Puzzles of Surface Segregation in Binary Pt–Pd Nanoparticles: Molecular Dynamics and Thermodynamic Simulations
Abstract
1. Introduction
- (i)
- Bond strengths, i.e., the relative strengths of the A–A, B–B, and A–B bonds between metal atoms. The components that form stronger bonds should tend to the core positions, while stronger A–B bonds will favor mixing (we believe that the bond strengths are determined, first of all, by the binding energies of components A and B);
- (ii)
- Surface energies: the metal with the lower surface energy (and the lower surface tension) will segregate to the NP surface;
- (iii)
- Atomic sizes that relate to the release of strains in the NPs with smaller atoms that tend to the core positions;
- (iv)
- Specific electronic effects for certain metals (i.e., the directionality of bonds, according to [27]);
- (v)
- Charge transfer effects: the most electronegative atom tends to occupy a surface site.
2. Atomistic Simulation of Pt–Pd Nanoparticles
2.1. Approaches to Molecular Dynamics Simulation
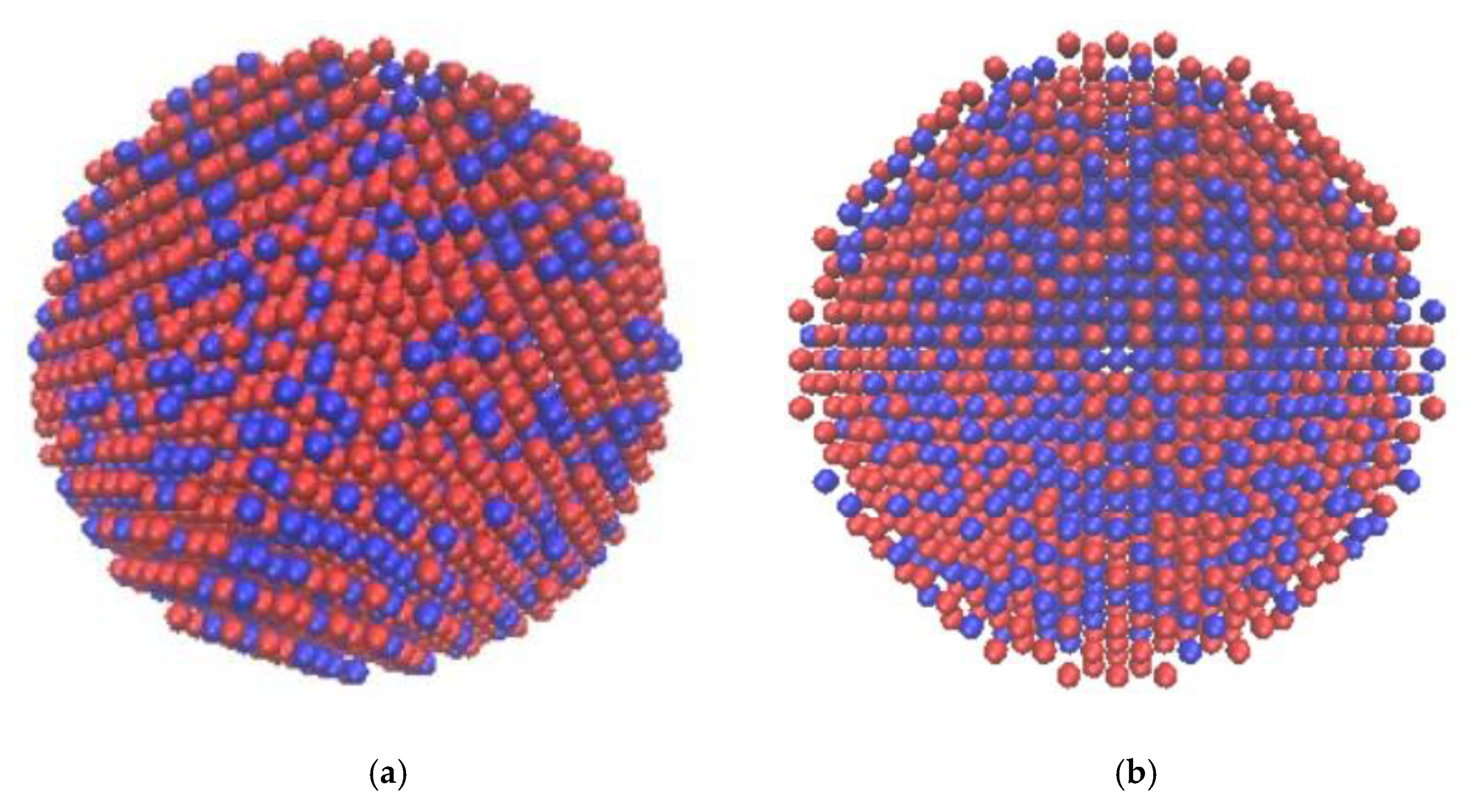
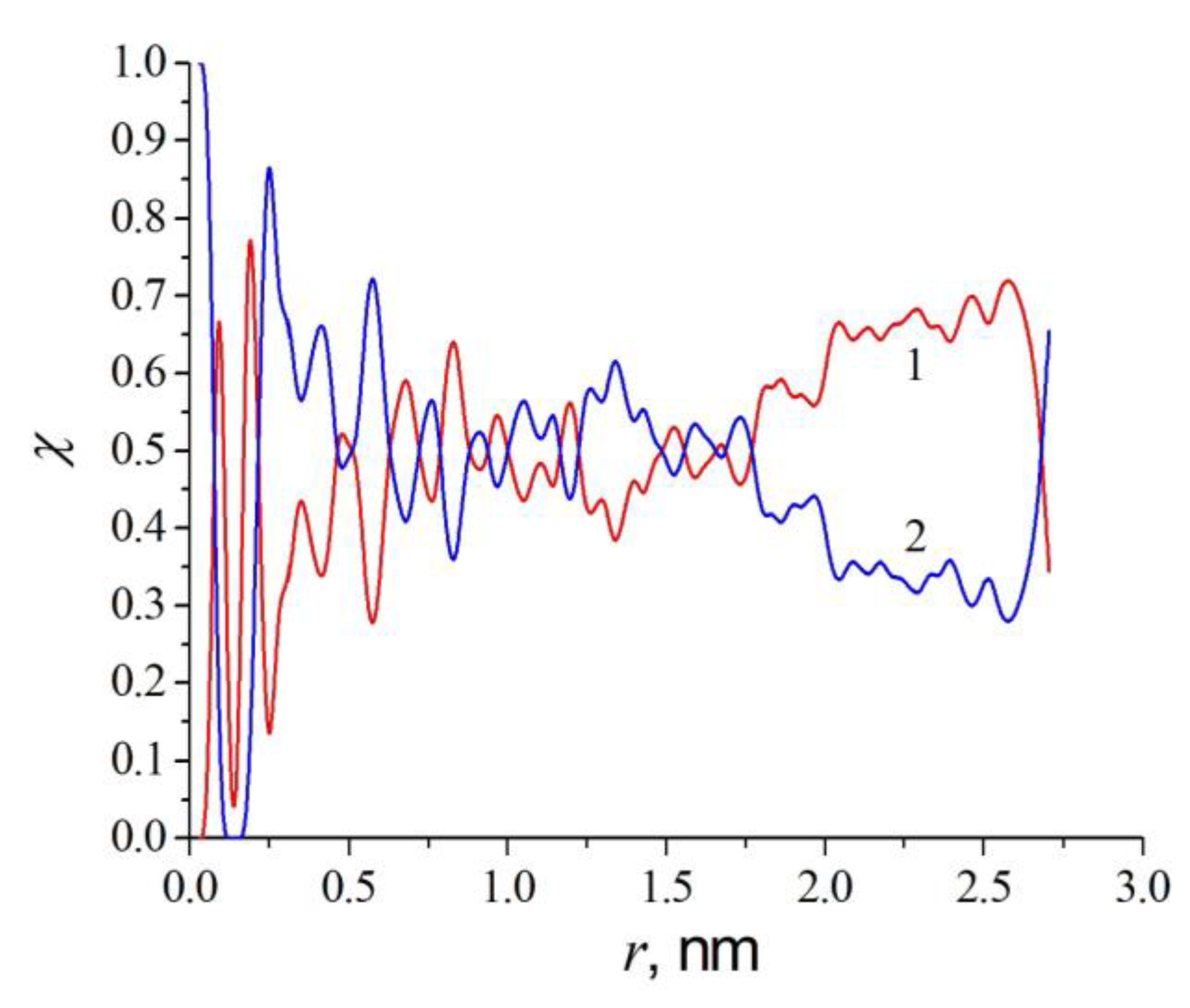
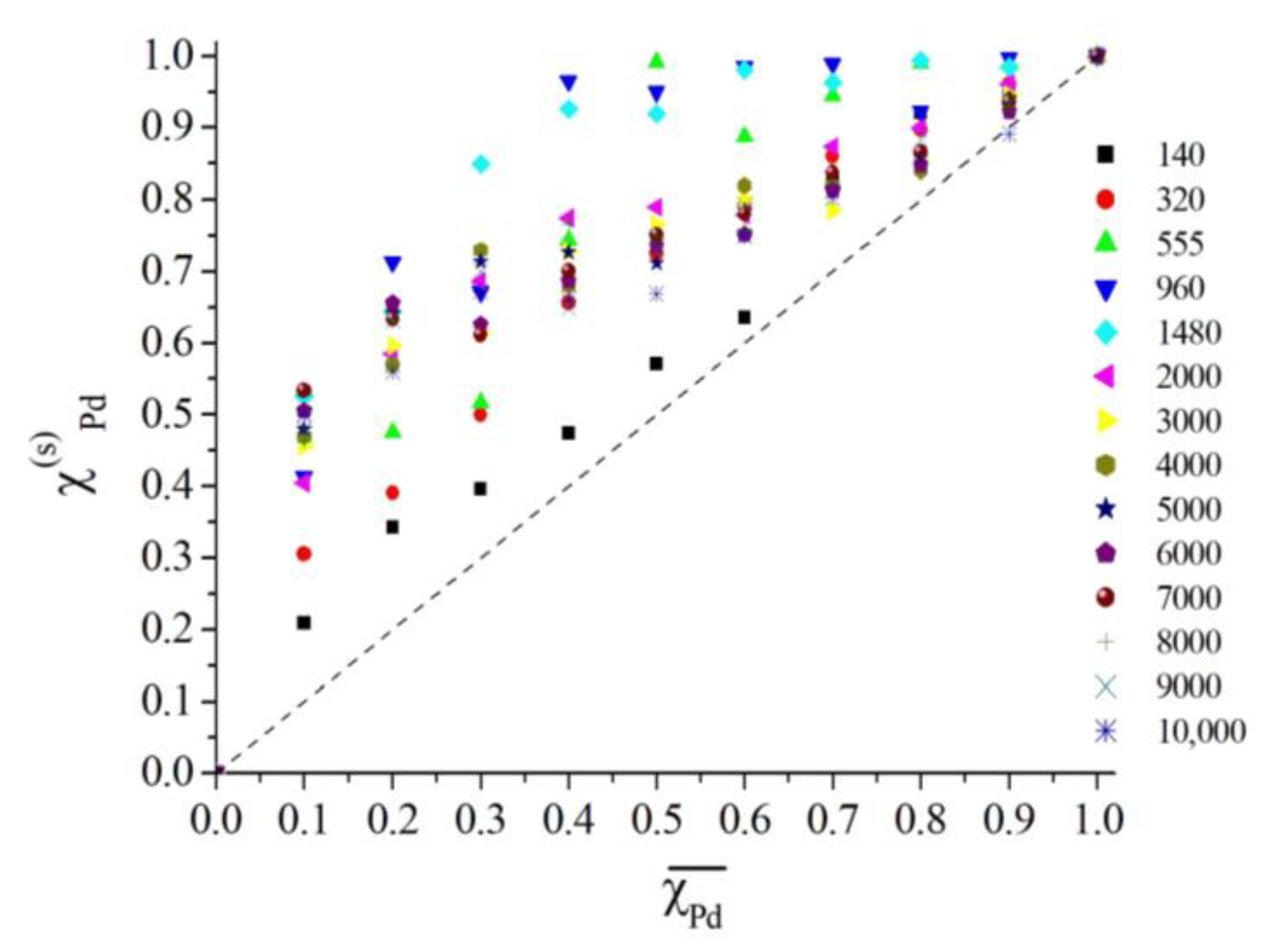
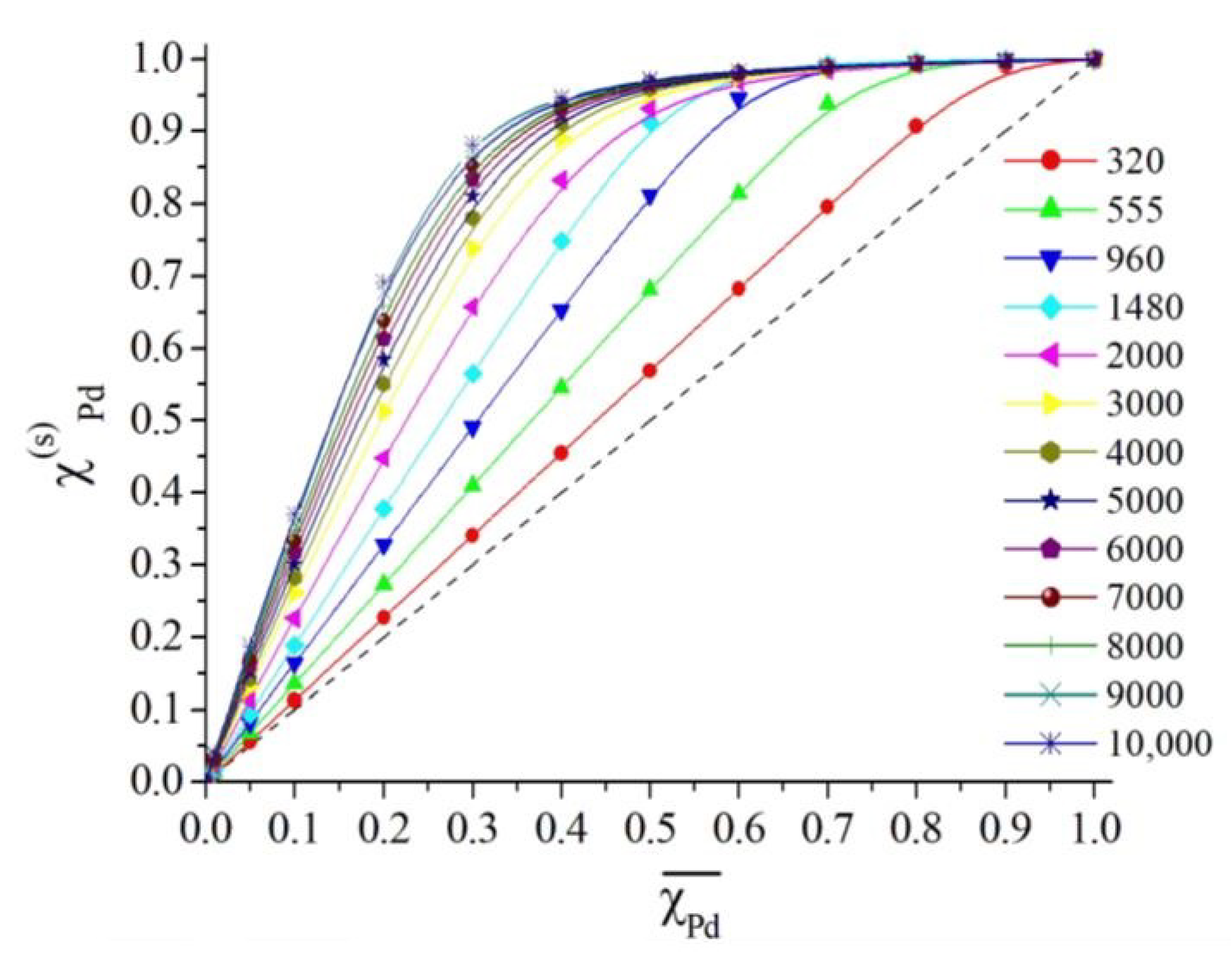
2.2. Results of Molecular Dynamics Simulation
3. Thermodynamic Simulation
3.1. Approach to Thermodynamic Simulation
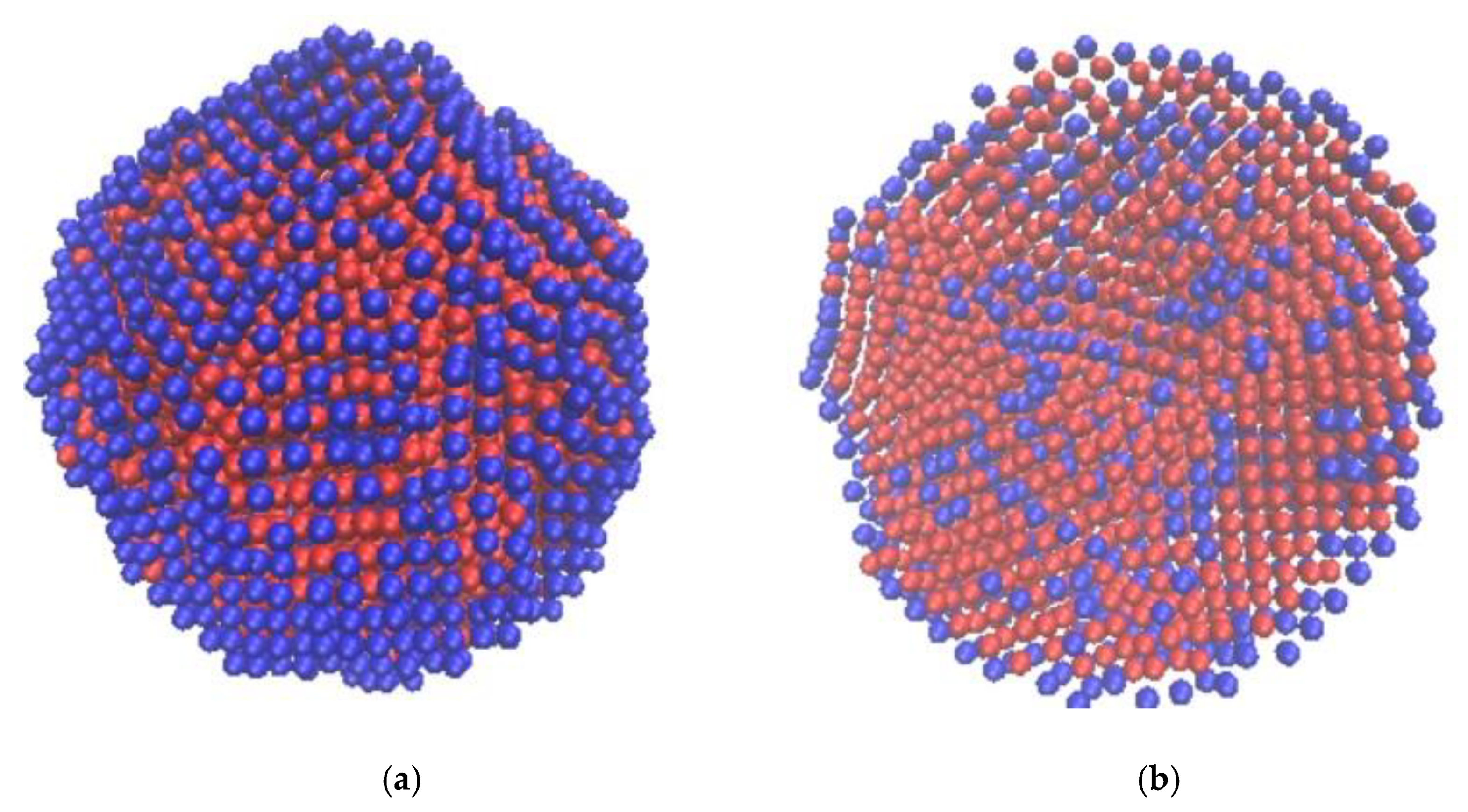
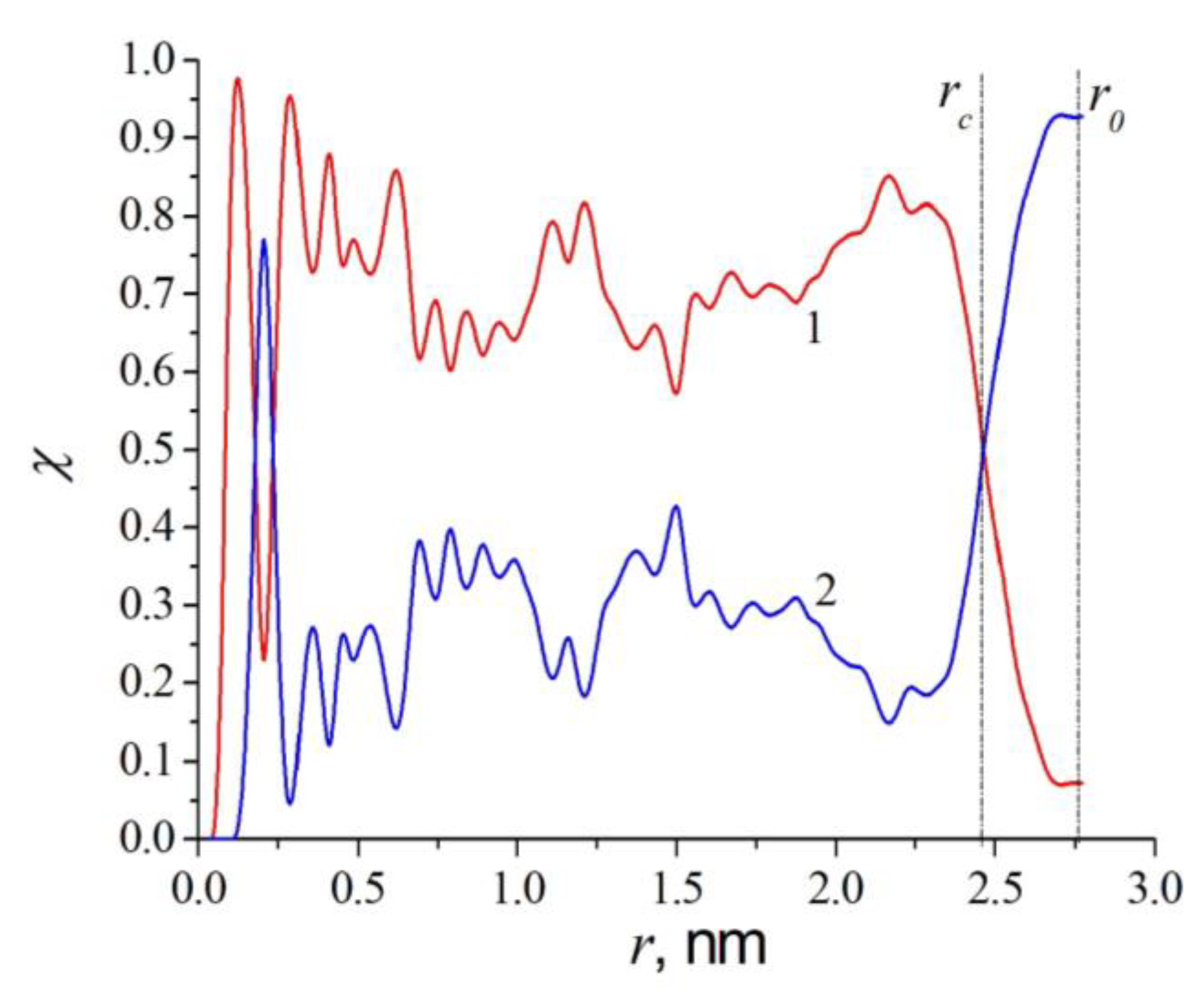
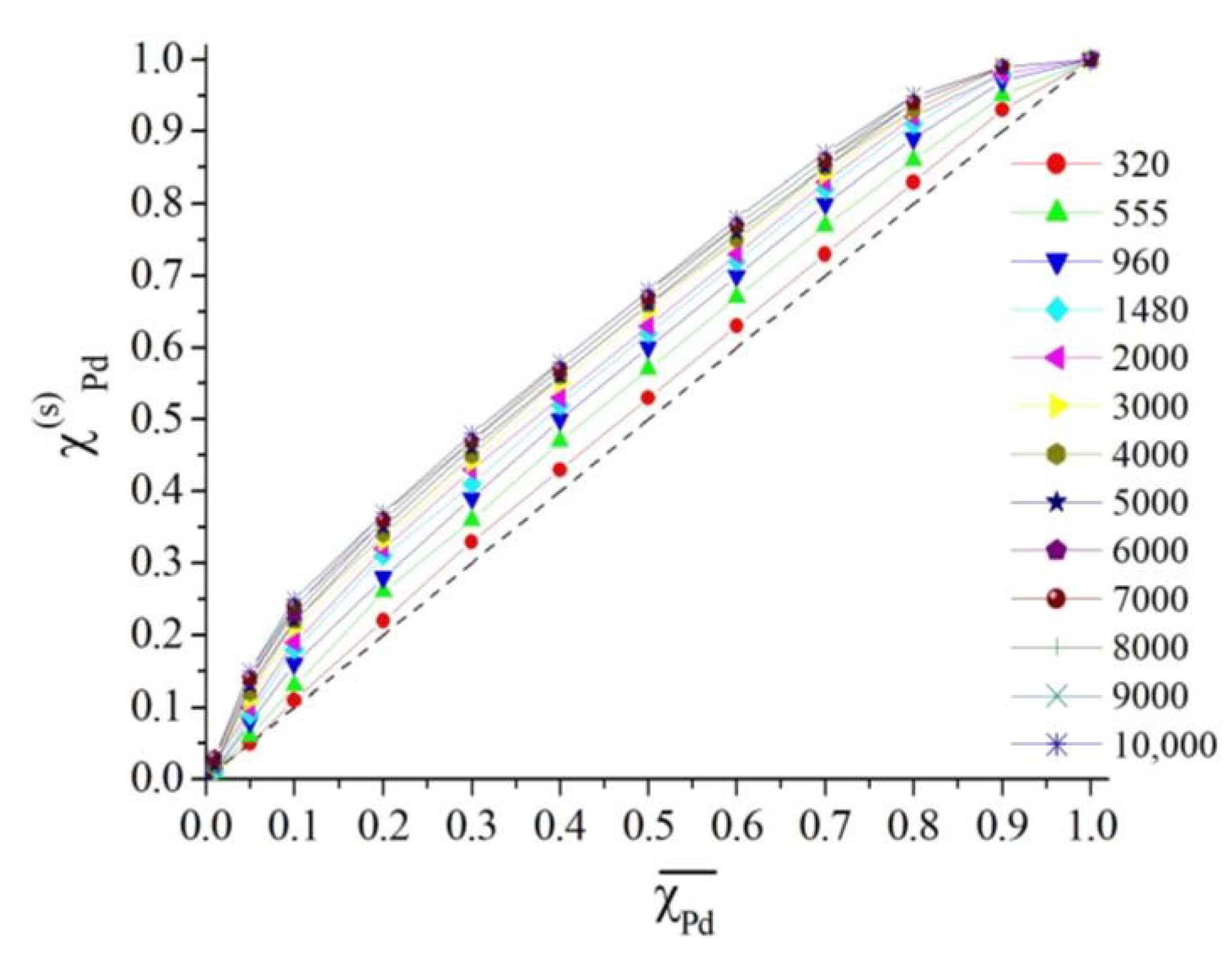
3.2. Results of Thermodynamic Simulation
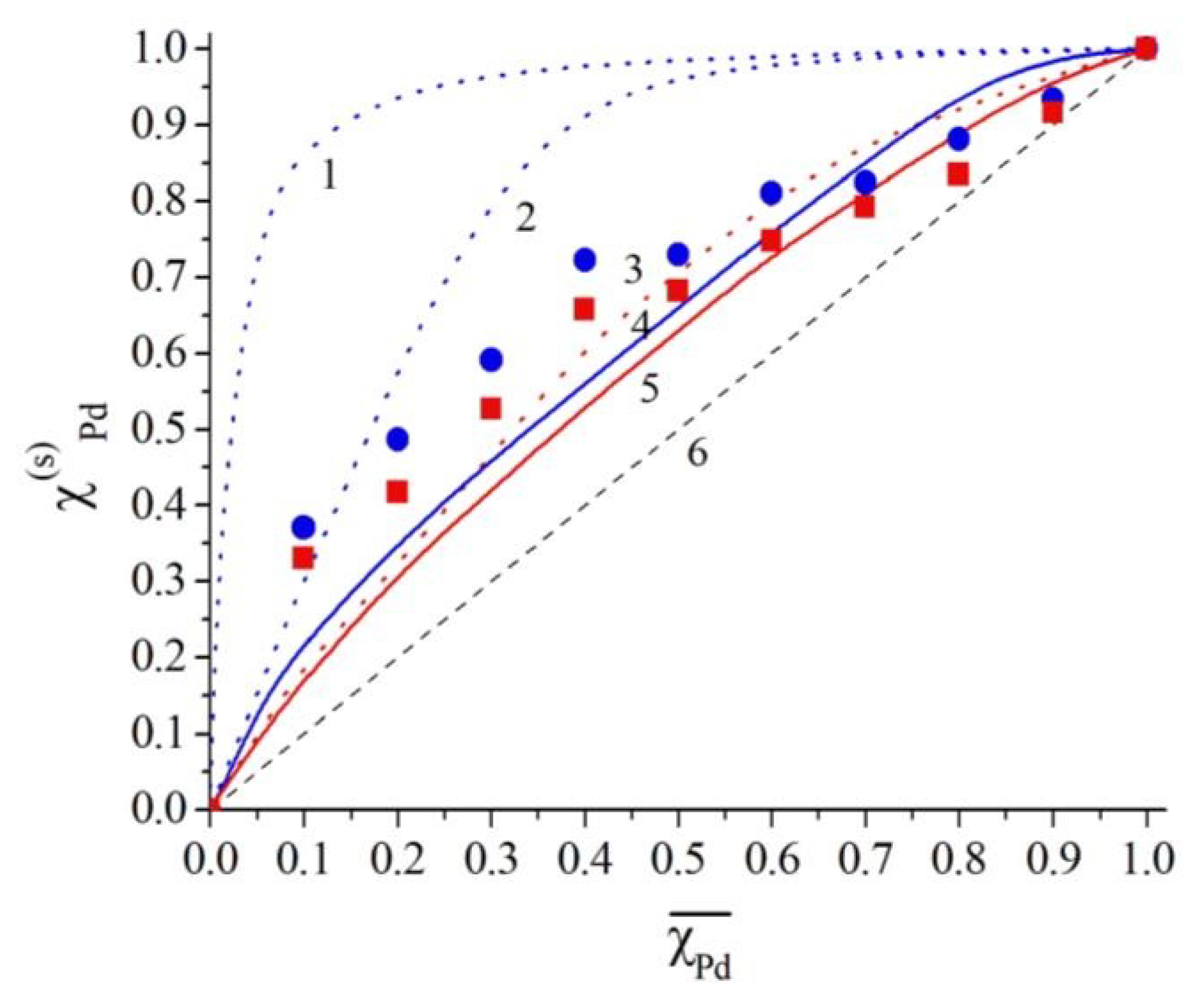
4. Discussion
- (i)
- If the bulk melting temperature of component A is larger than that of component B, then element A will segregate to the surface;
- (ii)
- If the bulk melting temperatures of both components have more or less the same magnitude, then the segregated element will be determined by a lower surface energy.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Algorithms of the Thermodynamic Simulation of the Surface Segregation in Binary NPs Based on Solving the Butler Equation
References
- Fendler, J.H. Nanoparticles and Nanostructured Films; Wiley-VCH: Weinheim, Germany, 1998; p. 468. [Google Scholar]
- Qazi, U.Y.; Javaid, R. A review on metal nanostructures: Preparation methods and their potential applications. Adv. Nanopart. 2016, 5, 27–43. [Google Scholar] [CrossRef]
- Hernando, A.; Crespo, P.; García, M.A. Metallic magnetic nanoparticles. Sci. World J. 2005, 5, 972–1001. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, M.D.; Pandey, R.; Blanco, M.A.; Khalkar, A. Magnetic properties of clusters with n = 0–13. J. Nanopart. Res. 2010, 12, 1129–1136. [Google Scholar] [CrossRef]
- Bartolomé, J.; Bartolomé, F.; García, L.M.; Figueroa, A.I.; Repollés, A.; Martínez-Pérez, M.J.; Luis, F.; Magén, C.; Selenska-Pobell, S.; Pobell, F.; et al. Strong paramagnetism of gold nanoparticles deposited on a sulfolobus acidocaldarius s layer. Phys. Rev. Lett. 2012, 109, 247203. [Google Scholar] [CrossRef]
- Karaagac, O.; Kockar, H. Optimisation of saturation magnetisation of iron nanoparticles synthesized by hydrogen reduction: Taguchi technique, response surface method, and multiple linear and quadratic regression analyses. J. Magn. Magn. Mater. 2018, 473, 190–197. [Google Scholar] [CrossRef]
- Samsonov, V.M.; Talyzin, I.V.; Puytov, V.V.; Vasilyev, S.A.; Romanov, A.A.; Alymov, M.I. When mechanisms of coalescence and sintering at the nanoscale fundamentally differ: Molecular dynamics study. J. Chem. Phys. 2022, 156, 214302. [Google Scholar] [CrossRef]
- Schmid, G.; Corain, B. Nanoparticulated gold: Syntheses, structures, electronics, and reactivities. Eur. J. Inorg. Chem. 2003, 2003, 3081–3098. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
- Zhai, Y.; Han, P.; Yun, Q.; Ge, Y.; Zhang, X.; Chen, Y.; Zhang, H. Phase engineering of metal nanocatalysts for electrochemical CO2 reduction. eScience 2022, 2, 467–485. [Google Scholar] [CrossRef]
- Yin, H.-J.; Zhou, J.-H.; Zhang, Y. Shaping well-defined noble metal nanostructures for highperformance electrocatalysts: Advances and perspectives. Inorg. Chem. Front. 2019, 6, 2582–2618. [Google Scholar] [CrossRef]
- Liang, S.; Chen, S.; Guo, Z.; Lan, Z.; Kobayashi, H.; Yan, X.; Li, R. In situ generated electron-deficient metallic copper as the catalytically active site for enhanced hydrogen production from alkaline formaldehyde solution. Catal. Sci. Technol. 2019, 9, 5292–5300. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Karthik, A.D.; Geetha, K. Applications of transition metal nanoparticles in antimicrobial therapy. Biomater. Tissue Eng. Bull. 2016, 3, 28–34. [Google Scholar]
- Balcerzak, M. Analytical methods for the determination of platinum in biological and environmental materials. A Review. Analyst 1997, 122, 67R–74R. [Google Scholar] [CrossRef]
- Troncoso, F.D.; Tonetto, G.M. Highly stable platinum monolith catalyst for the hydrogenation of vegetable oil. Chem. Eng. Process. 2022, 170, 108669. [Google Scholar] [CrossRef]
- Cha, G.; Hwang, I.; Hejazi, S.; Dobrota, A.S.; Pašti, I.A.; Osuagwu, B.; Kim, H.; Will, J.; Yokosawa, T.; Badura, Z.; et al. As a single atom Pd outperforms Pt as the most active co-catalyst for photocatalytic H2 evolution. iScience 2021, 24, 102938. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Huynh, K.-H.; Pham, X.-H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.-Y.; Jun, B.-H. Synthesis, properties, and biological applications of metallic alloy nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174. [Google Scholar] [CrossRef]
- Sytwu, K.; Vadai, M.; Dionne, J.A. Bimetallic nanostructures: Combining plasmonic and catalytic metals for photocatalysis. Adv. Phys. X 2019, 4, 1619480. [Google Scholar] [CrossRef]
- Xu, H.; Liu, P.; Zhang, W.; Wang, Q.; Yang, Y. Structure, stability, electronic and magnetic properties of monometallic Pd, Pt, and bimetallic Pd-Pt core-shell nanoparticles. Chem. Phys. 2020, 539, 110953. [Google Scholar] [CrossRef]
- Long, N.V.; Ohtaki, M.; Hien, T.D.; Randy, J.; Nogami, M. A comparative study of Pt and Pt-Pd core-shell nanocatalysts. Electrochim. Acta 2011, 56, 9133–9143. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Chen, J.; Huang, Y.; Chen, Z.-X. Surface segregation of PdM (M=Cu, Ag, Au) alloys and its implication to acetylene hydrogenation, DFT-based Monte Carlo simulations. Mater. Today Commun. 2021, 28, 102475. [Google Scholar] [CrossRef]
- Tello-Casas, F.F.; Cervantes-Aspeitia, E.Y.; Hernández-Pichardo, M.L.; Tufiño Velázquez, M.; Borja Urby, R.; del Angel, P.; González-Huerta, R. de G. Surface-enriched Pd/C electrocatalysts with a low load of Pt prepared by surface redox reactions for the oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 26005–26018. [Google Scholar] [CrossRef]
- Cai, X.; Lin, R.; Liu, X.; Zhao, Y. Effect of heat treatment on the surface structure of Pd@Pt–Ni core-shell catalysts for the oxygen reduction reaction. J. Alloys Compd. 2021, 884, 161059. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, K.; Gu, Q.; Jiang, Q.; Qin, J.; Leng, D.; Liu, Q.; Yang, B.; Yin, F. Optimized oxygen reduction activity by tuning shell component in Pd@Pt-based core-shell electrocatalysts. J. Colloid Interface Sci. 2021, 604, 301–309. [Google Scholar] [CrossRef]
- Ferrari, A.; Körmann, F. Design of compositionally complex catalysts: Role of surface segregation. J. Mater. Res. Technol. 2021, 14, 1830–1836. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Watanabe, F.; Honma, T.; Nakamura, I.; Poly, S.S.; Kawaguchi, T.; Tsuji, T.; Murayama, H.; Tokunaga, M.; Fujitani, T. Continuous-flow synthesis of Pd@Pt core-shell nanoparticles. Colloid Surf. A 2021, 620, 126607. [Google Scholar] [CrossRef]
- Akbarzadeh, H.; Mehrjouei, E.; Shamkhali, A.N.; Ramezanzadeh, S.; Abbaspour, M.; Salemi, S. Stability of Pd@void@M (M=Ni, Ag, and Pt) yolk shell nanoparticles controlled by structural factors: A molecular dynamics perspective. Colloid Surf. A 2021, 610, 125920. [Google Scholar] [CrossRef]
- Hang, N.T.N.; Yang, Y.; Nam, N.Q.T.; Nogami, M.; Phuc, L.H.; Long, N.V. Pt-based multimetal electrocatalysts and potential applications: Recent advancements in the synthesis of nanoparticles by modified polyol methods. Crystals 2022, 12, 375. [Google Scholar] [CrossRef]
- Wu, Z.-P.; Caracciolo, D.T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J.A.; Yan, S.; Hopkins, E.; Park, K.; et al. Alloying–realloying enabled high durability for Pt–Pd-3d-transition metal nanoparticle fuel cell catalysts. Nat. Commun. 2021, 12, 859. [Google Scholar] [CrossRef]
- Li, M.; Xia, Z.; Luo, M.; He, L.; Tao, L.; Yang, W.; Yu, Y.; Guo, S. Structural regulation of Pd-based nanoalloys for advanced electrocatalysis. Small Sci. 2021, 1, 2100061. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, F.; Fu, H.; Zeng, L.; Shang, C.; Zhu, L.; Guo, Z. Rational design of Pt-Pd-Ni trimetallic nanocatalysts for room-temperature benzaldehyde and styrene hydrogenation. Chem. Asian J. 2021, 16, 2298–2306. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, B.; Tielens, F.; Guesmi, H. Single Metal atoms embedded in the surface of Pt nanocatalysts: The effect of temperature and hydrogen pressure. Catalysts 2022, 12, 1669. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, J.; Dorakhan, R.; Nie, H.; Fu, G.; Quarantotto, A.; Howe, J.Y.; Chin, Y.-H. Structure and methane oxidation reactivity of bimetallic Pd and Pt nanoparticles. Appl. Catal. A-Gen. 2021, 629, 118290. [Google Scholar] [CrossRef]
- Schütz, J.; Störmer, H.; Lott, P.; Deutschmann, O. Effects of hydrothermal aging on CO and NO oxidation activity over monometallic and bimetallic Pt-Pd catalysts. Catalysts 2021, 11, 300. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Zhang, X.; Rao, S.; Li, J.; Wang, W.; Sun, Z.; Yang, J. Surface structure engineering of PtPd nanoparticles for boosting ammonia oxidation electrocatalysis. ACS Appl. Mater. Interfaces 2022, 14, 28816–28825. [Google Scholar] [CrossRef]
- Sarma, B.B.; Agostini, G.; Farpón, M.G.; Marini, C.; Pfänder, N.; Prieto, G. Bottom-up assembly of bimetallic nanocluster catalysts from oxide-supported single-atom precursors. J. Mater. Chem. A 2021, 9, 8401–8415. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Peres, L.; Collière, V.; Philippot, K.; Lecante, P.; Chen, Y.; Yan, N. Oxidation of methane to methanol over Pd@Pt nanoparticles under mild conditions in water. Catal. Sci. Technol. 2021, 11, 3493–3500. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, Q.; Duan, H.; Song, J.; Yang, H. Janus nanoparticles: From fabrication to (bio)applications. ACS Nano 2021, 15, 6147–6191. [Google Scholar] [CrossRef]
- Song, P.; Wen, D. Molecular dynamics simulation of a core-shell structured metallic nanoparticle. J. Phys. Chem. C 2010, 114, 8688–8696. [Google Scholar] [CrossRef]
- Deng, L.; Hu, W.; Deng, H.; Xiao, S. Surface segregation and structural features of bimetallic Au-Pt nanoparticles. J. Phys. Chem. C 2010, 114, 11026–11032. [Google Scholar] [CrossRef]
- Eom, N.; Messing, M.E.; Johansson, J.; Deppert, K. General trends in core−shell preferences for bimetallic nanoparticles. ACS Nano 2021, 15, 8883–8895. [Google Scholar] [CrossRef] [PubMed]
- Baletto, F.; Mottet, C.; Ferrando, R. Growth of three-shell onionlike bimetallic nanoparticles. Phys. Rev. Lett. 2003, 90, 135504. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, H.; Mehrjouei, E.; Abbaspour, M.; Shamkhali, A.N. Melting behavior of bimetallic and trimetallic nanoparticles: A review of md simulation studies. Top. Curr. Chem. 2021, 379, 22. [Google Scholar] [CrossRef]
- Paz-Borbón, L.O. Computational Studies of Transition Metal Nanoalloys; Springer: Berlin, Germany, 2011; p. 172. [Google Scholar]
- Butler, J.A.V. The thermodynamics of the surfaces of solutions. Proc. R. Soc. A Math. Phys. 1932, 135, 348–375. [Google Scholar]
- Samsonov, V.M.; Romanov, A.A.; Kartoshkin, A.Y.; Talyzin, I.V.; Puytov, V.V. Embedding functions for Pt and Pd: Recalculation and verification on properties of bulk phases, Pt, Pd, and Pt–Pd nanoparticles. Appl. Phys. A 2022, 128, 826. [Google Scholar] [CrossRef]
- Rousset, J.L.; Renouprez, A.J.; Cadrot, A.M. Ion-scattering study and Monte Carlo simulations of surface segregation in Pd-Pt nanoclusters obtained by laser vaporization of bulk alloys. Phys. Rev. B 1998, 58, 2150–2156. [Google Scholar] [CrossRef]
- Fiermans, L.; De Gryse, R.; De Doncker, G.; Jacobs, P.A.; Martensy, J.A. Pd segregation to the surface of bimetallic Pt–Pd particles supported on H-β zeolite evidenced with X-ray photoelectron spectroscopy and argon cation bombardment. J. Catal. 2000, 193, 108–114. [Google Scholar] [CrossRef]
- Bernardi, F.; Alves, M.C.M.; Traverse, A.; Silva, D.O.; Scheeren, C.W.; Dupont, J.; Morais, J. Monitoring atomic rearrangement in PtxPd1−x (x = 1, 0.7, or 0.5) nanoparticles driven by reduction and sulfidation processes. J. Phys. Chem. C 2009, 113, 3909–3916. [Google Scholar] [CrossRef]
- Mendoza-Pérez, R.; Guisbiers, G. Bimetallic Pt-Pd nano-catalyst: Size, shape and composition matter. Nanotechnology 2019, 30, 305702. [Google Scholar] [CrossRef]
- Ramirez Caballero, G.E.; Balbuena, P.B. Surface segregation phenomena in Pt–Pd nanoparticles: Dependence on nanocluster size. Mol. Simulat. 2006, 32, 297–303. [Google Scholar] [CrossRef]
- Wang, L.-L.; Johnson, D.D. Predicted Trends of Core-Shell Preferences for 132 LateTransition-Metal Binary-Alloy Nanoparticles. J. Am. Chem. Soc. 2009, 131, 14023–14029. [Google Scholar] [CrossRef]
- Mélinon, P.; Begin-Colin, S.; Duvail, J.L.; Gauffre, F.; Boime, N.H.; Ledoux, G.; Plain, J.; Reiss, P.; Silly, F.; Warot-Fonrose, B. Engineered inorganic core/shell nanoparticles. Phys. Rep. 2014, 543, 163–197. [Google Scholar] [CrossRef]
- Sutton, A.P.; Chen, J. Long-range Finnis–Sinclair potentials. Philos. Mag. Lett. 1990, 61, 139–146. [Google Scholar] [CrossRef]
- Zhou, X.W.; Johnson, R.A.; Wadley, H.N.G. Misfit-energy-increasing dislocations in vapor-deposited CoFe/NiFe multilayers. Phys. Rev. B 2004, 69, 144113. [Google Scholar] [CrossRef]
- An, H.M.; Zhao, Z.L.; Wang, Q.; Zhang, L.Y.; Gu, M.; Li, C.M. Ternary PtPdCu multicubes as a highly active and enhanced durable catalyst toward oxygen reduction reaction. Chemelectrochem 2018, 5, 1345–1349. [Google Scholar] [CrossRef]
- Akbarzadeh, H.; Mehrjouei, E.; Abbaspour, M.; Salemi, S.; Yaghoubi, H.; Kamrani, M. Thermal behavior of different types of Au–Pt–Pd nanoparticles: Dumbbell-like, three-shell, core-shell, and random-alloy. Mater. Chem. Phys. 2022, 294, 126955. [Google Scholar] [CrossRef]
- Lu, X.-Z.; Shao, G.-F.; Xu, L.-Y.; Liu, T.-D.; Wen, Y.-. H Structural optimization and segregation behavior of quaternary alloy nanoparticles based on simulated annealing algorithm. Chin. Phys. B 2016, 25, 053601. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in‘t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Clarendon Press: Oxford, UK, 1991; p. 641. [Google Scholar]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Grigoriev, S.; Meilikhov, E.Z.; Radzig, A.A. Handbook of Physical Quantities, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997; p. 1568. [Google Scholar]
- Crawley, A.F. Densities of liquid metals and alloys. Int. Metall. Rev. 1974, 19, 32–48. [Google Scholar] [CrossRef]
- Cleri, F.; Rosato, V. Tight-binding potentials for transition metals and alloys. Phys. Rev. B 1993, 48, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Nelli, D.; Ferrando, R.; Yuan, Y.; Li, Z.Y. Shape control of size-selected naked platinum nanocrystals. Nat. Commun. 2021, 12, 3019. [Google Scholar] [CrossRef] [PubMed]
- Nelli, D.; Roncaglia, C.; Ahearn, S.; Di Vece, M.; Ferrando, R.; Minnai, C. Octahedral Growth of PtPd Nanocrystals. Catalysts 2021, 11, 718. [Google Scholar] [CrossRef]
- Samsonov, V.M.; Talyzin, I.V.; Kartoshkin, A.Y.; Vasilyev, S.A. Surface segregation in binary Cu–Ni and Au–Co nanoalloys and the core–shell structure stability/instability: Thermodynamic and atomistic simulations. Appl. Nanosci. 2019, 9, 119–133. [Google Scholar] [CrossRef]
- Samsonov, V.M.; Bembel, A.G.; Kartoshkin, A.Y.; Vasilyev, S.A.; Talyzin, I.V. Molecular dynamics and thermodynamic simulations of segregation phenomena in binary metal nanoparticles. J. Therm. Anal. Calorim. 2018, 133, 1207–1217. [Google Scholar] [CrossRef]
- Samsonov, V.M.; Talyzin, I.V.; Kartoshkin, A.Y.; Vasiliev, S.A.; Alymov, M.I. On the problem of stability/instability of bimetallic core-shell nanostructures: Molecular dynamics and thermodynamic simulations. Comp. Mater. Sci. 2021, 199, 110710. [Google Scholar] [CrossRef]
- Kaptay, G. A coherent set of model equations for various surface and interface energies in systems with liquid and solid metals and alloys. Adv. Colloid Interface Sci. 2020, 283, 102212. [Google Scholar] [CrossRef]
- Kaptay, G. Modelling equilibrium grain boundary segregation, grain boundary energy and grain boundary segregation transition by the extended Butler equation. J. Mater. Sci. 2016, 51, 1738–1755. [Google Scholar] [CrossRef]
- Alchagirov, A.B.; Alchairov, B.B.; Taova, T.M.; Khokonov, K.B. Surface energy and surface tension of solid and liquid metals. Recommended values. Trans. Join. Weld. Res. Inst. Osaka Univ. 2001, 30, 287–291. [Google Scholar]
- Rodríguez-Proenza, C.; Palomares-Báez, J.; Chávez-Rojo, M.; García-Ruiz, A.; Azanza-Ricardo, C.; Santoveña-Uribe, A.; Luna-Bárcenas, G.; Rodríguez-López, J.L.; Esparza, R. Atomic surface segregation and structural characterization of PdPt bimetallic nanoparticles. Materials 2018, 11, 1882. [Google Scholar] [CrossRef]
- Chepkasov, I.V.; Gafner, Y.Y.; Vysotin, M.A.; Redel′, L.V. A study of melting of various types of Pt–Pd nanoparticles. Phys. Solid State 2017, 59, 2076–2081. [Google Scholar] [CrossRef]
- Chepkasov, I.V.; Visotin, M.A.; Kovaleva, E.A.; Manakhov, A.M.; Baidyshev, V.S.; Popov, Z.I. Stability and electronic properties of PtPd nanoparticles via MD and DFT calculations. J. Phys. Chem. C 2018, 122, 18070–18076. [Google Scholar] [CrossRef]
- Nelli, D.; Cerbelaud, M.; Ferrando, R.; Minnai, C. Tuning the coalescence degree in the growth of Pt–Pd nanoalloys. Nanoscale Adv. 2021, 3, 836–846. [Google Scholar] [CrossRef]
- Anderson, R.M.; Zhang, L.; Loussaert, J.A.; Frenkel, A.I.; Henkelman, G.; Crooks, R.M. An experimental and theoretical investigation of the inversion of Pd@Pt Core@Shell dendrimer-encapsulated nanoparticles. ACS Nano 2013, 7, 9345–9353. [Google Scholar] [CrossRef]
- Tománek, D.; Mukherjee, S.; Kumar, V.; Bennemann, K.H. Calculation of chemisorption and absorption induced surface segregation. Surf. Sci. 1982, 114, 11–22. [Google Scholar] [CrossRef]
- Guisbiers, G.; Mendoza-Cruz, R.; Bazán-Díaz, L.; Velázquez-Salazar, J.J.; Mendoza-Perez, R.; Robledo-Torres, J.A.; Rodriguez-Lopez, J.-L.; Montejano-Carrizales, J.M.; Whetten, R.L.; José-Yacamán, M. Electrum, the gold−silver alloy, from the bulk scale to the nanoscale: Synthesis, properties, and segregation rules. ACS Nano 2015, 10, 188–198. [Google Scholar] [CrossRef]
- Samsonov, V.M.; Vasilyev, S.A.; Nebyvalova, K.K.; Talyzin, I.V.; Sdobnyakov, N.Y.; Sokolov, D.N.; Alymov, M.I. Melting temperature and binding energy of metal nanoparticles: Size dependences, interrelation between them, and some correlations with structural stability of nanoclusters. J. Nanopart. Res. 2020, 22, 247. [Google Scholar] [CrossRef]
- Peters, K.F.; Cohen, J.B.; Chung, Y.W. Melting of Pb nanocrystals. Phys. Rev. B 1998, 57, 13430–13438. [Google Scholar] [CrossRef]
- Samsonov, V.M.; Vasilyev, S.A.; Bembel, A.G. Size dependence of the melting temperature of metallic nanoclusters from the viewpoint of the thermodynamic theory of similarity. Phys. Met Metallogr. 2016, 117, 749–755. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Q. Carbon induced segregation of Ni atoms in Cu-Ni alloy. Chem. Phys. Lett. 2022, 806, 139979. [Google Scholar] [CrossRef]
- Nelli, D.; Rossi, G.; Wang, Z.; Palmer, R.E.; Ferrando, R. Structure and orientation effects in the coalescence of Au clusters. Nanoscale 2020, 12, 7688–7699. [Google Scholar] [CrossRef] [PubMed]
- Cserháti, C.; Szabó, I.A.; Beke, D.L. Size effects in surface segregation. J. Appl. Phys. 1998, 83, 3021–3027. [Google Scholar] [CrossRef]
- Brongersma, H.; Sparnaay, M.; Buck, T. Surface segregation in Cu-Ni and Cu-Pt alloys; A comparison of low-energy ion-scattering results with theory. Surf. Sci. 1978, 71, 657–678. [Google Scholar] [CrossRef]
- Yan, X.L.; Wang, J.Y. Size effects on surface segregation in Ni–Cu alloy thin films. Thin Solid Film. 2013, 529, 483–487. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 1948, 40, 345–348. [Google Scholar] [CrossRef]
- Kaptay, G. The exponential excess Gibbs energy model revisited. Calphad 2017, 56, 169–184. [Google Scholar] [CrossRef]

| Metal | ||||||
|---|---|---|---|---|---|---|
| Embedding Functions | Experiment [65] | Embedding Functions | Experiment [66] | |||
| [58] | Our Functions | [58] | Our Functions | |||
| Pt Pd | 21.2 11.8 | 21.1 11.8 | 21.1 12.0 | 19.4 10.5 | 19.6 10.8 | 18.9 10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsonov, V.; Romanov, A.; Talyzin, I.; Lutsay, A.; Zhigunov, D.; Puytov, V. Puzzles of Surface Segregation in Binary Pt–Pd Nanoparticles: Molecular Dynamics and Thermodynamic Simulations. Metals 2023, 13, 1269. https://doi.org/10.3390/met13071269
Samsonov V, Romanov A, Talyzin I, Lutsay A, Zhigunov D, Puytov V. Puzzles of Surface Segregation in Binary Pt–Pd Nanoparticles: Molecular Dynamics and Thermodynamic Simulations. Metals. 2023; 13(7):1269. https://doi.org/10.3390/met13071269
Chicago/Turabian StyleSamsonov, Vladimir, Alexander Romanov, Igor Talyzin, Alexander Lutsay, Dmitriy Zhigunov, and Vladimir Puytov. 2023. "Puzzles of Surface Segregation in Binary Pt–Pd Nanoparticles: Molecular Dynamics and Thermodynamic Simulations" Metals 13, no. 7: 1269. https://doi.org/10.3390/met13071269
APA StyleSamsonov, V., Romanov, A., Talyzin, I., Lutsay, A., Zhigunov, D., & Puytov, V. (2023). Puzzles of Surface Segregation in Binary Pt–Pd Nanoparticles: Molecular Dynamics and Thermodynamic Simulations. Metals, 13(7), 1269. https://doi.org/10.3390/met13071269




