Oxidation Property of a Fourth-Generation Powder Metallurgy FGH4108 Nickel-Based Superalloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
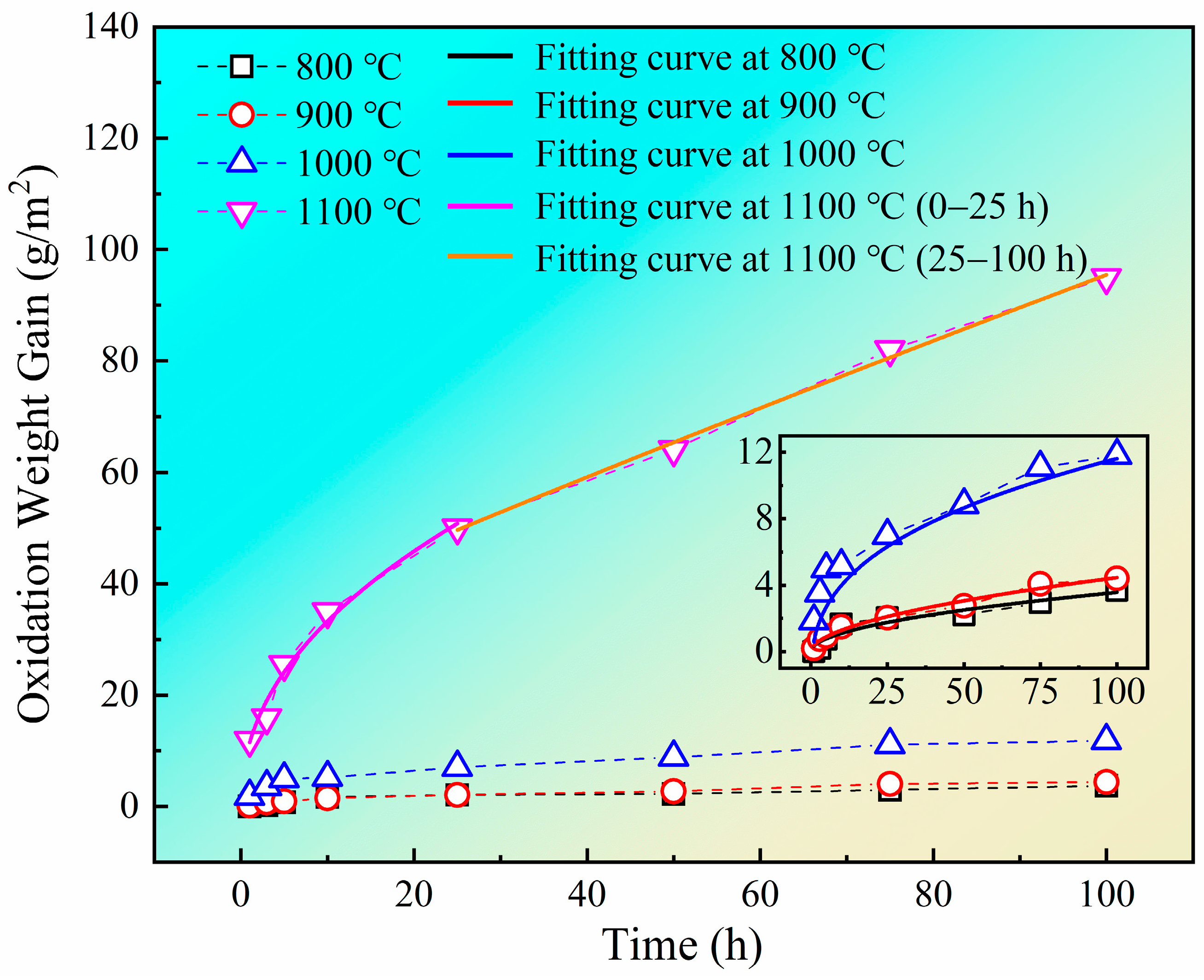
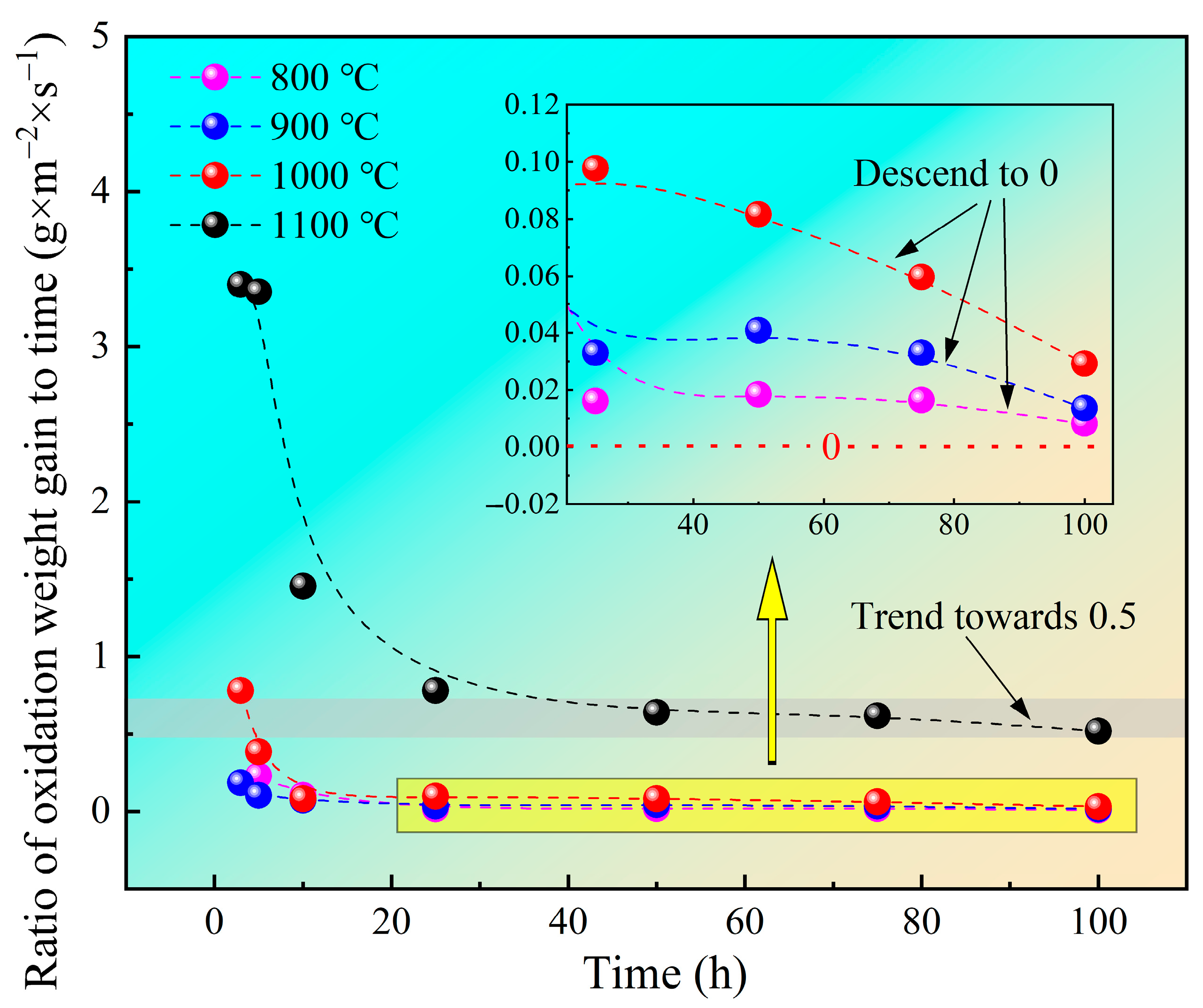
3.1. Oxidation Kinetics
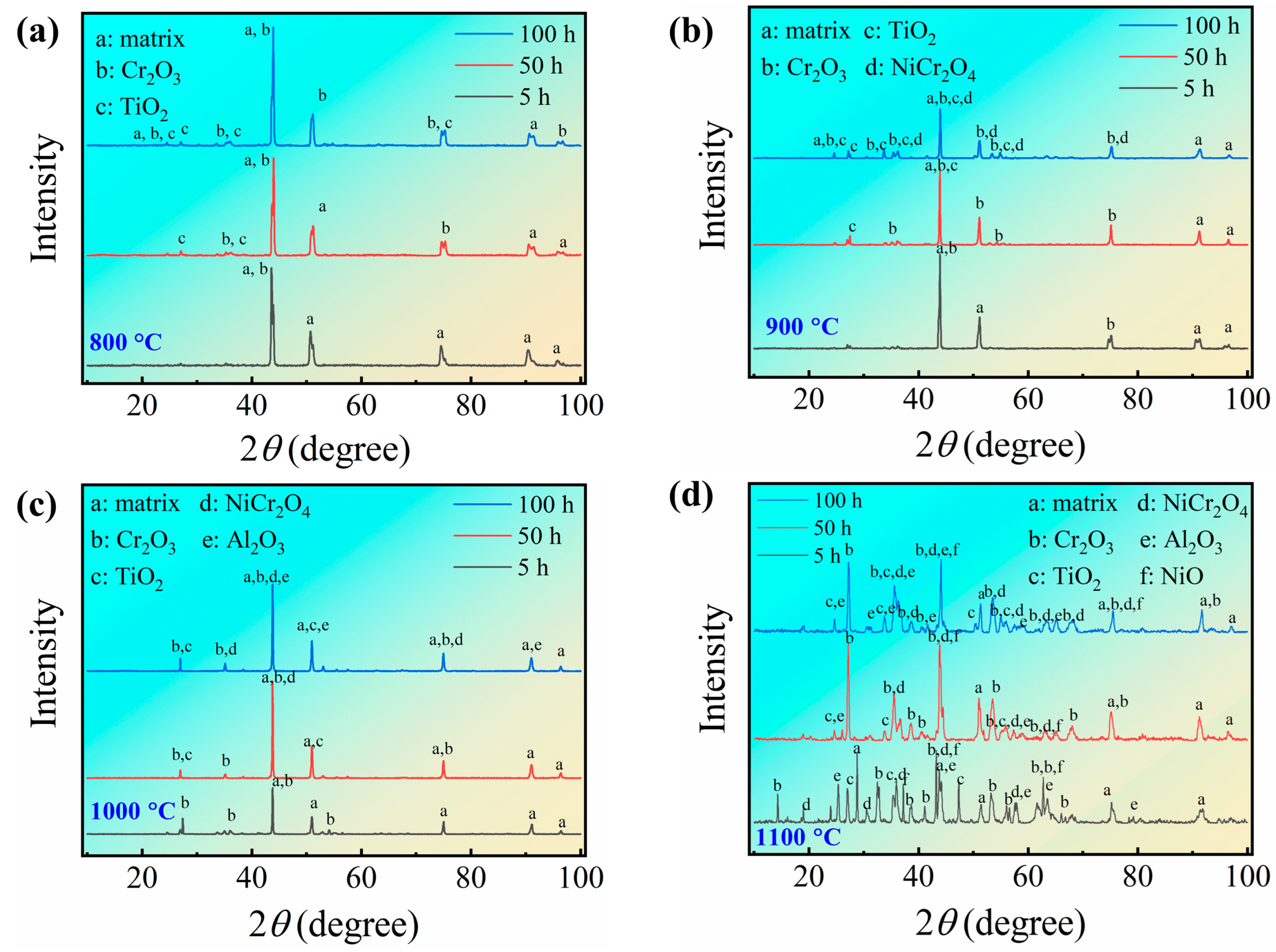
3.2. Surface Morphology and Phase Composition of the Oxide Layer
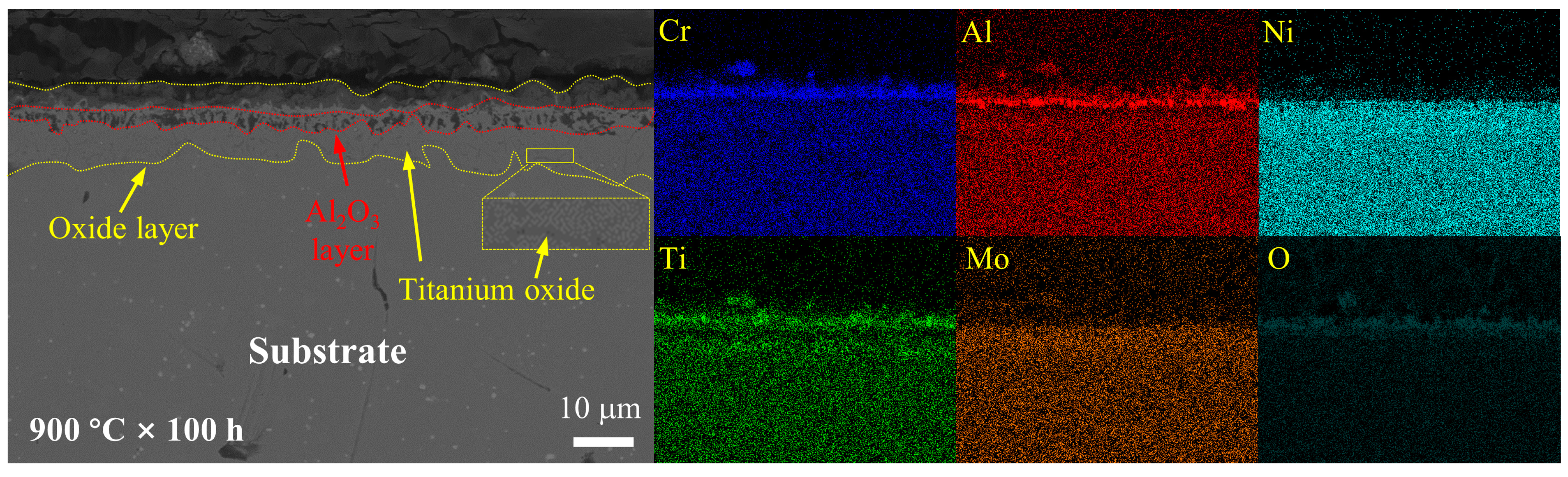
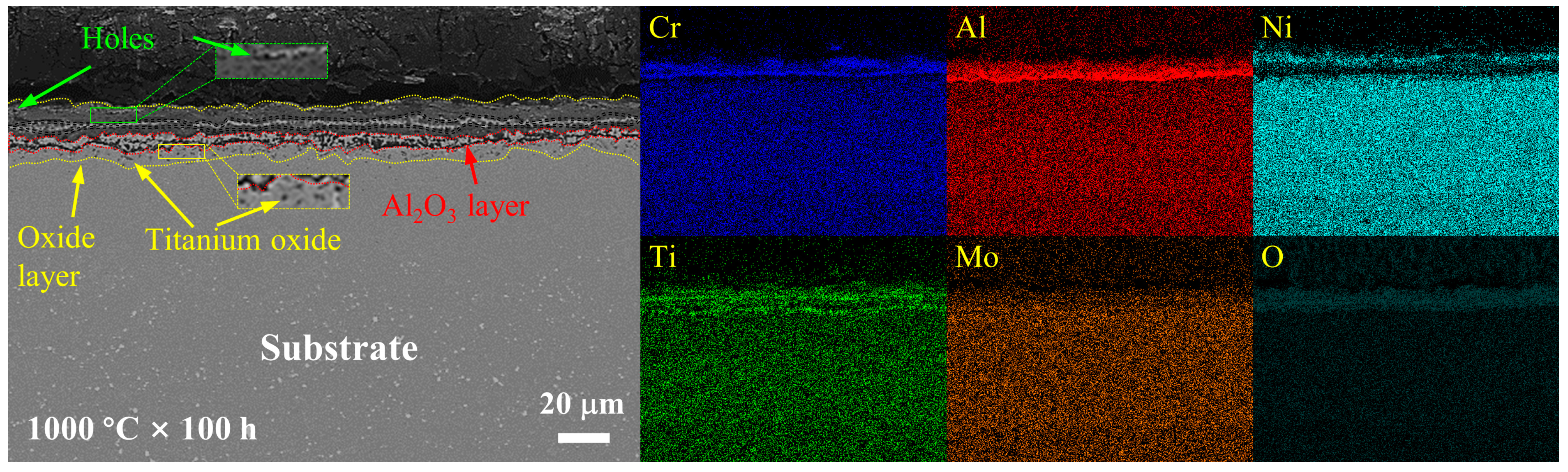
3.3. Cross-Sectional Analysis of the Oxide Layer
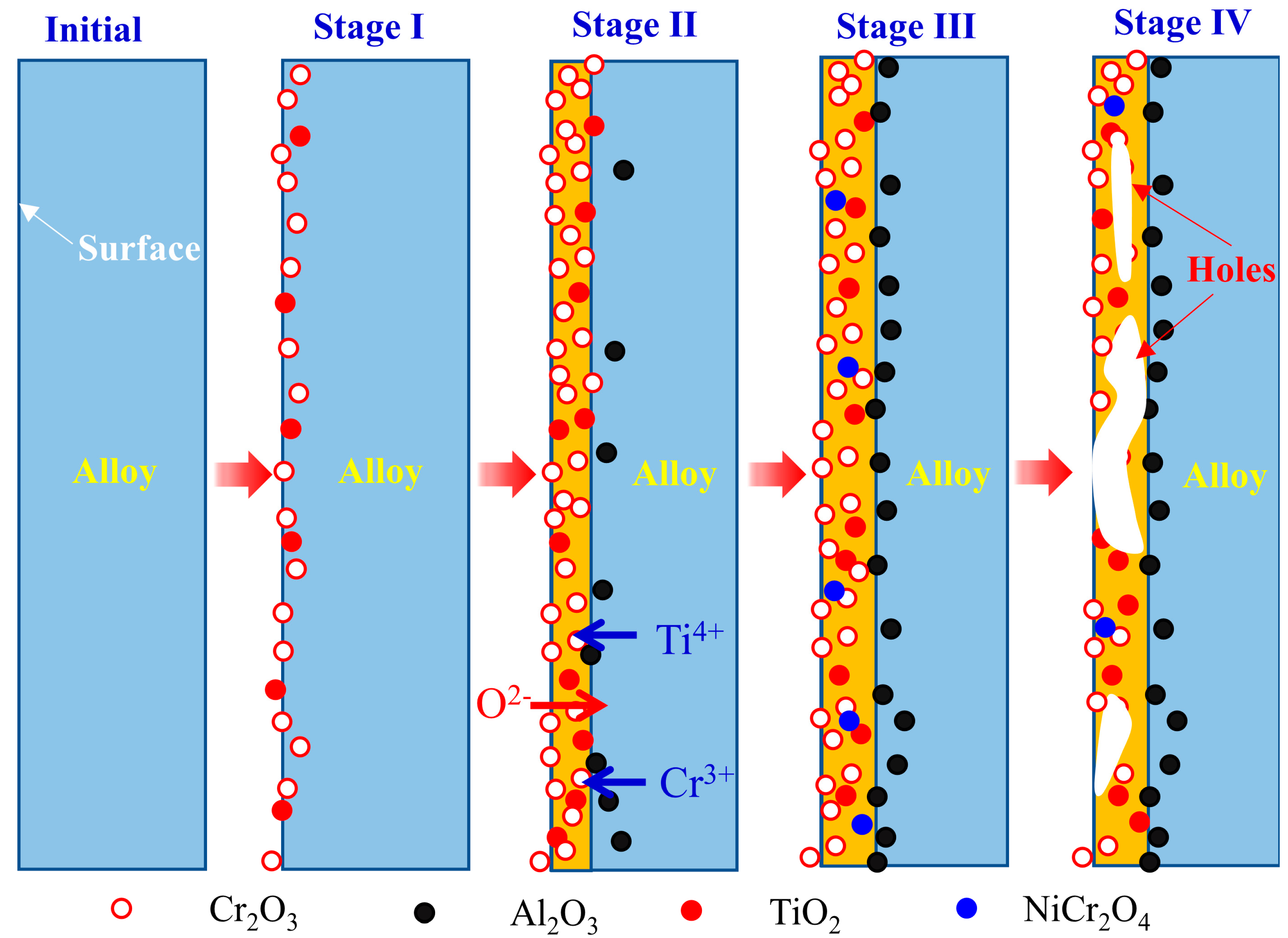
3.4. Oxidation Mechanism
3.4.1. Oxidation Kinetics
3.4.2. Selective Oxidation
3.4.3. Spalling of Oxides
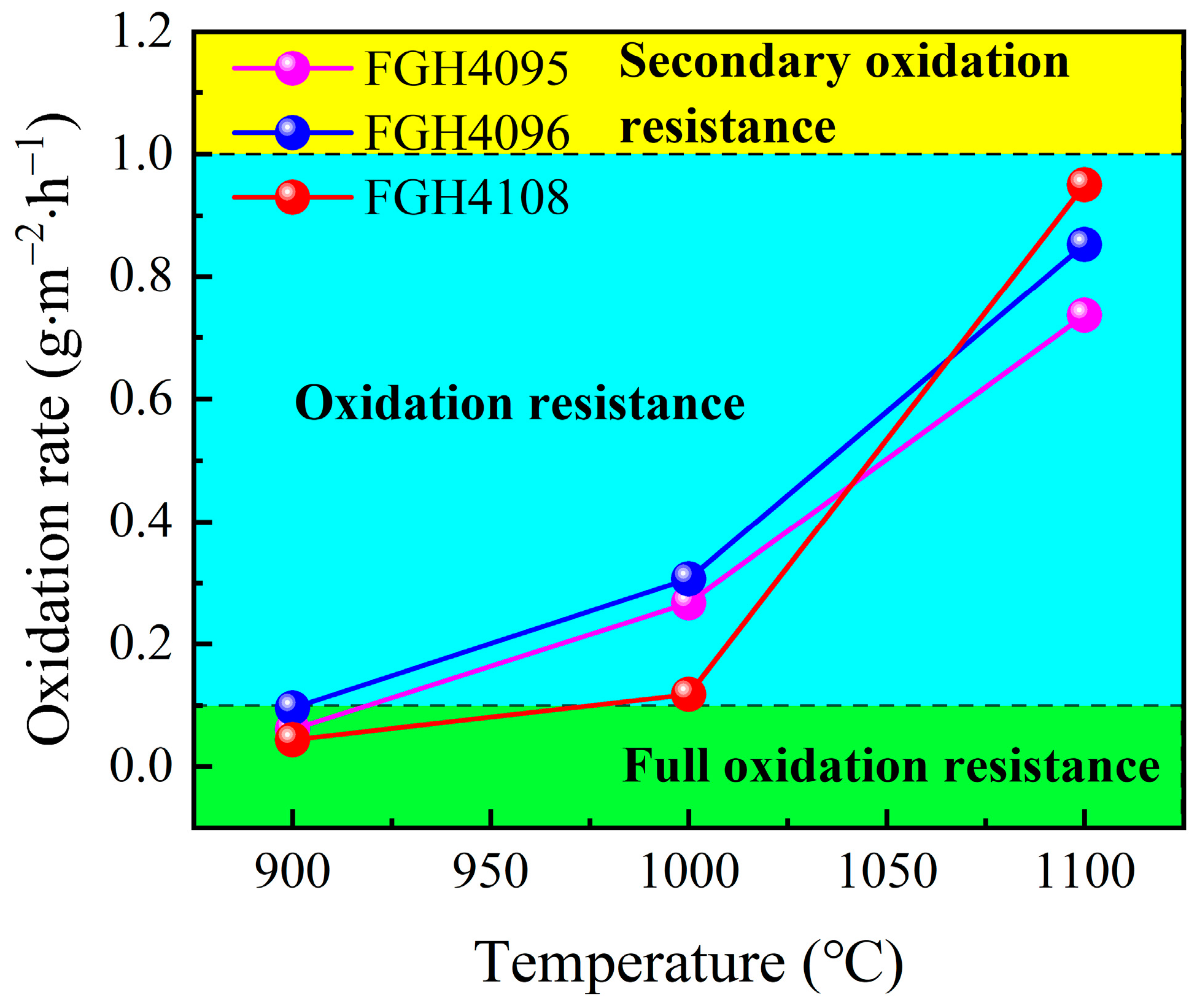
3.5. Comparison of Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, L.; Schmitz, G.; Meng, Y.; Chellali, R.; Schlesiger, R. Mechanism of Intermediate Temperature Embrittlement of Ni and Ni-based Superalloys. Crit. Rev. Solid State Mater. Sci. 2012, 37, 181–214. [Google Scholar] [CrossRef]
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Ouyang, X.; Liu, F.; Huang, L.; Ye, L.; Dong, H.; Tan, L.; Wang, L.; Jin, X.; Liu, Y. The Effects of Co on the Microstructure and Mechanical Properties of Ni-Based Superalloys Prepared via Selective Laser Melting. Materials 2023, 16, 2926. [Google Scholar] [CrossRef]
- Gao, Z.; Li, S.; Liu, G.; Shang, Z.; Song, D.; Yang, G.; Zou, J.; Liang, S. Microstructural Evolution and Tensile Properties of a Corrosion-Resistant Ni-Based Superalloys Used for Industrial Gas Turbines. Crystals 2023, 13, 669. [Google Scholar] [CrossRef]
- Alano, J.H.; Siqueira, R.L.; Júnior, C.B.M.; Silva, R.; Vacchi, G.D.S.; Della Rovere, C.A. A Survey on the Oxidation Behavior of a Nickel-Based Alloy Used in Natural Gas Engine Exhaust Valve Seats. Metals 2023, 13, 49. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Liu, X.; Lv, J.; Zheng, L. Deformation and damage mechanisms during stress rupture of Waspaloy after vacuum solution plus aging treatment. J. Alloys Compd. 2023, 946, 169461. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, L.; Wang, D.; Wei, X.; Min, H.; Jin, Y.; Tai, Q. Dissolution and Coarsening Kinetics of γ′ Precipitates in Waspaloy during Solution Treatment at Temperatures of 1000–1045 °C. J. Mater. Eng. Perform. 2022, 31, 7748–7756. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Dong, J.; Zheng, L. Inhomogeneous Planar Distribution of γ′ Precipitates in Waspaloy Caused by Local Spatial Consumption of MC Carbides. J. Mater. Eng. Perform. 2022, 31, 1397–1404. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, J.; Liu, H.; Zheng, L. Hot deformation behavior of a novel Ni–W–Co high-density Ni-based alloy with ul-tra-high W content. J. Alloys Compd. 2023, 950, 169937. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, M.; Xia, H.; Zheng, L.; Ta, N.; Meng, Y.; Cui, F. Pseudobinary Phase Diagrams of Eutectic Reaction for Pt-containing and Pt-free 718Plus Alloys. Adv. Eng. Mater. 2021, 23, 2001233. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Xu, M.; Meng, Y.; Xu, G.; Ta, N.; Zheng, L. Microstructure evolution dependence of work-hardening characteristic in cold deformation of a difficult-to-deform nickel-based superalloy. Mater. Sci. Eng. A 2021, 800, 140280. [Google Scholar] [CrossRef]
- Zhong, W.; Jiao, D.; Qiu, W.; Liu, Z.; Xu, W.; Li, Z.; Zhang, G. Oxidation Characteristics of Nickel-Based Superalloy Powders Exposed at Ambient Condition. Metals 2022, 12, 972. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.C.; Chellali, R.; Bouchikhaoui, H.; Dong, J.X. Oxidation property of powder metallurgy EP741NP Ni based superalloy at elevated temperatures. Mater. Technol. 2013, 28, 122–128. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.; Dong, J. Oxidation behavior and mechanism of powder metallurgy Rene95 nickel based superalloy between 800 and 1000 °C. Appl. Surf. Sci. 2010, 256, 7510–7515. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Sun, P.; Yang, G.; Song, W.; Wang, X. Effect of Solution Treatment on Microstructure Evolution of a Powder Metallurgy Nickel Based Superalloy with Incomplete Dynamic Recrystallization Microstructure. Metals 2023, 13, 239. [Google Scholar] [CrossRef]
- Li, G.; Sun, D.; Kang, J.; Gao, Y.; Yan, X.; Gao, Q.; Gao, K. The Effect of Hot Oscillatory Pressing Temperature on Microstructure and Tensile Behavior of Powder Metallurgy Superalloy. Metals 2022, 12, 1652. [Google Scholar] [CrossRef]
- Cobbinah, P.V.; Nzeukou, R.A.; Onawale, O.T.; Matizamhuka, W.R. Laser Powder Bed Fusion of Potential Superalloys: A Review. Metals 2020, 11, 58. [Google Scholar] [CrossRef]
- Sanchez, S.; Smith, P.; Xu, Z.; Gaspard, G.; Hyde, C.J.; Wits, W.W.; Ashcroft, I.A.; Chen, H.; Clare, A.T. Powder Bed Fusion of nickel-based superalloys: A review. Int. J. Mach. Tools Manuf. 2021, 165, 103729. [Google Scholar] [CrossRef]
- Brenneman, J.; Wei, J.; Sun, Z.; Liu, L.; Zou, G.; Zhou, Y. Oxidation behavior of GTD111 Ni-based superalloy at 900 °C in air. Corros. Sci. 2015, 100, 267–274. [Google Scholar] [CrossRef]
- Berthod, P.; Wora, S.A.O.; Aranda, L.; Medjahdi, G.; Etienne, E. Kinetic and Metallography Study of the Oxidation at 1250 °C of {Co+Ni}-Based Superalloys Containing Ti to Form MC Carbides. Metals 2021, 12, 10. [Google Scholar] [CrossRef]
- Moffat, J.P.; Whitfield, T.E.; Christofidou, K.A.; Pickering, E.J.; Jones, N.G.; Stone, H.J. The Effect of Heat Treatment on the Oxidation Resistance of Cobalt-Based Superalloys. Metals 2020, 10, 248. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Liu, D.; Zou, J. High temperature oxidation resistance of PM superalloys FGH4095 and FGH4096. J. Mater. Eng. 2023, 51, 122–131. [Google Scholar]
- Liu, C.; Sun, X.; Guan, H.; Hu, Z. Oxidation of the single-crystal Ni-base superalloy DD32 containing rhenium in air at 900 and 1000 °C. Surf. Coat. Technol. 2005, 197, 39–44. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, Q.; Yang, L.; Ren, P.; Yang, Y.; Wang, Q.; Li, W.; Zhu, S.; Wang, F. The cyclic oxidation behavior of a Pt modified γ’ nanocrystalline coating at 1150 °C. Corros. Sci. 2022, 208, 110638. [Google Scholar] [CrossRef]
- Zheng, J.; Hou, X.; Wang, X.; Meng, Y.; Zheng, X.; Zheng, L. Isothermal oxidation mechanism of a newly developed Nb–Ti–V–Cr–Al–W–Mo–Hf alloy at 800–1200 °C. Int. J. Refract. Met. Hard Mater. 2016, 54, 322–329. [Google Scholar] [CrossRef]
- Zheng, J.; Hou, X.; Wang, X.; Meng, Y.; Zheng, X.; Zheng, L. Isothermal oxidation mechanism of Nb–Ti–V–Al–Zr alloy at 700–1200 °C: Diffusion and interface reaction. Corros. Sci. 2015, 96, 186–195. [Google Scholar] [CrossRef]
- Busso, E.P.; Evans, H.E.; Qian, Z.Q.; Taylor, M.P. Effects of breakaway oxidation on local stresses in thermal barrier coatings. Acta Mater. 2010, 58, 1242–1251. [Google Scholar] [CrossRef]
- Mikkelsen, L.; Linderoth, S. High temperature oxidation of Fe–Cr alloy in O2–H2–H2O atmospheres; microstructure and kinetics. Mater. Sci. Eng. A 2003, 361, 198–212. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z.; Wu, Z.; Li, S.; He, Y. Oxidation behavior of Ni3Al and FeAl intermetallics under low oxygen partial pressures. Intermetallics 2002, 10, 263–270. [Google Scholar] [CrossRef]
- Liu, C.; Ma, J.; Sun, X. Oxidation behavior of a single-crystal Ni-base superalloy between 900 and 1000 °C in air. J. Alloys Compd. 2010, 491, 522–526. [Google Scholar] [CrossRef]
- Kamal, S.; Jayaganthan, R.; Prakash, S. High temperature oxidation studies of detonation-gun-sprayed Cr3C2–NiCr coating on Fe- and Ni-based superalloys in air under cyclic condition at 900 °C. J. Alloys Compd. 2009, 472, 378–389. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, X.; Smith, G.D.; Patel, S.J. Research and improvement on structure stability and corrosion resistance of nickel-base superalloy INCONEL alloy 740. Mater. Des. 2006, 27, 1120–1127. [Google Scholar] [CrossRef]
- Li, M. High Temperature Corrosion of Metals; Metallurgical Industry Press: Beijing, China, 2001. [Google Scholar]
- Liu, F.; Zhang, M.; Dong, J.; Zhang, Y. High-temperature oxidation of FGH96 P/M superalloy. Acta Met. Sin. Engl. Lett. 2007, 20, 102–110. [Google Scholar] [CrossRef]
- Ellingham, H. The physical chemistry of process metallurgy. J. Indian Chem. Soc. 1944, 63, 125–133. [Google Scholar]
- Zhao, S.; Xie, X.; Smith, G.D. The oxidation behavior of the new nickel-based superalloy Inconel 740 with and without Na2SO4 deposit. Surf. Coat. Technol. 2004, 185, 178–183. [Google Scholar] [CrossRef]
- Robino, C.V. Representation of mixed reactive gases on free energy (Ellingharn-Richardson) diagrams. Met. Mater. Trans. B 1996, 27, 65–69. [Google Scholar] [CrossRef]
- Gold, T. Hearing. II. The physical basis of the action of the cochlea. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 1948, 135, 492–498. [Google Scholar] [CrossRef]
- Duval, A.; Miserque, F.; Tabarant, M.; Nogier, J.P.; Gédéon, A. Influence of the oxygen partial pressure on the oxidation of Inconel 617 alloy at high temperature. Oxid. Met. 2010, 74, 215–238. [Google Scholar] [CrossRef]
- Xu, C.; Gao, W. Pilling-Bedworth ratio for oxidation of alloys. Mater. Res. Innov. 2000, 3, 231–235. [Google Scholar] [CrossRef]
- Feng, Y.; Dong, T.-S.; Li, G.-L.; Wang, R.; Ma, G.-Z.; Zhao, X.-W.; Liu, Q. The roles of stress in the thermal shock failure of YSZ TBCs before and after laser remelting. J. Alloys Compd. 2020, 828, 154417. [Google Scholar] [CrossRef]
- Evans, H.; Lobb, R. Conditions for the initiation of oxide-scale cracking and spallation. Corros. Sci. 1984, 24, 209–222. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Zhang, H.; Ni, N.; Li, L.; He, L.; Mu, R.; Zhao, X.; Guo, F. Y/Hf-doped AlCoCrFeNi high-entropy alloy with ultra oxidation and spallation resistance. Corros. Sci. 2020, 166, 108426. [Google Scholar] [CrossRef]





| Co | Cr | Al | Ti | C | B | Zr | Mo + W | Nb + Ta + Hf | Ni |
|---|---|---|---|---|---|---|---|---|---|
| 18.0 | 12.0 | 3.0 | 3.0 | 0.06 | 0.04 | 0.05 | 6.0 | 7.0 | Balance |
| Temperature (°C) | Time (h) | Kinetic Expressions | Correlation Coefficient R2 |
|---|---|---|---|
| 800 | 0–100 | 0.924 | |
| 900 | 0–100 | 0.949 | |
| 1000 | 0–100 | 0.966 | |
| 1100 | 0–25 | 0.978 | |
| 25–100 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wang, Z.; Lv, J.; Liu, X.; Zheng, L.; Liu, J. Oxidation Property of a Fourth-Generation Powder Metallurgy FGH4108 Nickel-Based Superalloy. Metals 2023, 13, 945. https://doi.org/10.3390/met13050945
Zhao X, Wang Z, Lv J, Liu X, Zheng L, Liu J. Oxidation Property of a Fourth-Generation Powder Metallurgy FGH4108 Nickel-Based Superalloy. Metals. 2023; 13(5):945. https://doi.org/10.3390/met13050945
Chicago/Turabian StyleZhao, Xin, Zhigang Wang, Jinjuan Lv, Xiao Liu, Lei Zheng, and Jiantao Liu. 2023. "Oxidation Property of a Fourth-Generation Powder Metallurgy FGH4108 Nickel-Based Superalloy" Metals 13, no. 5: 945. https://doi.org/10.3390/met13050945
APA StyleZhao, X., Wang, Z., Lv, J., Liu, X., Zheng, L., & Liu, J. (2023). Oxidation Property of a Fourth-Generation Powder Metallurgy FGH4108 Nickel-Based Superalloy. Metals, 13(5), 945. https://doi.org/10.3390/met13050945






