Acid-Assisted Separation of Cathodic Material from Spent Electric Vehicle Batteries for Recycling
Abstract
1. Introduction
2. Materials and Methods
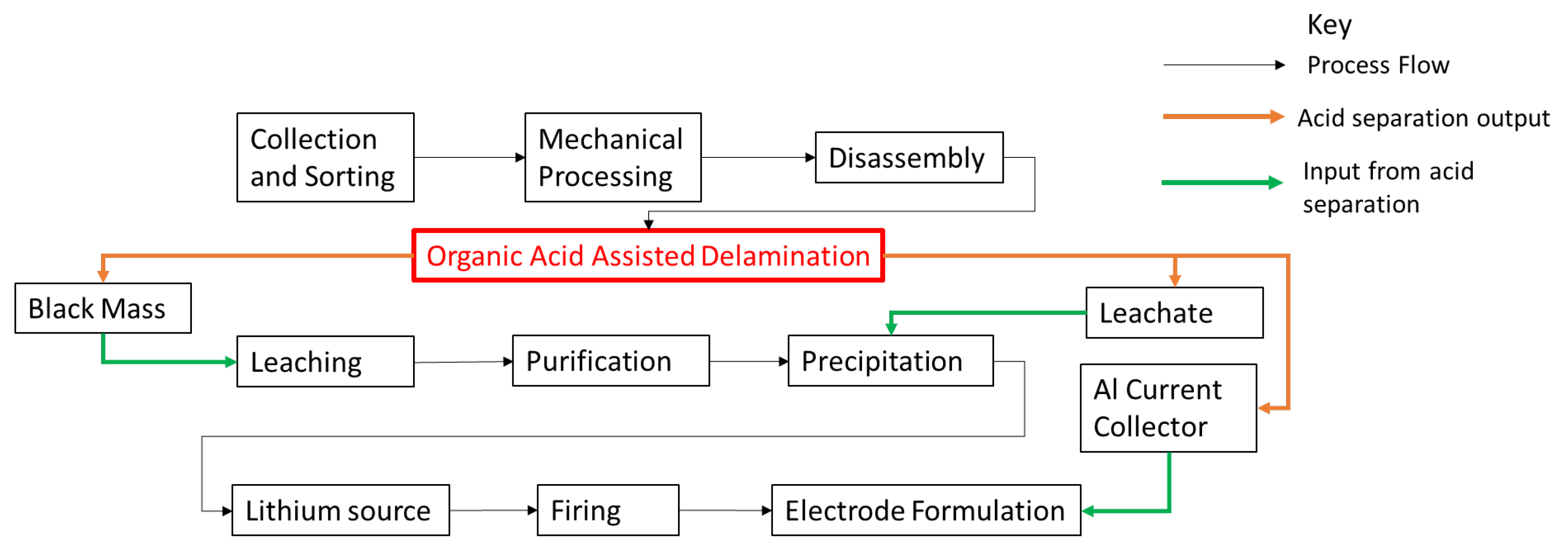
2.1. Separation Method
2.2. Material Characterisation
2.3. Electrochemical Testing
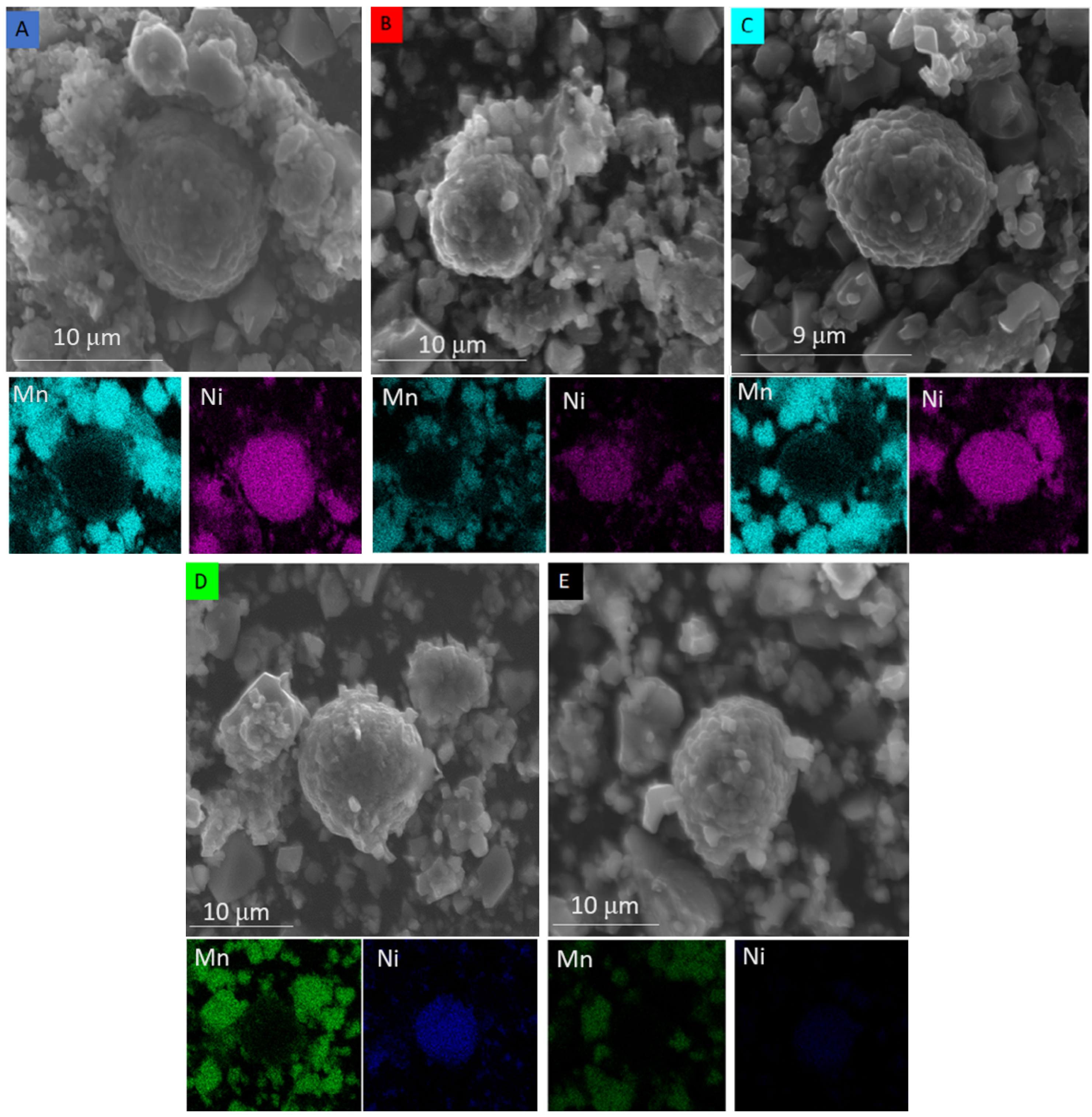
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef]
- International Energy Agency. Global Electric Vehicle Outlook 2022; Global EV Outlook 2022; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A Non-Academic Perspective on the Future of Lithium-Based Batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Lithium Battery Reusing and Recycling: A Circular Economy Insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef]
- Gaines, L. The Future of Automotive Lithium-Ion Battery Recycling: Charting a Sustainable Course. Sustain. Mater. Technol. 2014, 1–2, 2–7. [Google Scholar] [CrossRef]
- Ganter, M.J.; Landi, B.J.; Babbitt, C.W.; Anctil, A.; Gaustad, G. Cathode Refunctionalization as a Lithium Ion Battery Recycling Alternative. J. Power Sources 2014, 256, 274–280. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Gaines, L. To Recycle, or Not to Recycle, That Is the Question: Insights from Life-Cycle Analysis. MRS Bull. 2012, 37, 333–338. [Google Scholar] [CrossRef]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of Mixed Cathode Lithium-Ion Batteries for Electric Vehicles: Current Status and Future Outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, J.; Lyu, Z.; You, H.; Zhou, Y.; Gu, L.; Qu, J.; Xia, Z.; Zhang, Z.; Dai, H. Deep Learning Enhanced Lithium-Ion Battery Nonlinear Fading Prognosis. J. Energy Chem. 2023, 78, 565–573. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M. Cloud-Based in-Situ Battery Life Prediction and Classification Using Machine Learning. Energy Storage Mater. 2023, 57, 346–359. [Google Scholar] [CrossRef]
- Atalay, S.; Sheikh, M.; Mariani, A.; Merla, Y.; Bower, E.; Widanage, W.D. Theory of Battery Ageing in a Lithium-Ion Battery: Capacity Fade, Nonlinear Ageing and Lifetime Prediction. J. Power Sources 2020, 478, 229026. [Google Scholar] [CrossRef]
- Sommerville, R.; Shaw-Stewart, J.; Goodship, V.; Rowson, N.; Kendrick, E. A Review of Physical Processes Used in the Safe Recycling of Lithium Ion Batteries. Sustain. Mater. Technol. 2020, 25, e00197. [Google Scholar] [CrossRef]
- Sommerville, R.; Zhu, P.; Rajaeifar, M.A.; Heidrich, O.; Goodship, V.; Kendrick, E. A Qualitative Assessment of Lithium Ion Battery Recycling Processes. Resour. Conserv. Recycl. 2021, 165, 105219. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; He, Y.; Wang, H.; Zhang, T.; Xie, W. Recent Advances in Pretreating Technology for Recycling Valuable Metals from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2021, 406, 124332. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A Closed Loop Process for Recycling Spent Lithium Ion Batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for Recycling Mixed-Cathode Materials from Spent Lithium-Ion Batteries and Kinetics of Leaching. Waste Manag. 2018, 71, 362–371. [Google Scholar] [CrossRef]
- Gaines, L. Lithium-Ion Battery Recycling Processes: Research towards a Sustainable Course. Sustain. Mater. Technol. 2018, 17, e00068. [Google Scholar] [CrossRef]
- Sloop, S.; Crandon, L.; Allen, M.; Koetje, K.; Reed, L.; Gaines, L.; Sirisaksoontorn, W.; Lerner, M. A Direct Recycling Case Study from a Lithium-Ion Battery Recall. Sustain. Mater. Technol. 2020, 25, e00152. [Google Scholar] [CrossRef]
- Zhang, G.; Du, Z.; He, Y.; Wang, H.; Xie, W.; Zhang, T. A Sustainable Process for the Recovery of Anode and Cathode Materials Derived from Spent Lithium-Ion Batteries. Sustainability 2019, 11, 2363. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhu, X. Pyrolysis-Ultrasonic-Assisted Flotation Technology for Recovering Graphite and LiCoO2 from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10896–10904. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, T.; He, Y.; Zhao, Y.; Wang, S.; Zhang, G.; Zhang, Y.; Feng, Y. Recovery of Valuable Materials from Spent Lithium-Ion Batteries by Mechanical Separation and Thermal Treatment. J. Clean. Prod. 2018, 185, 646–652. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z. Recovery of Lithium from Spent Lithium-Ion Batteries Using Precipitation and Electrodialysis Techniques. Sep. Purif. Technol. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Microbial Production of Specialty Organic Acids from Renewable and Waste Materials. Crit. Rev. Biotechnol. 2015, 35, 497–513. [Google Scholar] [CrossRef]
- Calvo, L.; Vallejo, D. Formation of Organic Acids during the Hydrolysis and Oxidation of Several Wastes in Sub- and Supercritical Water. Ind. Eng. Chem. Res. 2002, 41, 6503–6509. [Google Scholar] [CrossRef]
- Son, J.; Joo, J.C.; Baritugo, K.A.; Jeong, S.; Lee, J.Y.; Lim, H.J.; Lim, S.H.; Yoo, J.I.; Park, S.J. Consolidated Microbial Production of Four-, Five-, and Six-Carbon Organic Acids from Crop Residues: Current Status and Perspectives. Bioresour. Technol. 2022, 351, 127001. [Google Scholar] [CrossRef]
- Fujii, K.; Aoki, M.; Kitayama, K. Biodegradation of Low Molecular Weight Organic Acids in Rhizosphere Soils from a Tropical Montane Rain Forest. Soil Biol. Biochem. 2012, 47, 142–148. [Google Scholar] [CrossRef]
- Kaya, M.; Kursunoglu, S.; Hussaini, S.; Gül, E. Leaching of Turkish Oxidized Pb–Zn Flotation Tailings by Inorganic and Organic Acids. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Siegmund, A., Alam, S., Grogan, J., Kerney, U., Shibata, E., Eds.; Springer International Publishing: Cham, Switerland, 2020; pp. 447–468. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Weng, Y.; Xu, S.; Huang, G.; Jiang, C. Synthesis and Performance of Li[(Ni1/3Co1/3Mn1/3)1-Mg]O2 Prepared from Spent Lithium Ion Batteries. J. Hazard. Mater. 2013, 246–247, 163–172. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Removal of Organics by Pyrolysis for Enhancing Liberation and Flotation Behavior of Electrode Materials Derived from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 2205–2214. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, W.; Han, J.; Jiao, F.; Qin, W.; Liu, T.; Zhao, C. Pyrolysis and Physical Separation for the Recovery of Spent LiFePO4 Batteries. Waste Manag. 2019, 89, 83–93. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, F.S. Innovative Leaching of Cobalt and Lithium from Spent Lithium-Ion Batteries and Simultaneous Dechlorination of Polyvinyl Chloride in Subcritical Water. J. Hazard. Mater. 2016, 316, 19–25. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Sun, K.; Qian, C.; Bao, W. Emerging Green Technologies for Recovery and Reuse of Spent Lithium-Ion Batteries—A Review. J. Mater. Chem. A 2022, 10, 17053–17076. [Google Scholar] [CrossRef]
- Maske, T.; Anwani, S.; Methekar, R. Development of Environmentally and Economically Sustainable Delamination Process for Spent Lithium-Ion Batteries. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 2572–2586. [Google Scholar] [CrossRef]
- Shin, H.; Zhan, R.; Dhindsa, K.S.; Pan, L.; Han, T. Electrochemical Performance of Recycled Cathode Active Materials Using Froth Flotation-based Separation Process. J. Electrochem. Soc. 2020, 167, 020504. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Hu, T.; Bai, X.; Wang, S.; Xie, W.; Hao, J.; He, Y. Recovery of LiCoO2 and Graphite from Spent Lithium-Ion Batteries by Cryogenic Grinding and Froth Flotation. Miner. Eng. 2020, 148, 106223. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Bai, X.; Wang, S.; Yang, D.; Fu, Y.; He, Y. Separation of the Cathode Materials from the Al Foil in Spent Lithium-Ion Batteries by Cryogenic Grinding. Waste Manag. 2019, 91, 89–98. [Google Scholar] [CrossRef]
- Marshall, J.; Gastol, D.; Sommerville, R.; Middleton, B.; Goodship, V.; Kendrick, E. Disassembly of Li Ion Cells—Characterization and Safety Considerations of a Recycling Scheme. Metals 2020, 10, 773. [Google Scholar] [CrossRef]
- Zhu, P.; Driscoll, E.H.; Dong, B.; Sommerville, R.; Zorin, A.; Slater, P.R.; Kendrick, E. Direct Reuse of Aluminium and Copper Current Collectors from Spent Lithium-Ion Batteries. Green Chem. 2023, 25, 3503–3514. [Google Scholar] [CrossRef]
- Gastol, D.; Marshall, J.; Cooper, E.; Mitchell, C.; Burnett, D.; Song, T.; Sommerville, R.; Middleton, B.; Crozier, M.; Smith, R.; et al. Reclaimed and Up-Cycled Cathodes for Lithium-Ion Batteries. Glob. Chall. 2022, 6, 2200046. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, L.L.; Slater, P.R.; Anderson, P.A. Battery Recycling. WO2022084668A1, 28 April 2022. [Google Scholar]
- Peeters, N.; Binnemans, K.; Riaño, S. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Higuchi, A.; Ankei, N.; Nishihama, S.; Yoshizuka, K. Selective Recovery of Lithium from Cathode Materials of Spent Lithium Ion Battery. JOM 2016, 68, 2624–2631. [Google Scholar] [CrossRef]
- Gerold, E.; Luidold, S.; Antrekowitsch, H. Selective Precipitation of Metal Oxalates from Lithium Ion Battery Leach Solutions. Metals 2020, 10, 1435. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Wu, J.; Yu, X.; Zhang, Y. Stearic Acid Modified Aluminum Surfaces with Controlled Wetting Properties and Corrosion Resistance. Corros. Sci. 2014, 83, 86–93. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Zhang, Y.; Lv, Q.; Xu, T.; Liu, T. Super-Hydrophobic Surface Treatment as Corrosion Protection for Aluminum in Seawater. Corros. Sci. 2009, 51, 1757–1761. [Google Scholar] [CrossRef]
- Li, J.; Wei, H.; Zhao, K.; Wang, M.; Chen, D.; Chen, M. Effect of Anodizing Temperature and Organic Acid Addition on the Structure and Corrosion Resistance of Anodic Aluminum Oxide Films. Thin Solid Film. 2020, 713, 138359. [Google Scholar] [CrossRef]
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective Recovery of Valuable Metals from Spent Lithium-Ion Batteries—Process Development and Kinetics Evaluation. J. Clean. Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Mu, Y.Y.; Song, X.F.; Yu, J.G. Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using L-Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic Acid-Based Leaching System: A Sustainable Process for Recovery of Valuable Metals from Spent Li-ion Batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of Cobalt and Lithium from Spent Lithium Ion Batteries Using Organic Citric Acid as Leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating Organic Acids as Alternative Leaching Reagents for Metal Recovery from Lithium Ion Batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Harper, G.D.J.; Kendrick, E.; Anderson, P.A.; Mrozik, W.; Christensen, P.; Lambert, S.; Greenwood, D.; Das, P.K.; Ahmeid, M.; Milojevic, Z.; et al. Roadmap for a Sustainable Circular Economy in Lithium-Ion and Future Battery Technologies. J. Phys. Energy 2023, 5, 021501. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Zou, H.; Gratz, E.; Apelian, D.; Wang, Y. A Novel Method to Recycle Mixed Cathode Materials for Lithium Ion Batteries. Green Chem. 2013, 15, 1183. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Zhang, L.; Xu, S. Cleaner Recycling of Cathode Material by In-Situ Thermite Reduction. J. Clean. Prod. 2020, 249, 119340. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Deng, X.; Bohu, T.; Zea, L.; Khaleque, H.N.; Gumulya, Y.; Boxall, N.J.; Morris, C.; Cheng, K.Y. Prospective Directions for Biohydrometallurgy. Hydrometallurgy 2020, 195, 105376. [Google Scholar] [CrossRef]
- Wittkowski, A.; Schirmer, T.; Qiu, H.; Goldmann, D.; Fittschen, U.E.A. Speciation of Manganese in a Synthetic Recycling Slag Relevant for Lithium Recycling from Lithium-Ion Batteries. Metals 2021, 11, 188. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a Low-Carbon Society: A Review of Lithium Resource Availability, Challenges and Innovations in Mining, Extraction and Recycling, and Future Perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
| Method | Advantage | Disadvantage |
|---|---|---|
| Mineral Acid Delamination [33] | Acids are readily available. | Acids may only be used once. Acids may be highly hazardous. Large amounts of waste generated. Inappropriate for direct recycling due to leaching. |
| Organic Acid Delamination [27,28,29] | Acids can be biologically derived and so can be renewable/green. Similar process to mineral acid delamination. | Acids may only be used once. Large amounts of waste generated. |
| Thermal Treatment [34,35] | Simple technology required. May be tailored to specific chemistries. | Release of toxic compounds. May not be chemistry agnostic |
| Solvent Dissolution [36,37,38] | Solvent can be recovered and reused. High recover efficiency. | Solvents may be toxic and flammable. Possible expense. |
| Mechanical Grinding [15,34,39] | Well-studied technology. Cheap. | Contaminated output requiring further separation. |
| Cryogenic Grinding [15,40,41] | Good peeling efficiency. Prevent surface chemical change. | Liquid nitrogen is expensive. Depending on size, aluminium may be difficult to recover. |
| Acid | Mass of Electrode Sample (g) | Mass of Separated Black Mass (g) | Al Mass (g) |
|---|---|---|---|
| Oxalic acid | 7.513 | 6.897 | 1.157 |
| Citric acid | 7.485 | 3.552 | 1.476 |
| Malic acid | 7.510 | 5.000 | 1.614 |
| Acetic acid | 7.517 | 5.563 | 1.494 |
| Lactic acid | 7.487 | 4.724 | 1.416 |
| Succinic acid | 7.488 | 5.085 | 1.742 |
| Pimelic acid | 7.519 | 5.000 | 1.786 |
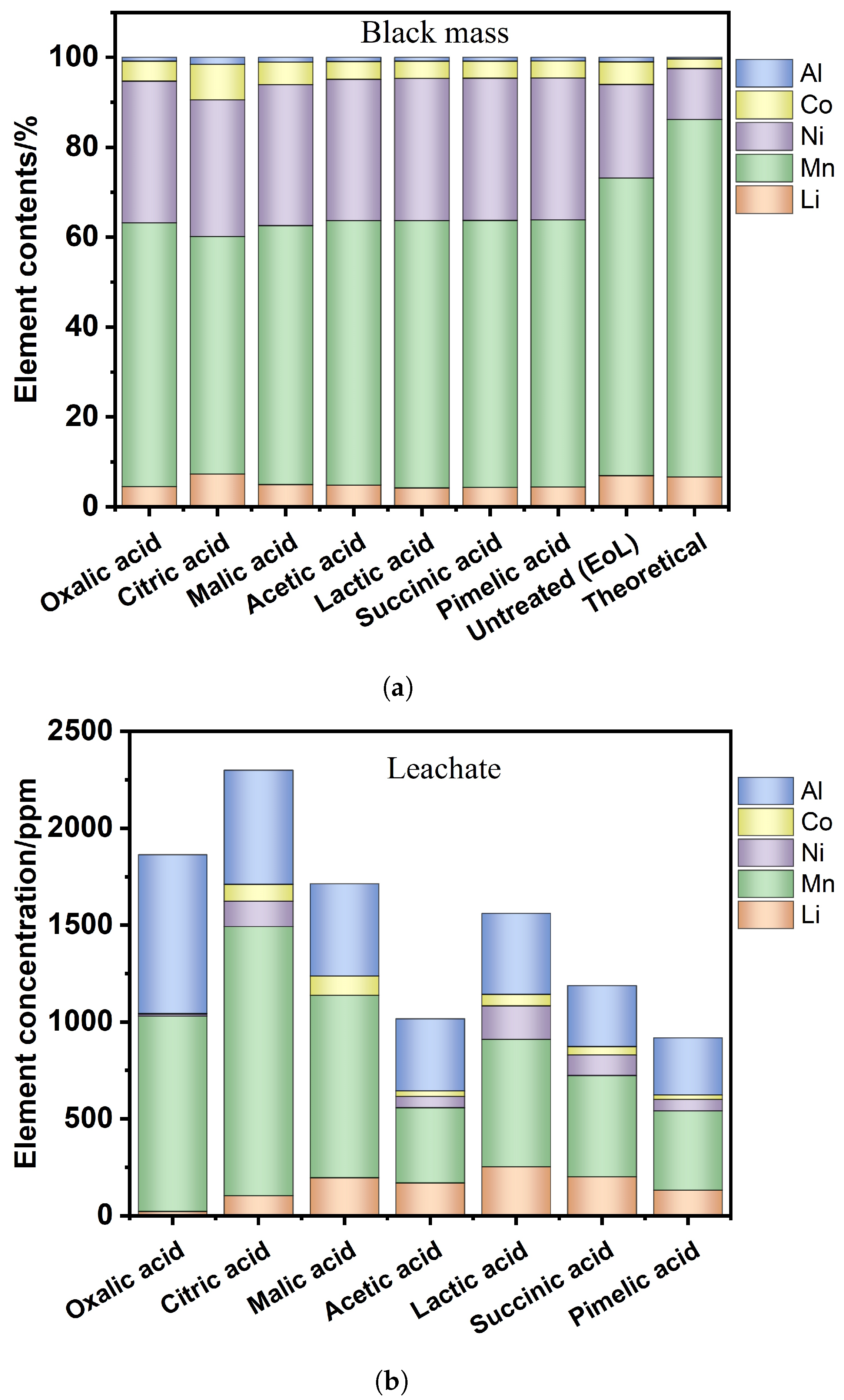
| Element | Oxalic | Citric | Malic | Acetic | Lactic | Succinic | Pimelic |
|---|---|---|---|---|---|---|---|
| Al | 10.5 | 12.8 | 11.9 | 12.0 | 11.7 | 11.8 | 11.3 |
| Co | 56.2 | 65.1 | 59.7 | 54.9 | 55.5 | 55.1 | 54.5 |
| Mn | 747.2 | 436.2 | 679.7 | 806.6 | 858.7 | 862.1 | 857.0 |
| Ni | 401.7 | 250.6 | 369.7 | 430.8 | 457.1 | 458.6 | 455.7 |
| Li | 57.5 | 60.4 | 58.7 | 66.8 | 61.3 | 62.6 | 64.4 |
| Element | Oxalic | Citric | Malic | Acetic | Lactic | Succinic | Pimelic |
|---|---|---|---|---|---|---|---|
| Al | 820.3 | 588.8 | 475.0 | 372.4 | 417.5 | 313.8 | 294.6 |
| Co | 1.6 | 87.1 | 100.6 | 28.7 | 60.0 | 43.7 | 24.4 |
| Mn | 12.3 | 131.0 | - | 57.8 | 172.4 | 106.9 | 58.6 |
| Ni | 22.8 | 104.0 | 196.3 | 169.7 | 254.0 | 201.5 | 132.3 |
| Li | 1007.7 | 1389.6 | 942.1 | 388.7 | 657.3 | 522.5 | 409.2 |
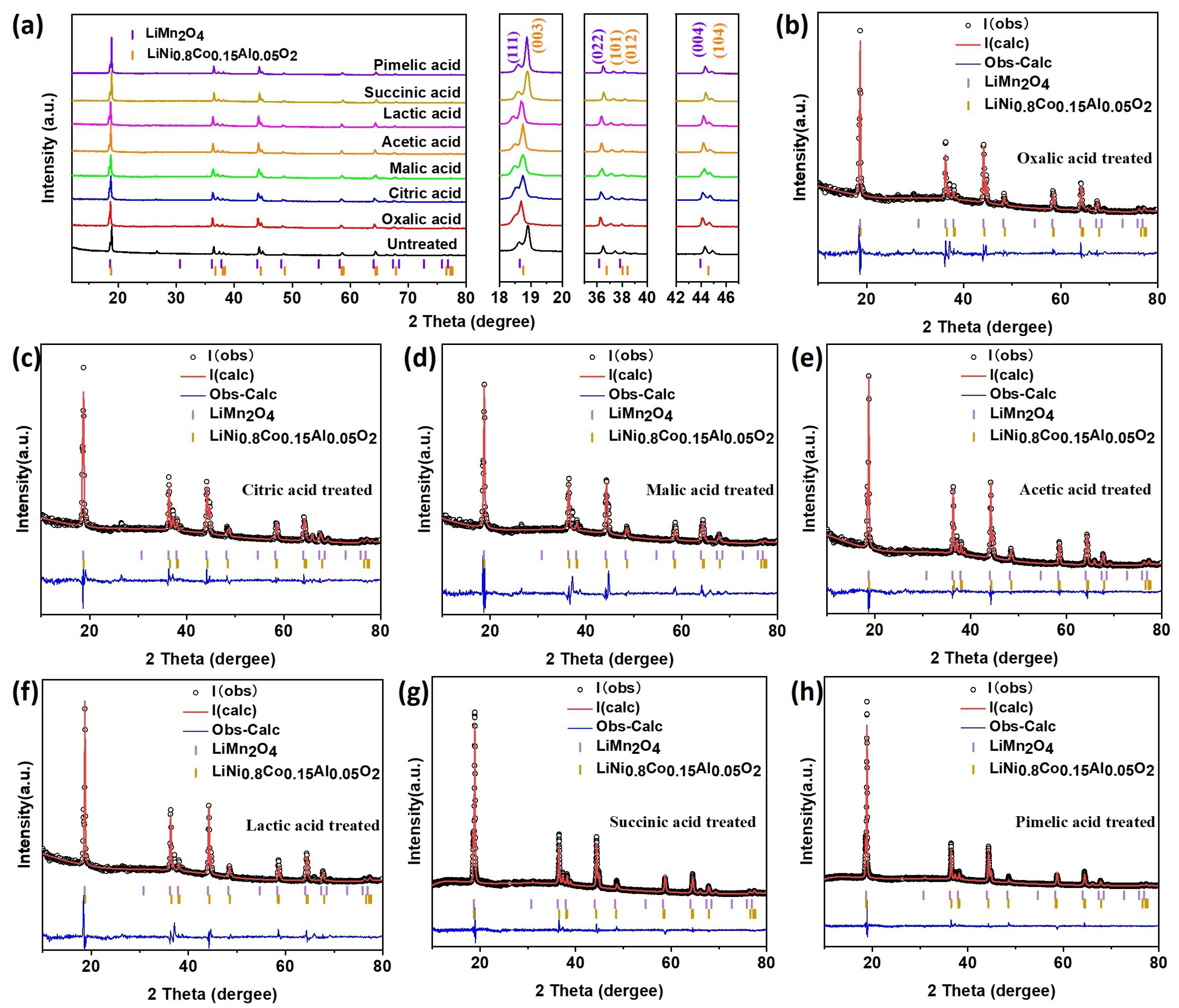
| Acid | Phase 1 Wt.Frac. | Phase 2 Wt.Frac. | Ratio Wt. Frac. 1/2 | Rwp % | Rp % | Chi2 |
|---|---|---|---|---|---|---|
| Oxalic acid | 0.75264 | 0.24736 | 3.04 | 5.02 | 3.21 | 10.15 |
| Citric acid | 0.75918 | 0.24082 | 3.15 | 5.90 | 4.08 | 4.577 |
| Malic acid | 0.76608 | 0.23392 | 3.27 | 6.21 | 3.48 | 15.63 |
| Acetic acid | 0.76843 | 0.23157 | 3.32 | 3.64 | 2.54 | 4.777 |
| Lactic acid | 0.81942 | 0.18058 | 4.54 | 5.72 | 3.27 | 11.82 |
| Succinic acid | 0.80778 | 0.19222 | 4.20 | 4.12 | 2.91 | 1.07 |
| Pimelic acid | 0.82649 | 0.17351 | 4.76 | 4.27 | 2.97 | 1.23 |
| Processing Acid | First-Cycle Capacity (mAh/g) | Peeling Efficiency (%) | Li+ Content (ppm) |
|---|---|---|---|
| Citric acid | 57.32 | 99.5 | 60.44 |
| Acetic Acid | 84.51 | 100.0 | 66.76 |
| Malic Acid | 54.23 | 86.4 | 58.66 |
| Oxalic Acid | 69.16 | 96.53 | 57.49 |
| Pimelic Acid | 67.26 | 100 | 64.41 |
| Succinic Acid | 60.15 | 100 | 62.57 |
| Lactic Acid | 66.04 | 96.50 | 61.26 |
| EoL Unprocessed | 107.62 | N/A | 71.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorin, A.; Song, T.; Gastol, D.; Kendrick, E. Acid-Assisted Separation of Cathodic Material from Spent Electric Vehicle Batteries for Recycling. Metals 2023, 13, 1276. https://doi.org/10.3390/met13071276
Zorin A, Song T, Gastol D, Kendrick E. Acid-Assisted Separation of Cathodic Material from Spent Electric Vehicle Batteries for Recycling. Metals. 2023; 13(7):1276. https://doi.org/10.3390/met13071276
Chicago/Turabian StyleZorin, Anton, Tengfei Song, Dominika Gastol, and Emma Kendrick. 2023. "Acid-Assisted Separation of Cathodic Material from Spent Electric Vehicle Batteries for Recycling" Metals 13, no. 7: 1276. https://doi.org/10.3390/met13071276
APA StyleZorin, A., Song, T., Gastol, D., & Kendrick, E. (2023). Acid-Assisted Separation of Cathodic Material from Spent Electric Vehicle Batteries for Recycling. Metals, 13(7), 1276. https://doi.org/10.3390/met13071276








