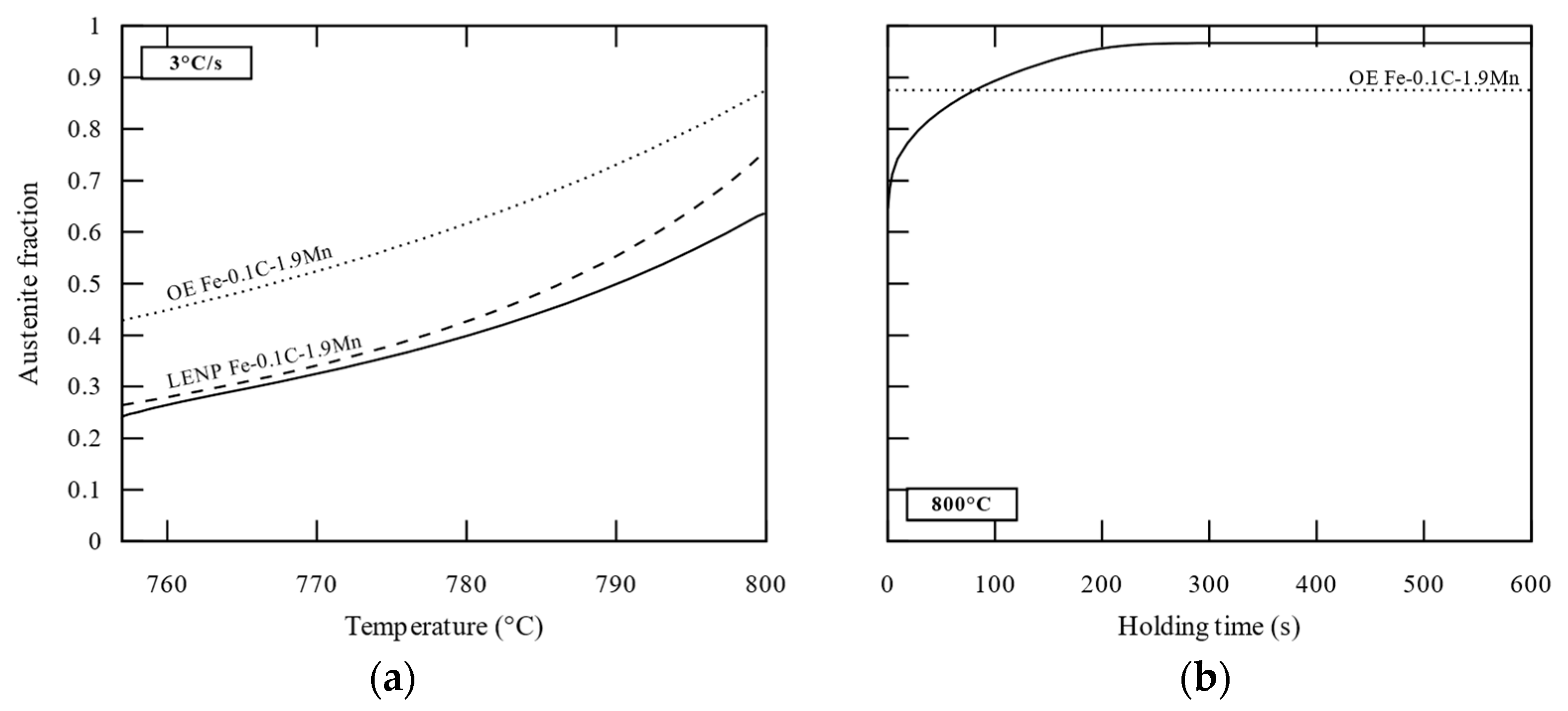
3.1. Austenite Planar Growth (APG Cell)
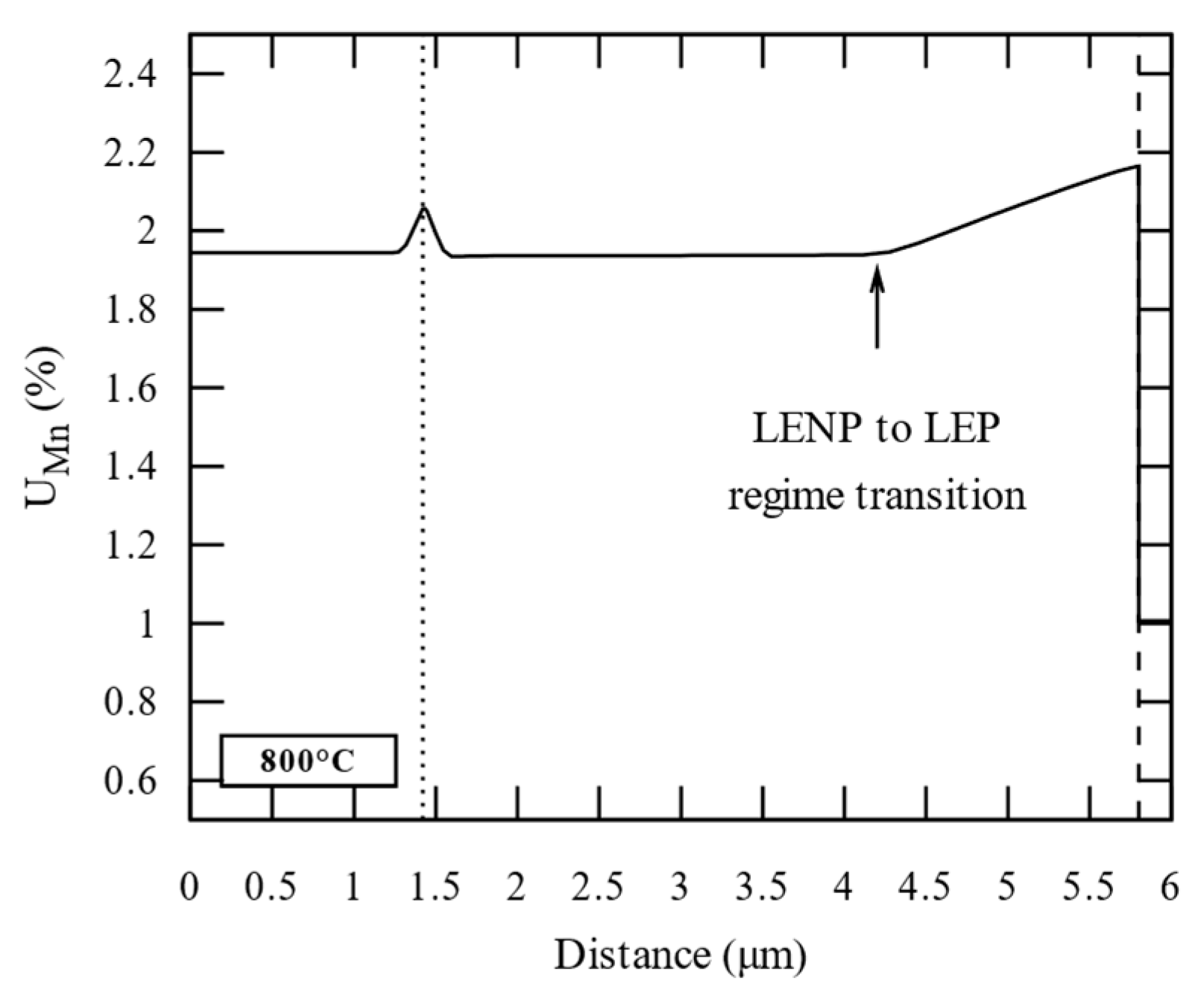
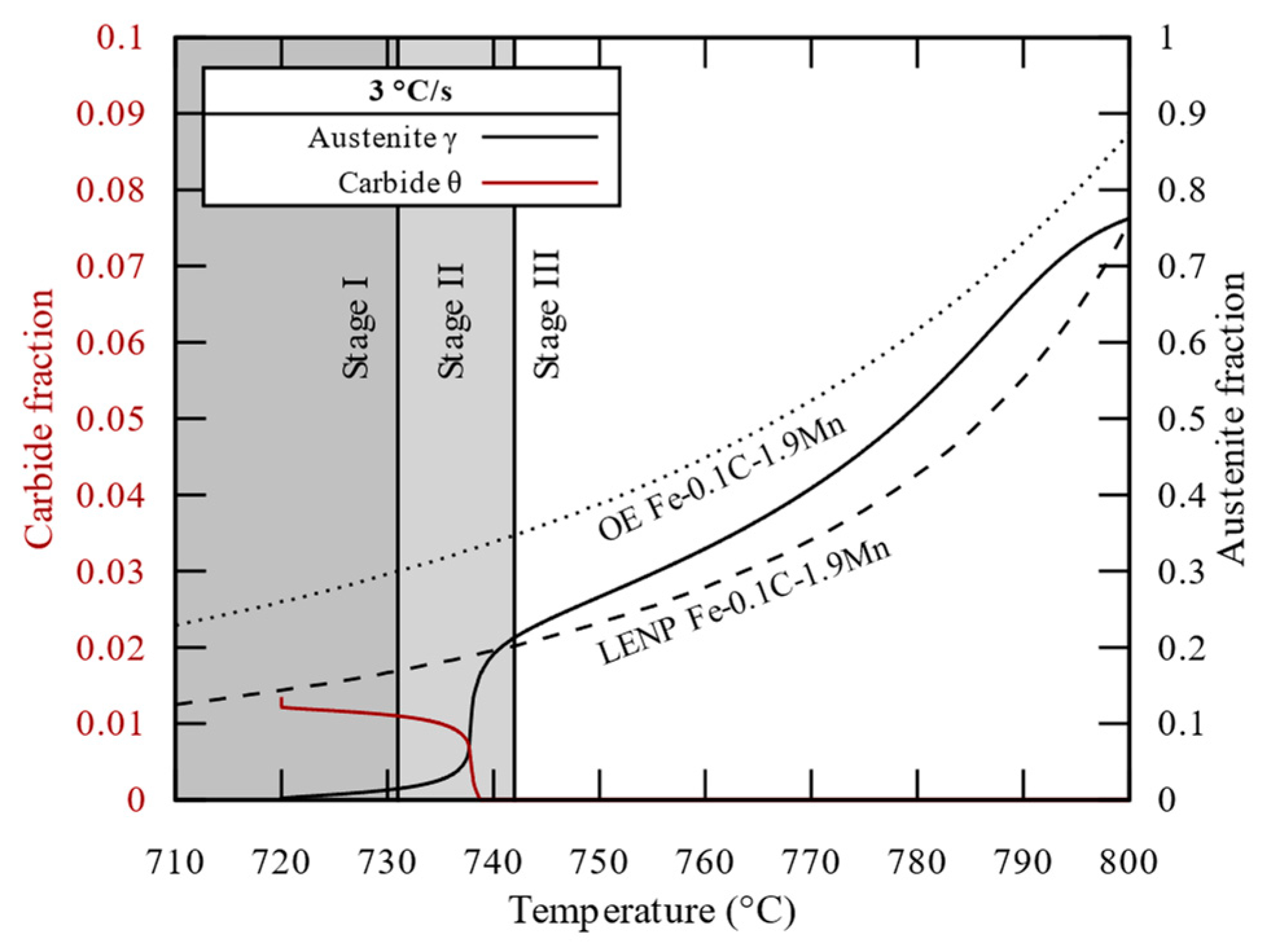
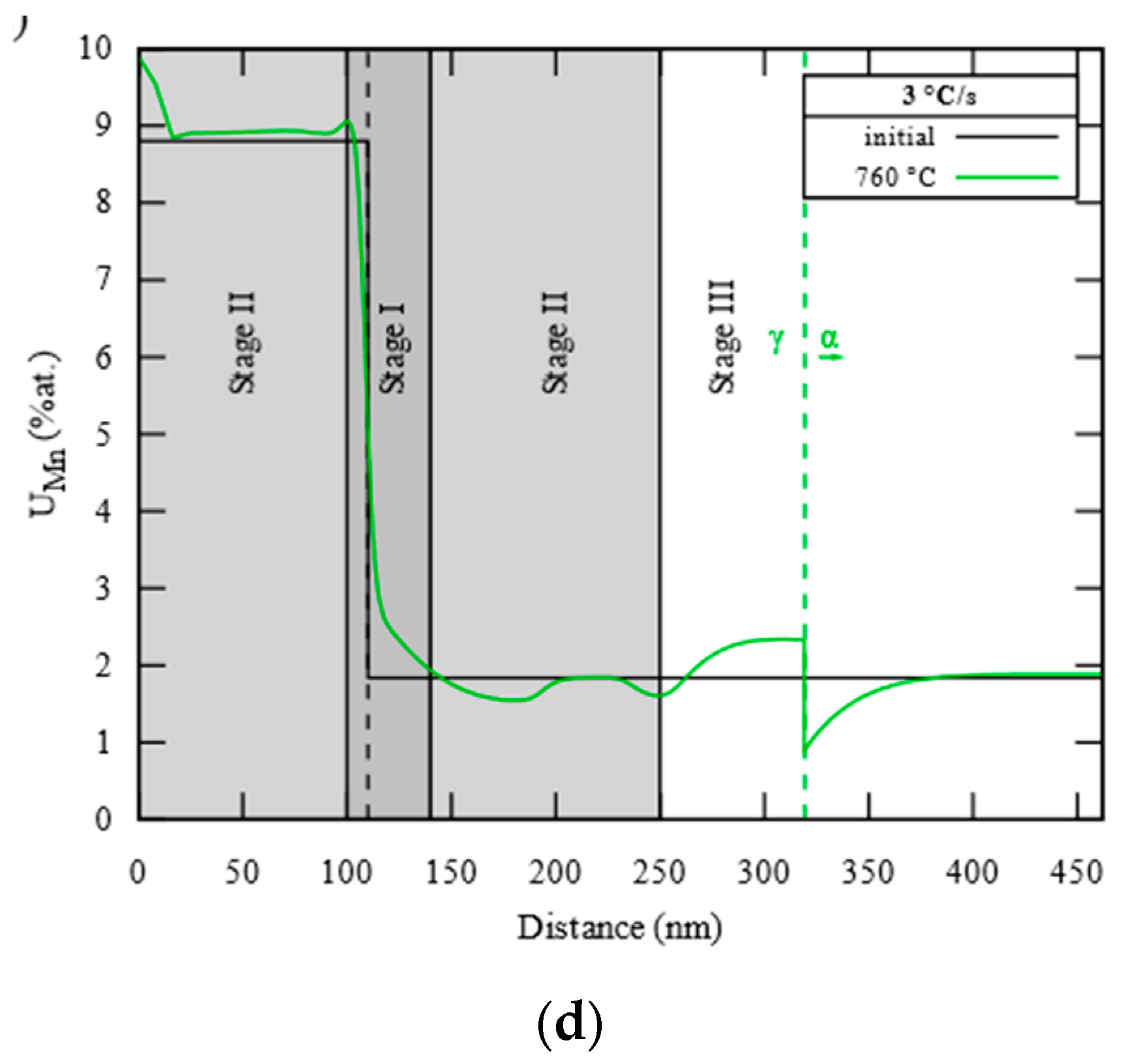
3.2. Carbide Dissolution and Austenite Growth Investigation (CD and AG Cell)
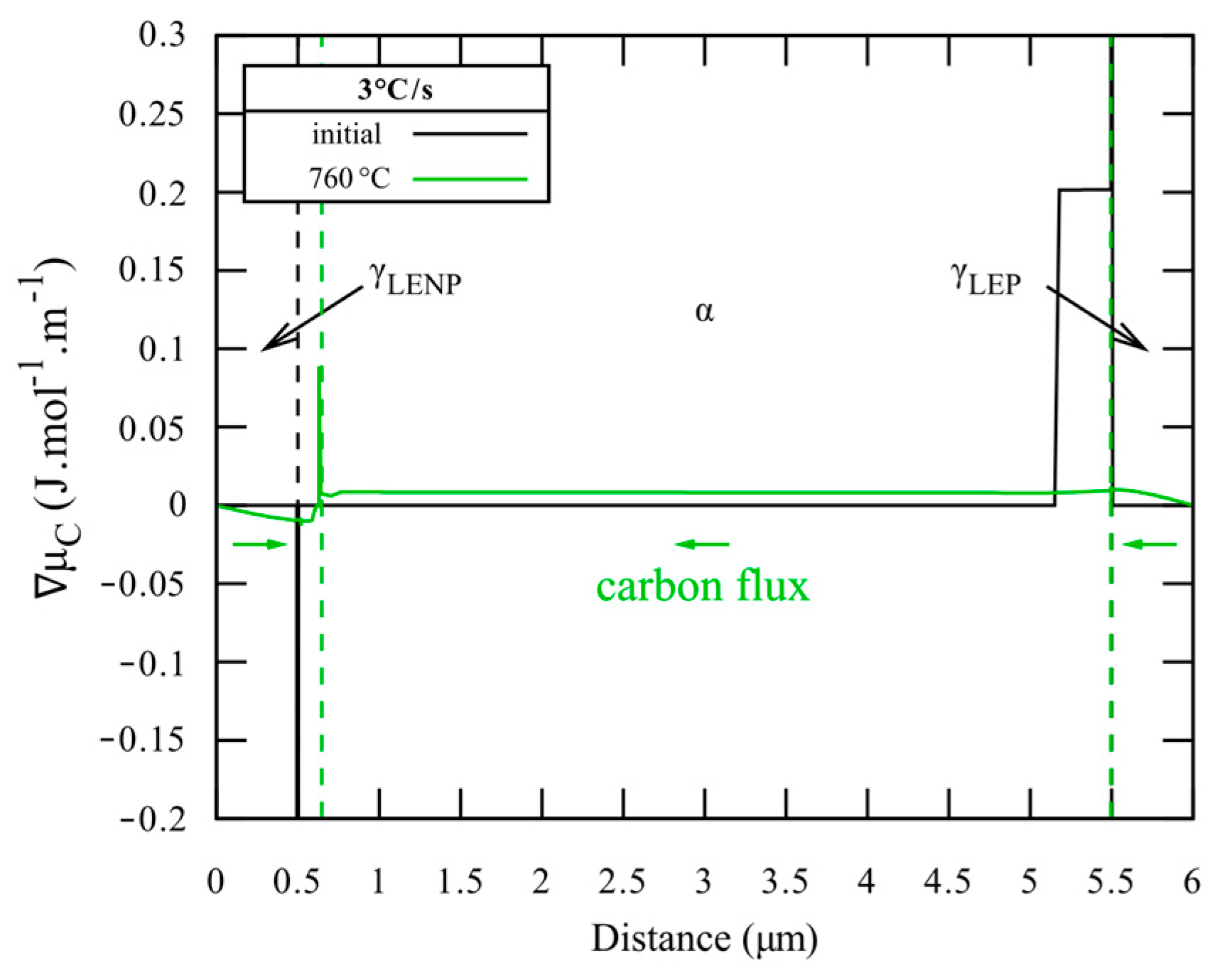
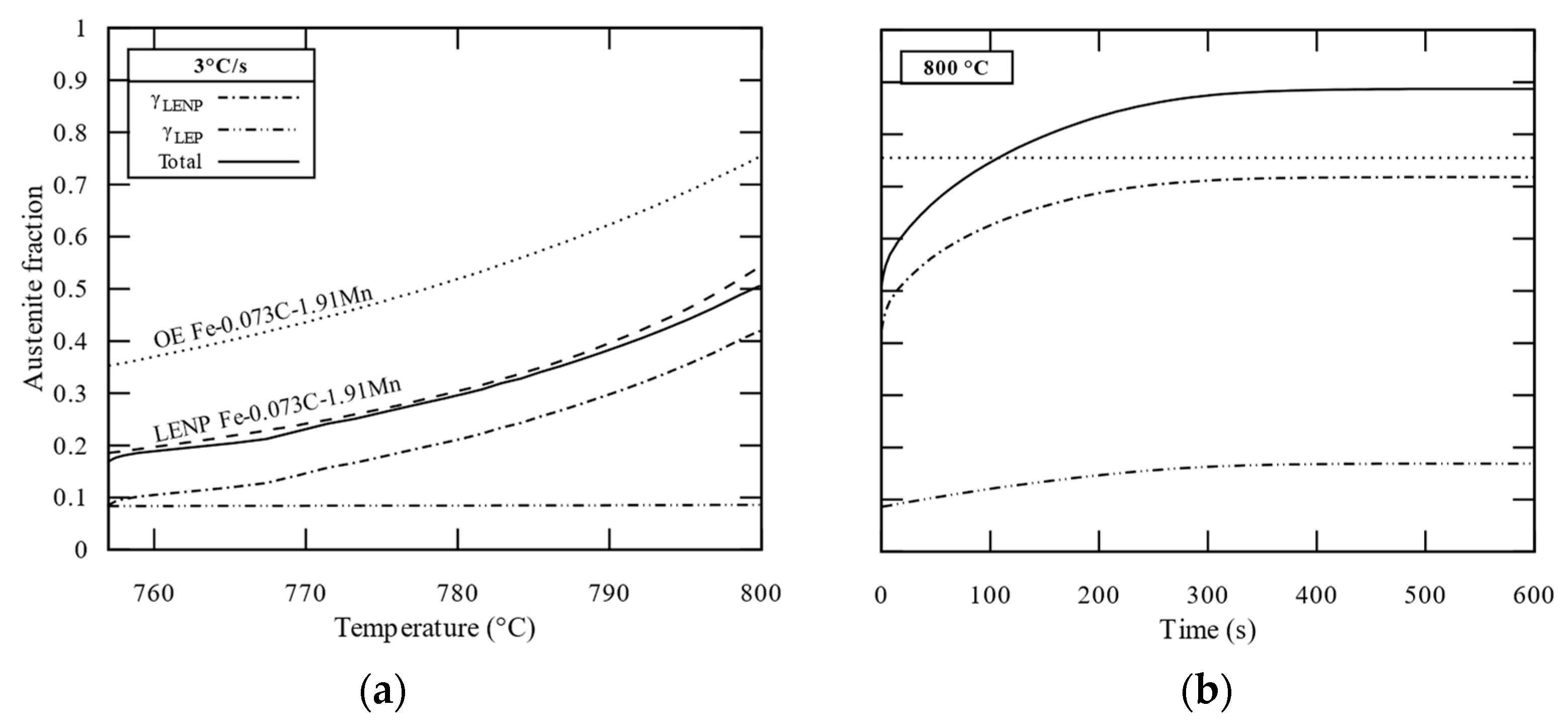
3.3. Interface Condition Competition (ICC Cell)
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allain, S.Y.P.; Moreno, M.; Lamari, M.; Zurob, H.; Teixeira, J.; Bonnet, F. A Physics-Based Mean-Field Model for Ferrite Recovery and Recrystallization. Metals 2020, 10, 622. [Google Scholar] [CrossRef]
- Pushkareva, I.; Allain, S.; Scott, C.; Redjaïmia, A.; Moulin, A. Relationship between Microstructure, Mechanical Properties and Damage Mechanisms in High Martensite Fraction Dual Phase Steels. ISIJ Int. 2015, 55, 2237–2246. [Google Scholar] [CrossRef]
- Allain, S.; Bouaziz, O. Microstructure Based Modeling for the Mechanical Behavior of Ferrite–Pearlite Steels Suitable to Capture Isotropic and Kinematic Hardening. Mater. Sci. Eng. A 2008, 496, 329–336. [Google Scholar] [CrossRef]
- Moreno, M. Metallurgical Mechanisms and Their Interactions during the Annealing of Cold-Rolled Ferrite-Pearlite Steels: Characterization and Modeling. Ph.D. Thesis, Université de Lorraine, Lorraine, France, 2019. [Google Scholar]
- Speich, G.R.; Demarest, V.A.; Miller, R.L. Formation of Austenite During Intercritical Annealing of Dual-Phase Steels. MTA 1981, 12, 1419–1428. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Bouaziz, O.; Pushkareva, I.; Scott, C.P. Towards the Microstructure Design of DP Steels: A Generic Size-Sensitive Mean-Field Mechanical Model. Mater. Sci. Eng. A 2015, 637, 222–234. [Google Scholar] [CrossRef]
- Scott, C.P.; Fazeli, F.; Shalchi Amirkhiz, B.; Pushkareva, I.; Allain, S.Y.P. Structure-Properties Relationship of Ultra-Fine Grained V-Microalloyed Dual Phase Steels. Mater. Sci. Eng. A 2017, 703, 293–303. [Google Scholar] [CrossRef]
- Kulakov, M.; Poole, W.J.; Militzer, M. The Effect of the Initial Microstructure on Recrystallization and Austenite Formation in a DP600 Steel. Metall. Mater. Trans. A 2013, 44, 3564–3576. [Google Scholar] [CrossRef]
- Teixeira, J.; Moreno, M.; Allain, S.Y.P.; Oberbillig, C.; Geandier, G.; Bonnet, F. Intercritical Annealing of Cold-Rolled Ferrite-Pearlite Steel: Microstructure Evolutions and Phase Transformation Kinetics. Acta Mater. 2021, 212, 116920. [Google Scholar] [CrossRef]
- Lai, Q.; Gouné, M.; Perlade, A.; Pardoen, T.; Jacques, P.; Bouaziz, O.; Bréchet, Y. Mechanism of Austenite Formation from Spheroidized Microstructure in an Intermediate Fe-0.1C-3.5Mn Steel. Metall. Mater. Trans. A 2016, 47, 3375–3386. [Google Scholar] [CrossRef]
- Wycliffe, P.A.; Purdy, G.R.; Embury, J.D. Growth of Austenite in the Intercritical Annealing of Fe-C-Mn Dual Phase Steels. Can. Metall. Q. 1981, 12, 1236. [Google Scholar] [CrossRef]
- Liu, Z.-K.; Höglund, L.; Jönsson, B.; Ågren, J. An Experimental and Theoretical Study of Cementite Dissolution in an Fe-Cr-C Alloy. MTA 1991, 22, 1745–1752. [Google Scholar] [CrossRef]
- Bourne, J.P.; Atkinson, C.; Reed, R.C. Diffusion-Controlled Growth in Ternary Systems. Metall. Mater. Trans. A 1994, 25, 2683–2694. [Google Scholar] [CrossRef]
- Miyamoto, G.; Usuki, H.; Li, Z.-D.; Furuhara, T. Effects of Mn, Si and Cr Addition on Reverse Transformation at 1073K from Spheroidized Cementite Structure in Fe–0.6 Mass% C Alloy. Acta Mater. 2010, 58, 4492–4502. [Google Scholar] [CrossRef]
- Gouné, M.; Maugis, P.; Drillet, J. A Criterion for the Change from Fast to Slow Regime of Cementite Dissolution in Fe–C–Mn Steels. J. Mater. Sci. Technol. 2012, 28, 728–736. [Google Scholar] [CrossRef]
- Wei, R.; Enomoto, M.; Hadian, R.; Zurob, H.S.; Purdy, G.R. Growth of Austenite from As-Quenched Martensite during Intercritical Annealing in an Fe–0.1C–3Mn–1.5Si Alloy. Acta Mater. 2013, 61, 697–707. [Google Scholar] [CrossRef]
- Wu, Y.X.; Wang, L.Y.; Sun, W.W.; Styles, M.J.; Studer, A.J.; Bréchet, Y.; Arlazarov, A.; Hutchinson, C.R. Austenite Formation Kinetics from Multicomponent Cementite-Ferrite Aggregates. Acta Mater. 2020, 18, 234. [Google Scholar] [CrossRef]
- Kulakov, M.; Poole, W.J.; Militzer, M. A Microstructure Evolution Model for Intercritical Annealing of a Low-Carbon Dual-Phase Steel. ISIJ Int. 2014, 54, 2627–2636. [Google Scholar] [CrossRef]
- Ollat, M. Characterization and Modeling of Microstructural Evolutions during the Thermal Treatment of Cold-Rolled Dual-Phase Steels. Ph.D. Thesis, Université de Lyon, Lyon, France, 2017. [Google Scholar]
- Gouné, M.; Danoix, F.; Ågren, J.; Bréchet, Y.; Hutchinson, C.R.; Militzer, M.; Purdy, G.; van der Zwaag, S.; Zurob, H. Overview of the Current Issues in Austenite to Ferrite Transformation and the Role of Migrating Interfaces Therein for Low Alloyed Steels. Mater. Sci. Eng. R Rep. 2015, 92, 1–38. [Google Scholar] [CrossRef]
- Marceaux dit Clément, A.; Hoummada, K.; Drillet, J.; Hébert, V.; Maugis, P. Effects of Cementite Size and Chemistry on the Kinetics of Austenite Formation during Heating of a High-Formability Steel. Comput. Mater. Sci. 2020, 182, 109786. [Google Scholar] [CrossRef]
- Christian, J.W. Deformation by Moving Interfaces. Metall. Trans. A 1982, 13, 509–538. [Google Scholar] [CrossRef]
- Mecozzi, M.G.; Bos, C.; Sietsma, J. A Mixed-Mode Model for the Ferrite-to-Austenite Transformation in a Ferrite/Pearlite Microstructure. Acta Mater. 2015, 88, 302–313. [Google Scholar] [CrossRef]
- Ollat, M.; Militzer, M.; Massardier, V.; Fabregue, D.; Buscarlet, E.; Keovilay, F.; Perez, M. Mixed-Mode Model for Ferrite-to-Austenite Phase Transformation in Dual-Phase Steel. Comput. Mater. Sci. 2018, 149, 282–290. [Google Scholar] [CrossRef]
- Bos, C.; Mecozzi, M.G.; Sietsma, J. A Microstructure Model for Recrystallisation and Phase Transformation during the Dual-Phase Steel Annealing Cycle. Comput. Mater. Sci. 2010, 48, 692–699. [Google Scholar] [CrossRef]
- Madej, L.; Sieradzki, L.; Sitko, M.; Perzynski, K.; Radwanski, K.; Kuziak, R. Multi Scale Cellular Automata and Finite Element Based Model for Cold Deformation and Annealing of a Ferritic–Pearlitic Microstructure. Comput. Mater. Sci. 2013, 77, 172–181. [Google Scholar] [CrossRef]
- Zheng, C.; Raabe, D. Interaction between Recrystallization and Phase Transformation during Intercritical Annealing in a Cold-Rolled Dual-Phase Steel: A Cellular Automaton Model. Acta Mater. 2013, 61, 5504–5517. [Google Scholar] [CrossRef]
- Zhu, B.; Militzer, M. Phase-Field Modeling for Intercritical Annealing of a Dual-Phase Steel. Metall. Mater. Trans. A 2015, 46, 1073–1084. [Google Scholar] [CrossRef]
- Azizi-Alizamini, H.; Militzer, M. Phase Field Modelling of Austenite Formation from Ultrafine Ferrite–Carbide Aggregates in Fe–C. Int. J. Mater. Res. 2010, 101, 534–541. [Google Scholar] [CrossRef]
- Lv, S.; Wang, S.; Wu, G.; Gao, J.; Yang, X.; Wu, H.; Mao, X. Application of Phase-Field Modeling in Solid-State Phase Transformation of Steels. J. Iron Steel Res. Int. 2022, 29, 867–880. [Google Scholar] [CrossRef]
- Lv, S.; Wu, H.-H.; Wang, K.; Zhu, J.; Wang, S.; Wu, G.; Gao, J.; Yang, X.-S.; Mao, X. The Austenite to Polygonal Ferrite Transformation in Low-Alloy Steel: Multi-Phase-Field Simulation. J. Mater. Res. Technol. 2023, 24, 9630–9643. [Google Scholar] [CrossRef]
- Rudnizki, J.; Böttger, B.; Prahl, U.; Bleck, W. Phase-Field Modeling of Austenite Formation from a Ferrite plus Pearlite Microstructure during Annealing of Cold-Rolled Dual-Phase Steel. Metall. Mater. Trans. A 2011, 42, 2516–2525. [Google Scholar] [CrossRef]
- Geng, X.; Wang, H.; Xue, W.; Xiang, S.; Huang, H.; Meng, L.; Ma, G. Modeling of CCT Diagrams for Tool Steels Using Different Machine Learning Techniques. Comput. Mater. Sci. 2020, 171, 109235. [Google Scholar] [CrossRef]
- Hart-Rawung, T.; Buhl, J.; Bambach, M. A Fast Approach for Optimization of Hot Stamping Based on Machine Learning of Phase Transformation Kinetics. Procedia Manuf. 2020, 47, 707–712. [Google Scholar] [CrossRef]
- Li, Y.; Li, S. Deep Learning Based Phase Transformation Model for the Prediction of Microstructure and Mechanical Properties of Hot-Stamped Parts. Int. J. Mech. Sci. 2022, 220, 107134. [Google Scholar] [CrossRef]
- Durodola, J.F. Machine Learning for Design, Phase Transformation and Mechanical Properties of Alloys. Prog. Mater. Sci. 2022, 123, 100797. [Google Scholar] [CrossRef]
- Diffusion Module (DICTRA) Documentation Set. 2020. Available online: https://thermocalc.com/wp-content/uploads/Documentation/Current_Static/diffusion-module-dictra-documentation-set.pdf (accessed on 10 July 2023).
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, Computational Tools for Materials Science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Hultgren, A. A Metallographic Study on Tungsten Steels; John Wiley: Hoboken, NJ, USA, 1920. [Google Scholar]
- Sun, W.W.; Wu, Y.X.; Yang, S.C.; Hutchinson, C.R. Advanced High Strength Steel (AHSS) Development through Chemical Patterning of Austenite. Scr. Mater. 2018, 146, 60–63. [Google Scholar] [CrossRef]
- Kasatkin, O.G.; Vinokur, B.B.; Pilyushenko, V.L. Calculation Models for Determining the Critical Points of Steel. Met. Sci. Heat Treat. 1984, 26, 27–31. [Google Scholar] [CrossRef]
- Yang, D.Z.; Brown, E.L.; Matlock, D.K.; Krauss, G. Ferrite Recrystallization and Austenite Formation in Cold-Rolled Intercritically Annealed Steel. MTA 1985, 16, 1385–1392. [Google Scholar] [CrossRef]
- Moreno, M.; Teixeira, J.; Ghanbaja, J.; Bonnet, F.; Allain, S. Evolution of Cementite Composition along the Processing of Cold-Rolled and Annealed Dual-Phase Steels. Materialia 2019, 6, 100179. [Google Scholar] [CrossRef]
- Huang, J.; Poole, W.J.; Militzer, M. Austenite Formation during Intercritical Annealing. Metall. Mater. Trans. A 2004, 35, 3363–3375. [Google Scholar] [CrossRef]
- Kamoutsi, H.; Gioti, E.; Haidemenopoulos, G.N.; Cai, Z.; Ding, H. Kinetics of Solute Partitioning During Intercritical Annealing of a Medium-Mn Steel. Metall. Mater. Trans. A 2015, 46, 4841–4846. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couchet, C.; Bonnet, F.; Teixeira, J.; Allain, S.Y.P. Numerical Investigations of Phase Transformations Controlled by Interface Thermodynamic Conditions during Intercritical Annealing of Steels. Metals 2023, 13, 1288. https://doi.org/10.3390/met13071288
Couchet C, Bonnet F, Teixeira J, Allain SYP. Numerical Investigations of Phase Transformations Controlled by Interface Thermodynamic Conditions during Intercritical Annealing of Steels. Metals. 2023; 13(7):1288. https://doi.org/10.3390/met13071288
Chicago/Turabian StyleCouchet, Clélia, Frédéric Bonnet, Julien Teixeira, and Sébastien Y. P. Allain. 2023. "Numerical Investigations of Phase Transformations Controlled by Interface Thermodynamic Conditions during Intercritical Annealing of Steels" Metals 13, no. 7: 1288. https://doi.org/10.3390/met13071288
APA StyleCouchet, C., Bonnet, F., Teixeira, J., & Allain, S. Y. P. (2023). Numerical Investigations of Phase Transformations Controlled by Interface Thermodynamic Conditions during Intercritical Annealing of Steels. Metals, 13(7), 1288. https://doi.org/10.3390/met13071288





