Reorientation Mechanisms of Graphene Coated Copper {001} Surfaces
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Materials Characterization
2.3. Molecular Dynamics Simulation
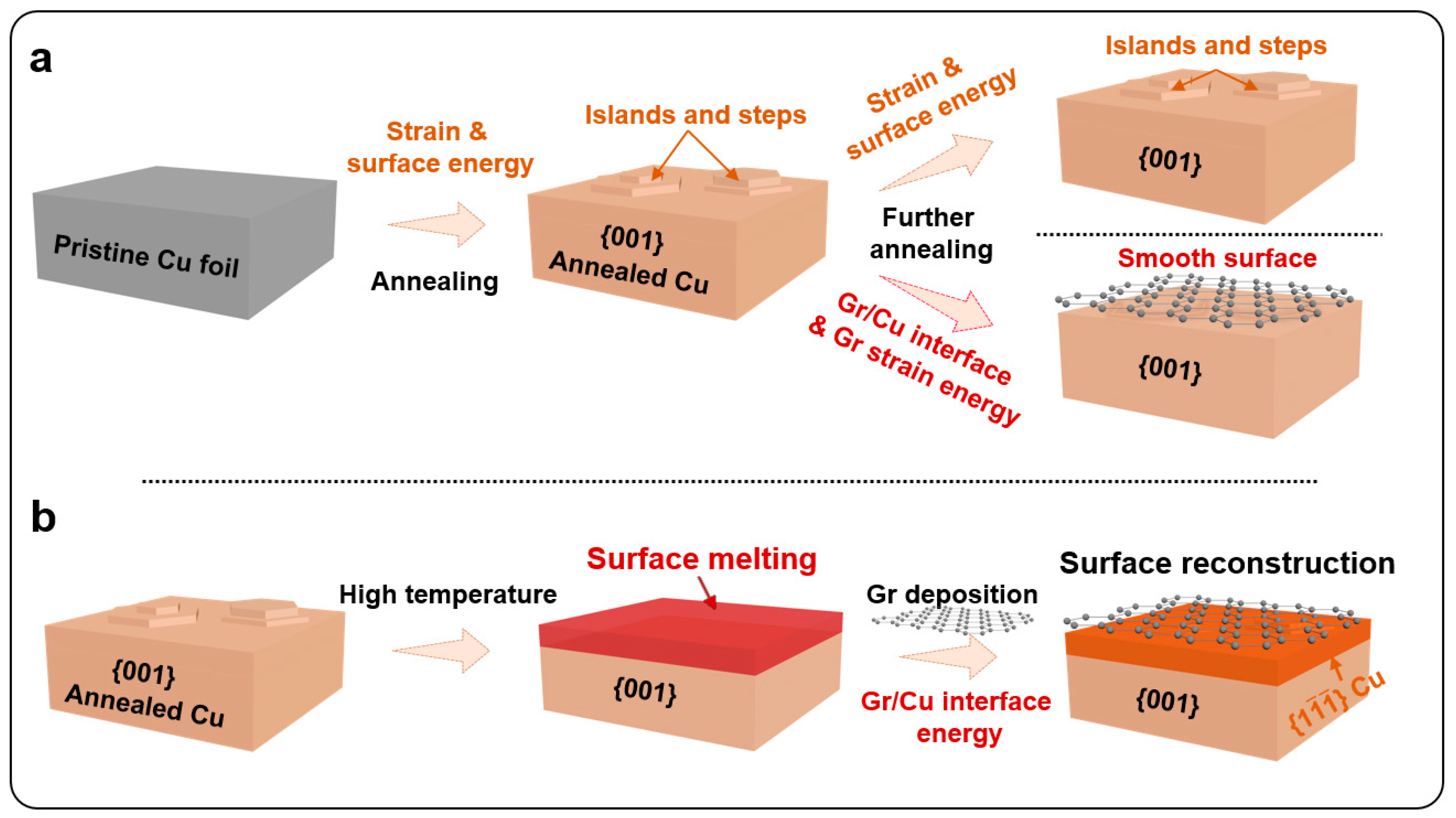
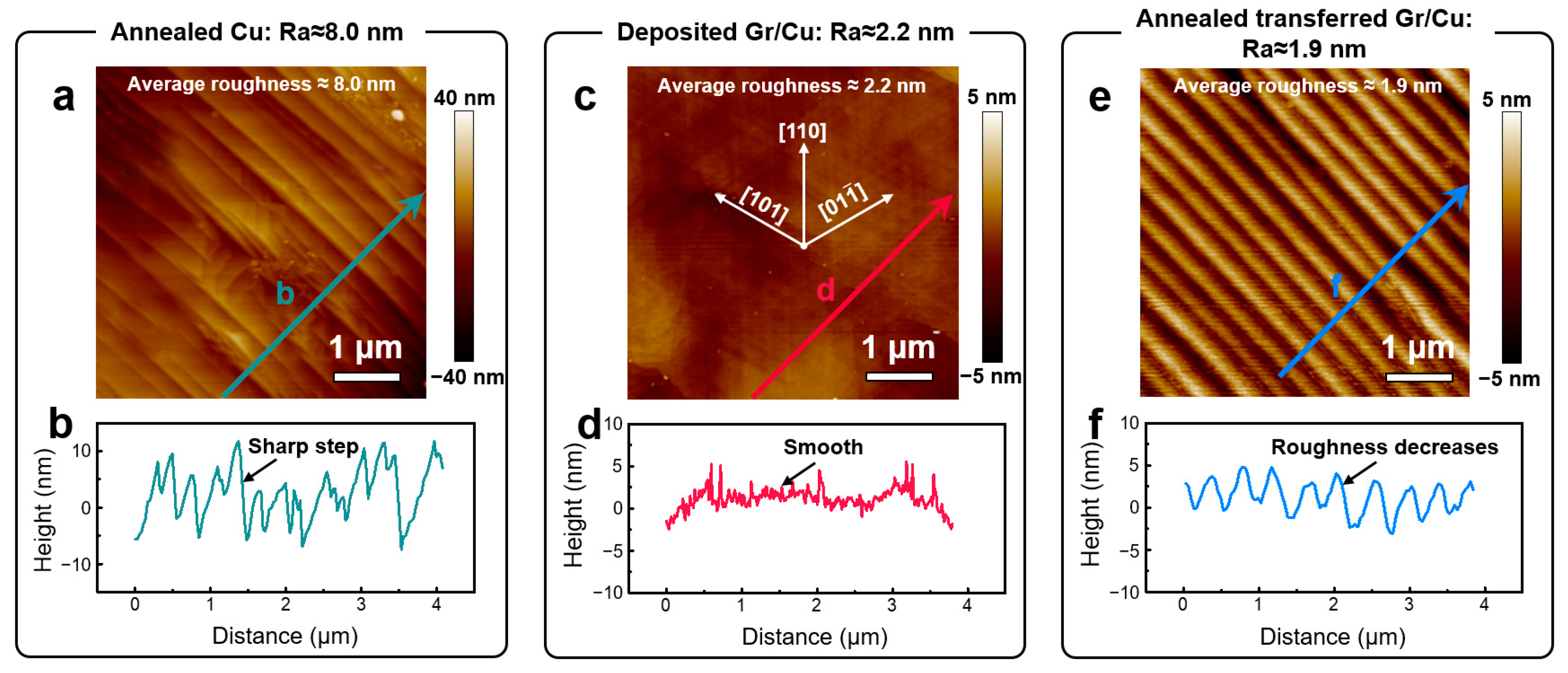
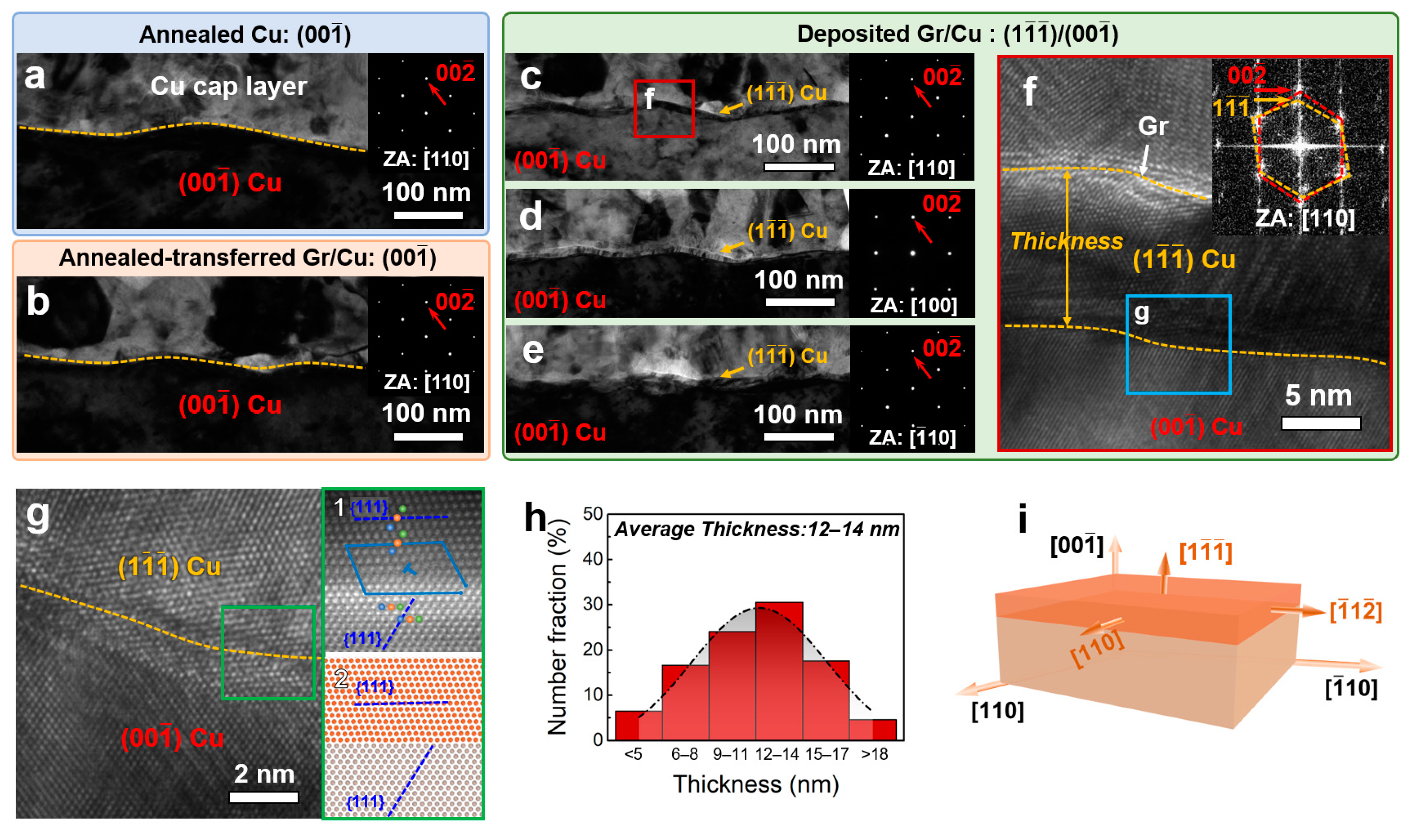
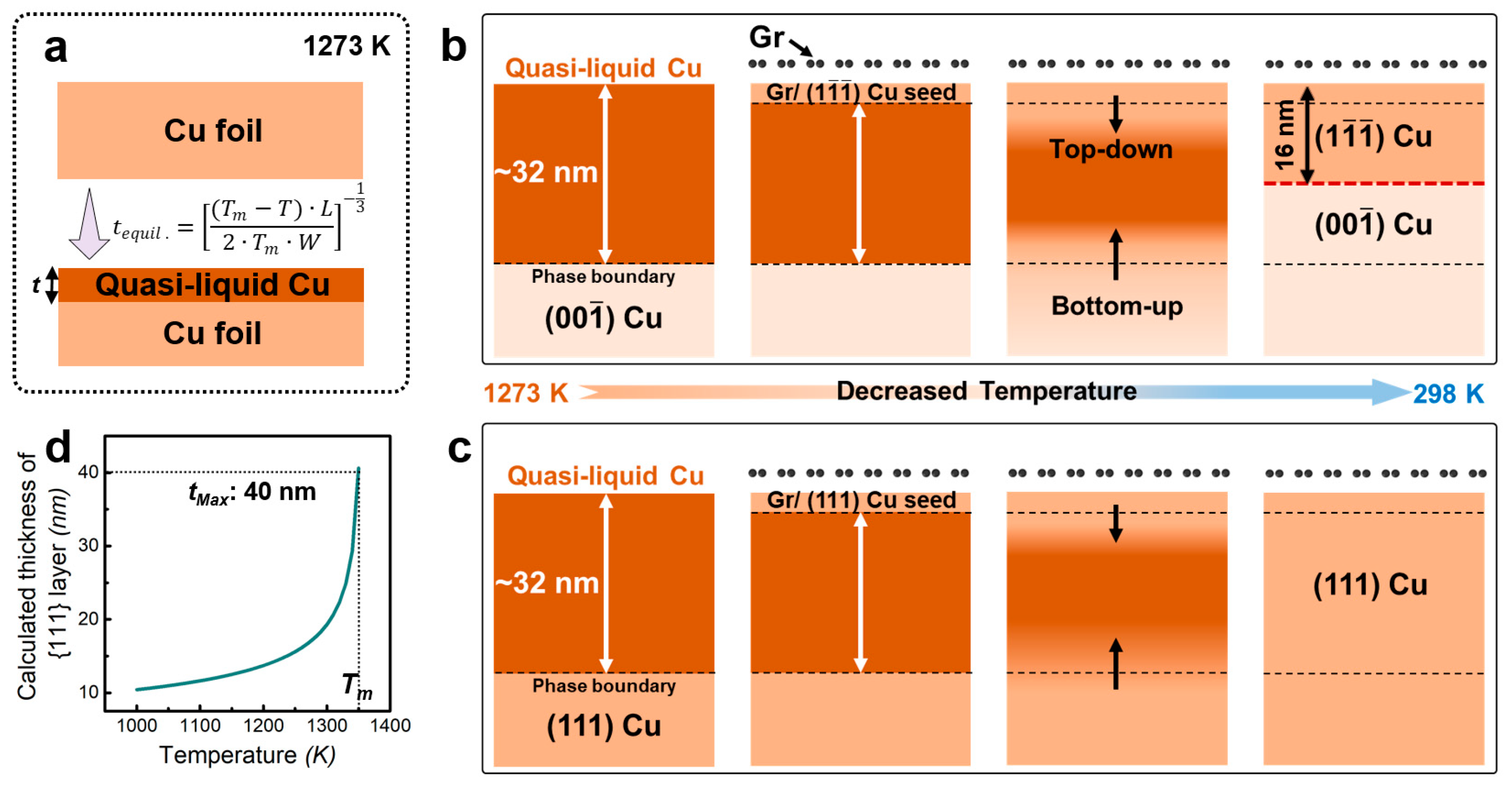
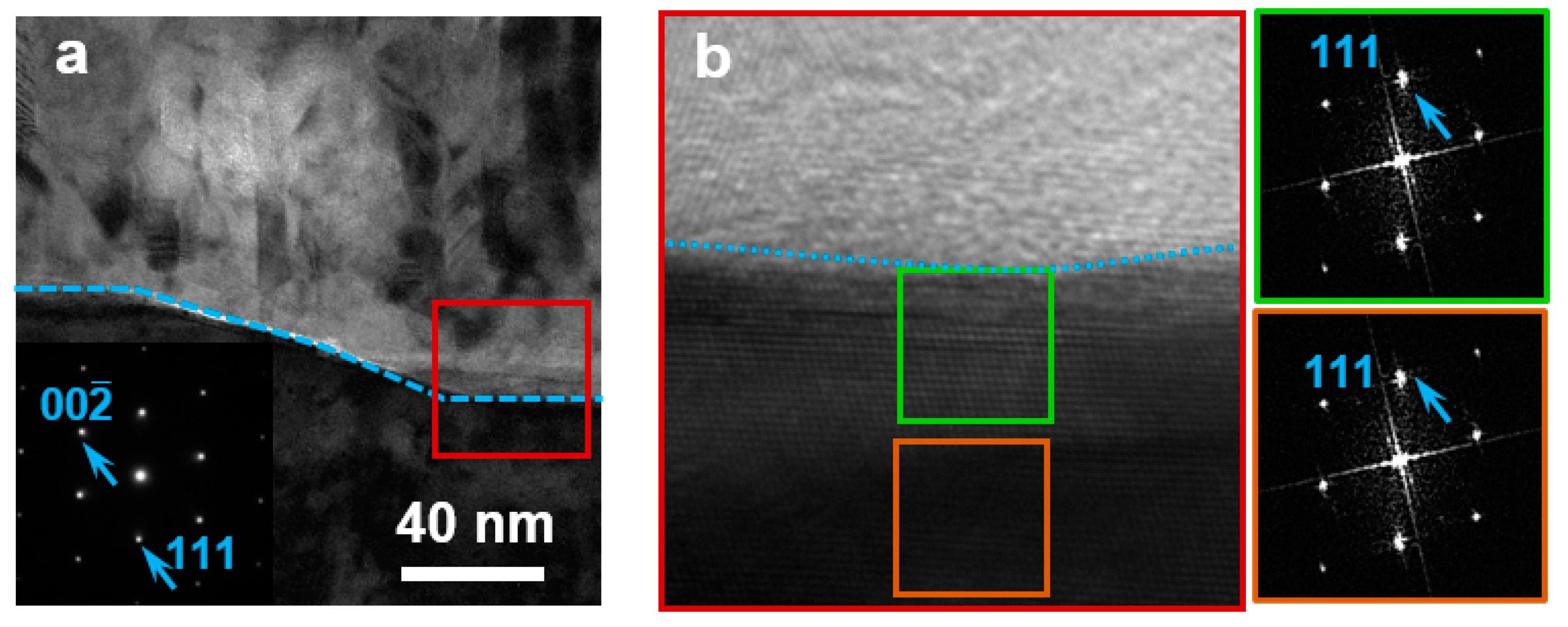
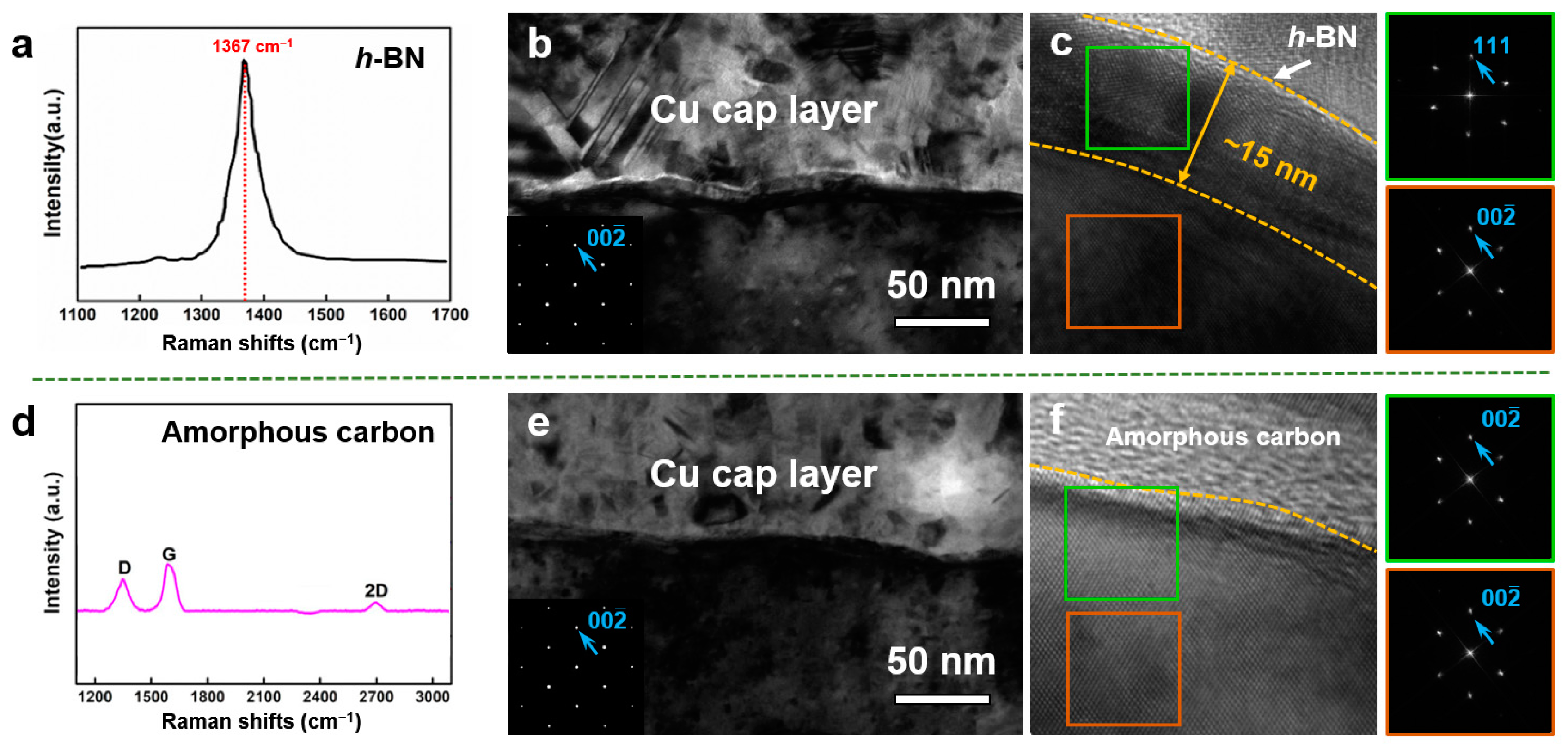
3. Results
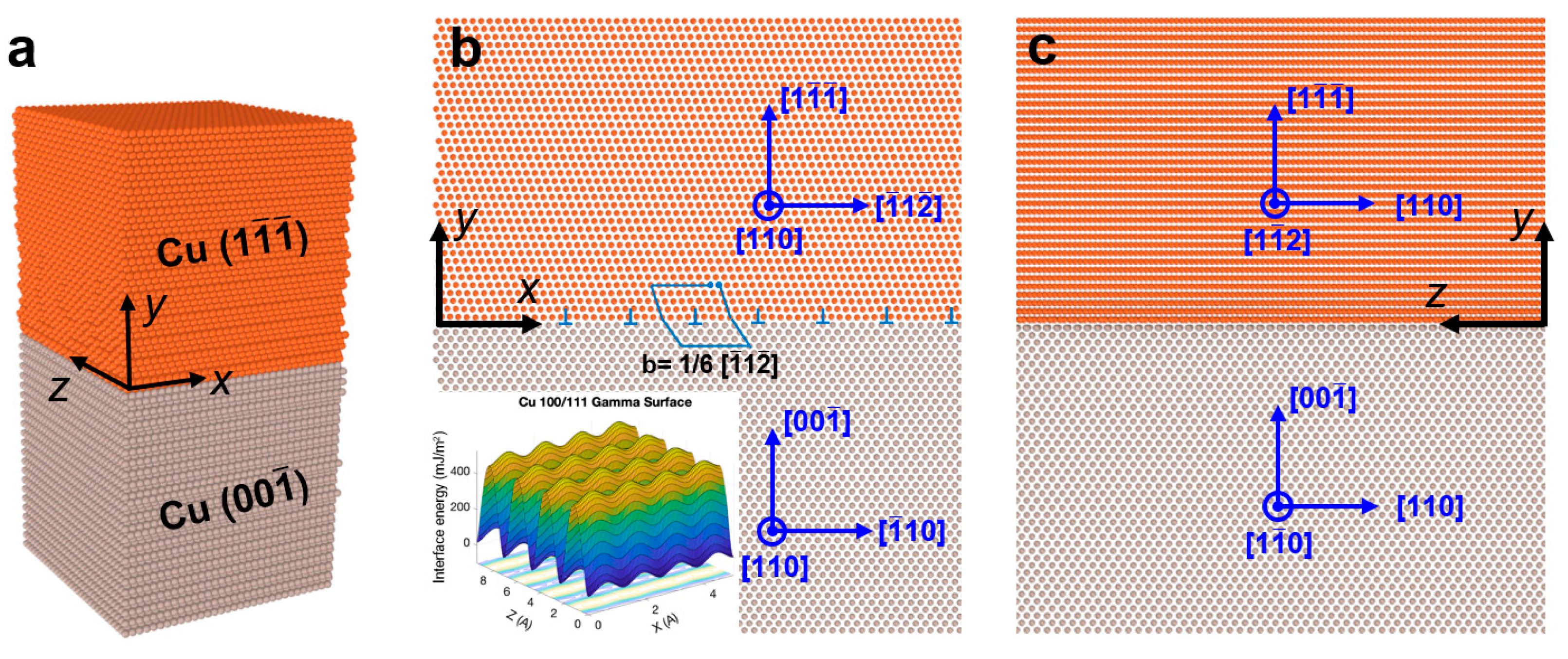
4. Discussion
5. Conclusions
- (1)
- The study confirmed the formation of a 12~14 nm thick {111} reconstructed layer on the high-temperature deposited Gr/{001} Cu surface.
- (2)
- By correlating the surface melting with {111} reconstructed layer, a melting-solidification mechanism was proposed to understand the formation mechanism of the surface reconstruction.
- (3)
- The proposed mechanism was then verified by coating h-BN and amorphous carbon on Cu surfaces.
- (4)
- The melting-solidification mechanism provides two important criteria that ensure the formation of close-packed {111} surface orientation in fcc metals: (i) an appropriate processing temperature that provides sufficient surface melting thickness, and (ii) a crystalline surface coated layer (including Gr and h-BN) that can modify the interfacial energy density.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hori, Y.; Takahashi, I.; Koga, O.; Hoshi, N. Selective Formation of C2 Compounds from Electrochemical Reduction of CO2 at a Series of Copper Single Crystal Electrodes. J. Phys. Chem. B 2001, 106, 15–17. [Google Scholar] [CrossRef]
- Quan, Z.; Wang, Y.; Fang, J. High-Index Faceted Noble Metal Nanocrystals. Acc. Chem. Res. 2012, 46, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Z.; Dong, J.; Yi, D.; Niu, J.; Wu, M.; Lin, L.; Yin, R.; Li, M.; Zhou, J.; et al. Ultrafast epitaxial growth of metre-sized single-crystal graphene on industrial Cu foil. Sci. Bull. 2017, 62, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Vitos, L.; Ruban, A.; Skriver, H.; Kollár, J. The surface energy of metals. Surf. Sci. 1998, 411, 186–202. [Google Scholar] [CrossRef]
- Tran, R.; Xu, Z.; Radhakrishnan, B.; Winston, D.; Sun, W.; Persson, K.A.; Ong, S.P. Surface energies of elemental crystals. Sci. Data 2016, 3, 160080. [Google Scholar] [CrossRef]
- Maurice, V.; Marcus, P. Progress in corrosion science at atomic and nanometric scales. Prog. Mater. Sci. 2018, 95, 132–171. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Lamichhane, B.; Kim, Y.; Lee, Y.; Cho, C.; Cheon, M.; Kim, J.; Jeong, H.; Ha, T.; et al. Flat-surface-assisted and self-regulated oxidation resistance of Cu(111). Nature 2022, 603, 434–438. [Google Scholar] [CrossRef]
- Hahn, C.; Hatsukade, T.; Kim, Y.-G.; Vailionis, A.; Baricuatro, J.H.; Higgins, D.C.; Nitopi, S.A.; Soriaga, M.P.; Jaramillo, T.F. Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl. Acad. Sci. USA 2017, 114, 5918–5923. [Google Scholar] [CrossRef]
- Huang, K.; Crooks, R.M. Enhanced electrocatalytic activity of Cu-modified, high-index single Pt NPs for formic acid oxidation. Chem. Sci. 2022, 13, 12479–12490. [Google Scholar] [CrossRef]
- Mayumi, S.; Murozono, I.; Nanatsue, H.; Ueda, S. Corrosion-Induced Contact Failures in Double-Level Al-Si-Cu Metallization. J. Electrochem. Soc. 1990, 137, 1861. [Google Scholar] [CrossRef]
- Zuo, Y.; Robador, A.; Wickham, M.; Mannan, S.H. Unraveling the complex oxidation effect in sintered Cu nanoparticle interconnects during high temperature aging. Corros. Sci. 2022, 209, 110713. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Diodati, S.; Pigato, M.; Bonesso, M.; Dabalà, M. Microstructural and Corrosion Properties of Hydroxyapatite Containing PEO Coating Produced on AZ31 Mg Alloy. Materials 2021, 14, 1531. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Shinde, K.; Oh, J.; Lee, S.; Chung, C.; Park, J. Microstructures and high temperature oxidation behaviors of AlMo0.5NbTa0.5TiZr high entropy alloys coated by silicon pack cementation. Corros. Sci. 2023, 219, 111203. [Google Scholar] [CrossRef]
- Miranda-Pérez, A.F.; Rodríguez-Vargas, B.R.; Calliari, I.; Pezzato, L. Corrosion Resistance of GMAW Duplex Stainless Steels Welds. Materials 2023, 16, 1847. [Google Scholar] [CrossRef]
- Wang, X.-M.; Zhang, F.-Q. Effects of soft sparking on micro/nano structure and bioactive components of microarc oxidation coatings on selective laser melted Ti6Al4V alloy. Surf. Coat. Technol. 2023, 462, 129478. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Jin, G.; Dong, Y.; Wang, Z.; Qi, Y. Microstructure evolution and performance enhancement of NbTaTiV-(Cr, Zr, W) single-phase refractory high-entropy alloy coatings: Role of additional elements. J. Alloys Compd. 2023, 951, 169918. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Z.; Zhang, P.; Xu, Z.; Lin, S.; He, M.; Lu, S.; Wu, X. Tailoring high-temperature oxidation resistance of FeCrMnVSi high entropy alloy coatings via building Si-rich dendrite microstructure. Appl. Surf. Sci. 2022, 606, 154862. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, G.; Wang, C.; Wang, T.; Zhang, Y.; Xin, T. Construction of Cr coatings with different columnar structure on Zircaloy-4 alloys to optimize the high-temperature steam oxidation behavior for accident tolerant fuel claddings. J. Alloys Compd. 2023, 946, 169385. [Google Scholar] [CrossRef]
- Yang, J.; Fang, K.; Xu, K.; Shen, X.; Xu, X. Effect of zinc or copper doping on corrosion resistance and anti-oxidative stress of strontium-based micro-arc oxidation coatings on titanium. Appl. Surf. Sci. 2023, 626, 157229. [Google Scholar] [CrossRef]
- Wei, X.X.; Zhang, B.; Wu, B.; Wang, Y.J.; Tian, X.H.; Yang, L.X.; Oguzie, E.E.; Ma, X.L. Enhanced corrosion resistance by engineering crystallography on metals. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Bufford, D.; Wang, H.; Sun, C.; Zhang, X. Mechanical properties of highly textured Cu/Ni multilayers. Acta Mater. 2011, 59, 1924–1933. [Google Scholar] [CrossRef]
- Che, J.G.; Chan, C.T.; Jian, W.-E.; Leung, T.C. Surface atomic structures, surface energies, and equilibrium crystal shape of molybdenum. Phys. Rev. B 1998, 57, 1875–1880. [Google Scholar] [CrossRef]
- Yang, K.; Ma, Y.; Zhang, Z.; Zhu, J.; Sun, Z.; Chen, J.; Zhao, H.; Song, J.; Li, Q.; Chen, N.; et al. Anisotropic thermal conductivity and associated heat transport mechanism in roll-to-roll graphene reinforced copper matrix composites. Acta Mater. 2020, 197, 342–354. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Chang, Z.; Liu, H.; Wang, Y.; Liang, Y.; Chen, B.; Ding, Q.; Zhao, Z.; Wang, R.; et al. Large Single-Crystal Cu Foils with High-Index Facets by Strain-Engineered Anomalous Grain Growth. Adv. Mater. 2020, 32, e2002034. [Google Scholar] [CrossRef]
- Zhang, W. Evolution of crystal morphologies to equilibrium by surface diffusion with anisotropic surface free energy in three dimensions. J. Cryst. Growth 2006, 297, 169–179. [Google Scholar] [CrossRef]
- Deng, B.; Pang, Z.; Chen, S.; Li, X.; Meng, C.; Li, J.; Liu, M.; Wu, J.; Qi, Y.; Dang, W.; et al. Wrinkle-Free Single-Crystal Graphene Wafer Grown on Strain-Engineered Substrates. ACS Nano 2017, 11, 12337–12345. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Z.; Xu, X.; Zhang, Z.; Duan, Y.; Dong, J.; Qiao, R.; You, S.; Wang, L.; Qi, J.; et al. Seeded growth of large single-crystal copper foils with high-index facets. Nature 2020, 581, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Huang, M.; Kwon, Y.; Zhang, L.; Li, B.-W.; Oh, S.; Dong, J.; Luo, D.; Biswal, M.; Cunning, B.V.; et al. Colossal grain growth yields single-crystal metal foils by contact-free annealing. Science 2018, 362, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.V.; Carel, R. Texture development in polycrystalline thin films. Mater. Sci. Eng. B 1995, 32, 211–219. [Google Scholar] [CrossRef]
- Sonnweber-Ribic, P.; Gruber, P.; Dehm, G.; Arzt, E. Texture transition in Cu thin films: Electron backscatter diffraction vs. X-ray diffraction. Acta Mater. 2006, 54, 3863–3870. [Google Scholar] [CrossRef]
- Thompson, C.V.; Carel, R. Stress and grain growth in thin films. J. Mech. Phys. Solids 1996, 44, 657–673. [Google Scholar] [CrossRef]
- Rhead, G.; McLean, M. Variation of surface energy with crystallographic orientation in silver. Acta Met. 1964, 12, 401–407. [Google Scholar] [CrossRef]
- Vlassak, J.J.; Nix, W. Measuring the elastic properties of anisotropic materials by means of indentation experiments. J. Mech. Phys. Solids 1994, 42, 1223–1245. [Google Scholar] [CrossRef]
- Yang, K.; Liu, G.; Ma, H.; Song, J.; Li, Q.; Chen, N.; Wang, Y.; Chen, D.; Liu, Y.; Fan, T. Formation of misfit dislocation arrays and helium nanochannels near copper surface assisted by high-temperature graphene deposition. Acta Mater. 2022, 237, 118134. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, M.; Ke, F. Shape Memory Effect in Cu Nanowires. Nano Lett. 2005, 5, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, Q.; Zhang, Q.; Liu, G.; Wang, J.; Yang, Y.; Guo, C.; Ni, J.; Song, J.; Zhang, J.; et al. Synergistically enhanced interface stability by graphene assisted copper surface reconstruction. Acta Mater. 2022, 226, 117638. [Google Scholar] [CrossRef]
- Takahashi, J.; Ueyama, T.; Kamei, K.; Kato, H.; Homma, Y. Experimental and theoretical studies on the surface morphology variation of a Ni substrate by graphene growth. J. Appl. Phys. 2021, 129, 024302. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Weinberg, G.; Zhang, Q.; Lunkenbein, T.; Klein-Hoffmann, A.; Kurnatowska, M.; Plodinec, M.; Li, Q.; Chi, L.; Schloegl, R.; et al. Direct Observation of Graphene Growth and Associated Copper Substrate Dynamics by in Situ Scanning Electron Microscopy. ACS Nano 2015, 9, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wu, J.; Zhang, S.; Qi, Y.; Zheng, L.; Yang, H.; Tang, J.; Tong, L.; Zhang, J.; Liu, Z.; et al. Anisotropic Strain Relaxation of Graphene by Corrugation on Copper Crystal Surfaces. Small 2018, 14, e1800725. [Google Scholar] [CrossRef]
- Yi, D.; Luo, D.; Wang, Z.-J.; Dong, J.; Zhang, X.; Willinger, M.-G.; Ruoff, R.S.; Ding, F. What Drives Metal-Surface Step Bunching in Graphene Chemical Vapor Deposition? Phys. Rev. Lett. 2018, 120, 246101. [Google Scholar] [CrossRef]
- Bienfait, M.; Gay, J.-M. Surface Melting and Diffusion. In Phase Transitions in Surface Films 2; Taub, H., Torzo, G., Lauter, H., Fain, S., Eds.; Springer: Boston, MA, USA, 1991; pp. 307–325. [Google Scholar]
- Levi, A. On Surface Melting. In Phase Transitions in Surface Films 2; Taub, H., Torzo, G., Lauter, H., Fain, S., Eds.; Springer: Boston, MA, USA, 1991; pp. 327–338. [Google Scholar]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Arenal, R.; Ferrari, A.C.; Reich, S.; Wirtz, L.; Mevellec, J.-Y.; Lefrant, S.; Rubio, A.; Loiseau, A. Raman Spectroscopy of Single-Wall Boron Nitride Nanotubes. Nano Lett. 2006, 6, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Foiles, S.M.; Baskes, M.I.; Daw, M.S. Embedded-atom-method functions for the fcc metals Cu, Ag, Au, Ni, Pd, Pt, and their alloys. Phys. Rev. B 1986, 33, 7983–7991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Yu, K.; Wang, H.; Chen, J.; Zhang, X. Stacking fault and partial dislocation dominated strengthening mechanisms in highly textured Cu/Co multilayers. Int. J. Plast. 2013, 49, 152–163. [Google Scholar] [CrossRef]
- Shao, S.; Wang, J. Relaxation, Structure, and Properties of Semicoherent Interfaces. JOM 2015, 68, 242–252. [Google Scholar] [CrossRef]
- Shao, S.; Akasheh, F.; Wang, J.; Liu, Y. Alternative misfit dislocations pattern in semi-coherent FCC {100} interfaces. Acta Mater. 2018, 144, 177–186. [Google Scholar] [CrossRef]
- Frenken, J.W.M.; van der Veen, J.F. Observation of Surface Melting. Phys. Rev. Lett. 1985, 54, 134–137. [Google Scholar] [CrossRef]
- van der Veen, J. Surface-Induced Melting of Solids. In Phase Transitions in Surface Films 2; Taub, H., Torzo, G., Lauter, H., Fain, S., Eds.; Springer: Boston, MA, USA, 1991; pp. 289–305. [Google Scholar]
- Eichenlaub, S.; Chan, C.; Beaudoin, S.P. Hamaker Constants in Integrated Circuit Metalization. J. Colloid Interface Sci. 2002, 248, 389–397. [Google Scholar] [CrossRef]
- Ferreira, I.L. On the Heat Capacity of Pure Elements and Phases. Mater. Res. 2021, 24, e20200529. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.; Liu, G.; Chen, J.; Wang, C.; Wang, H.; Wang, J.; Zhang, X. High Strength and Low Coercivity of Cobalt with Three-Dimensional Nanoscale Stacking Faults. Nano Lett. 2021, 21, 6480–6486. [Google Scholar] [CrossRef]
- Ananthakrishnan, G.; Surana, M.; Poss, M.; Yaacoub, J.J.; Zhang, K.; Admal, N.; Pochet, P.; Tawfick, S.; Johnson, H.T. Graphene-mediated stabilization of surface facets on metal substrates. J. Appl. Phys. 2021, 130, 165302. [Google Scholar] [CrossRef]
- Yang, K.; Tang, P.; Zhang, Q.; Ma, H.; Liu, E.; Li, M.; Zhang, X.; Li, J.; Liu, Y.; Fan, T.; et al. Enhanced defect annihilation capability of the graphene/copper interface: An In Situ study. Scr. Mater. 2021, 203, 114001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Yao, S.; Li, Q.; Ni, J.; Yan, Z.; Yang, K.; Liu, G.; Liu, Y.; Wang, J. Reorientation Mechanisms of Graphene Coated Copper {001} Surfaces. Metals 2023, 13, 910. https://doi.org/10.3390/met13050910
Song J, Yao S, Li Q, Ni J, Yan Z, Yang K, Liu G, Liu Y, Wang J. Reorientation Mechanisms of Graphene Coated Copper {001} Surfaces. Metals. 2023; 13(5):910. https://doi.org/10.3390/met13050910
Chicago/Turabian StyleSong, Jian, Songsong Yao, Quan Li, Jiamiao Ni, Zhuoxin Yan, Kunming Yang, Guisen Liu, Yue Liu, and Jian Wang. 2023. "Reorientation Mechanisms of Graphene Coated Copper {001} Surfaces" Metals 13, no. 5: 910. https://doi.org/10.3390/met13050910
APA StyleSong, J., Yao, S., Li, Q., Ni, J., Yan, Z., Yang, K., Liu, G., Liu, Y., & Wang, J. (2023). Reorientation Mechanisms of Graphene Coated Copper {001} Surfaces. Metals, 13(5), 910. https://doi.org/10.3390/met13050910










