Precipitation Behavior of the Second Phase in High-Nitrogen Austenitic Stainless Steel Alloyed with V and Nb
Abstract
:1. Introduction
2. Materials and Experimental Methods
3. Results
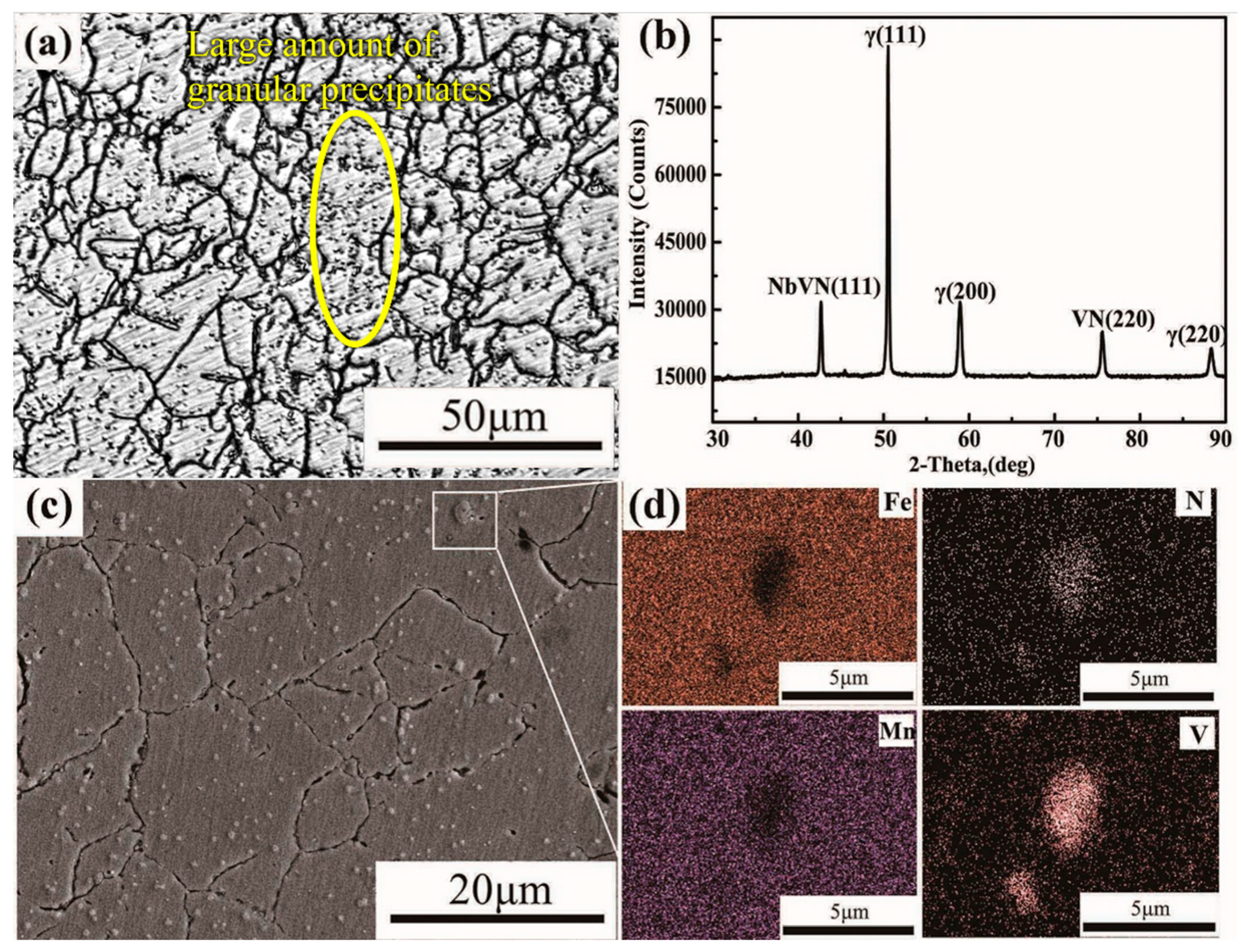
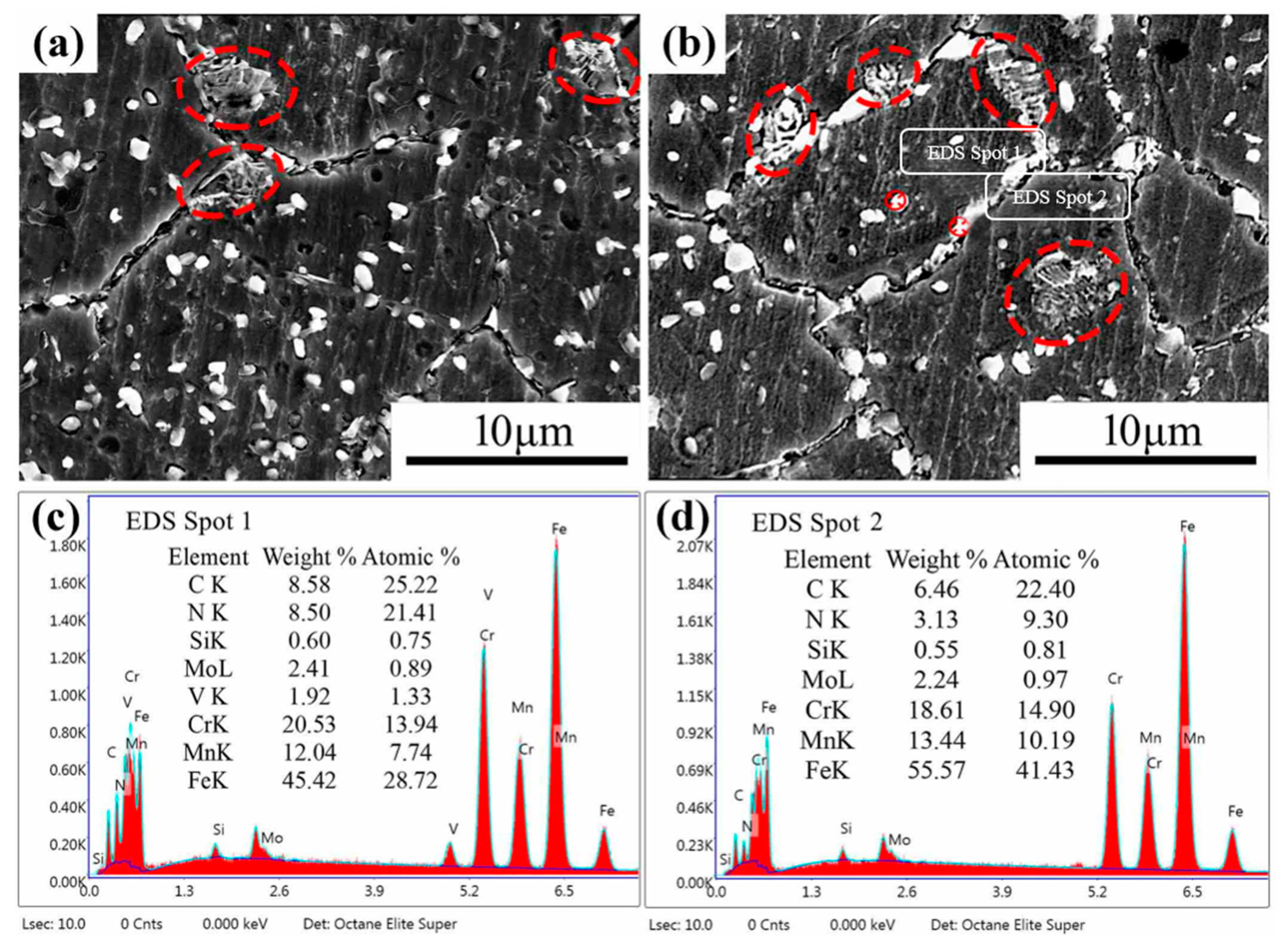
3.1. The Second Phase of the Original HNASS before Heat Treatment
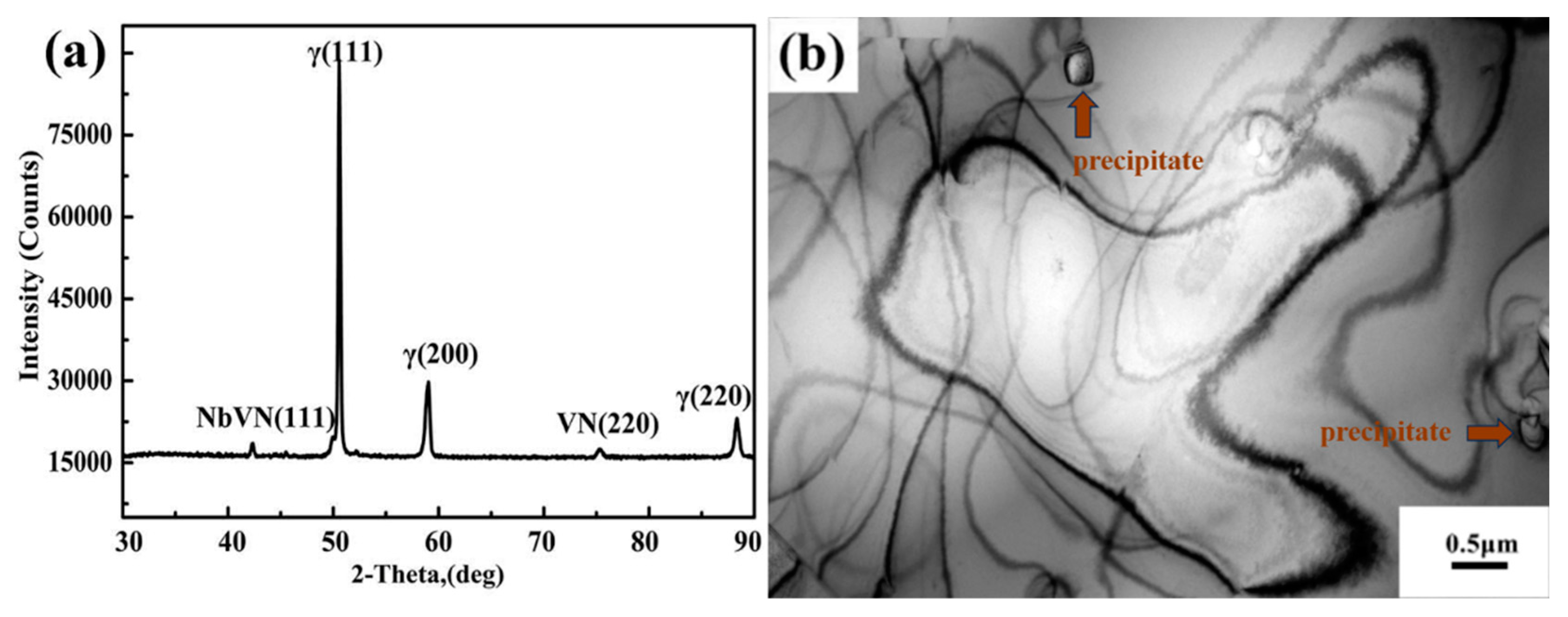
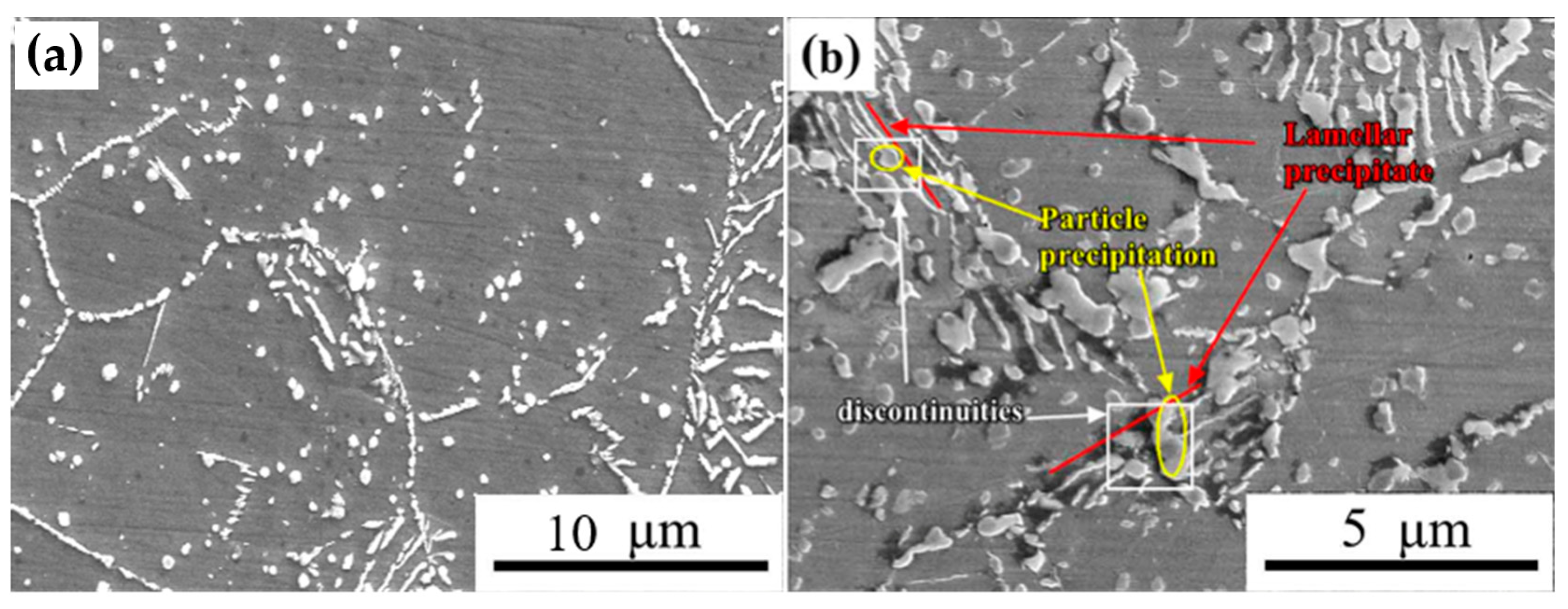
3.2. The Second Phase in Solution-Treated HNASS
3.3. Precipitated Phases in Aging HNASS
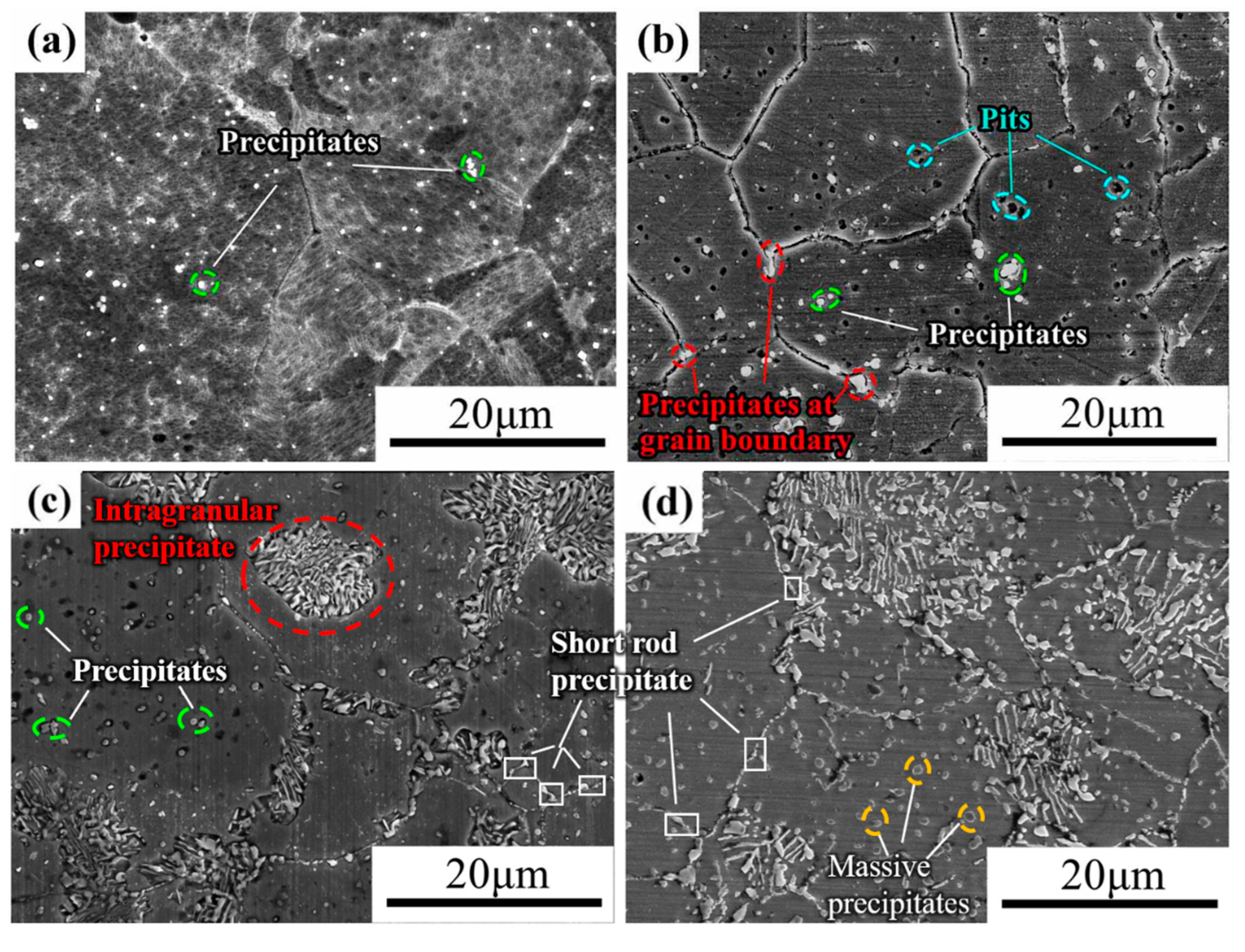
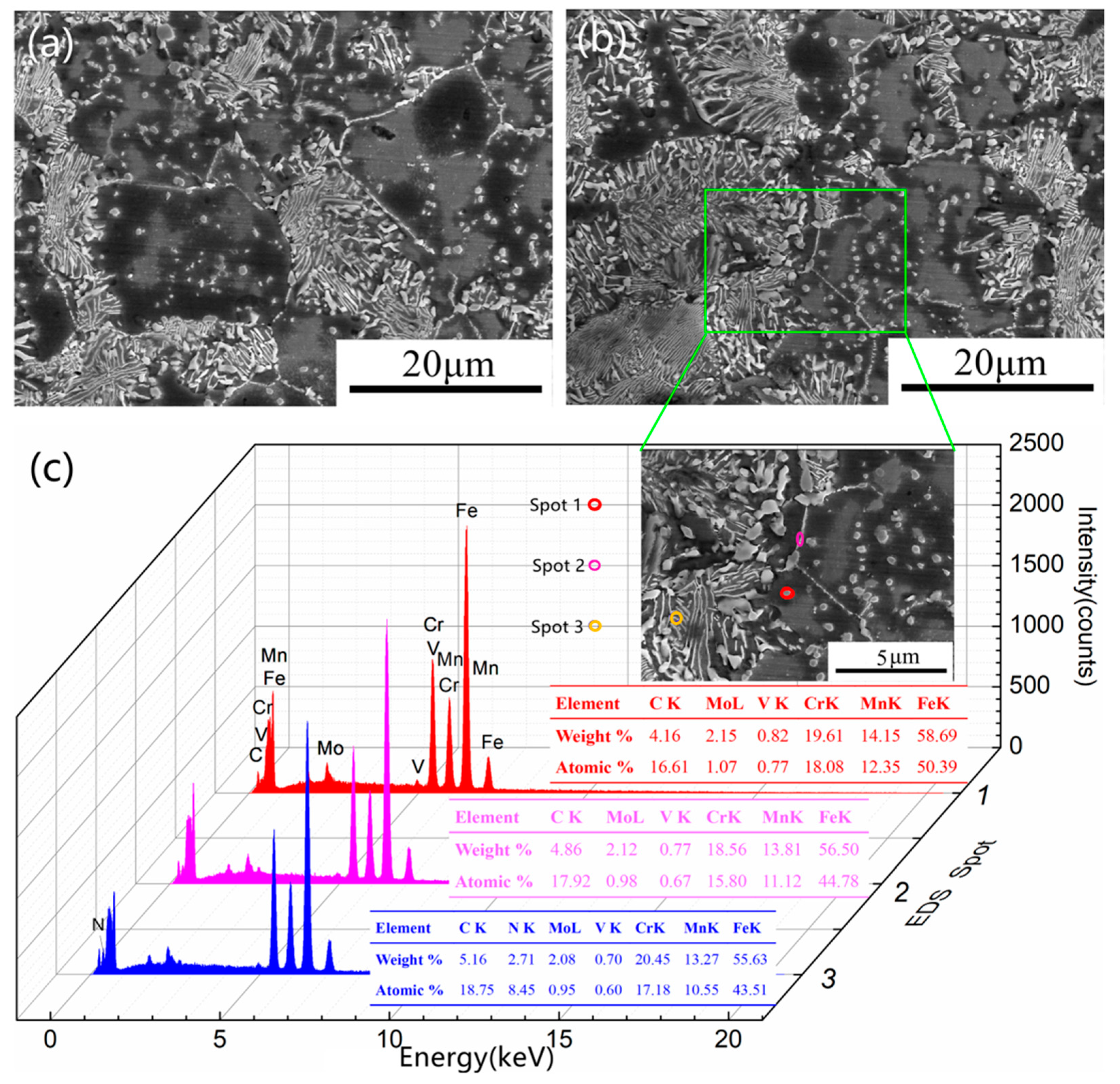
3.3.1. Precipitated Phase after 3 h of Aging
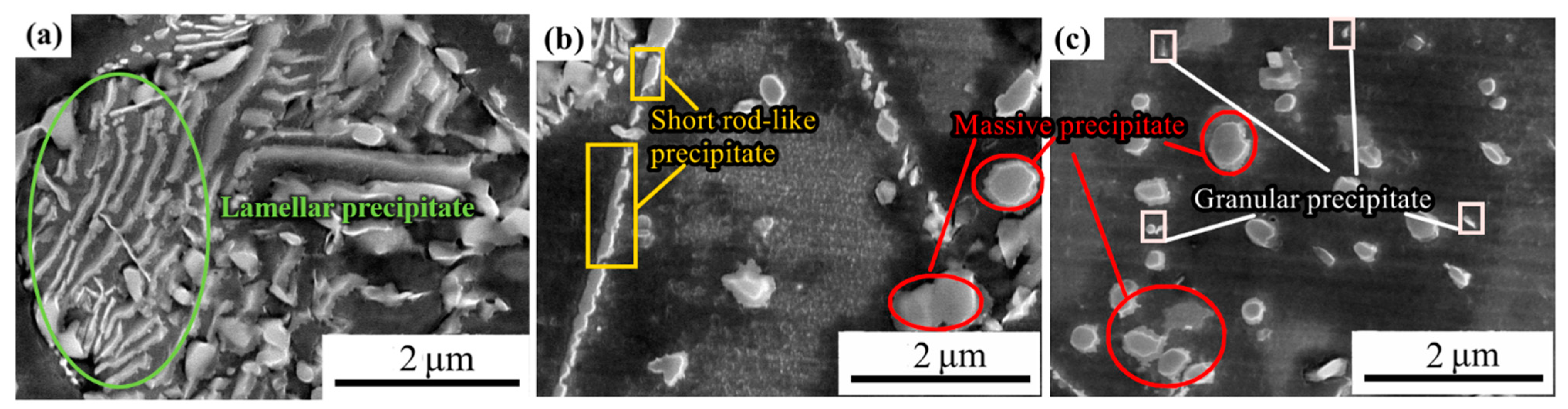
3.3.2. Precipitated Phase after 5 h of Aging
4. Discussion
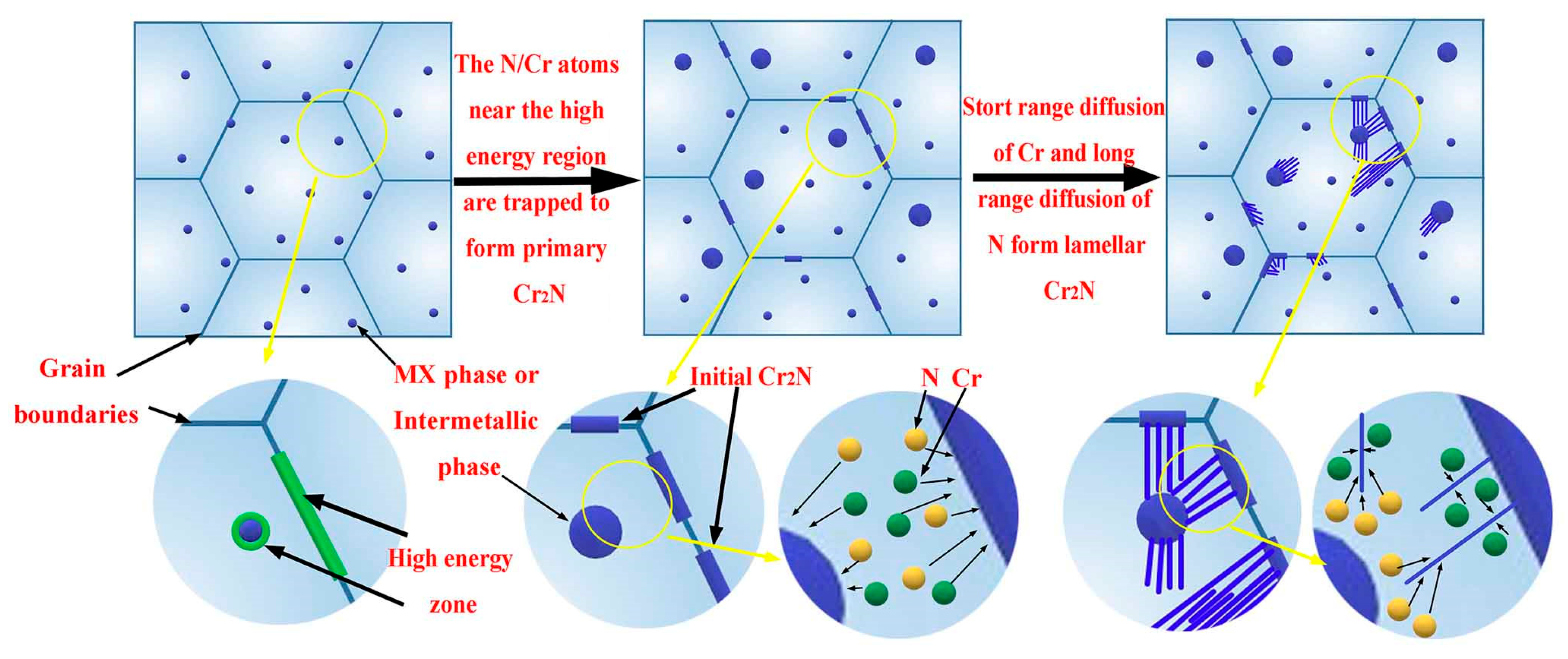
4.1. Characteristics and the Mechanism of Formation of Precipitates
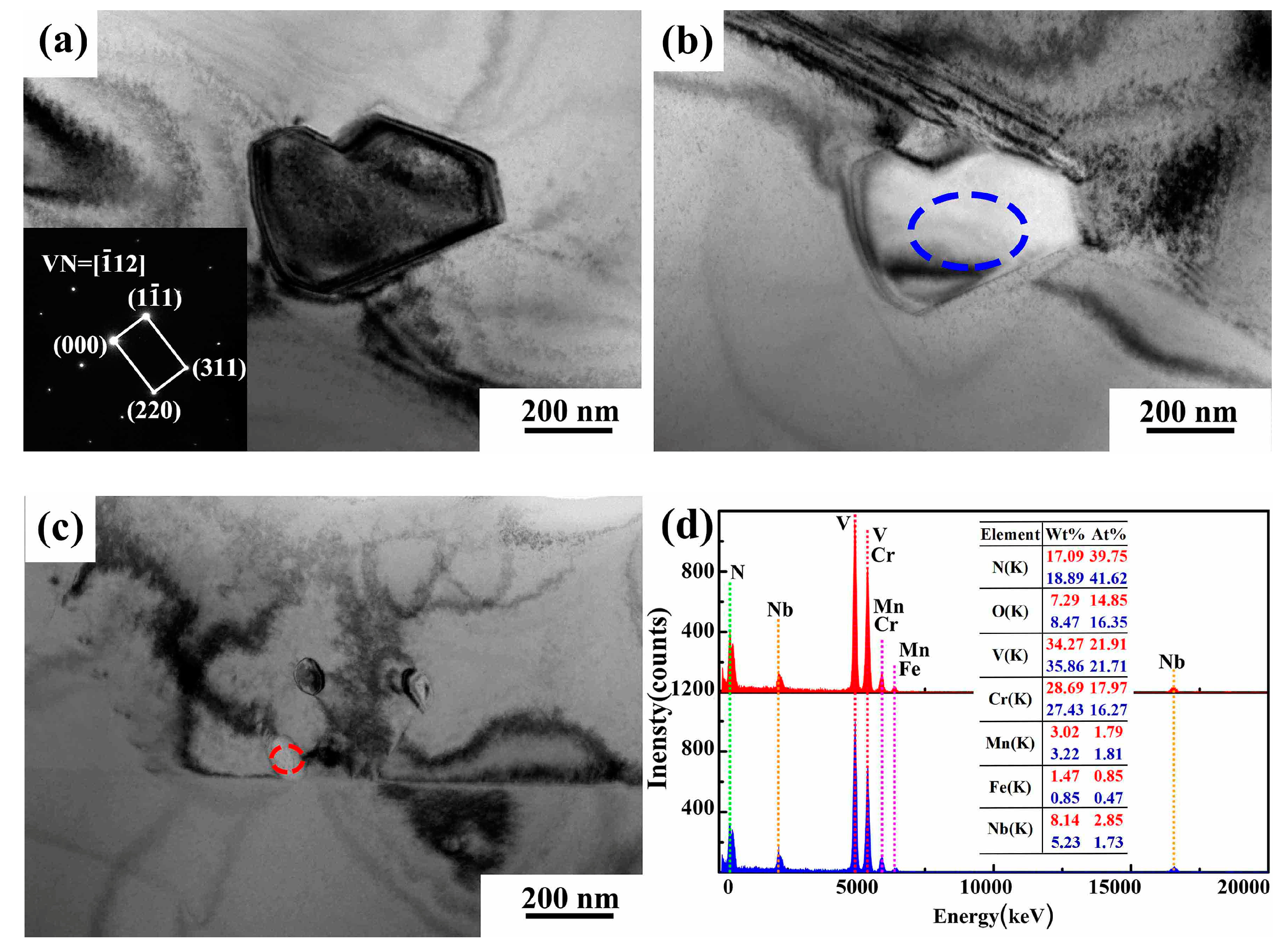
4.1.1. MX Phase
4.1.2. M2X Phase
4.1.3. Intermetallic Phase
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Toshihiro, A.; Lee, D.S.; Nie, Y. Bio-medical Materials. In Abstracts of IUMRS, Proceedings of the 11th International Conference in Asia (IUMRS-ICA2010), Qingdao, China, 25–28 September 2010; Chinese Society for Materials Research: Beijing, China, 2010; pp. 369–391. [Google Scholar]
- Gavriljuk, V.G.; Berns, H. High Nitrogen Steels: Structure, Properties, Manufacture, Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999. [Google Scholar] [CrossRef]
- Di Schino, A.; Kenny, J.M.; Mecozzi, M.G.; Barteri, M. Development of high nitrogen, low nickel, 18%Cr austenitic stainless steels. J. Mater. Sci. 2000, 35, 4803–4808. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.; Wang, Y.; Yin, T.; Gregersen, H.; Zhang, X.; Liao, X.; Wang, G. Immobilization of heparin/poly-l-lysine microspheres on medical grade high nitrogen nickel-free austenitic stainless steel surface to improve the biocompatibility and suppress thrombosis. Mater. Sci. Eng. C 2017, 73, 198–205. [Google Scholar] [CrossRef]
- Singh, B.B.; Sukumar, G.; Rao, P.P.; Kumar, K.S.; Madhu, V.; Kumar, R.A. Superior ballistic performance of high-nitrogen steels against deformable and non-deformable projectiles. Mater. Sci. Eng. A 2019, 751, 115–127. [Google Scholar] [CrossRef]
- Ichii, K.; Ueda, N.; Nakata, K. Low Temperature Ion-Nitriding of Austenitic High Nitrogen P900N Mo and X3MnCrNiMo16-16-16-4 Steel. In High Nitrogen Steel International Conference Papers; Dong, J., Su, J., Speidel, M.O., Eds.; Metallurgical Industry Press: Beijing, China, 2006; pp. 452–457. [Google Scholar]
- Kauss, N.; Heyn, A.; Michael, O.; Schymura, M.; Rosemann, P. Application limits and sensitisation behaviour of the manganese- and nitrogen-alloyed austenitic stainless steel P2000 (X13CrMnMoN18-14-3). Mater. Corros. 2021, 72, 1656–1667. [Google Scholar] [CrossRef]
- Xie, J.; Qu, X.; Jie, W.; Liu, X. Advanced Processing Technologies of Materials. In Abstracts of IUMRS, Proceedings of the 11th International Conference in Asia(IUMRS-ICA2010), Qingdao, China, 25–28 September 2010; Trans Tech Publications: Durnten, Switzerland, 2011; pp. 150–182. [Google Scholar]
- Luo, C.; Zhang, J.; Wen, J. Study on properties of aluminum alloy welded by friction stir welding. In Proceedings of the 2019 2nd International Conference on Advanced Electronic Materials, Computers and Materials Engineering (AEMCME 2019), Changsha, China, 19–21 April 2019; IOP Publishing: Bristol, UK, 2019; pp. 290–294. [Google Scholar]
- Li, H.-B.; Jiang, Z.-H.; Feng, H.; Ma, Q.-F.; Zhan, D.-P. Aging Precipitation Behavior of 18Cr-16Mn-2Mo-1.1N High Nitrogen Austenitic Stainless Steel and Its Influences on Mechanical Properties. J. Iron Steel Res. Int. 2012, 19, 43–51. [Google Scholar] [CrossRef]
- Lee, T.-H.; Kim, S.-J.; Shin, E.; Takaki, S. On the crystal structure of Cr2N precipitates in high-nitrogen austenitic stainless steel. III. Neutron diffraction study on the ordered Cr2N superstructure. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 979–986. [Google Scholar] [CrossRef]
- Ma, Y.; Rong, F.; Zhou, R.; Lang, Y.; Jiang, Y. Study on precipitation in austenitic stainless steel 18Cr18Mn2MoN during isothermal aging at 900 °C. Iron Steel 2007, 42, 64–68. (In Chinese) [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Y.; Wang, X.; Zhou, Y.; Ma, J.; Zhang, S. Corrosion Study on Ni-based Alloy in Acidic Environment. In Proceedings of the 2019 International Conference on Advanced Material Research and Processing Technology (AMRPT 2019), Wuhan, China, 19–21 July 2019; IOP Publishing: Bristol, UK, 2019; pp. 64–69. [Google Scholar]
- Xu, Y.; Yang, Y. Microstructure and Corrosion Properties 0Cr21Mn17Mo2N0.8 High Nitrogen Austenitic Stainless Steel. In Proceedings of the 2018 4th International Conference on Applied Materials and Manufacturing Technology (ICAMMT 2018), Nanchang, China, 25–27 May 2018; IOP Publishing: Bristol, UK, 2018; pp. 302–308. [Google Scholar]
- Hong, C.-M.; Shi, J.; Sheng, L.-Y.; Cao, W.-Q.; Hui, W.; Dong, H. Influence of hot working on microstructure and mechanical behavior of high nitrogen stainless steel. J. Mater. Sci. 2011, 46, 5097–5103. [Google Scholar] [CrossRef]
- Hu, G.; Wang, P.; Li, D.; Li, Y. Effects of nitrogen on precipitation and tensile behaviors of 25Cr 20Ni austenitic stainless steels at elevated temperatures. Mater. Sci. Eng. A 2019, 752, 93–100. [Google Scholar] [CrossRef]
- Hu, G.; Wang, P.; Li, D.; Li, Y. High-temperature Tensile Behavior in Coarse-grained and Fine-grained Nb-containing 25Cr–20Ni Austenitic Stainless Steel. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 1455–1465. [Google Scholar] [CrossRef]
- Fang, F.; Li, J.; Wang, Y. Characteristics and forming mechanism of precipitates in 18Mn18Cr high nitrogen steel. J. Univ. Sci. Technol. Beijing 2014, 36, 768–779. (In Chinese) [Google Scholar] [CrossRef]
- Qin, F.; Li, Y.; Zhao, X.; He, W.; Chen, H. Effect of nitrogen content on precipitation behavior and mechanical properties of Mn18Cr18N austenitic stainless steel. Acta Metall. Sin. 2018, 54, 55–64. (In Chinese) [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Zhang, G.; Qin, R.; Liu, W.; Xiong, H.; Shi, G.; Blackburn, J. Progress in additive manufacturing on new materials: A review. J. Mater. Sci. Technol. 2019, 35, 242–269. [Google Scholar] [CrossRef]
- Kamado, S.; Shin, K.S. Magnesium(Fourth International Conference on Magnesium). In Proceedings of the 11th International Conference in Asia (IUMRS-ICA2010), Qingdao, China, 25–28 September 2010; pp. 62–107. [Google Scholar]
- Shi, F.; Wang, L.; Cui, W.; Liu, C. Precipitation behavior of M2N in a high-nitrogen austenitic stainless steel during isothermal aging. Acta Met. Sin. (Engl. Lett.) 2007, 20, 95–101. [Google Scholar] [CrossRef]
- Kim, K.-S.; Kang, J.-H.; Kim, S.-J. Effects of carbon and nitrogen on precipitation and tensile behavior in 15Cr-15Mn-4Ni austenitic stainless steels. Mater. Sci. Eng. A 2018, 712, 114–121. [Google Scholar] [CrossRef]
- Kartik, B.; Veerababu, R.; Sundararaman, M.; Satyanarayana, D. Effect of high temperature ageing on microstructure and mechanical properties of a nickel-free high nitrogen austenitic stainless steel. Mater. Sci. Eng. A 2015, 642, 288–296. [Google Scholar] [CrossRef]
- Hua, J.; Wang, F.; Xue, X.; Ding, Z.; Chen, Z. Residual monotonic mechanical properties of bimetallic steel bar with fatigue damage. J. Build. Eng. 2022, 55, 104703. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Bao, L.; Gu, Y. Research Status and Control Measures of Transverse Cracks for Micro-alloyed Continuous Casting Billet. In Proceedings of the 4th International Conference on Manufacturing, Material and Metallurgical Engineering (ICMMME 2019), Chengdu, China, 22–25 March 2019; IOP Publishing: Bristol, UK, 2019; pp. 9–13. [Google Scholar]
- Xu, Y.; Li, W.; Wang, M.; Zhang, X.; Wu, Y.; Min, N.; Liu, W.; Jin, X. Nano-sized MX carbonitrides contribute to the stability of mechanical properties of martensite ferritic steel in the later stages of long-term aging. Acta Mater. 2019, 175, 148–159. [Google Scholar] [CrossRef]
- Characterization and measurement of nanostructure. In Abstracts Book of China International Conference on Nanoscience & Technology; Bulletin of Chinese Academic of Science: Beijing, China, 2005; pp. 199–260.
- Xu, W.; Sun, F. The Fine structure of Nb and V precipitates in Nb-V steel. Acta Metall. Sin. 1983, 6, 29–35, 117, 118. Available online: https://www.ams.org.cn/EN/abstract/abstract12108.shtml (accessed on 30 June 1983).
- Park, D.-B.; Huh, M.-Y.; Jung, W.-S.; Suh, J.-Y.; Shim, J.-H.; Lee, S.-C. Effect of vanadium addition on the creep resistance of 18Cr9Ni3CuNbN austenitic stainless heat resistant steel. J. Alloys Compd. 2013, 574, 532–538. [Google Scholar] [CrossRef]
- Erisir, E.; Prahl, U.; Bleck, W. Effect of precipitation on hot formability of high nitrogen steels. Mater. Sci. Eng. A 2010, 528, 519–525. [Google Scholar] [CrossRef]
- Wu, S.; Pan, T.; Cao, J.; He, D.; Xiao, Z. Barrier–corridor effect of longitudinal range–gorge terrain on monsoons in Southwest China. Geogr. Res. 2012, 31, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.D.; Wu, D.; Qu, W.Q.; Zhang, Z.F.; Zhang, W.F.; Gan, Y.; Long, S.G.; Wang, F.P.; Zhang, Z. Materials Characterization and Evaluation. In Abstracts of IUMRS, Proceedings of the 11th International Conference in Asia (IUMRS-ICA2010), Qingdao, China, 25–28 September 2010; Chinese Society for Materials Research: Beijing, China, 2010; pp. 417–437. [Google Scholar]
- Zhang, S.; Li, H.; Jiang, Z.; Li, Z.; Wu, J.; Zhang, B.; Duan, F.; Feng, H.; Zhu, H. Influence of N on precipitation behavior, associated corrosion and mechanical properties of super austenitic stainless steel S32654. J. Mater. Sci. Technol. 2020, 42, 143–155. [Google Scholar] [CrossRef]
- Kikuchi, M.; Kajihara, M.; Choi, S.-K. Cellular precipitation involving both substitutional and interstitial solutes: Cellular precipitation of Cr2N in Cr Ni austenitic steels. Mater. Sci. Eng. A 1991, 146, 131–150. [Google Scholar] [CrossRef]
- Li, X.; Hu, S. Research Progress of Springback in Multi-Point Forming of Sheet Metal. In Proceedings of the 2021 2nd International Conference on Mechanical Engineering and Materials (ICMEM 2021), Beijing, China, 19–20 November 2021; IOP Publishing: Bristol, UK, 2021; pp. 59–68. [Google Scholar]
- Lee, T.-H.; Oh, C.-S.; Gil Lee, C.; Kim, S.-J.; Takaki, S. Precipitation of σ-phase in high-nitrogen austenitic 18Cr–18Mn–2Mo–0.9N stainless steel during isothermal aging. Scr. Mater. 2004, 50, 1325–1328. [Google Scholar] [CrossRef]
- Lee, T.-H.; Kim, S.-J.; Takaki, S. Time-temperature-precipitation characteristics of high-nitrogen austenitic Fe−18Cr−18Mn−2Mo−0.9N steel. Met. Mater. Trans. A 2006, 37, 3445–3454. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Z.; Li, H.; Zhang, S.; Feng, H.; Li, H. Precipitation behavior and phase transformation of hyper duplex stainless steel UNS S32707 at nose temperature. Mater. Charact. 2017, 129, 31–39. [Google Scholar] [CrossRef]
- Maetz, J.-Y.; Douillard, T.; Cazottes, S.; Verdu, C.; Kléber, X. M23C6 carbides and Cr2N nitrides in aged duplex stainless steel: A SEM, TEM and FIB tomography investigation. Micron 2016, 84, 43–53. [Google Scholar] [CrossRef]
- Han, J.; Li, H.; Barbaro, F.; Jiang, L.; Zhu, Z.; Xu, H.; Ma, L. Precipitation and impact toughness of Nb–V stabilised 18Cr–2Mo ferritic stainless steel during isothermal aging. Mater. Sci. Eng. A 2014, 612, 63–70. [Google Scholar] [CrossRef]
- Xiao, C.; Feng, Q.; Chang, Y.J.O.; Liu, L.; Wu, Y. Advanced High Temperature Structural Materials. In Abstracts of IUMRS, Proceedings of the 11th International Conference in Asia (IUMRS-ICA2010), Qingdao, China, 25–28 September 2010; Trans Tech Publications: Durnten, Switzerland, 2011; pp. 108–132. [Google Scholar]
| Element | C | Si | Mn | Cr | Mo | Nb | V | N | S | P | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 0.06 | 0.63 | 15.66 | 18.07 | 3.02 | 0.07 | 0.66 | 1.02 | 0.005 | 0.009 | margin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Song, W.; Yang, C.; Zhao, X.; Liu, Q.; Zhang, Y.; Chen, L. Precipitation Behavior of the Second Phase in High-Nitrogen Austenitic Stainless Steel Alloyed with V and Nb. Metals 2023, 13, 1183. https://doi.org/10.3390/met13071183
Zhang R, Song W, Yang C, Zhao X, Liu Q, Zhang Y, Chen L. Precipitation Behavior of the Second Phase in High-Nitrogen Austenitic Stainless Steel Alloyed with V and Nb. Metals. 2023; 13(7):1183. https://doi.org/10.3390/met13071183
Chicago/Turabian StyleZhang, Ronghua, Wenzhi Song, Chuan Yang, Xin Zhao, Qian Liu, Yuan Zhang, and Liansheng Chen. 2023. "Precipitation Behavior of the Second Phase in High-Nitrogen Austenitic Stainless Steel Alloyed with V and Nb" Metals 13, no. 7: 1183. https://doi.org/10.3390/met13071183
APA StyleZhang, R., Song, W., Yang, C., Zhao, X., Liu, Q., Zhang, Y., & Chen, L. (2023). Precipitation Behavior of the Second Phase in High-Nitrogen Austenitic Stainless Steel Alloyed with V and Nb. Metals, 13(7), 1183. https://doi.org/10.3390/met13071183






