Dendritic Solidification and Physical Properties of Co-4.54%Sn Alloy with Broad Mushy Zone
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
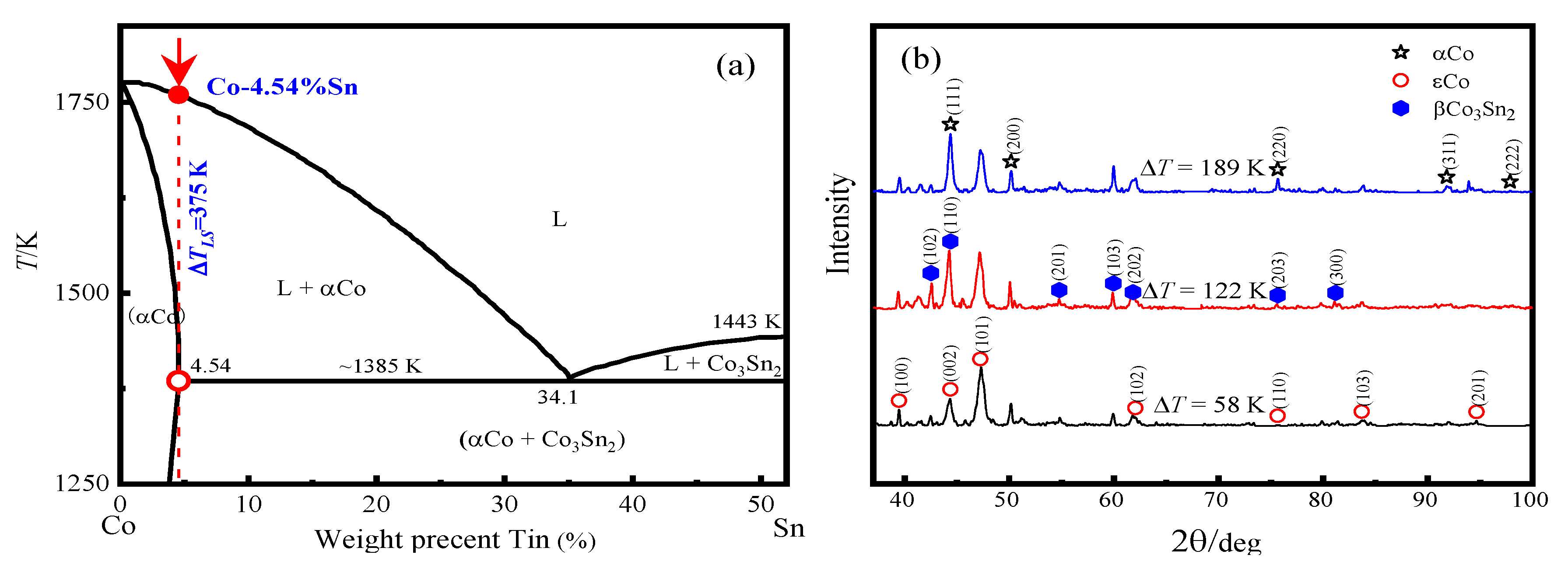
3.1. Rapid Solidification Structures
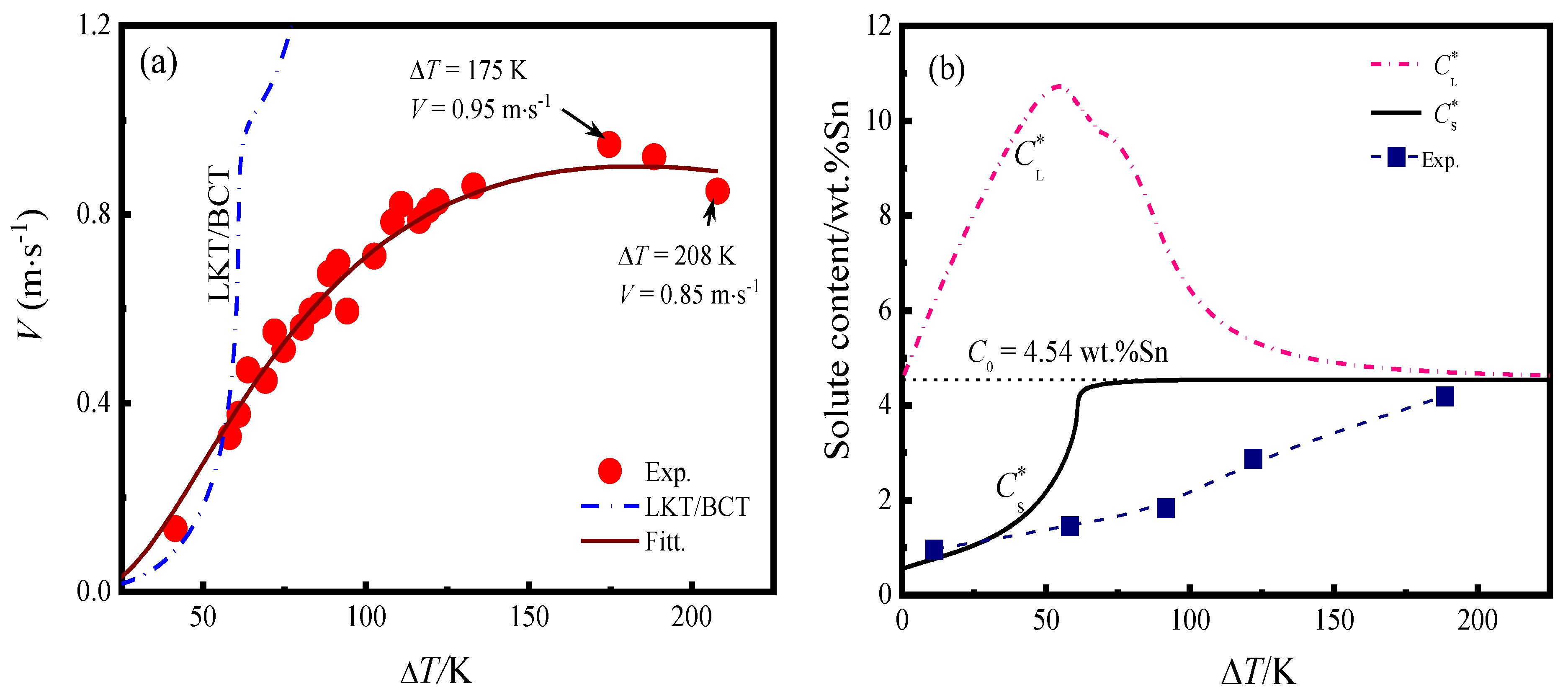
3.2. Dendritic Growth Kinetics
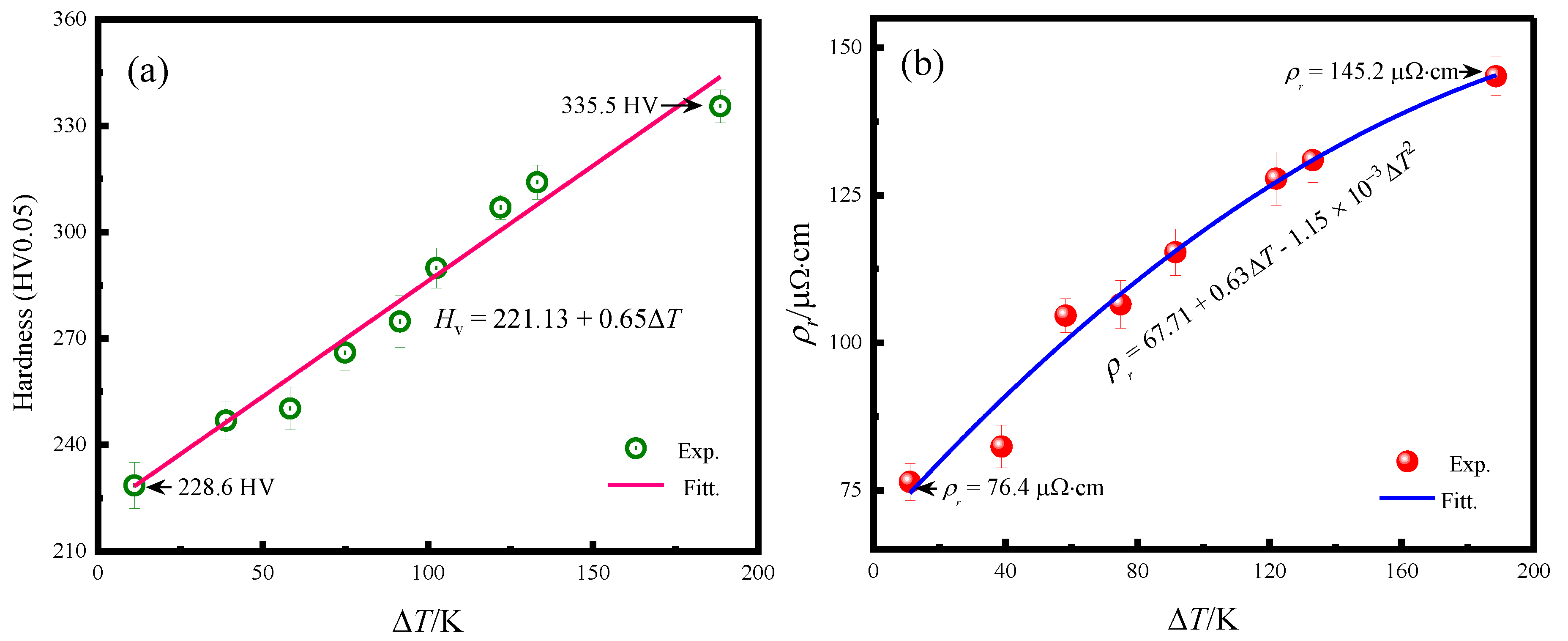
3.3. Microhardness and Electrical Resistivity
3.4. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sistaninia, M.; Terzi, S.; Phillion, A.; Drezet, J.-M.; Rappaz, M. 3-D granular modeling and in situ X-ray tomographic imaging: A comparative study of hot tearing formation and semi-solid deformation in Al–Cu alloys. Acta Mater. 2013, 61, 3831–3841. [Google Scholar] [CrossRef]
- Eskin, D.G.; Suyitno; Katgerman, L. Mechanical properties in the semi-solid state and hot tearing of aluminium alloys. Prog. Mater. Sci. 2004, 49, 629–711. [Google Scholar] [CrossRef]
- Kou, S. A criterion for cracking during solidification. Acta Mater. 2015, 88, 366–374. [Google Scholar] [CrossRef]
- Bordreuil, C.; Niel, A. Modelling of hot cracking in welding with a cellular automaton combined with an intergranular fluid flow model. Comput. Mater. Sci. 2013, 82, 442–450. [Google Scholar] [CrossRef]
- Rajani, H.Z.; Phillion, A. 3-D multi-scale modeling of deformation within the weld mushy zone. Mater. Des. 2016, 94, 536–545. [Google Scholar] [CrossRef]
- Wang, W.; Shen, C.; Luo, B.; Wei, B. Sluggish dendrite growth in substantially undercooled liquid Fe–Sb alloy. Philos. Mag. Lett. 2009, 89, 409–418. [Google Scholar] [CrossRef]
- Ramirez-Ledesma, A.L.; Lopez-Molina, E.; Lopez, H.F.; Juarez-Islas, J.A. Athermal ε-martensite transformation in a Co20Cr alloy: Effect of rapid solidification on plate nucleation. Acta Mater. 2016, 111, 138–147. [Google Scholar] [CrossRef]
- Spinelli, J.E.; Silva, B.L.; Garcia, A. Microstructure, phases morphologies and hardness of a Bi–Ag eutectic alloy for high temperature soldering applications. Mater. Des. 2014, 58, 482–490. [Google Scholar] [CrossRef]
- Wang, H.P.; Yao, W.J.; Wei, B. Remarkable solute trapping within rapidly growing dendrites. Appl. Phys. Lett. 2006, 89, 201905. [Google Scholar] [CrossRef]
- Kundin, J.; Mushongera, L.; Emmerich, H. Phase-field modeling of microstructure formation during rapid solidification in Inconel 718 superalloy. Acta Mater. 2015, 95, 343–356. [Google Scholar] [CrossRef]
- Lü, P.; Wang, H. Observation of the transition from primary dendrites to coupled growth induced by undercooling within Ni Zr hyperperitectic alloy. Scr. Mater. 2017, 137, 31–35. [Google Scholar] [CrossRef]
- Wang, W.L.; Hu, L.; Yang, S.J.; Wang, A.; Wang, L.; Wei, B. Liquid Supercoolability and Synthesis Kinetics of Quinary Refractory High-entropy Alloy. Sci. Rep. 2016, 16, 6. [Google Scholar] [CrossRef]
- Oloyede, O.; Cochrane, R.F.; Mullis, A.M. Effect of rapid solidification on the microstructure and microhardness of BS1452 grade 250 hypoeutectic grey cast iron. J. Alloys Compd. 2017, 707, 347–350. [Google Scholar] [CrossRef]
- Yakymovych, A.; Shtablavyi, I.; Mudry, S. Structural studies of liquid Co–Sn alloys. J. Alloys Compd. 2014, 610, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Sato, J.; Ohnuma, I.; Kainuma, R.; Ishida, K. A thermodynamic assessment of the Co–Sn system. Calphad 2004, 28, 213–220. [Google Scholar] [CrossRef]
- Fan, Q.; Chupas, P.J.; Whittingham, M.S. Characterization of Amorphous and Crystalline Tin–Cobalt Anodes. Electrochem. Solid-State Lett. 2007, 10, A274–A278. [Google Scholar] [CrossRef]
- Qiu, X.X.; Li, J.S.; Wang, J.; Guo, T.; Kou, H.C. Eric Beaugnon, Effect of liquideliquid structure transition on the nucleation in undercooled Co-Sn eutectic alloy. Mater. Chem. Phys. 2016, 170, 261–265. [Google Scholar] [CrossRef]
- Yakymovych, A.; Plevachuk, Y.; Mudry, S.; Brillo, J.; Kobatake, H.; Ipser, H. Viscosity of liquid Co–Sn alloys: Thermodynamic evaluation and experiment. Phys. Chem. Liq. 2013, 52, 562–570. [Google Scholar] [CrossRef]
- Yakymovych, A.; Fürtauer, S.; Elmahfoudi, A.; Ipser, H.; Flandorfer, H. Enthalpy of mixing of liquid Co–Sn alloys. J. Chem. Thermodyn. 2014, 74, 269–285. [Google Scholar] [CrossRef]
- Lipton, J.; Kurz, W.; Trivedi, R. Rapid dendrite growth in undercooled alloys. Acta Metall. 1987, 35, 957–964. [Google Scholar] [CrossRef]
- Boetinger, W.J.; Coriell, S.R.; Trivedi, R. Rapid Solidification Processing: Principles and Technoligies. In Proceedings of the Third Conference on Rapid Solidification Processing, Gaithersburg, MD, USA, 6–8 December 1982; p. 13. [Google Scholar]
- Cao, C.-D.; Lu, X.-Y.; Wei, B.-B. Solute Diffusion Controlled Dendritic Growth Under High Undercooling Conditions. Chin. Phys. Lett. 1998, 15, 840–842. [Google Scholar] [CrossRef]
- Lu, P.; Wang, H.P. Effects of undercooling and cooling rate on peritectic phase crystallization within Ni-Zr alloy melt. Metall. Mater. Trans. B-Proc. Metall. Mater. Proc. Sci. 2018, 49, 499–508. [Google Scholar] [CrossRef]
- Chou, H.P.; Chang, Y.-S.; Chen, S.-K.; Yeh, J.W. Microstructure, thermophysical and electrical properties in AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. Mater. Sci. Eng. B 2009, 63, 184–189. [Google Scholar] [CrossRef]
- Hamzaoui, R.; Elkedim, O.; Gaffet, E. Friction mode and shock mode effect on magnetic propeties of mechanically alloyed Fe-based nanocrystalline materials. J. Mater. Sci. 2004, 39, 5139–5142. [Google Scholar] [CrossRef]
- Billoni, O.V.; Bordone, E.E.; Urreta, S.E.; Fabietti, L.M.; Bertorello, H.R. Magnetic viscosity in a nanocrystalline two phase composite with enhanced remanence. J. Magn. Magn. Mater. 2000, 208, 1–12. [Google Scholar] [CrossRef]
- Amils, X.; Garitaonandia, J.; Nogués, J.; Suriñach, S.; Plazaola, F.; Muñoz, J.; Baró, M. Micro- and macroscopic magnetic study of the disordering (ball milling) and posterior reordering (annealing) of Fe-40 at.% Al. J. Non-Cryst. Solids 2001, 287, 272–276. [Google Scholar] [CrossRef]
- Herzer, G. Grain structure and magnetism of nanocry-stalline ferromagnets. IEEE Trans. Magn. 1989, 25, 3327–3329. [Google Scholar] [CrossRef]
- Han, L.; Maccari, F.; Filho, I.R.S.; Peter, N.J.; Wei, Y.; Gault, B.; Gutfleisch, O.; Li, Z.; Raabe, D. A mechanically strong and ductile soft magnet with extremely low coercivity. Nature 2022, 608, 310–316. [Google Scholar] [CrossRef]



| ΔT | Ms (emu/g) | Mr (emu/g) | Hc (kA/m) | Mr/Ms |
|---|---|---|---|---|
| 11K | 178.92 | 3.04 | 7.3 | 0.017 |
| 58K | 158.40 | 3.91 | 20.81 | 0.025 |
| 122K | 156.49 | 7.63 | 24.84 | 0.049 |
| 189K | 149.94 | 2.01 | 12.42 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, W.; Wang, A. Dendritic Solidification and Physical Properties of Co-4.54%Sn Alloy with Broad Mushy Zone. Metals 2023, 13, 1046. https://doi.org/10.3390/met13061046
Wang W, Li W, Wang A. Dendritic Solidification and Physical Properties of Co-4.54%Sn Alloy with Broad Mushy Zone. Metals. 2023; 13(6):1046. https://doi.org/10.3390/met13061046
Chicago/Turabian StyleWang, Weili, Wenhui Li, and Ao Wang. 2023. "Dendritic Solidification and Physical Properties of Co-4.54%Sn Alloy with Broad Mushy Zone" Metals 13, no. 6: 1046. https://doi.org/10.3390/met13061046
APA StyleWang, W., Li, W., & Wang, A. (2023). Dendritic Solidification and Physical Properties of Co-4.54%Sn Alloy with Broad Mushy Zone. Metals, 13(6), 1046. https://doi.org/10.3390/met13061046





